Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a highly fatal malignancy that has the worst 5-year survival rate of all of the common malignant tumors. Surgery, chemotherapy, and/or chemoradiation remain the main tactics for PDAC treatment. The efficacy of chemotherapy is often compromised because of the substantial risk of severe toxicities. In our study, we focused on identification of polymorphisms in the genes involved in drug metabolism, DNA repair and replication that are associated with inter-individual differences in drug-induced toxicities. Using the microarray, we genotyped 12 polymorphisms in the DPYD, XPC, GSTP1, MTHFR, ERCC1, UGT1A1, and TYMS genes in 78 PDAC patients treated with FOLFIRINOX. It was found that the TYMS rs11280056 polymorphism (6 bp-deletion in TYMS 3′-UTR) predicted grade 1–2 neurotoxicity (p = 0.0072 and p = 0.0019, according to co-dominant (CDM) and recessive model (RM), respectively). It is the first report on the association between TYMS rs11280056 and peripheral neuropathy. We also found that PDAC patients carrying the GSTP1 rs1695 GG genotype had a decreased risk for grade 3–4 hematological toxicity as compared to those with the AA or AG genotypes (p = 0.032 and p = 0.014, CDM and RM, respectively). Due to relatively high p-values, we consider that the impact of GSTP1 rs1695 requires further investigation in a larger sample size.
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly fatal malignancy that has the worst 5-year survival rate of all of the common malignant tumors [1,2]. Most patients with PDAC are asymptomatic until the disease reaches an advanced, and often unresectable, stage. Less than one-third of patients are only candidates for surgical resection at the time of their diagnosis, and surgery, chemotherapy, and/or chemoradiation remain the main tactics for treatment [3]. Despite progress in surgical procedures, the tumor recurrence rate exceeds 60% after radical surgical resection, and patients are further managed by chemotherapy [4,5].
Adjuvant chemotherapy may significantly improve disease-free survival (DFS), progression-free survival (PFS), and overall survival (OS). The current standard duration of adjuvant chemotherapy for PDAC is six months. A combination therapy of FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan, and oxaliplatin) has become one of the most common regimen for both (neo)adjuvant and advanced pancreatic cancer treatment [6,7]. In the neoadjuvant setting, the FOLFIRINOX regimen administered with or without radiotherapy was associated with improved tumor down-staging, converting initially unresectable tumors into successful resections with R0 margins in borderline resectable and locally advanced PDAC patients [8,9].
The efficacy of chemotherapy is often compromised because of the substantial risk for severe toxicities. Drug-induced adverse events (AEs) are responsible for treatment delay, reduction, cessation, or even the death of a patient. In particular, the FOLFIRINOX group has increased rates of grade 3–4 toxicities, especially neutropenia, diarrhea, and peripheral neuropathy [10].
Although the majority of cases of severe toxicity remain unexplained, a large subset of these AEs is associated with polymorphisms in metabolic, DNA replication and repair genes that are responsible for the differences in inter-individual drug response and toxicity. The FDA provides specific information regarding therapeutic management for some pharmacogenetic associations. However, most of the polymorphic variations have not been sufficiently evaluated in terms of the impact of genetic testing on therapeutic effectiveness or increased risk of specific AEs [11].
In the present work, we focused on the identification of polymorphisms in the genes involved in drug metabolism, DNA repair and replication that are associated with a substantial rate of toxicities. We genotyped 12 polymorphisms (DPYD rs3918290, rs75017182, rs55886062, rs67376798, rs2297595, XPC rs2228001, GSTP1 rs1695, MTHFR rs1801133, ERCC1 rs3212986, rs11615, UGT1A1 rs3064744, and TYMS rs11280056) using microarray-based analysis in a cohort of PDAC patients treated with FOLFIRINOX.
It is crucial to be aware of genetic biomarkers that are predictive or prognostic with respect to drug-induced toxicity, since it may aid in treatment selection and allow clinicians to improve treatment outcome for every individual patient.
2. Materials and Methods
2.1. Patients
A total of 78 patients affected by pancreatic ductal adenocarcinoma and treated at the N. N. Blokhin Cancer Research Center (Moscow) with FOLFIRINOX between 2019 and 2020 were enrolled in the study. Main inclusion criteria were: PDAC histology, treatment with FOLFIRINOX regimen, availability of data regarding toxicity, and availability of blood samples.
All patients started their treatment with standard dosage. Dose reductions were made according to local practice. In general, any grade 3–4 adverse events and grade 1–2 neurotoxicity that caused a delay of the next cycle resulted in approximately 20% dose reduction for subsequent cycles. Patient characteristics are shown in Table 1.

Table 1.
Clinical information of PDAC patients.
The research was carried out in accordance with the Declaration of Helsinki and followed Good Clinical Practice guidelines. Approval of the study was obtained from the Ethics Committee of N. N. Blokhin Cancer Research Center in Moscow (Protocol code No 5, Date of approval: 27 May 2019). All subjects were required to read, understand, and sign an IRB-approved informed consent form. Laboratory investigators were blinded to the clinical information on patients’ data.
2.2. Assessment and Management of Chemotherapy Toxicity
A number of hematologic toxicities (anemia, leukopenia, neutropenia, and thrombocytopenia) and non-hematologic toxicities (asthenia, diarrhea, mucositis, nausea, stomatitis, vomiting, hepatic, skin, and neurotoxicity) were ascertained at the beginning of each chemotherapy cycle using Common Toxicity Criteria for Adverse Events (CTCAE), version 5.0 [12]. During the course of the treatment, all AEs were monitored, and maximum toxicity grade was reported.
2.3. Clinical Specimens and DNA Isolation
Between 3 and 5 mL of venous blood was collected in EDTA-containing tubes. The samples were stored at +4 °C during 3–10 days before DNA isolation. A QIAamp DNA Micro Kit (Qiagen, Hilden, Germany) was used to isolate DNA, according to the manufacturer’s instructions.
2.4. Control Samples
A total of 38 DNA samples with known genotypes were used as controls to verify the specificity of the microarray interrogating probes. The control sample panel included WT DNA samples as well as DNA samples harboring all corresponding variations to be analyzed. The control samples had been extracted from the whole blood earlier and had undergone preliminary genotype identification by Sanger sequencing.
2.5. Sequencing
Sequencing was performed on the Applied Biosystems 3730 DNA Analyzer (Applied Biosystems, Carlsbad, CA, USA), using the ABI PRISM® BigDye™ Terminator v. 3.1 Kit (Applied Biosystems).
2.6. Oligonucleotide Probes, Primers, and Microarray Fabrication
Oligonucleotide probes for immobilization on a microarray support and PCR primers for multiplex amplification were synthesized using an automated 394 DNA/RNA synthesizer (Applied Biosystems). Oligonucleotides were designed with Oligo v. 6.31 (Molecular Biology Insights, Cascade, CO, USA) and BioEdit v. 7.09 software (Ibis Biosciences, Carlsbad, CA, USA). The oligonucleotide probes had a spacer with a free amino group 3′-Amino-Modifier C6 CPG 500 (Glen Research, Sterling, VA, USA) for subsequent copolymerization with microarray hydrogel components. The LNA residues (Qiagen, Hilden, Germany) were incorporated into UGT1A1 genotyping probes to increase the specificity of hybridization analysis [13]. The nucleotide sequences of the probes and primers are shown in Tables S1 and S2, respectively. Biological microarrays were manufactured by the procedure described in detail earlier [14]. Each gel pad was in triplicate to improve the reliability of the analysis.
2.7. Genotyping of DNA Samples by Hybridization on Microarrays
DNA samples for hybridization on the microarray were prepared using two-stage asymmetric multiplex PCR. The reaction mixture contained 0.25 mM each of dATP, dCTP, dGTP, 0.125 mM of dUTP and dTTP (Evrogene, Moscow, Russia), 6.6 μM of Sulfo-Cyanine 5 dUTP (Lumiprobe, Moscow, Russia), 2.5 U Taq DNA polymerase (Qiagen, Hilden, Germany), 1 μL of primer mix containing 1 pmol/μL of each specific primer, 1 pmol/μL forward adapter primer and 100 pmol/μL reverse adapter primer, 3.3 μL 10 × PCR Buffer (Qiagen), 5.1 mM MgCl2 (Qiagen), and at least 10 ng of genomic DNA in a final volume of 33 μL. Amplification was performed on a C1000 Touch Thermal Cycler (BioRad, Hercules, CA, USA). The reaction mixture was incubated at 95 °C for 10 min followed by 25 cycles of 30 s at 95 °C, 70 °C, and 72 °C at the first PCR stage and then 49 cycles of 30 s at 95 °C, 54 °C, and 72 °C at the second PCR stage. Finally, the mixture was incubated for 5 min at 72 °C.
The mixture for hybridization analysis contained 33 μL of the PCR reaction mixture and 9 μL of a solution containing 4 M guanidine thiocyanate, 200 mM HEPES, and 20 mM EDTA (Sigma-Aldrich, St. Louis, MO, USA), pH 7.5. The resulting mixture was infused into a microarray chamber and incubated for 6 h at 37 °C. After hybridization, the microarray was washed twice with distilled water for 30 s at 37 °C and then dried.
2.8. Image Acquisition and Processing
Hybridization images were acquired and processed using a fluorescence analyzer setup with specialized software “ImaGel Studio” (Biochip-IMB, Moscow, Russia). To evaluate the discriminating ratio of hybridization probes, fluorescence signal intensities were acquired in each gel pad and processed using the software. The fluorescence signals produced by the microarray gel elements were used as the input data as follows: Jm = (Im − I0)/(Bm − I0), where Im was the fluorescence signal intensity per unit area in the internal part of a gel element, Bm was the counterpart background intensity, I0 was the dark current in the charge-coupled device (CCD), and m was the gel element number. As every gel pad was in triplicate, the signal intensities were averaged.
Identification of single nucleotide variation (SNV) and deletions was based on differences in fluorescence signals acquired from gel pads bearing immobilized oligonucleotide probes to the major and minor allele variants. If the target contains any polymorphisms, a corresponding gel pad in which a perfect hybridization duplex has formed results in a significant increase in its fluorescence intensity.
2.9. Validation of the Microarray
The specificity of microarray genotyping was verified by comparative analysis with Sanger sequencing. The interrogating oligonucleotide probes corresponding to every allele variant were validated by hybridization of pre-sequenced samples. To be sure that signal intensity ratios are reproducible from one assay to another, each unique control genotype was analyzed three times.
2.10. Statistical Analysis
We classified the patients enrolled in the study into three genotype groups: individuals with the more frequent homozygous or a major genotype (AA), the heterozygous genotype (Aa), and the minor homozygous genotype (aa). The influence of each genotype on endpoints was estimated in accordance with three genetic models: (i) the co-dominant model, in which every effect of Aa and aa genotypes compared to AA were estimated; (ii) the dominant model, which assumes that the presence of one or two minor alleles has an equal effect, therefore, patients with Aa and aa variants were grouped together and compared to patients with the AA genotype; (iii) the recessive model, which supposes that the only one minor allele does not affect the clinical endpoints significantly. This model was used to estimate the effect of the aa genotype compared to Aa or AA genotypes pooled together. Testing for Hardy-Weinberg equilibrium was applied for detecting genotyping errors.
Two-sided Pearson’s χ2 tests and Cochran-Armitage trend tests were used to determine correlations between the qualitative characteristics. IBM SPSS Statistics software, version 26 (IBM Corp., Armonk, NY, USA) was used to perform statistical analysis.
3. Results
3.1. Design of Multiplex PCR
A single tube asymmetric multiplex PCR was developed for simultaneous amplification of 12 target sequences of DPYD rs3918290, rs75017182, rs55886062, rs67376798, rs2297595, XPC rs2228001, GSTP1 rs1695, MTHFR rs1801133, ERCC1 rs3212986, rs11615, UGT1A1 rs3064744, and TYMS rs11280056. Primers containing specific and universal sequences complimentary to adapter primers were designed for target amplification on the first PCR stage (Table S1). The universal sequence part of forward and reverse primers was different. In the first PCR stage, targets of interest were amplified in a standard exponential manner, and the resulting PCR products contained universal sequences at their 5′ and 3′-ends. Shorter universal adapter primers were used in the second asymmetric PCR stage. At that stage, the annealing temperature was lower, and the concentration of universal reverse primers was significantly higher (see Materials and Methods). Due to this methodical approach, the reaction yielded predominantly single-stranded and fluorescently labeled amplification products suitable for subsequent hybridization analysis.
3.2. Microarray for Genotyping Analysis
A microarray for the simultaneous identification of DPYD rs3918290, rs75017182, rs55886062, rs67376798, rs2297595, XPC rs2228001, GSTP1 rs1695, MTHFR rs1801133, ERCC1 rs3212986, rs11615, UGT1A1 rs3064744, and TYMS rs11280056 was developed, validated, and applied for genotyping PDAC patients’ DNA samples. The genes and polymorphisms were selected as being potentially predictive of 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin toxicity and were analyzed according to probable biological function of the genes as well as the clinical annotations reported in the PharmGKB database (www.pharmgkb.org (accessed on 17 September 2021)).
The genetic variations and genotypes analyzed by the microarray are listed in Table 2.

Table 2.
Genes, genetic variations, genotype, and allele frequencies in PDAC patients (n = 78).
The microarray contained three identical gel pads for each polymorphic allele and six gel pads containing no probes to estimate background fluorescence signals. The microarray layout and an example of a hybridization image are shown in Figure 1. The gel pads were arranged in six vertical columns. The first three columns contained probes specific to the major alleles, and the last three included probes to the minor allele variants. Horizontal rows corresponded to the individual polymorphisms.
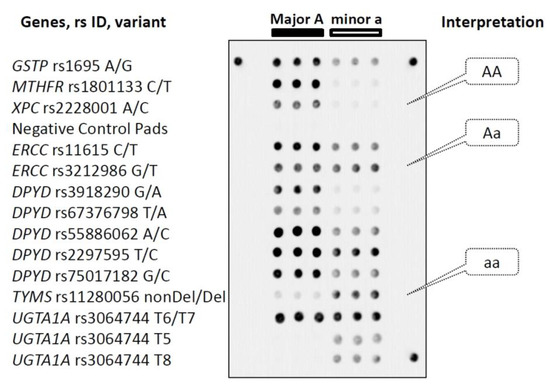
Figure 1.
Layout of the genotyping microarray and an example of interpretation of hybridization images. Every horizontal row of gel elements corresponds to the gene variant indicated on the left. Each gel pad harboring a unique interrogating oligonucleotide probe is in triplicate. Gel elements corresponding to the major (A) and minor (a) alleles are arranged in triple left and right columns, respectively. Illustration of identification of homo- and heterozygote genotypes is in the right callouts. Three peripheral gel spots are positioning markers. See the text for details.
Genotyping analysis performed on the microarray was based on differences in fluorescence signals acquired from gel pads relevant to the corresponding target polymorphism. The difference occurs because the fluorescently labeled amplification products form perfect hybridization duplexes only with probes that are fully complementary to the target sequences. Otherwise, if the target contains minor polymorphisms (SNP, deletion), a perfect duplex turns into an imperfect duplex, resulting in a significant decrease in the intensity of the corresponding fluorescence signal. The specificity of polymorphism identification by the interrogating oligonucleotide probes was verified by hybridization of pre-sequenced samples. The control DNA samples harboring all allele variations were analyzed by hybridization. Relevant fluorescence signals corresponding to all target polymorphisms were observed for every control sample that confirmed the ability of the developed technique to identify the genetic variants accurately.
3.3. Genotyping of Clinical Samples
A total of 78 DNA samples isolated from PDAC patients’ blood that were treated with first-line FOLFIRINOX were genotyped with the microarray-based technique described above. Genotyping was aimed at identification of 12 polymorphisms (SNV and deletions) in 7 genes: DPYD (rs3918290, rs75017182, rs55886062, rs67376798, and rs2297595); XPC (rs2228001); GSTP1 (rs1695); MTHFR (rs1801133); ERCC1 (rs3212986 and rs11615); UGT1A1 (rs3064744); and TYMS (rs11280056). A complete set of associations between each polymorphism and adverse events were considered. In particularly, hematological toxicity included anemia, leukopenia, neutropenia, and thrombocytopenia; gastrointestinal toxicity included diarrhea, vomiting, nausea, stomatitis, and mucositis; neurological toxicity included ototoxicity, central neurotoxicity, and peripheral sensory/motor polyneuropathy. Skin and hepatic toxicities were also monitored. Some other patients’ clinical indicators including age, gender, cancer staging, comorbidity, and specific markers (carcinoembryonic (CEA) and carbohydrate (CA19-9) antigens) were taken into consideration.
3.3.1. Neurological Toxicity
Among 78 PDAC patients administered with FOLFIRINOX chemotherapy, neurological toxicity occurred in 25 (32%) patients (Table 3). In all cases, neurotoxicity manifested as peripheral neuropathy.

Table 3.
TYMS rs11280056 and GSTP1 rs1695 genotype frequencies identified in PDAC patients treated with FOLFIRINOX (n = 78).
It was found that the allele 6 bp-deletion of the thymidylate synthase gene (TYMS rs11280056) predicted grade 1–2 neurological toxicity in advanced PDAC patients (p = 0.0072 and p = 0.0019, according to co-dominant and recessive model, respectively).
The homozygous TYMS rs11280056 6 bp-deletion genotype was identified in 6/25 (24%) patients and the heterozygous genotype was observed in 10/25 (40%) patients with neurological toxicity. The heterozygous TYMS rs11280056 genotype was also identified in 24/53 (45%) patients who did not have any AEs including neurological toxicity.
It was reported that one PDAC patient carrying the homozygous TYMS rs11280056 6 bp-deletion genotype had no drug–induced neurological toxicity. During retrospective analysis, we found that the patient had received only three cycles of therapy because of tumor progression.
Thus, patients carrying TYMS rs11280056 6 bp-deletion homozygous genotype were at significantly increased risk for grades 1–2 neurological toxicity compared with wild type patients without the deletion.
The genetic variants of the DPYD, XPC, GSTP1, MTHFR, and UGT1A1 genes were not associated with neurotoxicity.
3.3.2. Hematological Toxicity
Differential associations with grade 3–4 hematological toxicity were seen across the treatment arms. Anemia, leukopenia, neutropenia, and thrombocytopenia were pooled into one group, and any of these events was considered as the occurrence of hematological toxicity.
Grade 3–4 hematological toxicity was reported in 31/78 (40%) PDAC patients treated with FOLFIRINOX (Table 3). The presence of the homozygous AA genotype (GSTP1 rs1695) was observed in 13/31 (42%) patients with hematological adverse events. The heterozygote AG was identified in 16/31 (52%) patients. Only 2/31 (6%) patients with hematological toxicity were homozygous GG genotype carriers.
In a cohort of 47 patients without any hematological toxicity, the allele distribution was as follows: 19/47 (40%) patients harbored the homozygous AA genotype, 15 (32%) patients had the heterozygous AG, and 13 (28%) patients carried the homozygous GG genotype.
According to co-dominant and recessive models, it was found that the GG genotype (also known as GSTP1*B) was associated with a lower risk for grade 3–4 hematological toxicity in advanced PDAC patients (p = 0.032 and p = 0.014, respectively).
We did not observe any associations between hematological toxicity and other genetic variants in the DPYD, XPC, GSTP1, MTHFR, and UGT1A1 genes.
3.3.3. Allele Frequencies
Allele frequencies obtained by microarray genotyping are summarized in Table 2 and were calculated in a cohort of 78 patients who were representatives of the Eastern Slavs to a great extent. Alleles were designated as major and minor variants according to the 1000 Genomes Project allele’s frequencies for the global population. The allele frequencies obtained from the analyzed samples were comparable to the relevant data of the 1000 Genomes Project (http://www.internationalgenome.org/data (accessed on 7 September 2021)).
4. Discussion
Most cytotoxic anticancer drugs are characterized by a dose-related effect and a narrow therapeutic index, that requires AEs monitoring and individual dose selection in some patients. Dose selection is usually based on age and body surface area (BSA) with or without including Therapeutic Drug Monitoring (TDM). However, these characteristics are not always sufficient to determine an optimal therapeutic dose since inter-individual variations remain beyond consideration. For example, in spite of the fact that 5-fluorouracil (5-FU) administration is individualized to the extent of BSA-based dosing, BSA does not correlate accurately with any pharmacokinetic parameters in adults [15]. Thus, pharmacogenomic, pharmacokinetic, and pharmacodynamic factors should be taken into consideration to optimize the benefit for patients, in terms of both antitumor activity and treatment tolerance.
In the current study, we retrospectively evaluated associations between AEs and polymorphic variation in the genes involved in drug metabolism, DNA repair and replication in PDAC patients treated with FOLFIRINOX chemotherapy.
The genetic variants selected as potential predictors of toxicity induced by combination therapy of FOLFIRINOX were as follows: DPYD rs3918290, rs75017182, rs55886062, rs67376798, rs2297595, XPC rs2228001, GSTP1 rs1695, MTHFR rs1801133, ERCC1 rs3212986, rs11615, UGT1A1 rs3064744, and TYMS rs11280056. Genotyping was performed on the specialized microarray that was developed and validated in the current study. The approach utilizes a low-density hydrogel microarray platform that had been successfully applied in a number of diagnostic applications (for review, see [16,17]).
The present study is the first report demonstrating the significant association between TYMS genetic polymorphism, specifically TYMS rs11280056, and neurotoxicity among pancreatic cancer patients treated with FOLFIRINOX. To the best of our knowledge, this association has not been reported earlier. The increased risk for grade 1–2 neurotoxicity was disclosed in PDAC patients with 6 bp-deletion in the TYMS gene compared with patients without the deletion (p = 0.0072 and p = 0.0019, according to co-dominant and recessive model, respectively). Notably, grade 3–4 neurotoxicity has not been reported in the course of the study due to a prompt dose reduction immediately following the early signs of neurological toxicity.
The thymidylate synthase (TYMS) gene is a prospective marker of clinical response and toxicity to 5-FU since this enzyme is a molecular target of fluoropyirimidines [18,19], and polymorphisms in the regulatory regions of the TYMS gene may affect its level of expression [20,21]. However, a number of studies contain conflicting results that do not allow proper evaluation the clinical value of these allele variants [22,23,24]. Particularly, some studies report significant association between the clinical response and/or AEs and variations in the TYMS 5′UTR and 3′UTR regulatory regions, while other studies contain contradictory results [25,26,27,28].
Notably, Castro-Rojas C. et al. reported that the TYMS rs45445694 polymorphism (5’VNTR 2R/2R) was associated with severe toxicity to the 5-FU-based chemotherapy in colorectal cancer patients. In their case, the main types of toxicity were gastrointestinal (46%) and, specifically, neurological toxicity (38%). The latter manifested as dysesthesia and sensory neuropathy [29].
Based on the results of the above study and our findings, it can be assumed that TYMS genetic polymorphisms probably could be associated with fluoropyrimidine-induced neurotoxicity in other cancer types as well.
Another noteworthy result of the study was that the GSTP1 rs1695 homozygous GG genotype was associated with a decreased risk for grades 3–4 hematological toxicity compared with the heterozygous AG allele variant and the homozygous AA type (p = 0.032 and p = 0.014, according to co-dominant and recessive models, respectively).
GSTP1 (Glutathione S-Transferase) encoded by the GSTP1 gene is an enzyme that plays an important role in the detoxification of xenobiotics, including platinum-based anticancer drugs [30,31]. GSTP1 is considered to be an indicator of response to chemotherapy and drug-induced AEs [32], although no definite conclusions have been derived.
A number of studies have reported the association between GSTP1 rs1695 and hematological toxicity, and patients carrying the homozygous GG genotype (GSTP1*B) had a decreased risk for drug-induced hematological toxicity [33,34]. The results obtained in the current study fit fairly well with these findings. We have found that among 31 patients with hematological toxicity only 2 (6%) carried the GG genotype. By contrast, in the patient cohort without hematological toxicity, 13/47 (28%) patients harbored the GG genotype. Nevertheless, we suppose the rs1695 GG genotype probably should not be considered a strong predictive biomarker for a lower risk for hematological toxicity, due to relatively high p-values and the limited sample size. The impact of this allele variant requires further investigation in a larger sample size by well-designed prospective clinical trials.
Besides the gene variations mentioned above, we have analyzed other genetic variants in the DPYD, UGT1A1, ERCC1, XPC, and MTHFR genes. We did not find any associations between AEs and genetic polymorphisms in these genes.
DPYD and UGT1A1 were included in the genotyping microarray since these genes encode two key drug metabolizing enzymes, dihydropyrimidine dehydrogenase (DPD) and uridine diphosphate glucuronosyltransferases (UGT1A1), that are involved in the catabolic pathways of 5-FU and irinotecan [35,36]. Genotyping of germline variations in these genes is strongly required to establish an effective dose of 5-FU and irinotecan, respectively.
According to the Guideline for Fluoropyrimidines and DPYD issued by Clinical Pharmacogenetics Implementation Consortium (CPIC), the frequency of DPYD allele variants significantly associated with decreased function of the enzyme is low enough and in most variants does not exceed <0.005 [37]. Nevertheless, deficiency in the DPD enzyme leads to increased exposure to the cytotoxic agent and its active metabolites and, consequently, an increased risk of related AEs.
The most well-studied genetic variants of UGT1A1 are UGT1A1*28 and UGT1A1*6. The UGT1A1*28 gene variant is a tandem TA repeat polymorphism [A(TA)7TAA] in the UGT1A1 promoter region that leads to decreased gene expression. Homozygous carriers of the variants T7/T7 (patients with so called Gilbert’s syndrome) have decreased UGT1A1 expression by approximately 70% [38,39].
The ERCC1 and XPC gene polymorphisms were analyzed since the genes are involved in the nucleotide-excision repair (NER) DNA-repair pathway. Excision repair cross-complementation group 1 (ERCC1) encoded by the ERCC1 gene is a key protein in the NER pathway. Together with xeroderma pigmentosum complementation group F (XPF), ERCC1 forms a heterodimer complex and participates in the elimination of different DNA adducts induced by UV light, reactive oxygen species, environmental mutagens, and especially cancer chemotherapy drugs during NER [40,41]. The XPF/ERCC1 complex is also involved in double strand break repair (DSBR) [42].
Multiple studies are devoted to investigating polymorphisms in the ERCC1 gene (e.g., see review [43]). The most commonly investigated SNV in the ERCC1 gene is rs11615. The majority of studies reported a significant association between the mutant CC genotype and better DFS, PFS, and OS [44,45,46,47,48,49]. However, some studies showed contradictory results and reported that patients with the CC genotype had worse treatment outcome in terms of PFS and OS [50,51,52].
Another primary NER initiating protein is the xeroderma pigmentosum group C (XPC). This protein plays an essential role in the first steps of NER, particularly in damage recognition as well as open complex formation and reparation [53,54,55]. It was reported that SNV in the XPC gene are potential markers of treatment response to oxaliplatin-based therapy in cancer patients [56,57,58]. Therefore, genetic variations in ERCC1 and XPC may have prospective value for predicting response to oxaliplatin-based chemotherapy, and further investigation of these polymorphisms seems essential and reasonable.
Methylenetetrahydrofolate reductase (MTHFR) encoded by the MTHFR gene is the most critical enzyme in the metabolism of folate and fluoropyrimidines [59]. Therefore, the MTHFR enzymatic activity may hypothetically predict the clinical responses and toxicity to 5-FU. One of the most frequent functional SNV in the MTHFR gene is rs1801133 (C > T; Ala222Val). This variant occurs in the homozygous state in 10–15% of many North American and European populations and correlates with reduced enzyme activity [59,60]. However, the evidence of genetic association remains relatively weak, and the results reported in systematic reviews are not consistent [61,62,63]. Therefore, further well-designed prospective investigations are needed to validate the present findings.
Multiple studies have reported significant world-wide variation in cancer prognosis between ethnic groups [64,65,66]. In the current study, microarray genotyping resulted in allele frequency determination in a cohort of 78 patients who were representatives of the Eastern Slavs to a great extent. We suppose this data will also contribute to further investigation of drug-induced AEs associated with different ethnic groups.
This study has some strengths and limitations. Our study’s main strength is the identification of the novel association between TYMS rs11280056 genetic polymorphism and neurotoxicity that could be taken into consideration for estimation of the increased risk of drug-induced AEs. The microarray developed in the study seems a promising genotyping tool to simultaneously identify a dozen of gene polymorphic variants accurately and relatively quickly (in one day). Additional data related to the impact of the GSTP1 rs1695 genetic polymorphism on the risk for hematological drug-induced toxicity was also reported.
The present study has some limitations: (i) in statistical analysis, we pooled drug-induced anemia, leukopenia, neutropenia, and thrombocytopenia into one group (hematological toxicity) and did not consider each syndrome separately; (ii) the number of patients enrolled in the study was not very large, and further prospective investigations are desirable to confirm and quantitate the findings; and (iii) every association identified in the current study is related to the whole combination therapy of FOLFIRINOX that includes four chemotherapy agents. We did not investigate the effects of each agent separately.
5. Conclusions
In summary, the results of the present study indicate that the TYMS genetic polymorphism rs11280056 is significantly associated with grade 1–2 neurotoxicity among pancreatic cancer patients treated with FOLFIRINOX. This association is reported for the first time and TYMS rs11280056 polymorphic variant could be considered a promising prognostic marker of neurotoxicity. Another association between the GSTP1 rs1695 GG genotype and the decreased risk for grade 3–4 hematological toxicity was also identified but it was not as strong and requires further validation.
Further validation studies on a larger number of patients are desirable to confirm and quantitate these associations. It may help clinicians to identify patients who have a high risk of FOLFIRINOX-induced AEs and improve treatment decision for cancer patients.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pharmaceutics14010077/s1: Table S1: Multiplex PCR primers used to prepare DNA samples for microarray genotyping; Table S2: Sequences of oligonucleotide probes immobilized on the microarray.
Author Contributions
Conceptualization, V.M., M.E. and R.H.; methodology, R.H. and I.S.; software, I.P. and R.H.; validation, V.M., M.E., E.K. and V.L.; formal analysis, V.M. and M.E.; investigation, M.E., I.S. and I.P.; resources, I.P. and V.L.; data curation, V.M. and E.K.; writing—original draft preparation, V.M.; writing—review and editing, M.E., E.K. and V.M.; visualization, M.E., R.H. and E.K.; supervision, V.M.; project administration, V.M.; funding acquisition, V.M. and V.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 16-15-00257.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of N. N. Blokhin Cancer Research Center (Moscow), (Protocol code No 5, Date of approval: 27 May 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Riall, T.S.; Lillemoe, K.D. Underutilization of Surgical Resection in Patients With Localized Pancreatic Cancer. Ann. Surg. 2007, 246, 181–182. [Google Scholar] [CrossRef]
- Thomas, R.M.; Truty, M.J.; Nogueras-Gonzalez, G.M.; Fleming, J.B.; Vauthey, J.-N.; Pisters, P.W.T.; Lee, J.E.; Rice, D.C.; Hofstetter, W.L.; Wolff, R.A.; et al. Selective Reoperation for Locally Recurrent or Metastatic Pancreatic Ductal Adenocarcinoma Following Primary Pancreatic Resection. J. Gastrointest. Surg. 2012, 16, 1696–1704. [Google Scholar] [CrossRef]
- Torre, M.L.; Nigri, G.; Conte, A.L.; Mazzuca, F.; Tierno, S.M.; Salaj, A.; Marchetti, P.; Ziparo, V.; Ramacciato, G. Is a Preoperative Assessment of the Early Recurrence of Pancreatic Cancer Possible after Complete Surgical Resection? Gut Liver 2014, 8, 102–108. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Chone, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. Unicancer GI PRODIGE 24/CCTG PA.6 trial: A multicenter international randomized phase III trial of adjuvant mFOLFIRINOX versus gemcitabine (gem) in patients with resected pancreatic ductal adenocarcinomas. J. Clin. Oncol. 2018, 36, LBA4001. [Google Scholar] [CrossRef]
- Gillen, S.; Schuster, T.; Meyer zum Büschenfelde, C.; Friess, H.; Kleeff, J. Preoperative/Neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta-analysis of Response and Resection Percentages. PLoS Med. 2010, 7, e1000267. [Google Scholar] [CrossRef]
- Conroy, T.; Bachet, J.-B.; Ayav, A.; Huguet, F.; Lambert, A.; Caramella, C.; Maréchal, R.; Van Laethem, J.-L.; Ducreux, M. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur. J. Cancer 2016, 57, 10–22. [Google Scholar] [CrossRef]
- Goldstein, D.; Hassan El-Maraghi, R.; Hammel, P.; Heinemann, V.; Kunzmann, V.; Sastre, J.; Scheithauer, W.; Siena, S.; Tabernero, J.; Teixeira, L.; et al. nab-Paclitaxel Plus Gemcitabine for Metastatic Pancreatic Cancer: Long-Term Survival From a Phase III Trial. J. Natl. Cancer Inst. 2015, 107, 413. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Table of Pharmacogenetic Associations. Available online: https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations (accessed on 15 May 2021).
- Cancer Therapy Evaluation Program (CTEP) Common Terminology Criteria for Adverse Events (CTCAE).v.5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed on 17 September 2021).
- Fesenko, E.E.; Heydarov, R.N.; Stepanova, E.V.; Abramov, M.E.; Chudinov, A.V.; Zasedatelev, A.S.; Mikhailovich, V.M. Microarray with LNA-probes for genotyping of polymorphic variants of Gilbert’s syndrome gene UGT1A1(TA)n. Clin. Chem. Lab. Med. 2013, 51, 1177–1184. [Google Scholar] [CrossRef]
- Rubina, A.Y.; Pan’kov, S.; Dementieva, E.; Pen’kov, D.; Butygin, A.; Vasiliskov, V.; Chudinov, A.; Mikheikin, A.; Mikhailovich, V.; Mirzabekov, A. Hydrogel drop microchips with immobilized DNA: Properties and methods for large-scale production. Anal. Biochem. 2004, 325, 92–106. [Google Scholar] [CrossRef]
- Wilhelm, M.; Mueller, L.; Miller, M.C.; Link, K.; Holdenrieder, S.; Bertsch, T.; Kunzmann, V.; Stoetzer, O.J.; Suttmann, I.; Braess, J.; et al. Prospective, Multicenter Study of 5-Fluorouracil Therapeutic Drug Monitoring in Metastatic Colorectal Cancer Treated in Routine Clinical Practice. Clin. Colorectal Cancer 2016, 15, 381–388. [Google Scholar] [CrossRef]
- Mikhailovich, V.; Gryadunov, D.; Kolchinsky, A.; Makarov, A.A.; Zasedatelev, A. DNA microarrays in the clinic: Infectious diseases. BioEssays 2008, 30, 673–682. [Google Scholar] [CrossRef]
- Gryadunov, D.; Dementieva, E.; Mikhailovich, V.; Nasedkina, T.; Rubina, A.; Savvateeva, E.; Fesenko, E.; Chudinov, A.; Zimenkov, D.; Kolchinsky, A.; et al. Gel-based microarrays in clinical diagnostics in Russia. Expert Rev. Mol. Diagn. 2011, 11, 839–853. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Pullarkat, S.T.; Stoehlmacher, J.; Ghaderi, V.; Xiong, Y.-P.; Ingles, S.A.; Sherrod, A.; Warren, R.; Tsao-Wei, D.; Groshen, S.; Lenz, H.-J. Thymidylate synthase gene polymorphism determines response and toxicity of 5-FU chemotherapy. Pharm. J. 2001, 1, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Koda, K.; Miyauchi, H.; Kosugi, C.; Kaiho, T.; Takiguchi, N.; Kobayashi, S.; Maruyama, T.; Matsubara, H. Tumor 5-fu-related mRNA expression and efficacy of oral fluoropyrimidines in adjuvant chemotherapy of colorectal cancer. Anticancer Res. 2016, 36, 5325–5331. [Google Scholar] [CrossRef] [PubMed]
- Toffoli, G.; Cecchin, E. Pharmacogenetics and stomach cancer: An update. Pharmacogenomics 2007, 8, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H. Predictive diagnostics in colorectal cancer: Impact of genetic polymorphisms on individual outcomes and treatment with fluoropyrimidine-based chemotherapy. EPMA J. 2010, 1, 485–494. [Google Scholar] [CrossRef]
- Gusella, M.; Padrini, R. G > C SNP of thymidylate synthase with respect to colorectal cancer. Pharmacogenomics 2007, 8, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.; Azevedo, R.; Sousa, H.; Seabra, V.; Medeiros, R. Current approaches for TYMS polymorphisms and their importance in molecular epidemiology and pharmacogenetics. Pharmacogenomics 2013, 14, 1337–1351. [Google Scholar] [CrossRef]
- Hitre, E.; Budai, B.; Adleff, V.; Czeglédi, F.; Horváth, Z.; Gyergyay, F.; Lövey, J.; Kovács, T.; Orosz, Z.; Láng, I.; et al. Influence of thymidylate synthase gene polymorphisms on the survival of colorectal cancer patients receiving adjuvant 5-fluorouracil. Pharmacogenet. Genom. 2005, 15, 723–730. [Google Scholar] [CrossRef]
- Park, C.-M.; Lee, W.Y.; Chun, H.-K.; Cho, Y.B.; Yun, H.R.; Heo, J.S.; Yun, S.H.; Kim, H.C. Relationship of polymorphism of the tandem repeat sequence in the thymidylate synthase gene and the survival of stage III colorectal cancer patients receiving adjuvant 5-flurouracil-based chemotherapy. J. Surg. Oncol. 2010, 101, 22–27. [Google Scholar] [CrossRef]
- Afzal, S.; Gusella, M.; Jensen, S.A.; Vainer, B.; Vogel, U.; Andersen, J.T.; Brødbæk, K.; Petersen, M.; Jimenez-Solem, E.; Adleff, V.; et al. The association of polymorphisms in 5-fluorouracil metabolism genes with outcome in adjuvant treatment of colorectal cancer. Pharmacogenomics 2011, 12, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Sulzyc-Bielicka, V.; Bielicki, D.; Binczak-Kuleta, A.; Kaczmarczyk, M.; Pioch, W.; Machoy-Mokrzynska, A.; Ciechanowicz, A.; Gołębiewska, M.; Drozdzik, M. Thymidylate Synthase Gene Polymorphism and Survival of Colorectal Cancer Patients Receiving Adjuvant 5-Fluorouracil. Genet. Test. Mol. Biomark. 2013, 17, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rojas, C.A.; Esparza-Mota, A.R.; Hernandez-Cabrera, F.; Romero-Diaz, V.J.; Gonzalez-Guerrero, J.F.; Maldonado-Garza, H.; Garcia-Gonzalez, I.S.; Buenaventura-Cisneros, S.; Sanchez-Lopez, J.Y.; Ortiz-Lopez, R.; et al. Thymidylate synthase gene variants as predictors of clinical response and toxicity to fluoropyrimidine-based chemotherapy for colorectal cancer. Drug Metab. Pers. Ther. 2017, 32, 209–218. [Google Scholar] [CrossRef]
- Moscow, J.A.; Fairchild, C.R.; Madden, M.J.; Ransom, D.T.; Wieand, H.S.; O’Brien, E.E.; Poplack, D.G.; Cossman, J.; Myers, C.E.; Cowan, K.H. Expression of Anionic Glutathione-S-transferase and P-Glycoprotein Genes in Human Tissues and Tumors. Cancer Res. 1989, 49, 1422–1428. [Google Scholar]
- Stoehlmacher, J. Association between Glutathione S-Transferase P1, T1, and M1 Genetic Polymorphism and Survival of Patients with Metastatic Colorectal Cancer. Cancer Spectr. Knowl. Environ. 2002, 94, 936–942. [Google Scholar] [CrossRef]
- Le Morvan, V.; Smith, D.; Laurand, A.; Brouste, V.; Bellott, R.; Soubeyran, I.; Mathoulin-Pelissier, S.; Robert, J. Determination of ERCC2 Lys751Gln and GSTP1 Ile105Val gene polymorphisms in colorectal cancer patients: Relationships with treatment outcome. Pharmacogenomics 2007, 8, 1693–1703. [Google Scholar] [CrossRef]
- Booten, R.; Ward, T.; Heighway, J.; Ashcroft, L.; Morris, J.; Thatcher, N. Glutathione-S-Transferase P1 Isoenzyme Polymorphisms, Platinum-Based Chemotherapy, and Non-small Cell Lung Cancer. J. Thorac. Oncol. 2006, 1, 679–683. [Google Scholar] [CrossRef][Green Version]
- Agostini, M.; Pasetto, L.M.; Pucciarelli, S.; Terrazzino, S.; Ambrosi, A.; Bedin, C.; Galdi, F.; Friso, M.L.; Mescoli, C.; Urso, E.; et al. Glutathione S-transferase P1 Ile105Val polymorphism is associated with haematological toxicity in elderly rectal cancer patients receiving preoperative chemoradiotherapy. Drugs Aging 2008, 25, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Amstutz, U.; Froehlich, T.K.; Largiadèr, C.R. Dihydropyrimidine dehydrogenase gene as a major predictor of severe 5-fluorouracil toxicity. Pharmacogenomics 2011, 12, 1321–1336. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, D.; Kuang, Q.; Liu, G.; Xu, W. Association of UGT1A1*28 polymorphisms with irinotecan-induced toxicities in colorectal cancer: A meta-analysis in Caucasians. Pharm. J. 2014, 14, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Amstutz, U.; Henricks, L.M.; Offer, S.M.; Barbarino, J.; Schellens, J.H.M.; Swen, J.J.; Klein, T.E.; McLeod, H.L.; Caudle, K.E.; Diasio, R.B.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin. Pharmacol. Ther. 2018, 103, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Gelbart, T.; Demina, A. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: A balanced polymorphism for regulation of bilirubin metabolism? Proc. Natl. Acad. Sci. USA 1998, 95, 8170–8174. [Google Scholar] [CrossRef]
- Bosma, P.J.; Chowdhury, J.R.; Bakker, C.; Gantla, S.; de Boer, A.; Oostra, B.A.; Lindhout, D.; Tytgat, G.N.J.; Jansen, P.L.M.; Elferink, R.P.J.O.; et al. The Genetic Basis of the Reduced Expression of Bilirubin UDP-Glucuronosyltransferase 1 in Gilbert’s Syndrome. N. Engl. J. Med. 1995, 333, 1171–1175. [Google Scholar] [CrossRef]
- Sijbers, A.M.; de Laat, W.L.; Ariza, R.R.; Biggerstaff, M.; Wei, Y.-F.; Moggs, J.G.; Carter, K.C.; Shell, B.K.; Evans, E.; de Jong, M.C.; et al. Xeroderma Pigmentosum Group F Caused by a Defect in a Structure-Specific DNA Repair Endonuclease. Cell 1996, 86, 811–822. [Google Scholar] [CrossRef]
- Scharer, O.D. Nucleotide Excision Repair in Eukaryotes. Cold Spring Harb. Perspect. Biol. 2013, 5, a012609. [Google Scholar] [CrossRef]
- Ahmad, A.; Robinson, A.R.; Duensing, A.; van Drunen, E.; Beverloo, H.B.; Weisberg, D.B.; Hasty, P.; Hoeijmakers, J.H.J.; Niedernhofer, L.J. ERCC1-XPF Endonuclease Facilitates DNA Double-Strand Break Repair. Mol. Cell. Biol. 2008, 28, 5082–5092. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, E.C.; Lim, L.; de Hingh, I.H.J.T.; Gelderblom, H.; Guchelaar, H.-J.; Deenen, M.J. Genetic Variants in DNA Repair Pathways as Potential Biomarkers in Predicting Treatment Outcome of Intraperitoneal Chemotherapy in Patients with Colorectal Peritoneal Metastasis: A Systematic Review. Front. Pharmacol. 2020, 11, 577968. [Google Scholar] [CrossRef] [PubMed]
- Stoehlmacher, J.; Park, D.J.; Zhang, W.; Yang, D.; Groshen, S.; Zahedy, S.; Lenz, H.-J. A multivariate analysis of genomic polymorphisms: Prediction of clinical outcome to 5-FU/oxaliplatin combination chemotherapy in refractory colorectal cancer. Br. J. Cancer 2004, 91, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.M.-H.; Tzeng, C.-H.; Chen, P.-M.; Lin, J.-K.; Lin, T.-C.; Chen, W.-S.; Jiang, J.-K.; Wang, H.-S.; Wang, W.-S. ERCC1 codon 118 C→T polymorphism associated with ERCC1 expression and outcome of FOLFOX-4 treatment in Asian patients with metastatic colorectal carcinoma. Cancer Sci. 2009, 100, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Chua, W.; Goldstein, D.; Lee, C.K.; Dhillon, H.; Michael, M.; Mitchell, P.; Clarke, S.J.; Iacopetta, B. Molecular markers of response and toxicity to FOLFOX chemotherapy in metastatic colorectal cancer. Br. J. Cancer 2009, 101, 998–1004. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Tzeng, C.-H.; Chen, P.-M.; Lin, J.-K.; Lin, T.-C.; Chen, W.-S.; Jiang, J.-K.; Wang, H.-S.; Wang, W.-S. Influence of GSTP1 I105V polymorphism on cumulative neuropathy and outcome of FOLFOX-4 treatment in Asian patients with colorectal carcinoma. Cancer Sci. 2010, 101, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-Y.; Huang, M.-L.; Chen, M.-J.; Lu, C.-Y.; Chen, C.-F.; Tsai, P.-C.; Chuang, S.-C.; Hou, M.-F.; Lin, S.-R.; Wang, J.-Y. Multiple genetic polymorphisms in the prediction of clinical outcome of metastatic colorectal cancer patients treated with first-line FOLFOX-4 chemotherapy. Pharmacogenet. Genom. 2011, 21, 18–25. [Google Scholar] [CrossRef]
- Li, H.-Y.; Ge, X.; Huang, G.-M.; Li, K.-Y.; Zhao, J.-Q.; Yu, X.-M.; Bi, W.-S.; Wang, Y.-L. GSTP1, ERCC1 and ERCC2 Polymorphisms, Expression and Clinical Outcome of Oxaliplatin-based Adjuvant Chemotherapy in Colorectal Cancer in Chinese Population. Asian Pac. J. Cancer Prev. 2012, 13, 3465–3469. [Google Scholar] [CrossRef]
- Martinez-Balibrea, E.; Abad, A.; Aranda, E.; Sastre, J.; Manzano, J.L.; Díaz-Rubio, E.; Gómez-España, A.; Aparicio, J.; García, T.; Maestu, I.; et al. Pharmacogenetic approach for capecitabine or 5-fluorouracil selection to be combined with oxaliplatin as first-line chemotherapy in advanced colorectal cancer. Eur. J. Cancer 2008, 44, 1229–1237. [Google Scholar] [CrossRef]
- Paré, L.; Marcuello, E.; Altés, A.; Del Río, E.; Sedano, L.; Salazar, J.; Cortés, A.; Barnadas, A.; Baiget, M. Pharmacogenetic prediction of clinical outcome in advanced colorectal cancer patients receiving oxaliplatin/5-fluorouracil as first-line chemotherapy. Br. J. Cancer 2008, 99, 1050–1055. [Google Scholar] [CrossRef]
- Rao, D.; Mallick, A.B.; Augustine, T.; Daroqui, C.; Jiffry, J.; Merla, A.; Chaudhary, I.; Seetharam, R.; Sood, A.; Gajavelli, S.; et al. Excision repair cross-complementing group-1 (ERCC1) induction kinetics and polymorphism are markers of inferior outcome in patients with colorectal cancer treated with oxaliplatin. Oncotarget 2019, 10, 5510–5522. [Google Scholar] [CrossRef]
- Sugasawa, K.; Ng, J.M.Y.; Masutani, C.; Iwai, S.; van der Spek, P.J.; Eker, A.P.M.; Hanaoka, F.; Bootsma, D.; Hoeijmakers, J.H.J. Xeroderma Pigmentosum Group C Protein Complex Is the Initiator of Global Genome Nucleotide Excision Repair. Mol. Cell 1998, 2, 223–232. [Google Scholar] [CrossRef]
- Hoogstraten, D.; Bergink, S.; Ng, J.M.Y.; Verbiest, V.H.M.; Luijsterburg, M.S.; Geverts, B.; Raams, A.; Dinant, C.; Hoeijmakers, J.H.J.; Vermeulen, W.; et al. Versatile DNA damage detection by the global genome nucleotide excision repair protein XPC. J. Cell Sci. 2008, 121, 2850–2859. [Google Scholar] [CrossRef] [PubMed]
- Shell, S.M.; Hawkins, E.K.; Tsai, M.-S.; Hlaing, A.S.; Rizzo, C.J.; Chazin, W.J. Xeroderma pigmentosum complementation group C protein (XPC) serves as a general sensor of damaged DNA. DNA Repair 2013, 12, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, H.; Zhang, Y.; Kang, H.; Sun, M.; Wang, E.; Yang, X.; Lian, M.; Yu, Z.; Zhao, L.; et al. DNA repair genes XPC, XPG polymorphisms: Relation to the risk of colorectal carcinoma and therapeutic outcome with oxaliplatin-based adjuvant chemotherapy. Mol. Carcinog. 2012, 51, E83–E93. [Google Scholar] [CrossRef]
- Kap, E.J.; Seibold, P.; Richter, S.; Scherer, D.; Habermann, N.; Balavarca, Y.; Jansen, L.; Becker, N.; Pfütze, K.; Popanda, O.; et al. Genetic variants in DNA repair genes as potential predictive markers for oxaliplatin chemotherapy in colorectal cancer. Pharm. J. 2015, 15, 505–512. [Google Scholar] [CrossRef]
- Hu, X.; Qin, W.; Li, S.; He, M.; Wang, Y.; Guan, S.; Zhao, H.; Yao, W.; Wei, M.; Liu, M.; et al. Polymorphisms in DNA repair pathway genes and ABCG2 gene in advanced colorectal cancer: Correlation with tumor characteristics and clinical outcome in oxaliplatin-based chemotherapy. Cancer Manag. Res. 2018, 11, 285–297. [Google Scholar] [CrossRef]
- Frosst, P.; Blom, H.J.; Milos, R.; Goyette, P.; Sheppard, C.A.; Matthews, R.G.; Boers, G.J.H.; den Heijer, M.; Kluijtmans, L.A.J.; van den Heuve, L.P.; et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995, 10, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Schettert, I.T.; Morandini Filho, A.A.F.; Guerra-Shinohara, E.M.; Krieger, J.E. Methylenetetrahydrofolate reductase (MTHFR) c677t gene variant modulates the homocysteine folate correlation in a mild folate-deficient population. Clin. Chim. Acta 2004, 340, 99–105. [Google Scholar] [CrossRef]
- Zintzaras, E.; Ziogas, D.C.; Kitsios, G.D.; Papathanasiou, A.A.; Lau, J.; Raman, G. MTHFR gene polymorphisms and response to chemotherapy in colorectal cancer: A meta-analysis. Pharmacogenomics 2009, 10, 1285–1294. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Kong, X. MTHFR C677T Polymorphism is Associated with Tumor Response to Preoperative Chemoradiotherapy: A Result Based on Previous Reports. Med. Sci. Monit. 2015, 21, 3068–3076. [Google Scholar] [CrossRef][Green Version]
- Zhong, L.; He, X.; Zhang, Y.; Chuan, J.-L.; Chen, M.; Zhu, S.-M.; Peng, Q. Relevance of methylenetetrahydrofolate reductase gene variants C677T and A1298C with response to fluoropyrimidine-based chemotherapy in colorectal cancer: A systematic review and meta-analysis. Oncotarget 2018, 9, 31291–31301. [Google Scholar] [CrossRef] [PubMed]
- Abou Alaiwi, S.; Nassar, A.H.; Adib, E.; Groha, S.M.; Akl, E.W.; McGregor, B.A.; Esplin, E.D.; Yang, S.; Hatchell, K.; Fusaro, V.; et al. Trans-ethnic variation in germline variants of patients with renal cell carcinoma. Cell Rep. 2021, 34, 108926. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.-M.; Borno, H.T.; Cheng, H.H.; Zhou, A.Y.; Small, E.J. Ethnic disparities among men with prostate cancer undergoing germline testing. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 80.e1–80.e7. [Google Scholar] [CrossRef]
- Ho, W.-K.; Tan, M.-M.; Mavaddat, N.; Tai, M.-C.; Mariapun, S.; Li, J.; Ho, P.-J.; Dennis, J.; Tyrer, J.P.; Bolla, M.K.; et al. European polygenic risk score for prediction of breast cancer shows similar performance in Asian women. Nat. Commun. 2020, 11, 3833. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).