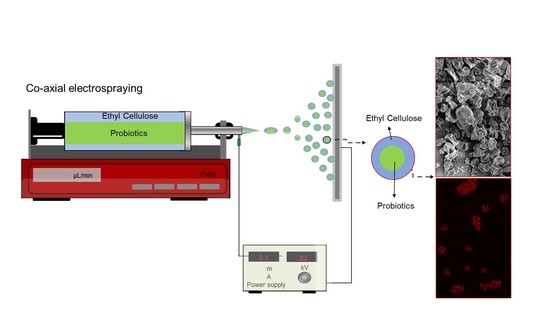
Electrosprayed Ethyl Cellulose Core-Shell Microcapsules for the Encapsulation of Probiotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacteria Culture
2.3. Electrospray Solutions Preparation
2.4. Co-Axial Electrospray of ETC Core–Shell Capsules Encapsulating Bifido
2.5. Microscopy Analysis
2.6. Fourier Transform Infrared Spectroscopy (FT-IR)
2.7. Water Activity (aw)
2.8. Cell Viability, Encapsulation Efficiency, and Storage Stability
2.9. Data Analysis
3. Results and Discussion
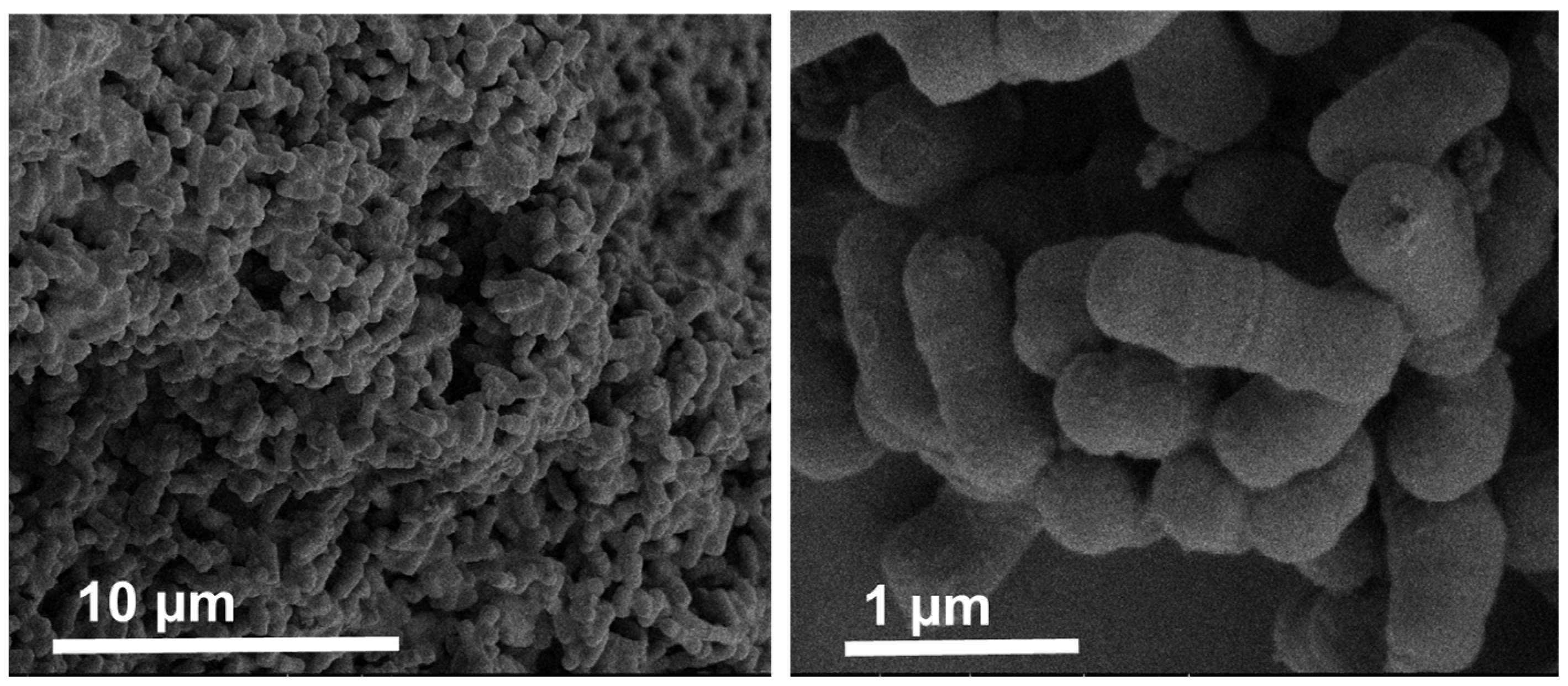
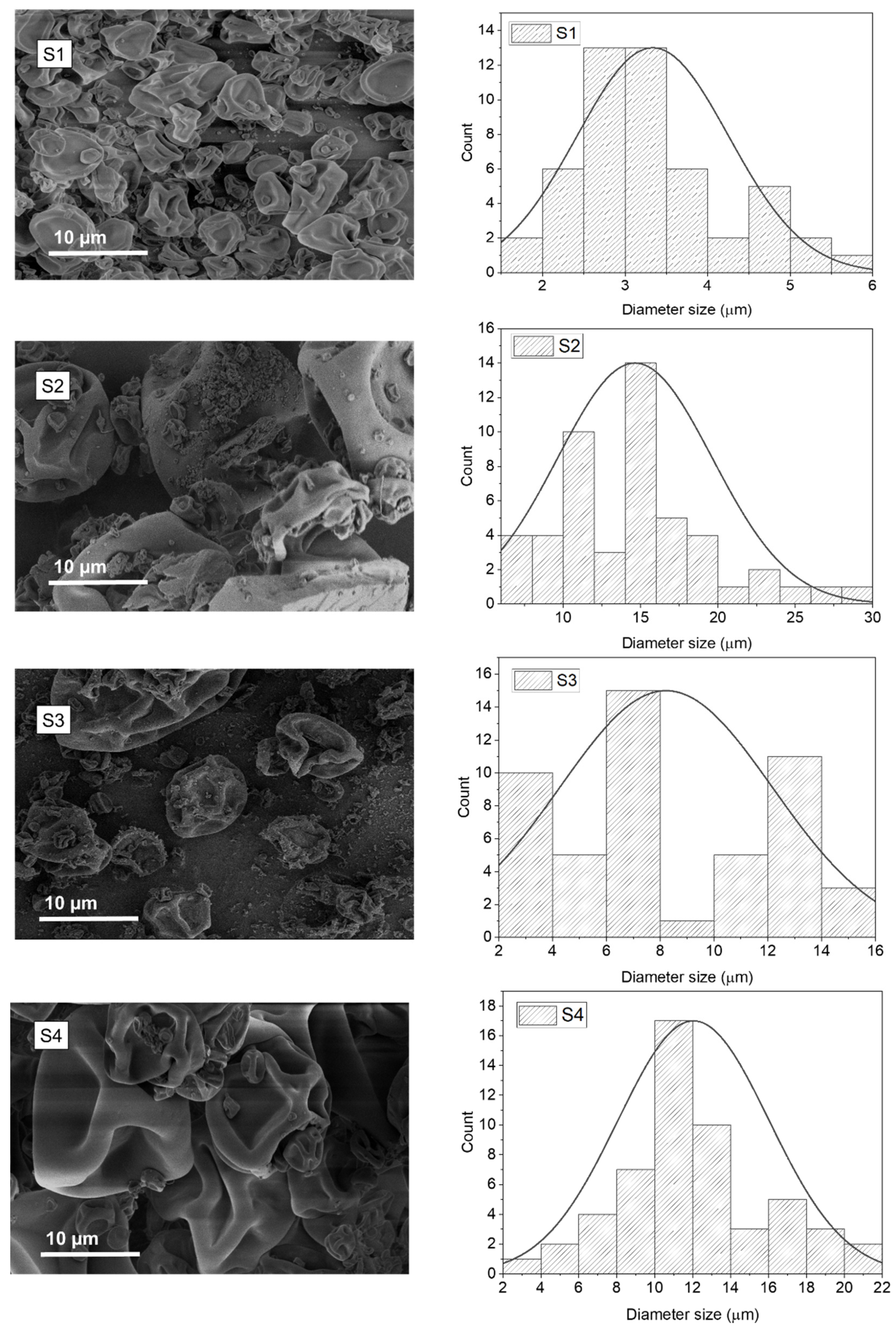
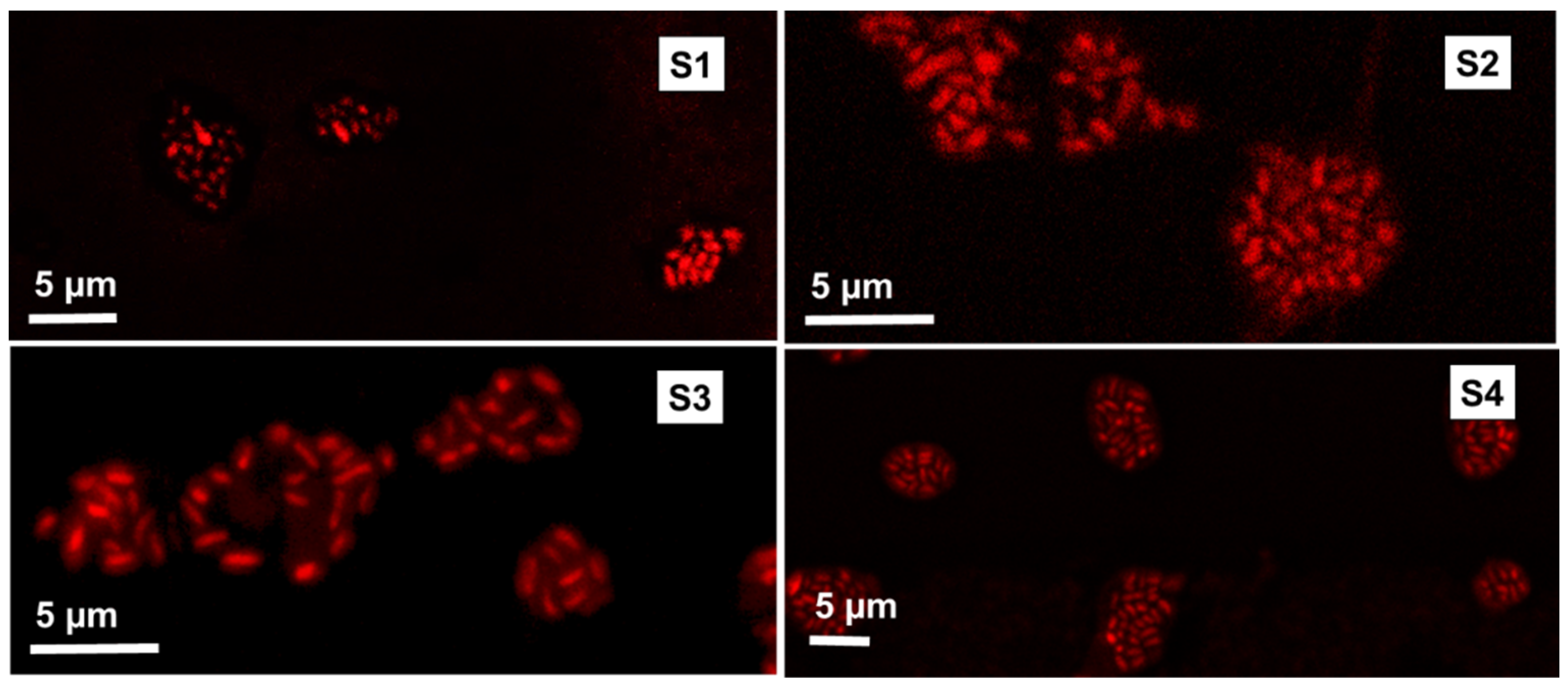
3.1. Microcapsules and Cells Size and Morphology
3.2. Water Activity (aw)
3.3. FTIR
3.4. Cell Viability and Stability over Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendes, A.C.; Chronakis, I.S. Electrohydrodynamic Encapsulation of Probiotics: A Review. Food Hydrocoll. 2021, 117, 106688. [Google Scholar] [CrossRef]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R.; Tromp, R.H.H. Electrospray-Assisted Drying of Live Probiotics in Acacia Gum Microparticles Matrix. Carbohydr. Polym. 2018, 183, 183–191. [Google Scholar] [CrossRef]
- Jiménez, M.; Flores-Andrade, E.; Pascual-Pineda, L.A.; Beristain, C.I. Effect of Water Activity on the Stability of Lactobacillus Paracasei Capsules. LWT—Food Sci. Technol. 2015, 60, 346–351. [Google Scholar] [CrossRef]
- Chávarri, M.; Marañón, I.; Villarán, M.C. Encapsulation Technology to Protect Probiotic Bacteria. Probiotics 2012, 501–540. [Google Scholar] [CrossRef] [Green Version]
- García-Moreno, P.J.; Mendes, A.C.; Jacobsen, C.; Chronakis, I.S. Biopolymers for the Nano-Microencapsulation of Bioactive Ingredients by Electrohydrodynamic Processing; Springer: Cham, Switzerland, 2018; ISBN 9783319946252. [Google Scholar]
- Pitigraisorn, P.; Srichaisupakit, K.; Wongpadungkiat, N.; Wongsasulak, S. Encapsulation of Lactobacillus Acidophilus in Moist-Heat-Resistant Multilayered Microcapsules. J. Food Eng. 2017, 192, 11–18. [Google Scholar] [CrossRef]
- Mendes, A.C.; Stephansen, K.; Chronakis, I.S. Electrospinning of Food Proteins and Polysaccharides. Food Hydrocoll. 2017, 68, 53–68. [Google Scholar] [CrossRef]
- López-Rubio, A.; Sanchez, E.; Wilkanowicz, S.; Sanz, Y.; Lagaron, J.M. Electrospinning as a Useful Technique for the Encapsulation of Living Bifidobacteria in Food Hydrocolloids. Food Hydrocoll. 2012, 28, 159–167. [Google Scholar] [CrossRef]
- Laelorspoen, N.; Wongsasulak, S.; Yoovidhya, T.; Devahastin, S. Microencapsulation of Lactobacillus Acidophilus in Zein-Alginate Core-Shell Microcapsules via Electrospraying. J. Funct. Foods 2014, 7, 342–349. [Google Scholar] [CrossRef]
- Lancuški, A.; Abu Ammar, A.; Avrahami, R.; Vilensky, R.; Vasilyev, G.; Zussman, E. Design of Starch-Formate Compound Fibers as Encapsulation Platform for Biotherapeutics. Carbohydr. Polym. 2017, 158, 68–76. [Google Scholar] [CrossRef]
- Librán, C.M.M.; Castro, S.; Lagaron, J.M.M. Encapsulation by Electrospray Coating Atomization of Probiotic Strains. Innov. Food Sci. Emerg. Technol. 2017, 39, 216–222. [Google Scholar] [CrossRef]
- Arca, H.C.; Mosquera-Giraldo, L.I.; Bi, V.; Xu, D.; Taylor, L.S.; Edgar, K.J. Pharmaceutical Applications of Cellulose Ethers and Cellulose Ether Esters. Biomacromolecules 2018, 19, 2351–2376. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Barbut, S.; Marangoni, A.G. Physical Structure and Thermal Behavior of Ethylcellulose. Cellulose 2014, 21, 3243–3255. [Google Scholar] [CrossRef]
- Huang, L.Y.; Yu, D.G.; Branford-White, C.; Zhu, L.M. Sustained Release of Ethyl Cellulose Micro-Particulate Drug Delivery Systems Prepared Using Electrospraying. J. Mater. Sci. 2012, 47, 1372–1377. [Google Scholar] [CrossRef]
- Wasilewska, K.; Winnicka, K. Ethylcellulose-a Pharmaceutical Excipient with Multidirectional Application in Drug Dosage Forms Development. Materials 2019, 12, 3386. [Google Scholar] [CrossRef] [Green Version]
- Eltayeb, M.; Stride, E.; Edirisinghe, M. Electrosprayed Core-Shell Polymer-Lipid Nanoparticles for Active Component Delivery. Nanotechnology 2013, 24, 465604. [Google Scholar] [CrossRef]
- Brady, J.; Du, T.; Lee, I.; Li, J. Polymer Properties and Characterization; Academic Press: Cambridge, MA, USA, 2017; ISBN 9780128024478. [Google Scholar]
- Wu, X.; Wang, L.; Yu, H.; Huang, Y. Effect of Solvent on Morphology of Electrospinning Ethyl Cellulose Fibers. J. Appl. Polym. Sci. 2005, 97, 1292–1297. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, X.; Wang, L.; Huang, Y. Electrospinning of Ethyl—Cyanoethyl Cellulose / Tetrahydrofuran Solutions. J. Appl. Polym. Sci. 2003, 91, 242–246. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.H.; Yu, D.G.; Williams, G.R. Tunable Biphasic Drug Release from Ethyl Cellulose Nanofibers Fabricated Using a Modified Coaxial Electrospinning Process. Nanoscale Res. Lett. 2014, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Zaitoon, A.; Lim, L.T. Effect of Poly(Ethylene Oxide) on the Electrospinning Behavior and Characteristics of Ethyl Cellulose Composite Fibers. Materialia 2020, 10, 100649. [Google Scholar] [CrossRef]
- Niu, B.; Zhan, L.; Shao, P.; Xiang, N.; Sun, P.; Chen, H.; Gao, H. Electrospinning of Zein-Ethyl Cellulose Hybrid Nanofibers with Improved Water Resistance for Food Preservation. Int. J. Biol. Macromol. 2020, 142, 592–599. [Google Scholar] [CrossRef]
- Kutzli, I.; Beljo, D.; Gibis, M.; Baier, S.K.; Weiss, J. Effect of Maltodextrin Dextrose Equivalent on Electrospinnability and Glycation Reaction of Blends with Pea Protein Isolate. Food Biophys. 2020, 15, 206–215. [Google Scholar] [CrossRef]
- Kang, C.H.; Han, S.H.; Kim, J.S.; Kim, Y.; Jeong, Y.; Park, H.M.; Paek, N.S. Inhibition of Nitric Oxide Production, Oxidative Stress Prevention, and Probiotic Activity of Lactic Acid Bacteria Isolated from the Human Vagina and Fermented Food. Microorganisms 2019, 7, 109. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.N.; Clasen, C.; Van den Mooter, G. Pharmaceutical Applications of Electrospraying. J. Pharm. Sci. 2016, 105, 2601–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verruck, S.; de Liz, G.R.; Dias, C.O.; de Mello Castanho Amboni, R.D.; Prudencio, E.S. Effect of Full-Fat Goat’s Milk and Prebiotics Use on Bifidobacterium BB-12 Survival and on the Physical Properties of Spray-Dried Powders under Storage Conditions. Food Res. Int. 2019, 119, 643–652. [Google Scholar] [CrossRef]
- Nakagawa, H.; Oyama, T. Molecular Basis of Water Activity in Glycerol–Water Mixtures. Front. Chem. 2019, 7, 731. [Google Scholar] [CrossRef] [Green Version]
- Vodnar, D.C.; Socaciu, C.; Rotar, A.M.; Stãnilã, A. Morphology, FTIR Fingerprint and Survivability of Encapsulated Lactic Bacteria (Streptococcus Thermophilus and Lactobacillus Delbrueckii Subsp. Bulgaricus) in Simulated Gastric Juice and Intestinal Juice. Int. J. Food Sci. Technol. 2010, 45, 2345–2351. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Branton, A.; Trivedi, D.; Nayak, G.; Mishra, R.K.; Jana, S. Characterization of Physicochemical and Thermal Properties of Biofield Treated Ethyl Cellulose and Methyl Cellulose. Int. J. Biomed. Mater. Res. 2015, 3, 83–91. [Google Scholar] [CrossRef]
- Santos, M.; Gerbino, E.; Tymczyszyn, E.; Gomez-Zavaglia, A. Applications of Infrared and Raman Spectroscopies to Probiotic Investigation. Foods 2015, 4, 283–305. [Google Scholar] [CrossRef] [Green Version]
- Huq, T.; Khan, A.; Khan, R.A.; Riedl, B.; Lacroix, M. Encapsulation of Probiotic Bacteria in Biopolymeric System. Crit. Rev. Food Sci. Nutr. 2013, 53, 909–916. [Google Scholar] [CrossRef] [Green Version]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [Green Version]

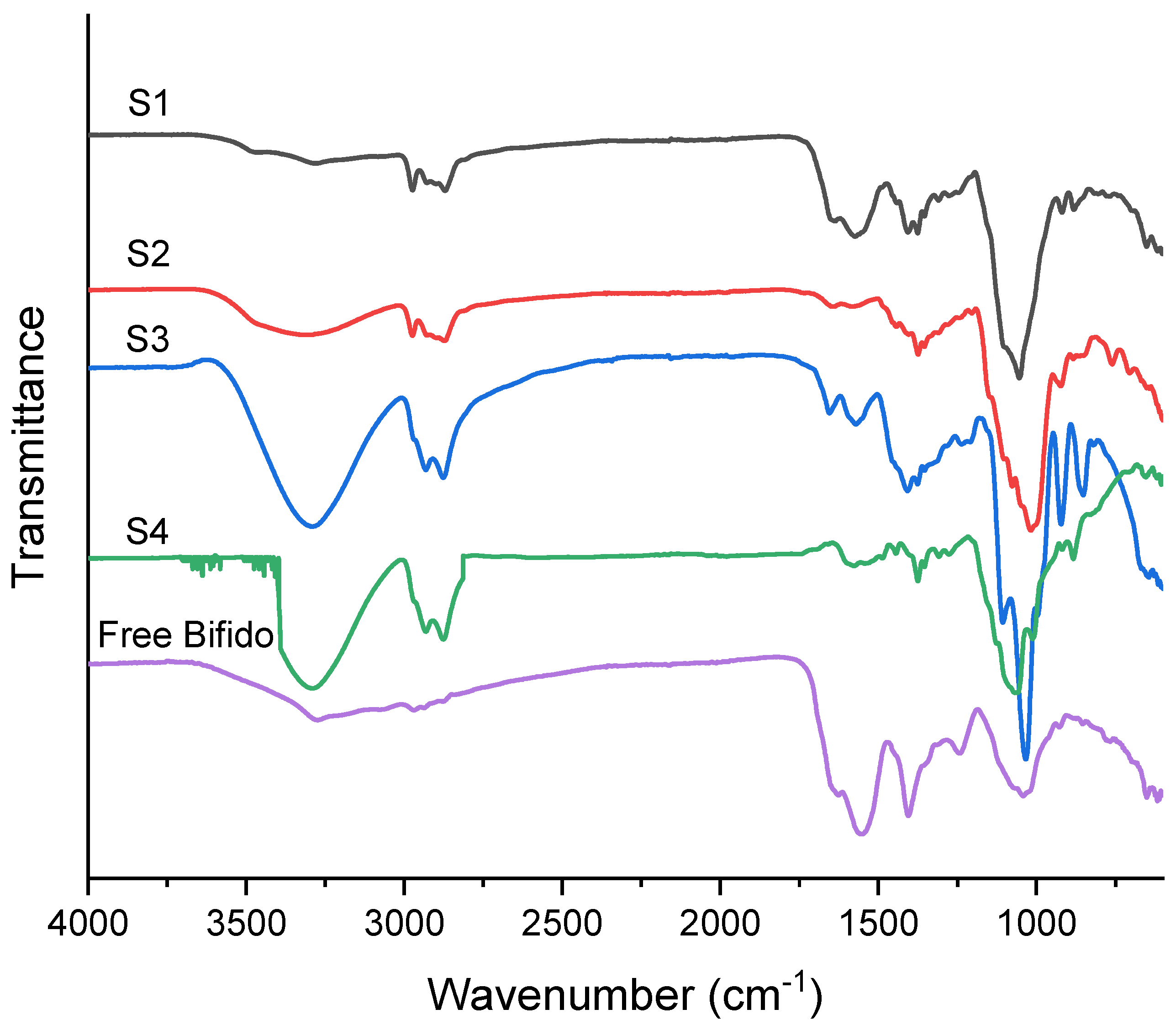
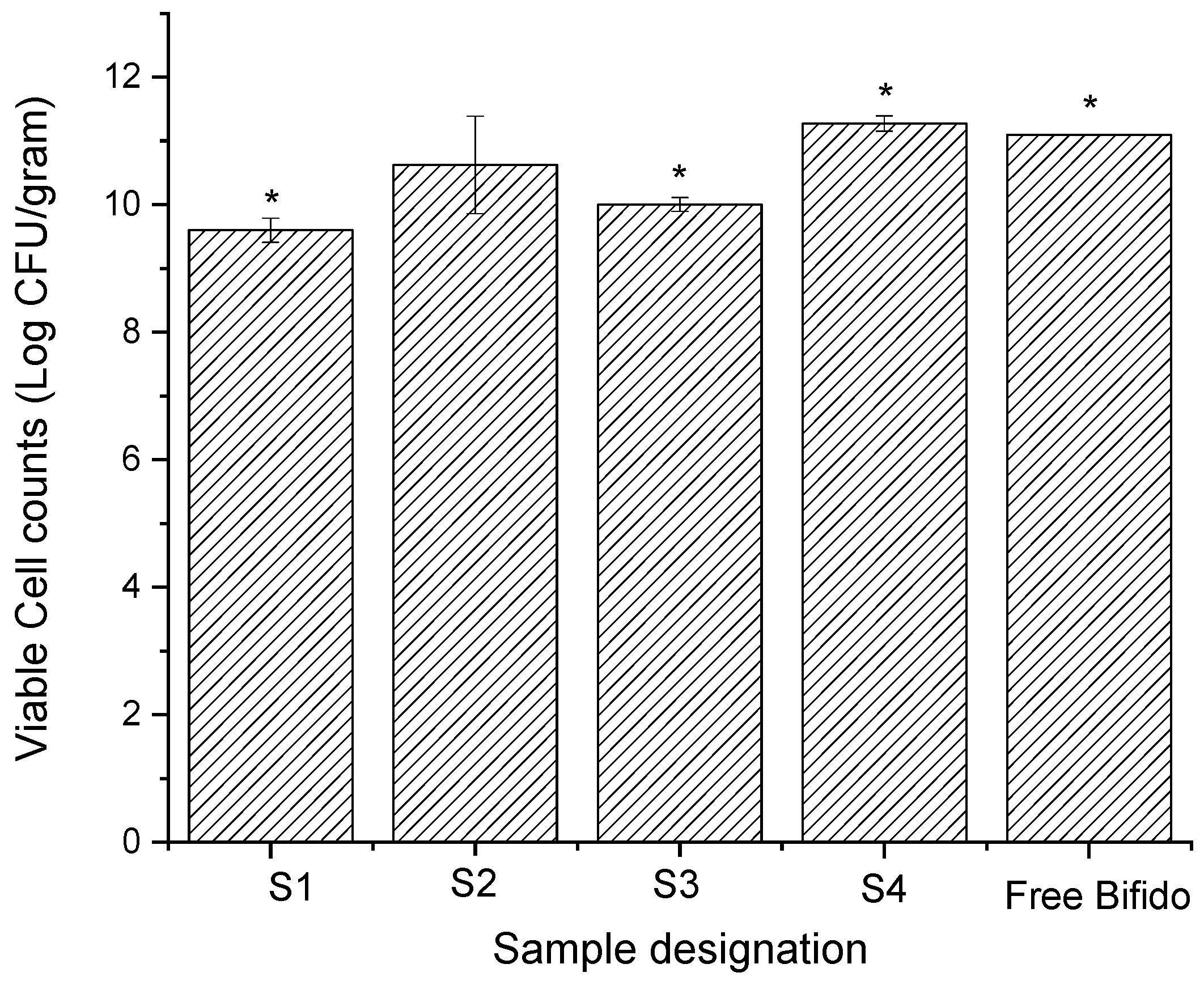
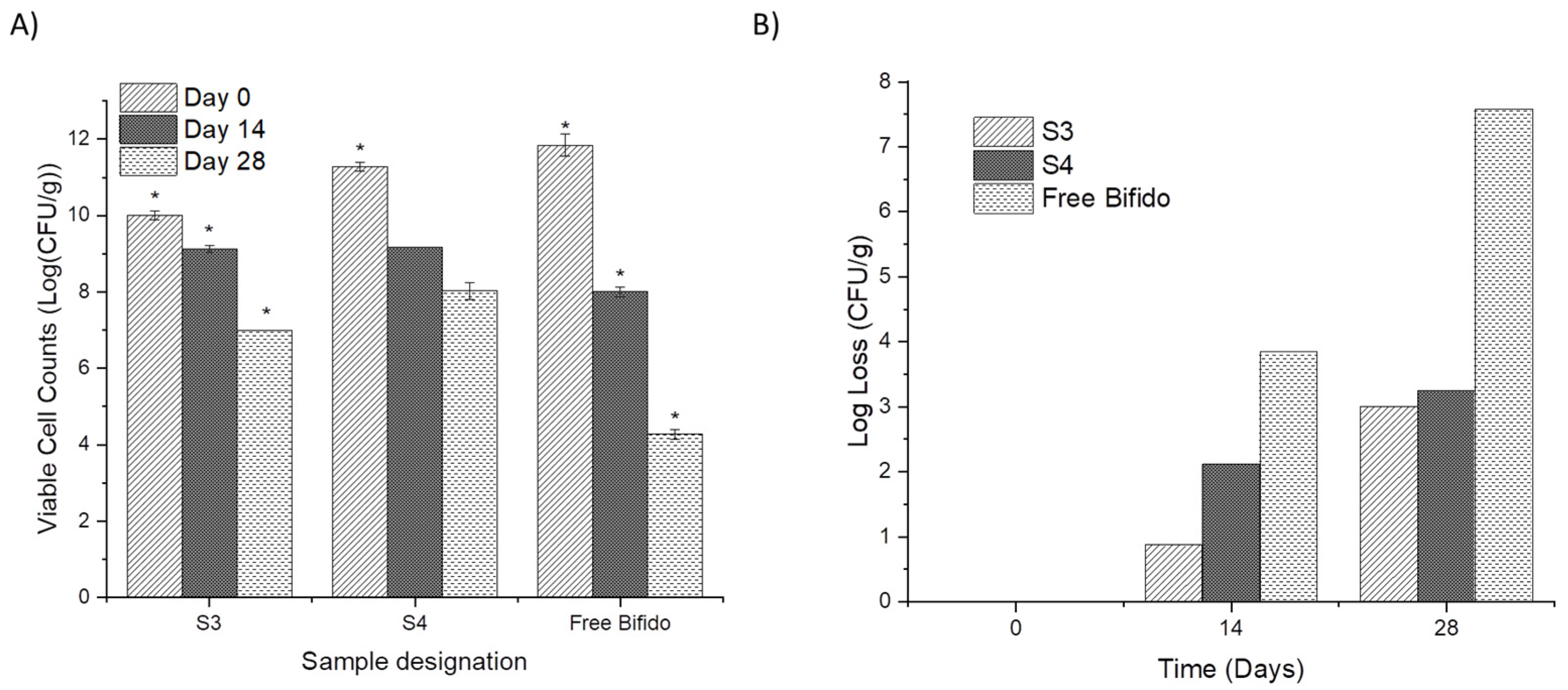
| Sample Designation | Solvent in Shell | Core Composition |
|---|---|---|
| S1 | Ethanol | Bifido |
| S2 | Ethanol | Bifido–maltodextrin |
| S3 | Ethanol | Bifido–glycerol |
| S4 | Acetone | Bifido-–glycerol |
| Sample Designation | Diameter Size /µm | PDI | aw | EE% |
|---|---|---|---|---|
| S1 | 3.33 ± 1.18 * | 0.083 | 0.13 ± 0.028 | 39.6 |
| S2 | 14.63 ± 4.99 * | 0.12 | 0.16 ± 0.010 | 44.8 |
| S3 | 8.21 ± 3.96 * | 0.23 | 0.20 ± 0.029 | 77.6 |
| S4 | 12.04 ± 3.98 * | 0.09 | 0.18 ± 0.066 | 87.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, J.S.; Dima, P.; Chronakis, I.S.; Mendes, A.C. Electrosprayed Ethyl Cellulose Core-Shell Microcapsules for the Encapsulation of Probiotics. Pharmaceutics 2022, 14, 7. https://doi.org/10.3390/pharmaceutics14010007
Moreno JS, Dima P, Chronakis IS, Mendes AC. Electrosprayed Ethyl Cellulose Core-Shell Microcapsules for the Encapsulation of Probiotics. Pharmaceutics. 2022; 14(1):7. https://doi.org/10.3390/pharmaceutics14010007
Chicago/Turabian StyleMoreno, Jorge Sevilla, Panagiota Dima, Ioannis S. Chronakis, and Ana C. Mendes. 2022. "Electrosprayed Ethyl Cellulose Core-Shell Microcapsules for the Encapsulation of Probiotics" Pharmaceutics 14, no. 1: 7. https://doi.org/10.3390/pharmaceutics14010007
APA StyleMoreno, J. S., Dima, P., Chronakis, I. S., & Mendes, A. C. (2022). Electrosprayed Ethyl Cellulose Core-Shell Microcapsules for the Encapsulation of Probiotics. Pharmaceutics, 14(1), 7. https://doi.org/10.3390/pharmaceutics14010007