Ten Years of Experience Support Pharmacogenetic Testing to Guide Individualized Drug Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
- Monotherapy: a patient’s PGx test was requested for a specific gene.
- Polytherapy: patients on polypharmacy. A model designed to analyze polymedicated patients, called 5SPM, was applied to this patient group.
2.2. 5-Step Precision Medicine Model
2.2.1. Step 1: Clinical, Epidemiological and Therapeutic Data Collection
2.2.2. Step 2: Predictions of Drug–Drug Interactions and Pharmacokinetic Specific Pathways
2.2.3. Step 3: Pharmacogenetic Analysis of Selected Genes
2.2.4. Step 4: Rationalized PGx-Guided Adjustments of Drug Therapy
2.2.5. Step 5: Assessment of the Intervention and Model Reevaluation
2.3. Statistical Analysis
3. Results
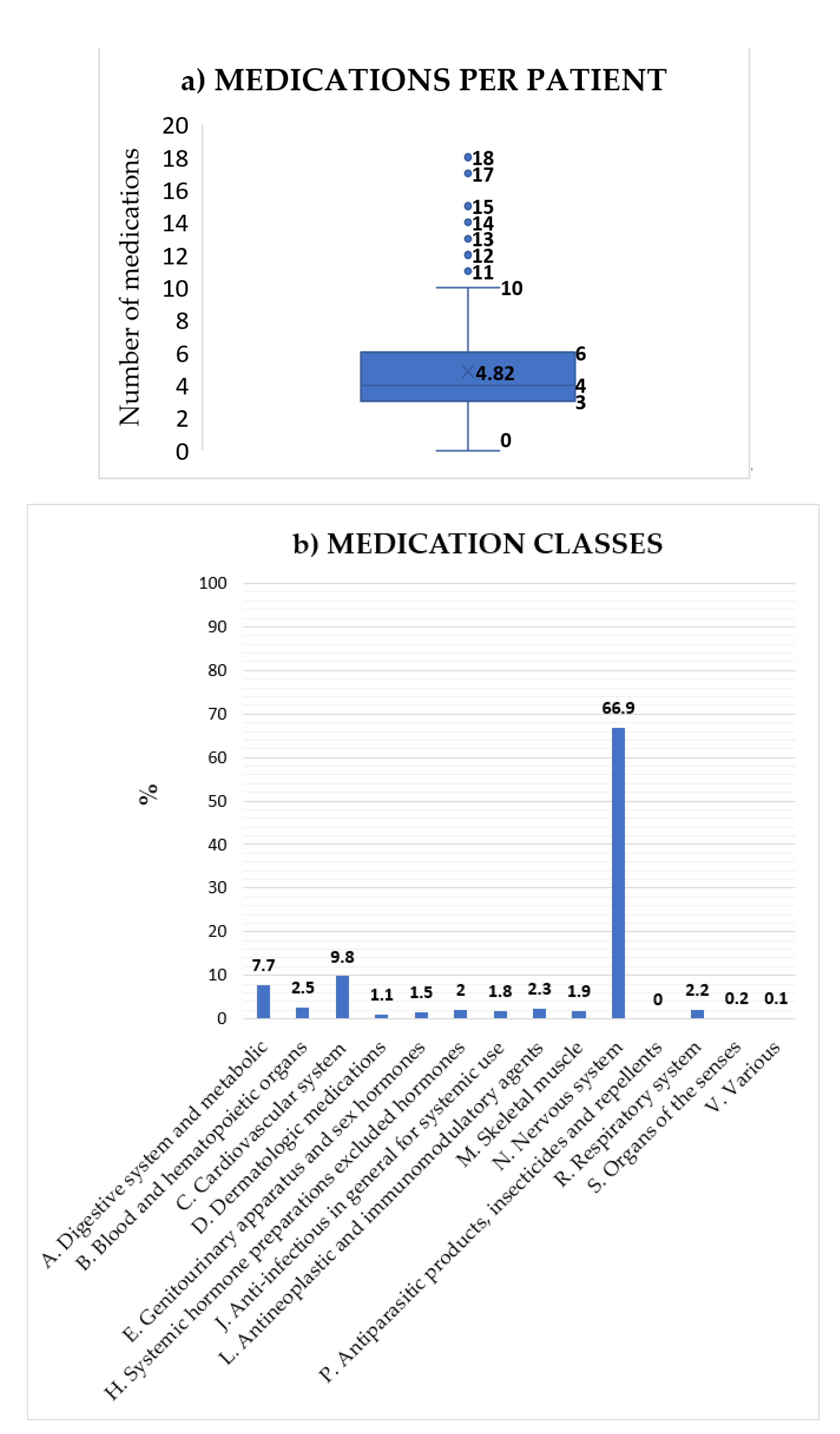
3.1. Clinical Data Collection
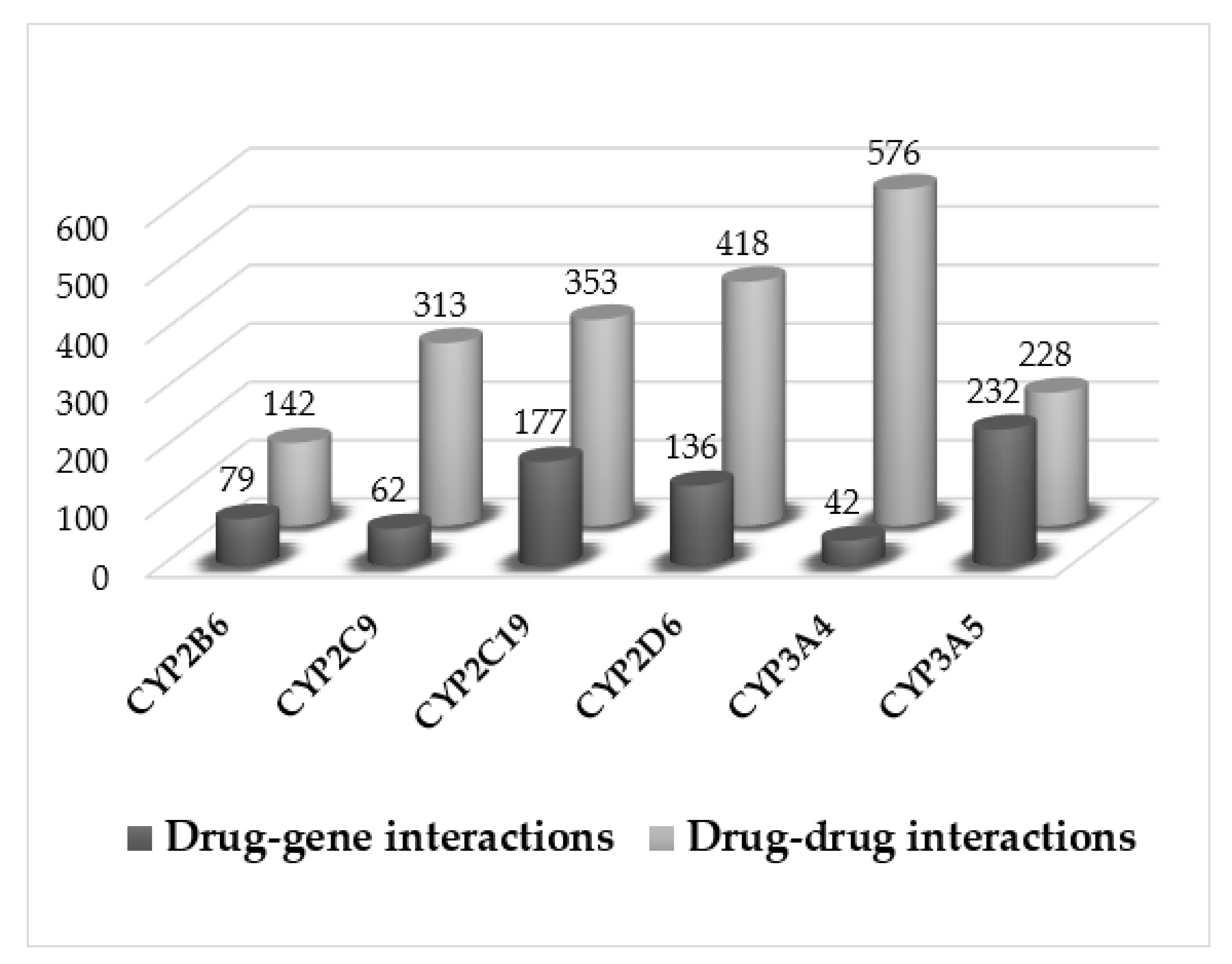
3.2. Pharmacological Interactions
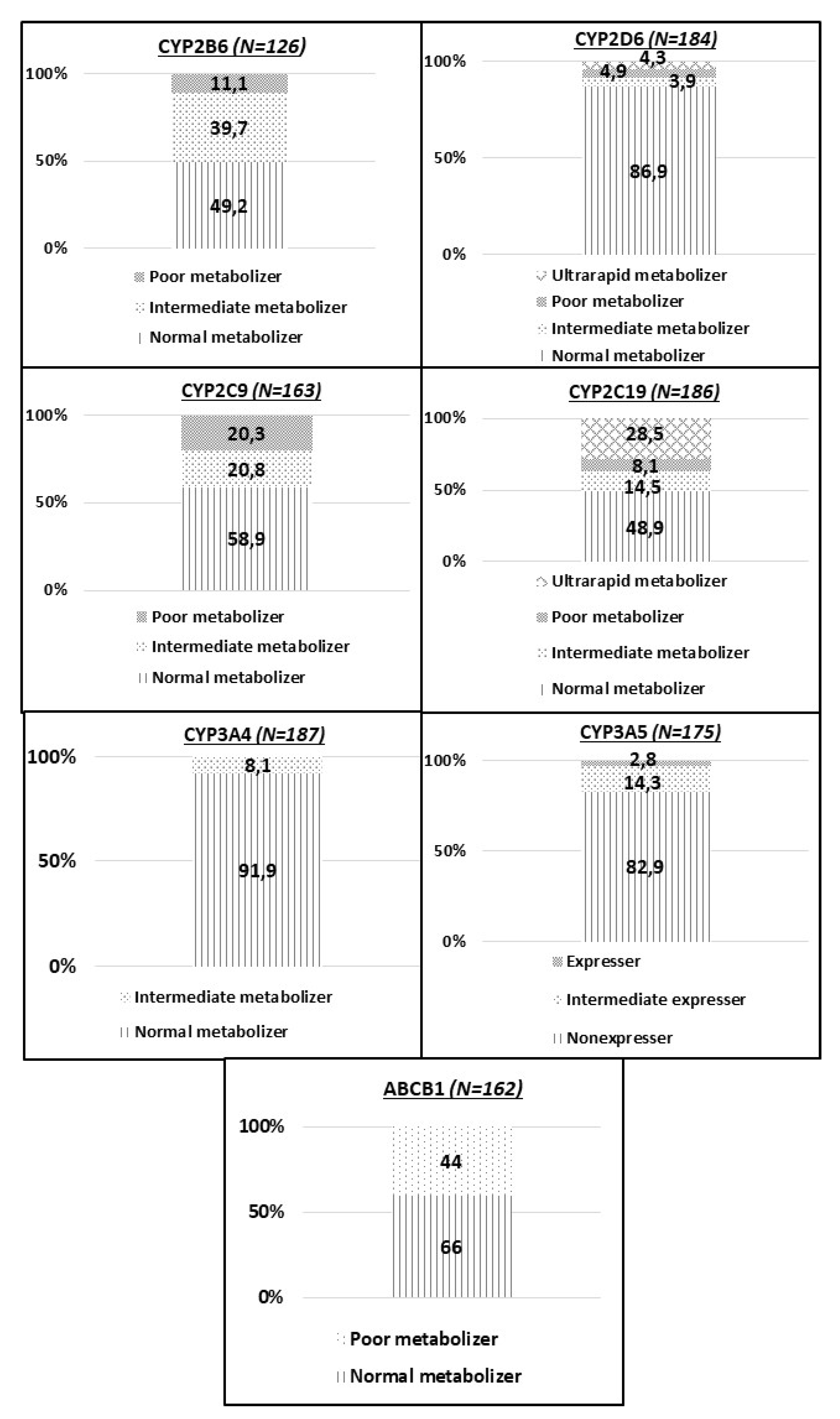
3.3. Pharmacogenetic Analysis
3.4. Identification of Novel Allelic Variants
3.5. Clinical Results
3.6. Economic Implications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahar, M.A.; Setiawan, D.; Hak, E.; Wilffert, B. Pharmacogenetics of drug–drug interaction and drug–drug–gene interaction: A systematic review on CYP2C9, CYP2C19 and CYP2D6. Pharmacogenomics 2017, 18, 701–739. [Google Scholar] [CrossRef]
- Elliott, L.S.; Henderson, J.C.; Neradilek, M.B.; Moyer, N.A.; Ashcraft, K.C.; Thirumaran, R.K. Clinical im-pact of pharmacogenetic profiling with a clinical decision support tool in polypharmacy home health patients: A prospective pilot randomized controlled trial. PLoS ONE 2017, 12, e0170905. [Google Scholar] [CrossRef] [Green Version]
- Lewis, D.F. 57 varieties: The human cytochromes P450. Pharmacogenomics 2004, 5, 305–318. [Google Scholar] [CrossRef]
- Zhou, S.-F. Polymorphism of Human Cytochrome P450 2D6 and Its Clinical Significance. Part I. Clin. Pharmacokinet. 2009, 48, 689–723. [Google Scholar] [CrossRef]
- Zhou, S.-F. Polymorphism of Human Cytochrome P450 2D6 and Its Clinical Significance. Part II. Clin. Pharmacokinet. 2009, 48, 761–804. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, A.; Sadighi, A.; Ryu, R.; Akhlaghi, F. Physicochemical Properties, Biotransformation, and Transport Pathways of Established and Newly Approved Medications: A Systematic Review of the Top 200 Most Prescribed Drugs vs. the FDA-Approved Drugs Between 2005 and 2016. Clin. Pharmacokinet. 2019, 58, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Turpeinen, M.; Klein, K.; Schwab, M. Functional pharmacogenetics/genomics of human cyto-chromes P450 involved in drug biotransformation. Anal. Bioanal. Chem. 2008, 392, 1093–1108. [Google Scholar] [CrossRef] [PubMed]
- Ingelman-Sundberg, M.; Sim, S.C.; Gomez, A.; Rodriguez-Antona, C. Influence of cytochrome P450 polymor-phisms on drug therapies: Pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol. Ther. 2007, 116, 496–526. [Google Scholar] [CrossRef] [PubMed]
- Waring, R.H. Cytochrome P450: Genotype to phenotype. Xenobiotica 2020, 50, 9–18. [Google Scholar] [CrossRef]
- Hocum, B.T.; White, J.R.; Heck, J.W.; Thirumaran, R.K.; Moyer, N.; Newman, R.; Ashcraft, K. Cytochrome P–450 gene and drug interaction analysis in patients referred for pharmaco-genetic testing. Am. J. Health Syst. Pharm. 2016, 73, 61–67. [Google Scholar] [CrossRef]
- Brockmöller, J.; Kirchheiner, J.; Meisel, C.; Roots, I. Pharmacogenetic diagnostics of cytochrome P450 poly-morphisms in clinical drug development and in drug treatment. Pharmacogenomics 2000, 1, 125–151. [Google Scholar] [CrossRef]
- Zhou, Z.-W.; Chen, X.-W.; Sneed, K.B.; Yang, Y.-X.; Zhang, X.; He, Z.-X.; Chow, K.; Yang, T.; Duan, W.; Zhou, S.-F. Clinical Association between Pharmacogenomics and Adverse Drug Reactions. Drugs 2015, 75, 589–631. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Dasgupta, A. Therapeutic Drug Monitoring: Newer Drugs and Biomarkers; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Wang, D.; Guo, Y.; Wrighton, S.A.; Cooke, G.E.; Sadee, W. Intronic polymorphism in CYP3A4 affects hepatic expression and response to statin drugs. Pharm. J. 2011, 11, 274–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaedigk, A.; Ingelman-Sundberg, M.; Miller, N.A.; Leeder, J.S.; Whirl-Carrillo, M.; Klein, T.E. The Pharmacogene Variation (PharmVar) Consortium: Incorporation of the Human Cyto-chrome P450 (CYP) Allele Nomenclature Database. Clin. Pharmacol. Ther. 2018, 103, 399–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nofziger, C.; Turner, A.J.; Sangkuhl, K.; Whirl-Carrillo, M.; Agúndez, J.A.G.; Black, J.L.; Dunnenberger, H.M.; Ruano, G.; Kennedy, M.A.; Phillips, M.S.; et al. PharmVar GeneFocus: CYP2D6. Clin. Pharmacol. Ther. 2020, 107, 154–170. [Google Scholar] [CrossRef] [Green Version]
- Kalman, L.V.; Agundez, J.; Appell, M.L.; Black, J.L.; Bell, G.C.; Boukouvala, S.; Zanger, U.M. Pharmaco-genetic allele nomenclature: International workgroup recommendations for test result reporting. Clin. Pharmacol. Ther. 2016, 99, 172–185. [Google Scholar] [CrossRef]
- Caudle, K.E.; Sangkuhl, K.; Whirl-Carrillo, M.; Swen, J.J.; Haidar, C.E.; Klein, T.E.; Gammal, R.S.; Relling, M.V.; Scott, S.A.; Hertz, D.L.; et al. Standardizing CYP 2D6 Genotype to Phenotype Translation: Consensus Recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clin. Transl. Sci. 2020, 13, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Mutawi, T.M.; Zedan, M.M.; Yahya, R.S.; Zakria, M.M.; El-Sawi, M.R.; Gaedigk, A. Genetic variability of CYP2D6, CYP3A4 and CYP3A5 among the Egyptian population. Pharmacogenomics 2021, 22, 323–334. [Google Scholar] [CrossRef]
- Ingelman-Sundberg, M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): Clinical consequences, evolutionary aspects and functional diversity. Pharm. J. 2005, 5, 6–13. [Google Scholar] [CrossRef]
- Radford, H.; Simpson, K.H.; Rogerson, S.; Johnson, M.I. A Single Site Population Study to Investigate CYP2D6 Phenotype of Patients with Persistent Non-Malignant Pain. Medicina 2019, 55, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The International Transporter Consortium. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [CrossRef] [PubMed]
- SEARCH Collaborative Group. SLCO1B1 Variants and Statin-Induced Myopathy—A Genomewide Study. N. Engl. J. Med. 2008, 359, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Pasanen, M.K.; Fredrikson, H.; Neuvonen, P.J.; Niemi, M. Different Effects of SLCO1B1 Polymorphism on the Pharmacokinetics of Atorvastatin and Rosuvastatin. Clin. Pharmacol. Ther. 2007, 82, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Vadillo, E.; Garcia-Berrocal, B.; Sanchez-Martín, A.; Isidoro-García, M. Análisis de coste-efectividad del genotipado de CYP3A4/CYP3A5 en pacientes polimedicados. Gest. Eval. Costes Sanit. 2014, 15, 521–534. [Google Scholar]
- Chan, H.T.; Chin, Y.M.; Low, S.-K. The Roles of Common Variation and Somatic Mutation in Cancer Pharmacogenomics. Oncol. Ther. 2019, 7, 1–32. [Google Scholar] [CrossRef]
- World Health Organization. The Anatomical Therapeutic Chemical Classification System with Defined Daily Doses (ATC/DDD). Available online: http://www.who.int/classifications/atcddd/en/ (accessed on 3 June 2018).
- PharmGKB. The Pharmacogenomics Knowledge Base. Available online: https://www.pharmgkb.org (accessed on 3 June 2018).
- PubMed Homepage. Available online: http://www.ncbi.nlm.nih.gov/pubmed/ (accessed on 3 June 2018).
- SuperCYP. Bioinformatics.charite.de. Available online: http://bioinformatics.charite.de/supercyp/ (accessed on 3 June 2018).
- PharmVar. Pharmvar.org. Available online: https://www.pharmvar.org/ (accessed on 26 January 2020).
- Rebsamen, M.C.; Desmeules, J.; Daali, Y.; Chiappe, A.; Diemand, A.; Rey, C.; Chabert, J.; Dayer, P.; Hochstrasser, D.; Rossier, M.F. The AmpliChip CYP450 test: Cytochrome P450 2D6 genotype assessment and phenotype prediction. Pharm. J. 2009, 9, 34–41. [Google Scholar] [CrossRef]
- AutoGenomics. Autogenomics.com. Available online: http://www.autogenomics.com/ (accessed on 4 March 2020).
- Sánchez-Iglesias, S.; García-Solaesa, V.; García-Berrocal, B.; Sanchez-Martín, A.; Lorenzo-Romo, C.; Martín-Pinto, T.; Gaedigk, A.; González-Buitrago, J.M.; Isidoro-García, M. Role of Pharmacogenetics in Improving the Safety of Psychiatric Care by Predicting the Potential Risks of Mania in CYP2D6 Poor Metabolizers Diagnosed with Bipolar Disorder. Medicine 2016, 95, e2473. [Google Scholar] [CrossRef] [PubMed]
- Gaedigk, A.; Isidoro-García, M.; Pearce, R.E.; Sánchez, S.; García-Solaesa, V.; Lorenzo-Romo, C.; Gonzalez-Tejera, G.; Corey, S. Discovery of the nonfunctional CYP2D6*31 allele in Spanish, Puerto Rican, and US Hispanic populations. Eur. J. Clin. Pharmacol. 2010, 66, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Gaedigk, A.; Hernandez, J.; García-Solaesa, V.; Sánchez, S.; Isidoro-García, M. Detection and characterization of the CYP2D6*9x2 gene duplication in two Spanish populations: Resolution of AmpliChip CYP450 test no-calls. Pharmacogenomics 2011, 12, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Gaedigk, A.; Riffel, A.K.; Berrocal, M.B.G.; Solaesa, V.G.; Dávila, I.; Isidoro-Garcia, M. Characterization of a complex CYP2D6 genotype that caused an AmpliChip CYP450 Test® no-call in the clinical setting. Clin. Chem. Lab. Med. 2014, 52. [Google Scholar] [CrossRef] [PubMed]
- Isidoro-García, M.; Sánchez-Martín, A.; García-Berrocal, B.; Román-Curto, C. Primun non nocere, polypharmacy and pharmacogenetics. Pharmacogenomics 2015, 16, 1903–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, A.S.; Iglesias Gomez, A.; Garcia-Berrocal, B.; Cabrera Figueroa, S.; Cardero Sanchez, M.; Calvo Hernandez, M.V.; Gonzalez-Buitrago, J.M.; Valverde Merino, M.P.; Bustos Tovar, C.; Fuertes Marin, A.; et al. Dose reduction of efavirenz: An observational study describing cost–effectiveness, pharmacokinetics and pharmacogenetics. Pharmacogenomics 2014, 15, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Carrascal-Laso, L.; Franco-Martín, M.; Marcos-Vadillo, E.; Ramos-Gallego, I.; García-Berrocal, B.; Mayor-Toranzo, E.; Sánchez-Iglesias, S.; Lorenzo, C.; Sevillano-Jiménez, A.; Sánchez-Martín, A.; et al. Economic Impact of the Application of a Precision Medicine Model (5SPM) on Psychotic Patients. Pharm. Pers. Med. 2021, 14, 1015–1025. [Google Scholar] [CrossRef]
- Stingl, J.C.; Brockmöller, J.; Viviani, R. Genetic variability of drug-metabolizing enzymes: The dual impact on psychiatric therapy and regulation of brain function. Mol. Psychiatry 2013, 18, 273–287. [Google Scholar] [CrossRef]
- Isvoran, A.; Louet, M.; Vladoiu, D.L.; Craciun, D.; Loriot, M.-A.; Villoutreix, B.; Miteva, M.A. Pharmacogenomics of the cytochrome P450 2C family: Impacts of amino acid variations on drug metabolism. Drug Discov. Today 2017, 22, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Woodside, B. Is there a role for pharmacogenetics in the treatment of anorexia nervosa? Pharmacogenomics 2016, 17, 1381–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tracy, T.S.; Chaudhry, A.S.; Prasad, B.V.S.S.S.; Thummel, K.E.; Schuetz, E.G.; Zhong, X.-B.; Tien, Y.-C.; Jeong, H.; Pan, X.; Shireman, L.M.; et al. Interindividual Variability in Cytochrome P450-Mediated Drug Metabolism. Drug Metab. Dispos. 2016, 44, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Ingelman-Sundberg, M. Pharmacogenetics of cytochrome P450 and its applications in drug therapy: The past, present and future. Trends Pharmacol. Sci. 2004, 25, 193–200. [Google Scholar] [CrossRef]
| Variable | Value |
|---|---|
| PATIENTS | |
| Total number of caucasian patients included: | 210 |
| - Average age (range; years) | 48 (9–91) |
| - Male: Female (%) | 50.5: 49.5 |
| PHARMACOGENETIC ANALYSIS REQUEST | |
| Application request (% of total) | |
| - Adverse events | 47.1 |
| - Poor response to treatment | 19.0 |
| - Others | 33.9 |
| Medical specialties applicants (% of total) | |
| - Psychiatry | |
| - Eating disorders unit | 65.2 |
| - Allergy | 9.5 |
| - Others (rheumatology, pediatric, oncology, neurology, pharmacy, hematology, infectious disease) | 4.7 |
| 20.6 | |
| Rank | Drug | Drug-Gene Interaction Counts |
|---|---|---|
| 1 | Omeprazole | 53 |
| 2 | Quetiapine | 47 |
| 3 | Olanzapine | 42 |
| 4 | Risperidone | 37 |
| 5 | Venlafaxine | 34 |
| 6 | Aripiprazole | 33 |
| 7 | Sertraline | 33 |
| 8 | Valproic Acid | 24 |
| 9 | Paracetamol/Acetaminophen | 24 |
| 10 | Clonazepam | 20 |
| 11 | Haloperidol | 20 |
| 12 | Clozapine | 19 |
| 13 | Fluoxetine | 18 |
| 14 | Alprazolam | 15 |
| 15 | Escitalopram | 15 |
| 16 | Methadone | 15 |
| 17 | Zolpidem | 15 |
| 18 | Atorvastatin | 13 |
| 19 | Diazepam | 11 |
| 20 | Cholecalciferol | 9 |
| 21 | Trazodone | 9 |
| 22 | Carbamazepine | 8 |
| 23 | Paroxetine | 8 |
| 24 | Rosuvastatin | 8 |
| 25 | Bupropion | 7 |
| Rank | Drug | Drug-Drug Interaction Counts |
|---|---|---|
| 1 | Omeprazole | 141 |
| 2 | Olanzapine | 138 |
| 3 | Quetiapine | 113 |
| 4 | Sertraline | 100 |
| 5 | Valproic Acid | 83 |
| 6 | Aripiprazole | 80 |
| 7 | Venlafaxine | 70 |
| 8 | Clozapine | 68 |
| 9 | Risperidone | 65 |
| 10 | Fluoxetine | 62 |
| 11 | Paracetamol/Acetaminophen | 59 |
| 12 | Clonazepam | 59 |
| 13 | Escitalopram | 38 |
| 14 | Methadone | 35 |
| 15 | Zolpidem | 35 |
| 16 | Haloperidol | 32 |
| 17 | Mirtazapine | 31 |
| 18 | Diazepam | 27 |
| 19 | Trazodone | 27 |
| 20 | Atorvastatin | 25 |
| 21 | Bupropion | 24 |
| 22 | Simvastatin | 24 |
| 23 | Cholecalciferol | 23 |
| 24 | Paroxetine | 19 |
| Gene | Variants (SNPs) |
|---|---|
| CYP2C9 | rs1799853 (CYP2C9*2) |
| rs1057910 (CYP2C9*3) | |
| CYP2C19 | rs4244285 (CYP2C19*2) |
| rs4986893 (CYP2C19*3) | |
| rs12248560 (CYP2C19*17) | |
| CYP3A4 | rs2740574 (CYP3A4*1b) |
| CYP3A5 | rs776746 (CYP3A5*3) |
| ABCB1 | rs1045642 (C3435T) |
| CYP2D6 | rs1080985 (CYP2D6*2A) |
| rs1065852 (CYP2D6*10 and *4) | |
| rs28371706 (CYP2D6*17, *40, *58 and *64) | |
| rs5030655 (CYP2D6*6) | |
| rs5030865 (CYP2D6*8 and *14) | |
| rs3892097 (CYP2D6*4) | |
| rs5030862 (CYP2D6*12) | |
| rs61736512 (CYP2D6*1, *1xN, *2xN, *3xN, *4xN, *6xN, *9x2, *10x2, *17x2, *29, *29x2, *35xN, *36xN, *41x2, *43xN, *45xN, *70, *107 and *149) | |
| rs28371725 (CYP2D6*41) | |
| rs35742686 (CYP2D6*3) | |
| rs5030656 (CYP2D6*9) | |
| rs16947 (CYP2D6*2) | |
| rs5030867 (CYP2D6*7) | |
| CYP2B6 | rs3745274 (CYP2B6*6) |
| CYP1A2 | rs762551 (CYP1A2*1F) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Martín, M.C.; García-Berrocal, B.; Sánchez-Martín, A.; Marcos-Vadillo, E.; García-Salgado, M.J.; Sánchez, S.; Lorenzo, C.; González-Parra, D.; Sans, F.; Franco, M.; et al. Ten Years of Experience Support Pharmacogenetic Testing to Guide Individualized Drug Therapy. Pharmaceutics 2022, 14, 160. https://doi.org/10.3390/pharmaceutics14010160
Peña-Martín MC, García-Berrocal B, Sánchez-Martín A, Marcos-Vadillo E, García-Salgado MJ, Sánchez S, Lorenzo C, González-Parra D, Sans F, Franco M, et al. Ten Years of Experience Support Pharmacogenetic Testing to Guide Individualized Drug Therapy. Pharmaceutics. 2022; 14(1):160. https://doi.org/10.3390/pharmaceutics14010160
Chicago/Turabian StylePeña-Martín, María Celsa, Belén García-Berrocal, Almudena Sánchez-Martín, Elena Marcos-Vadillo, María Jesús García-Salgado, Santiago Sánchez, Carolina Lorenzo, David González-Parra, Francisco Sans, Manuel Franco, and et al. 2022. "Ten Years of Experience Support Pharmacogenetic Testing to Guide Individualized Drug Therapy" Pharmaceutics 14, no. 1: 160. https://doi.org/10.3390/pharmaceutics14010160
APA StylePeña-Martín, M. C., García-Berrocal, B., Sánchez-Martín, A., Marcos-Vadillo, E., García-Salgado, M. J., Sánchez, S., Lorenzo, C., González-Parra, D., Sans, F., Franco, M., Gaedigk, A., Mateos-Sexmero, M. J., Sanz, C., & Isidoro-García, M. (2022). Ten Years of Experience Support Pharmacogenetic Testing to Guide Individualized Drug Therapy. Pharmaceutics, 14(1), 160. https://doi.org/10.3390/pharmaceutics14010160







