Effects of Slow-Acting Metformin Treatment on the Hormonal and Morphological Thyroid Profile in Patients with Insulin Resistance
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Protocol
2.2. Patient Selection
2.3. Hormonal Measurements
2.4. Ultrasound Scan
2.5. Statistical Analysis
2.6. Ethical Approval
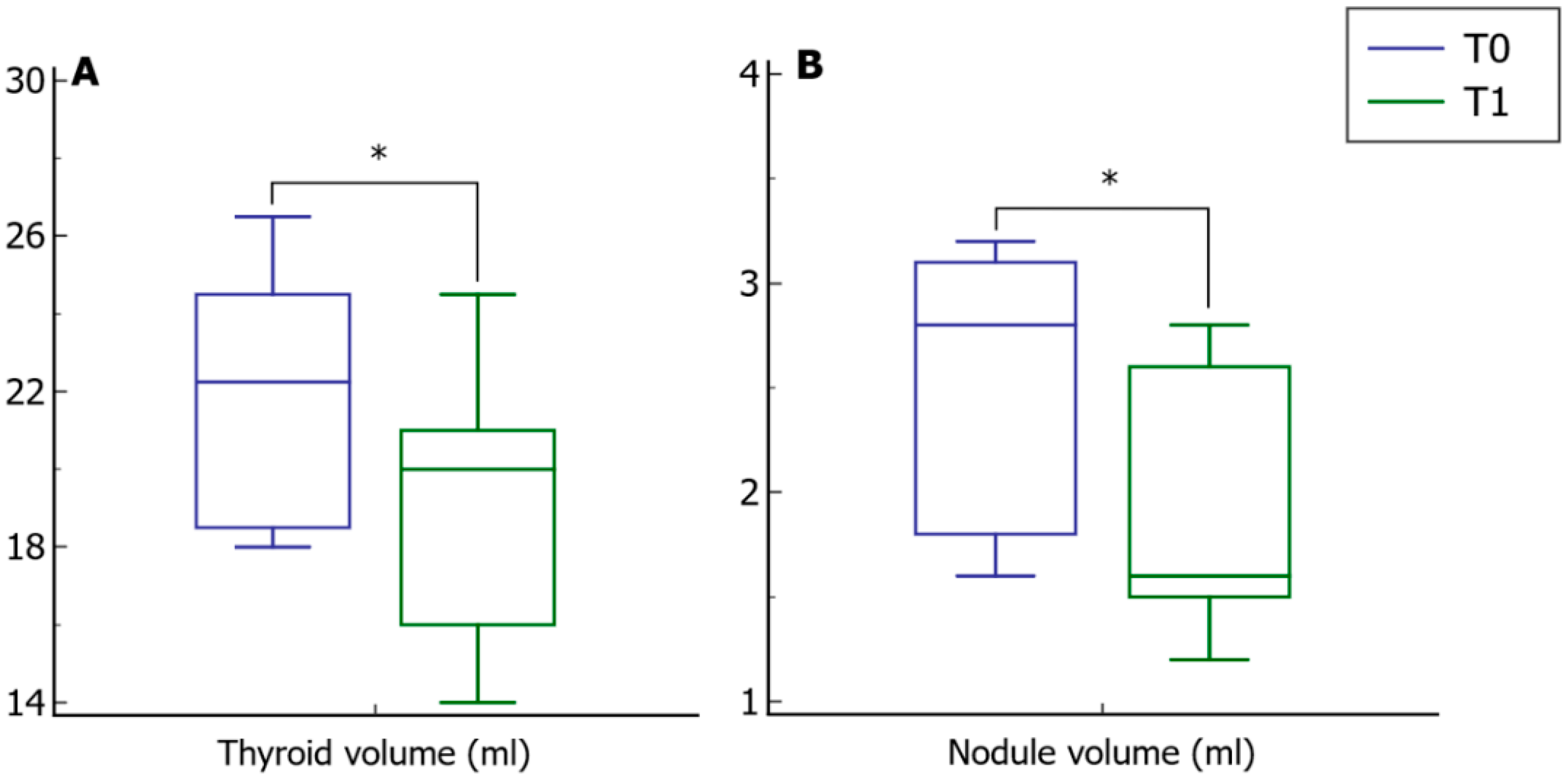
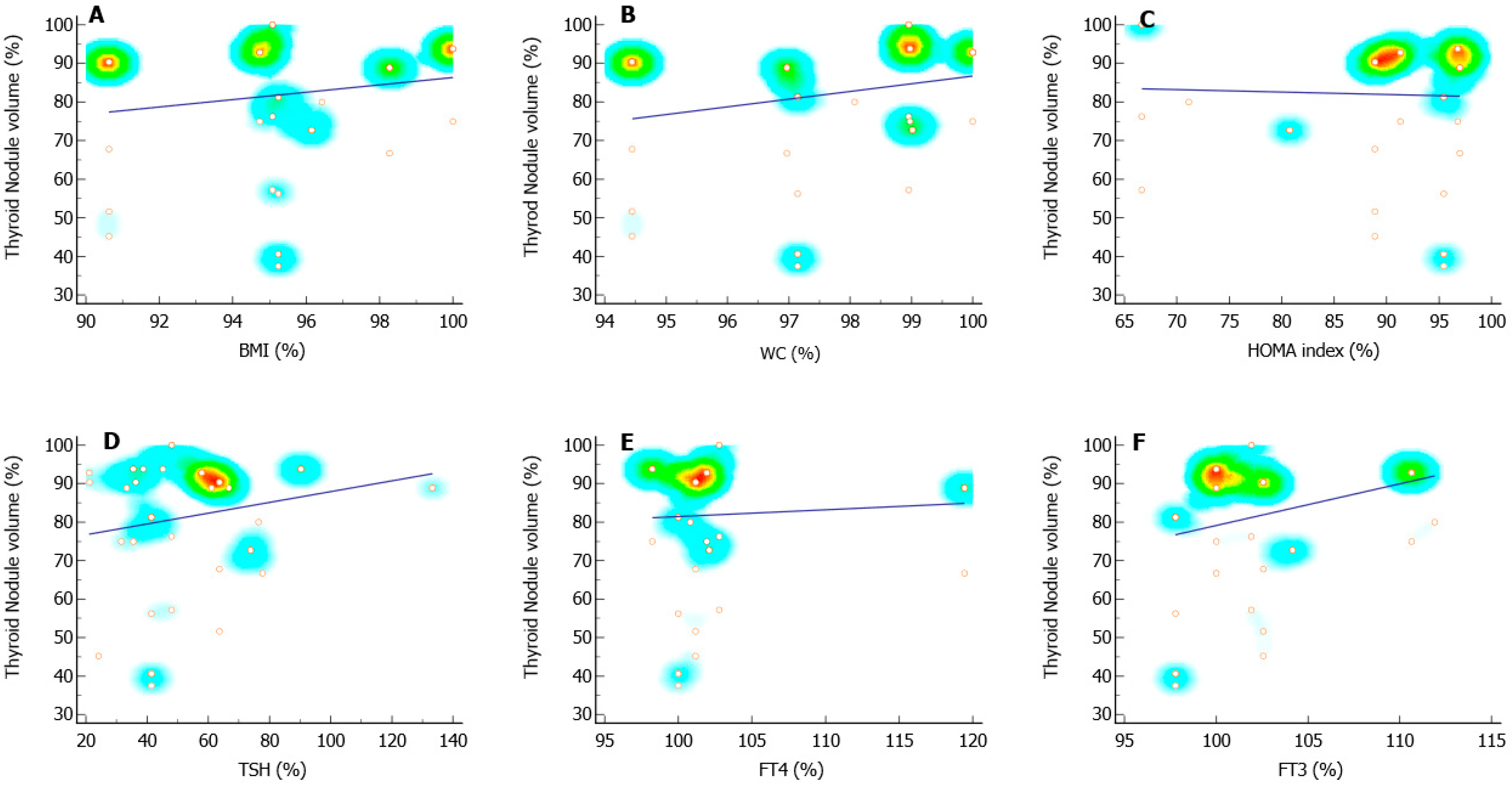
3. Results
3.1. Before and after Analysis
3.2. Correlation Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crafa, A.; Calogero, A.E.; Cannarella, R.; Mongioì, L.M.; Condorelli, R.A.; Greco, E.A.; Aversa, A.; La Vignera, S. The Burden of Hormonal Disorders: A Worldwide Overview with a Particular Look in Italy. Front. Endocrinol. 2021, 12, 694325. [Google Scholar] [CrossRef] [PubMed]
- Altomare, M.; La Vignera, S.; Asero, P.; Recupero, D.; Condorelli, R.A.; Scollo, P.; Gulisano, A.; Magro, E.; Tumino, D.; Tumino, S.; et al. High prevalence of thyroid dysfunction in pregnant women. J. Endocrinol. Investig. 2013, 36, 407–411. [Google Scholar]
- Chubb, S.A.; Davis, W.A.; Inman, Z.; Davis, T.M. Prevalence and progression of subclinical hypothyroidism in women with type 2 diabetes: The Fremantle Diabetes Study. Clin. Endocrinol. 2005, 62, 480–486. [Google Scholar] [CrossRef]
- Dowling, R.J.; Niraula, S.; Stambolic, V.; Goodwin, P.J. Metformin in cancer: Translational challenges. J. Mol. Endocrinol. 2012, 48, R31–R43. [Google Scholar] [CrossRef] [PubMed]
- Buczynska, A.; Sidorkiewicz, I.; Kretowski, A.J.; Zbucka-Kretowska, M.; Adamska, A. Metformin intervention—A panacea for cancer treatment? Cancers 2022, 14, 1336. [Google Scholar] [CrossRef] [PubMed]
- Vigersky, R.A.; Filmore-Nassar, A.; Glass, A.R. Thyrotropin suppression by metformin. J. Clin. Endocrinol. Metab. 2006, 91, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Al-Alusi, M.A.; Du, L.; Li, N.; Yeh, M.W.; He, X.; Braverman, L.E.; Leung, A.M. Metformin Does Not Suppress Serum Thyrotropin by Increasing Levothyroxine Absorption. Thyroid 2015, 25, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Haroon, S.M.; Khan, K.; Maqsood, M.; Iqbal, S.; Aleem, M.; Khan, T.U. Exploring the Effect of Metformin to Lower Thyroid-Stimulating Hormone in Euthyroid and Hypothyroid Type-2 Diabetic Patients. Cureus 2021, 13, e13283. [Google Scholar] [CrossRef] [PubMed]
- Isidro, M.L.; Penín, M.A.; Nemiña, R.; Cordido, F. Metformin reduces thyrotropin levels in obese, diabetic women with primary hypothyroidism on thyroxine replacement therapy. Endocrine 2007, 32, 79–82. [Google Scholar] [CrossRef]
- Cappelli, C.; Rotondi, M.; Pirola, I.; Agosti, B.; Gandossi, E.; Valentini, U.; De Martino, E.; Cimino, A.; Chiovato, L.; Agabiti-Rosei, E.; et al. TSH-lowering effect of metformin in type 2 diabetic patients: Differences between euthyroid, untreated hypothyroid, and euthyroid on L-T4 therapy patients. Diabetes Care 2009, 32, 1589–1590. [Google Scholar] [CrossRef]
- Rotondi, M.; Cappelli, C.; Magri, F.; Botta, R.; Dionisio, R.; Iacobello, C.; De Cata, P.; Nappi, R.E.; Castellano, M.; Chiovato, L. Thyroidal effect of metformin treatment in patients with polycystic ovary syndrome. Clin. Endocrinol. 2011, 75, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Morteza Taghavi, S.; Rokni, H.; Fatemi, S. Metformin decreases thyrotropin in overweight women with polycystic ovarian syndrome and hypothyroidism. Diab. Vasc. Dis. Res. 2011, 8, 47–48. [Google Scholar] [CrossRef] [PubMed]
- Severo, M.D.; Andrade, T.S.; Correa, V., Jr.; Naujorks, A.A.; Gus, M.; Schaan, B.D. Metformin effect on TSH in subclinical hypothyroidism: Randomized, double-blind, placebo-controlled clinical trial. Endocrine 2018, 59, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, C.; Rotondi, M.; Pirola, I.; Agosti, B.; Formenti, A.; Zarra, E.; Valentini, U.; Leporati, P.; Chiovato, L.; Castelano, M. Thyreotropin levels in diabetic patients on metformin treatment. Eur. J. Endocrinol. 2012, 167, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Palui, R.; Sahoo, J.; Kamalanathan, S.; Kar, S.S.; Sridharan, K.; Durgia, H.; Raj, H.; Patil, M. Effects of metformin on thyroid function tests in patients with subclinical hypothyroidism: An open-label randomized controlled trial. J. Endocrinol. Investig. 2019, 42, 1451–1458. [Google Scholar] [CrossRef]
- Lupoli, R.; Di Minno, A.; Tortora, A.; Ambrosino, P.; Lupoli Gelsy, A.; Di Minno, M.N.D. Effects of treatment with metformin on TSH levels: A meta-analysis of literature studies. J. Clin. Endocrinol. Metab. 2014, 99, E143–E148. [Google Scholar] [CrossRef]
- Santos-Palacios, S.; Brugos-Larumbe, A.; Guilln-Grima, F.; Garmendia-Madariaga, A.; Galofré, J.C. Does metformin have a “buffer effect” on serum TSH levels in euthyroid diabetic patients? Hormones 2015, 14, 280–285. [Google Scholar] [CrossRef]
- Karimifar, M.; Aminorroaya, A.; Amini, M.; Mirfendereski, T.; Iraj, B.; Feizi, A.; Norozi, A. Effect of metformin on thyroid stimulating hormone and thyroid volume in patients with prediabetes: A randomized placebo-controlled clinical trial. J. Res. Med. Sci. 2014, 19, 1019–1026. [Google Scholar]
- Krysiak, R.; Szkrobka, W.; Okopien, B. The effect of metformin on the hypothalamic-pituitary-thyroid axis in patients with type 2 diabetes and subclinical hyperthyroidism. Exp. Clin. Endocrinol. Diabetes 2015, 123, 205–208. [Google Scholar] [CrossRef]
- Anil, C.; Kut, A.; Atesagaoglu, B.; Nar, A.; Bascil Tutuncu, N.; Gursoy, A. Metformin Decreases Thyroid Volume and Nodule Size in Subjects with Insulin Resistance: A Preliminary Study. Med. Princ. Pract. 2016, 25, 233–236. [Google Scholar] [CrossRef]
- Oleandri, S.E.; Maccario, M.; Rossetto, R.; Procopio, M.; Grottoli, S.; Avogadri, E.; Gauna, C.; Ganzaroli, C.; Ghigo, E. Three-month treatment with metformin or dexfenfluramine does not modify the effects of diet on anthropometric and endocrine-metabolic parameters in abdominal obesity. J. Endocrinol. Investig. 1999, 22, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Rezzónico, J.; Rezzónico, M.; Pusiol, E.; Pitoia, F.; Niepomniszcze, H. Metformin treatment for small benign thyroid nodules in patients with insulin resistance. Metab. Syndr. Relat. Disord. 2011, 9, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, N.; Laurberg, P.; Rasmussen, L.B.; Bülow, I.; Perrild, H.; Ovesen, L.; Jørgensen, T. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J. Clin. Endocrinol. Metab. 2005, 90, 4019–4024. [Google Scholar] [CrossRef] [PubMed]
- Kok, P.; Roelfsema, F.; Langendonk, J.G.; Frölich, M.; Burggraaf, J.; Meinders, A.E.; Pijl, H. High circulating thyrotropin levels in obese women are reduced after body weight loss induced by caloric restriction. J. Clin. Endocrinol. Metab. 2005, 90, 4659–4663. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Paganelli, M.; Morabito, A.; Vedani, P.; Barbieri, M.; Paolisso, G.; Folli, F.; Pontiroli, A.E. Weight loss through gastric banding: Effects on TSH and thyroid hormones in obese subjects with normal thyroid function. Obesity 2010, 18, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Fournier, J.P.; Yin, H.; Yu, O.H.; Azoulay, L. Metformin and low levels of thyroid-stimulating hormone in patients with type 2 diabetes mellitus. CMAJ 2014, 186, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, J.; Fan, Q.; Li, H.; Di, Y. The Effect of Metformin on Thyroid-Associated Serum Hormone Levels and Physiological Indexes: A Meta-Analysis. Curr. Pharm. Des. 2019, 25, 3257–3265. [Google Scholar] [CrossRef]
- Díez, J.J.; Iglesias, P. Relationship between serum thyrotropin concentrations and metformin therapy in euthyroid patients with type 2 diabetes. Clin. Endocrinol. 2013, 78, 505–511. [Google Scholar] [CrossRef]
- Dimic, D.; Golubovic, M.V.; Radenkovic, S.; Radojkovic, D.; Pesic, M. The effect of metformin on TSH levels in euthyroid and hypothyroid newly diagnosed diabetes mellitus type 2 patients. Bratisl. Lek. Listy 2016, 117, 433–435. [Google Scholar] [CrossRef]
- Blanc, E.; Ponce, C.; Brodschi, D.; Nepote, A.; Barreto, A.; Schnitman, M.; Fossati, P.; Salgado, P.; Cejas, C.; Faingold, C.; et al. Association between worse metabolic control and increased thyroid volume and nodular disease in elderly adults with metabolic syndrome. Metab. Syndr. Relat. Disord. 2015, 13, 221–226. [Google Scholar] [CrossRef]
- Răcătăianu, N.; Bolboacă, S.D.; Sitar-Tăut, A.V.; Mârza, S.; Moga, D.; Valea, A.; Ghervan, C. The effect of Metformin treatment in obese insulin-resistant patients with euthyroid goiter. Acta Clin. Belg. 2018, 73, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ayturk, S.; Gursoy, A.; Kut, A.; Anil, C.; Nar, A.; Bascil Tutuncu, N. Metabolic syndrome and its components are associated with increased thyroid volume and nodule prevalence in a mild-to-moderate iodine-deficient area. Eur. J. Endocrinol. 2009, 161, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Van Keymeulen, A.; Golstein, J.; Fusco, A.; Dumont, J.E.; Roger, P.P. Regulation of thyroid cell proliferation by TSH and other factors: A critical evaluation of in vitro models. Endocr. Rev. 2001, 22, 631–656. [Google Scholar] [CrossRef]
- Dowling, R.J.; Goodwin, P.J.; Stambolic, V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011, 9, 33. [Google Scholar] [CrossRef]
- Dos Santos, P.B.; Gertrudes, L.N.; Conceição, F.L.; de Andrade, B.M.; de Carvalho, D.P.; Vaisman, M.; Teixeira, P.F.D.S. Effects of Metformin on TSH Levels and Benign Nodular Goiter Volume in Patients Without Insulin Resistance or Iodine Insufficiency. Front. Endocrinol. 2019, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Okopien, B. The effect of metformin on the hypothalamic-pituitary-thyroid axis in women with polycystic ovary syndrome and subclinical hypothyroidism. J. Clin. Pharmacol. 2015, 55, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Okopien, B. Thyrotropin-lowering effect of metformin in a patient with resistance to thyroid hormone. Clin. Endocrinol. 2011, 75, 404–406. [Google Scholar] [CrossRef]
- Łabuzek, K.; Suchy, D.; Gabryel, B.; Bielecka, A.; Liber, S.; Okopień, B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010, 62, 956–965. [Google Scholar] [CrossRef]
- Duntas, L.H.; Orgiazzi, J.; Brabant, G. The interface between thyroid and diabetes mellitus. Clin. Endocrinol. 2011, 75, 1–9. [Google Scholar] [CrossRef]
- Alevizaki, M. Metformin and the thyroid: Some questions still remain. Clin. Endocrinol. 2013, 78, 503–504. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkrobka, W.; Okopien, B. The Effect of Metformin on Hypothalamic-Pituitary-Thyroid Axis Activity in Women with Interferon-Induced Hypothyroidism: A Pilot Study. Exp. Clin. Endocrinol. Diabetes 2016, 124, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Gilowska, M.; Szkróbka, W.; Okopień, B. The effect of metformin on the hypothalamic-pituitary-thyroid axis in patients with type 2 diabetes and amiodarone-induced hypothyroidism. Pharmacol. Rep. 2016, 68, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Klubo-Gwiezdzinska, J.; Costello, J., Jr.; Patel, A.; Bauer, A.; Jensen, K.; Mete, M.; Burman, K.D.; Wartofsky, L.; Vasko, V. Treatment with metformin is associated with higher remission rate in diabetic patients with thyroid cancer. J. Clin. Endocrinol. Metab. 2013, 98, 3269–3279. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.K.; Kim, W.G.; Kwon, H.; Choi, Y.M.; Jeon, M.J.; Kim, T.Y.; Shong, Y.K.; Kim, W.B.; Kim, E.Y. Metformin Is Associated with a Favorable Outcome in Diabetic Patients with Cervical Lymph Node Metastasis of Differentiated Thyroid Cancer. Eur. Thyroid J. 2015, 4, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xu, S.; Renko, K.; Derwahl, M. Metformin inhibits growth of thyroid carcinoma cells, suppresses self-renewal of derived cancer stem cells, and potentiates the effect of chemotherapeutic agents. J. Clin. Endocrinol. Metab. 2012, 97, E510–E520. [Google Scholar] [CrossRef] [PubMed]


| Overall Cohort | Male | Female | |
|---|---|---|---|
| n | 50 | 10 | 40 |
| Age (years) | 36.9 ± 12.8 | 30.5 ± 12.1 | 38.5 ± 12.6 |
| BMI (Kg/m2) | 30.6 ± 2.0 | 30.3 ± 1.8 | 30.7 ± 2.1 |
| WC (cm) | 102.1 ± 4.1 | 105.0 ± 3.2 | 101.4 ± 4.1 |
| HOMA index | 4.5 ± 1.2 | 5.5 ± 0.9 | 4.2 ± 1.1 |
| TSH (µIU/mL) | 2.9 ± 0.6 | 3.6 ± 0.3 | 2.8 ± 0.6 |
| FT4 (pmol/L) | 10.1 ± 1.8 | 9.5 ± 1.0 | 10.2 ± 1.9 |
| FT3 (pmol/L) | 4.7 ± 0.6 | 4.3 ± 0.4 | 4.9 ± 0.6 |
| Thyroid volume (mL) | 22.2 ± 2.8 | 21.3 ± 3.4 | 22.4 ± 2.6 |
| Nodule volume (mL) | 2.5 ± 0.6 | 3.0 ± 0.6 | 2.3 ± 0.6 |
| Independent Variables | Coeff. | Std. Error | t | p | rpartial | rsemipartial | VIF | |
|---|---|---|---|---|---|---|---|---|
| (Constant) | 14.7623 | |||||||
| Ratio (%) | BMI | −0.5055 | 0.6815 | −0.742 | 0.4623 | −0.1124 | 0.08816 | 7.013 |
| WC | 1.7123 | 1.0770 | 1.590 | 0.1192 | 0.2356 | 0.1890 | 6.757 | |
| HOMA index | 0.2338 | 0.1068 | 2.188 | 0.0341 | 0.3165 | 0.2601 | 1.476 | |
| TSH | 0.1062 | 0.04047 | 2.625 | 0.0119 | 0.3716 | 0.3120 | 1.365 | |
| FT4 | −0.5227 | 0.1531 | −3.414 | 0.0014 | −0.4618 | 0.4057 | 1.382 | |
| FT3 | −0.2007 | 0.3421 | −0.587 | 0.5604 | −0.08913 | 0.06974 | 3.114 | |
| (Constant) | 0.6420 | |||||||
| Difference (Δ) | BMI | −0.8474 | 0.6184 | −1.370 | 0.1777 | −0.2045 | 0.1569 | 9.362 |
| WC | 0.6245 | 0.2729 | 2.288 | 0.0271 | 0.3295 | 0.2621 | 8.017 | |
| HOMA index | 1.1012 | 0.7080 | 1.555 | 0.1272 | 0.2308 | 0.1782 | 1.939 | |
| TSH | 0.7785 | 0.3806 | 2.045 | 0.0470 | 0.2978 | 0.2343 | 1.880 | |
| FT4 | −1.9855 | 0.5246 | −3.785 | 0.0005 | −0.4999 | 0.4335 | 1.373 | |
| FT3 | −0.5183 | 1.7888 | −0.290 | 0.7734 | −0.04415 | 0.03319 | 2.860 |
| Independent Variables | Coeff. | Std. Error | t | p | rpartial | rsemipartial | VIF | |
|---|---|---|---|---|---|---|---|---|
| (Constant) | −96.9390 | |||||||
| Ratio (%) | BMI | 2.9434 | 1.8960 | 1.552 | 0.1279 | 0.2304 | 0.2135 | 7.013 |
| WC | −2.9136 | 2.9963 | −0.972 | 0.3363 | −0.1467 | 0.1337 | 6.757 | |
| HOMA index | −0.1986 | 0.2972 | −0.668 | 0.5075 | −0.1014 | 0.09191 | 1.476 | |
| TSH | 0.07272 | 0.1126 | 0.646 | 0.5218 | 0.09802 | 0.08882 | 1.365 | |
| FT4 | −0.1454 | 0.4260 | −0.341 | 0.7345 | −0.05198 | 0.04693 | 1.382 | |
| FT3 | 2.0563 | 0.9516 | 2.161 | 0.0363 | 0.3130 | 0.2972 | 3.114 | |
| (Constant) | 0.4233 | |||||||
| Difference (Δ) | BMI | 0.4208 | 0.2032 | 2.070 | 0.0445 | 0.3011 | 0.2698 | 9.362 |
| WC | −0.2482 | 0.2327 | −1.067 | 0.2921 | −0.1606 | 0.1390 | 1.939 | |
| HOMA index | −0.1214 | 0.08968 | −1.354 | 0.1828 | −0.2022 | 0.1765 | 8.017 | |
| TSH | 0.05472 | 0.1251 | 0.437 | 0.6640 | 0.06656 | 0.05702 | 1.880 | |
| FT4 | −0.02535 | 0.1724 | −0.147 | 0.8838 | −0.02242 | 0.01917 | 1.373 | |
| FT3 | 1.5362 | 0.5879 | 2.613 | 0.0123 | 0.3702 | 0.3406 | 2.860 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Condorelli, R.A.; Cannarella, R.; Aversa, A.; Basile, L.; Avola, O.; Calogero, A.E.; La Vignera, S. Effects of Slow-Acting Metformin Treatment on the Hormonal and Morphological Thyroid Profile in Patients with Insulin Resistance. Pharmaceutics 2022, 14, 1987. https://doi.org/10.3390/pharmaceutics14101987
Condorelli RA, Cannarella R, Aversa A, Basile L, Avola O, Calogero AE, La Vignera S. Effects of Slow-Acting Metformin Treatment on the Hormonal and Morphological Thyroid Profile in Patients with Insulin Resistance. Pharmaceutics. 2022; 14(10):1987. https://doi.org/10.3390/pharmaceutics14101987
Chicago/Turabian StyleCondorelli, Rosita A., Rossella Cannarella, Antonio Aversa, Livia Basile, Ottavia Avola, Aldo E. Calogero, and Sandro La Vignera. 2022. "Effects of Slow-Acting Metformin Treatment on the Hormonal and Morphological Thyroid Profile in Patients with Insulin Resistance" Pharmaceutics 14, no. 10: 1987. https://doi.org/10.3390/pharmaceutics14101987
APA StyleCondorelli, R. A., Cannarella, R., Aversa, A., Basile, L., Avola, O., Calogero, A. E., & La Vignera, S. (2022). Effects of Slow-Acting Metformin Treatment on the Hormonal and Morphological Thyroid Profile in Patients with Insulin Resistance. Pharmaceutics, 14(10), 1987. https://doi.org/10.3390/pharmaceutics14101987











