Microfluidic Synthesis and Analysis of Bioinspired Structures Based on CaCO3 for Potential Applications as Drug Delivery Carriers
Abstract
1. Introduction
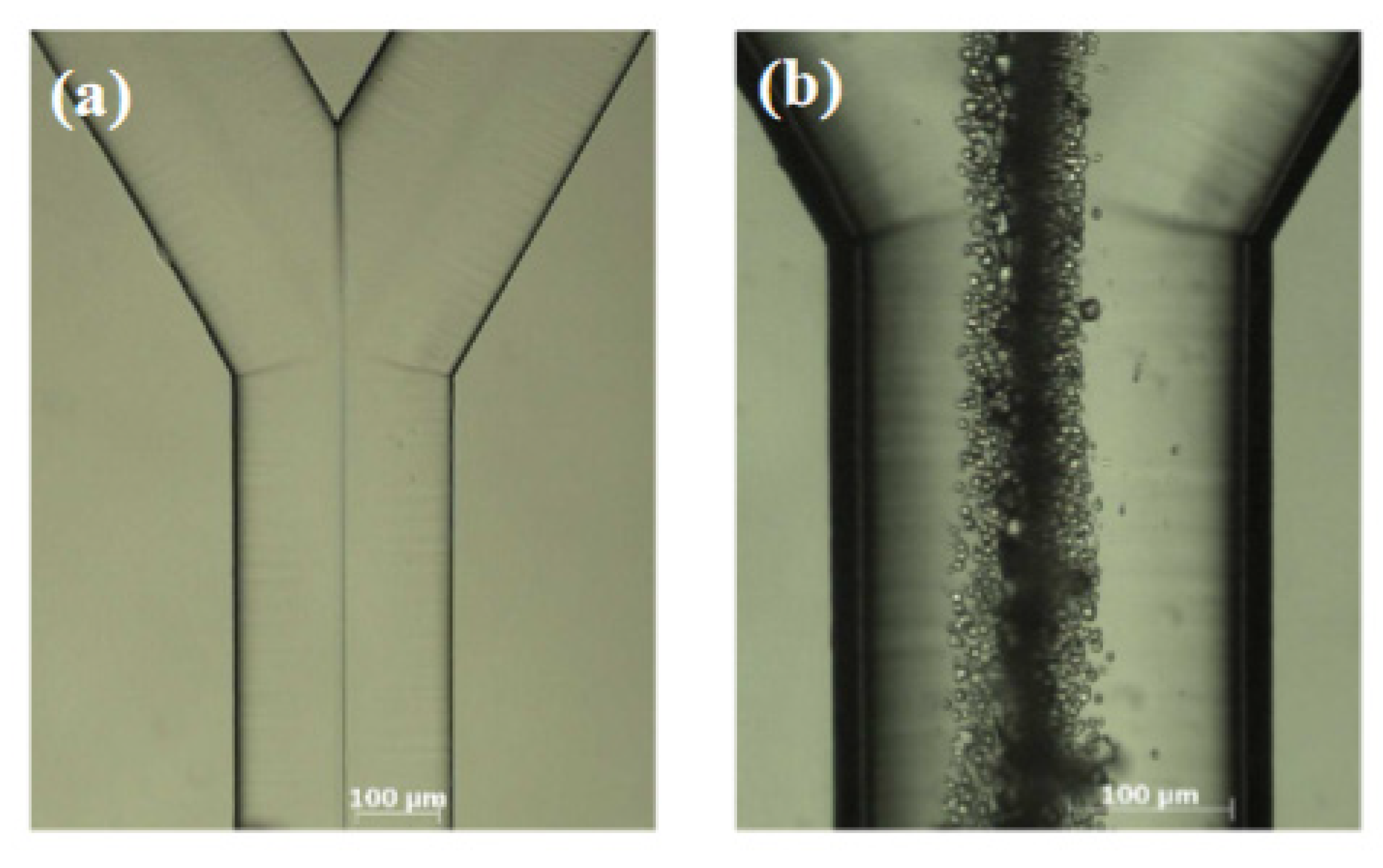
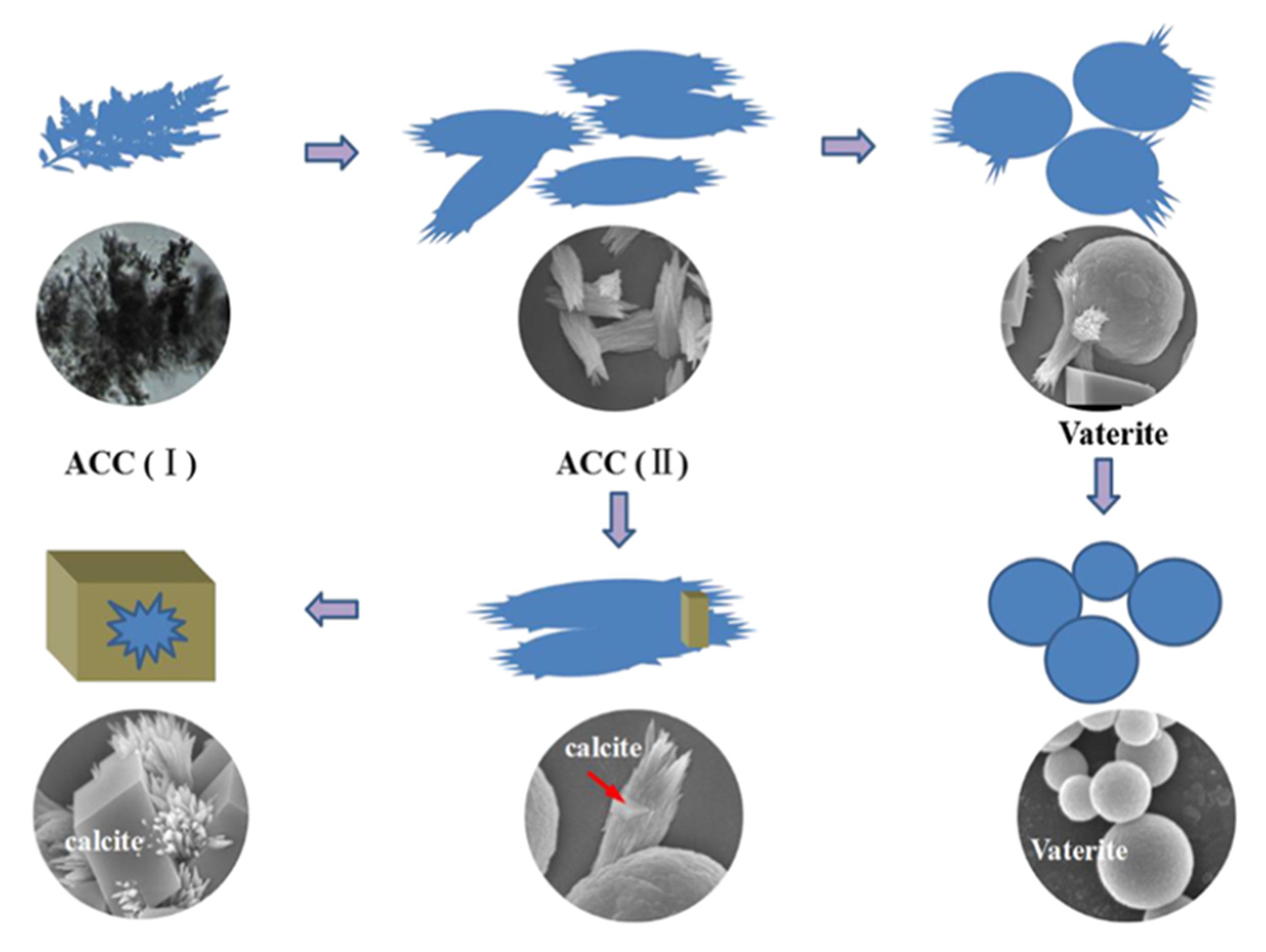
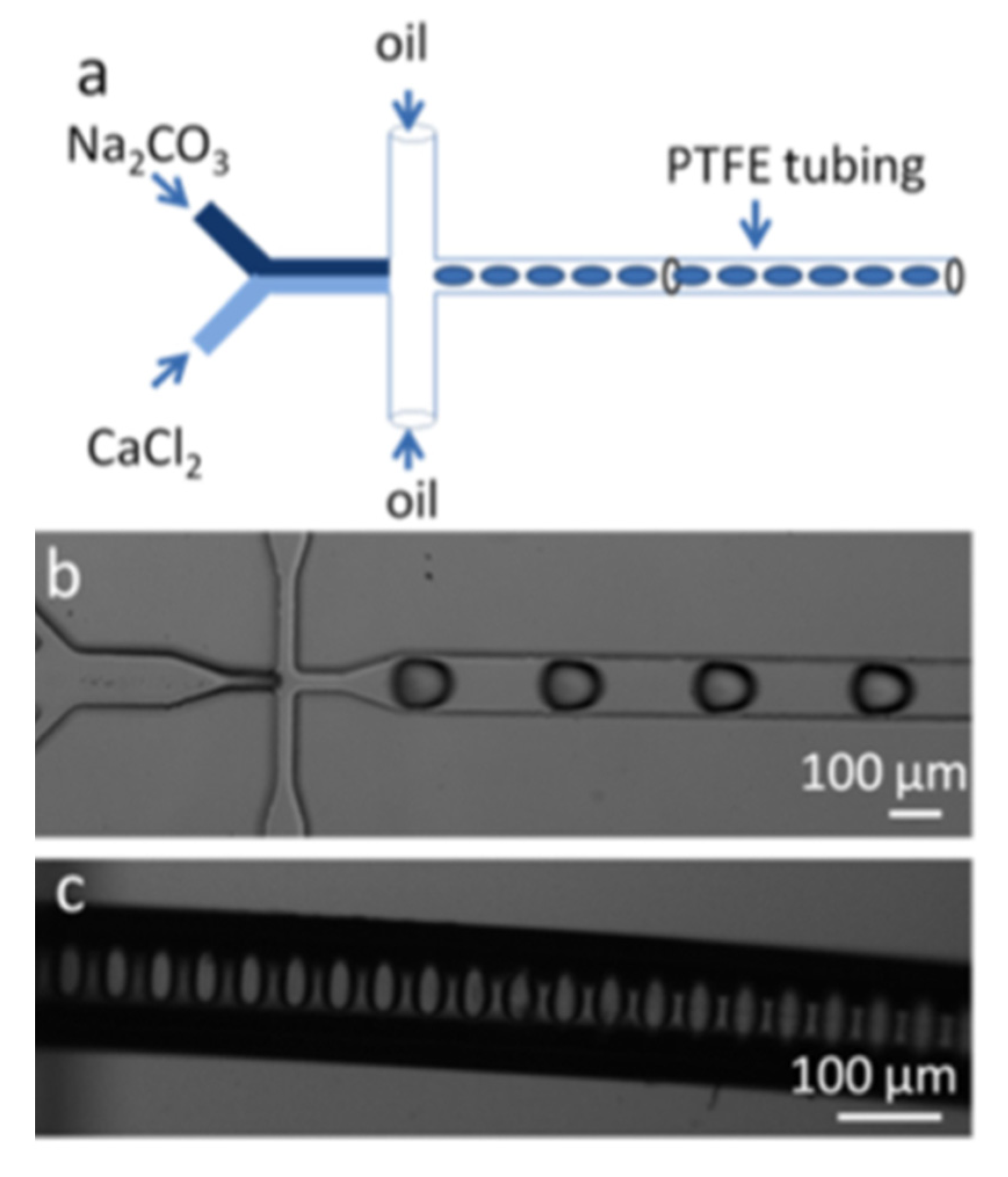
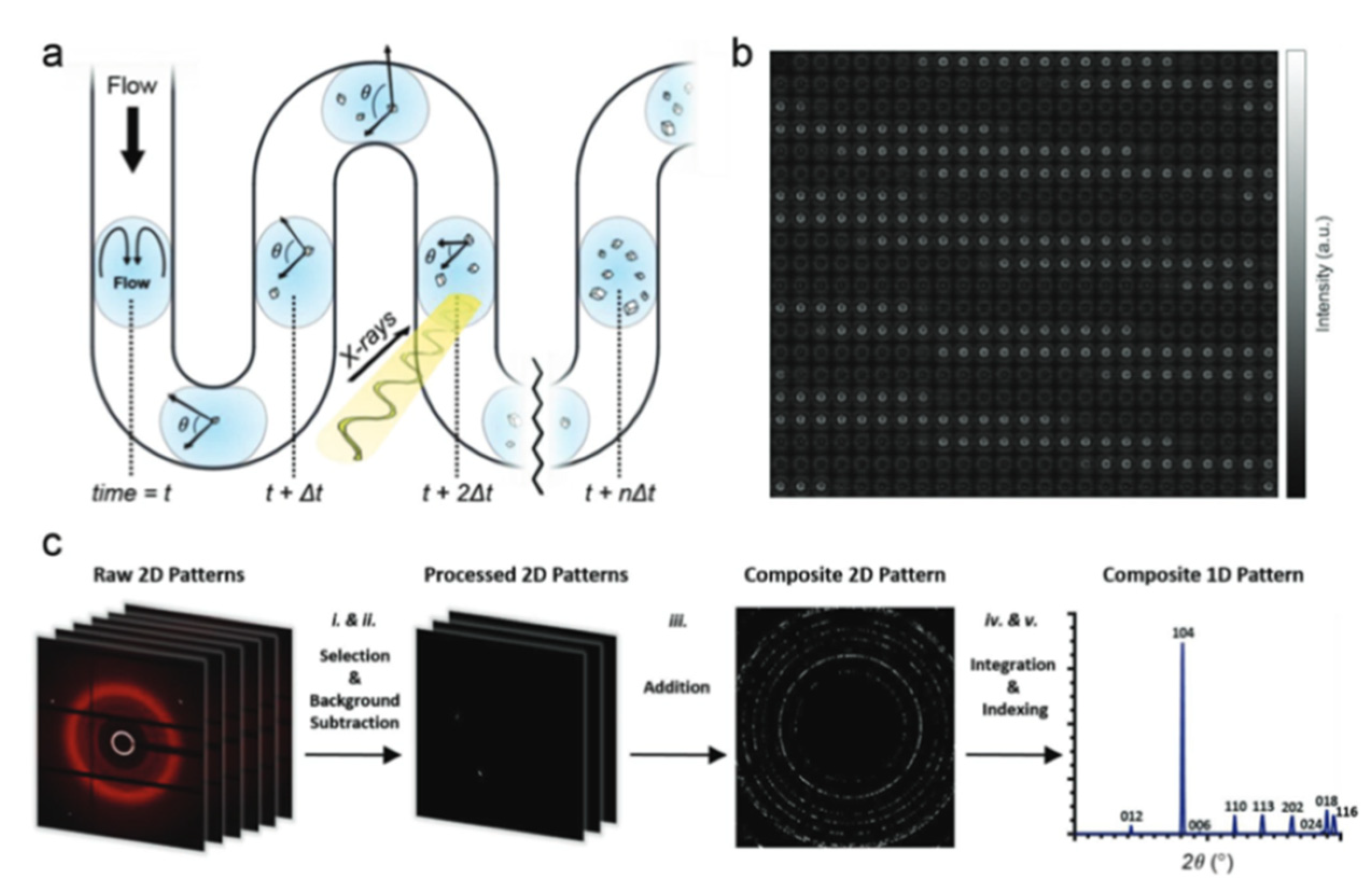
2. Microfluidic CaCO3 Synthesis
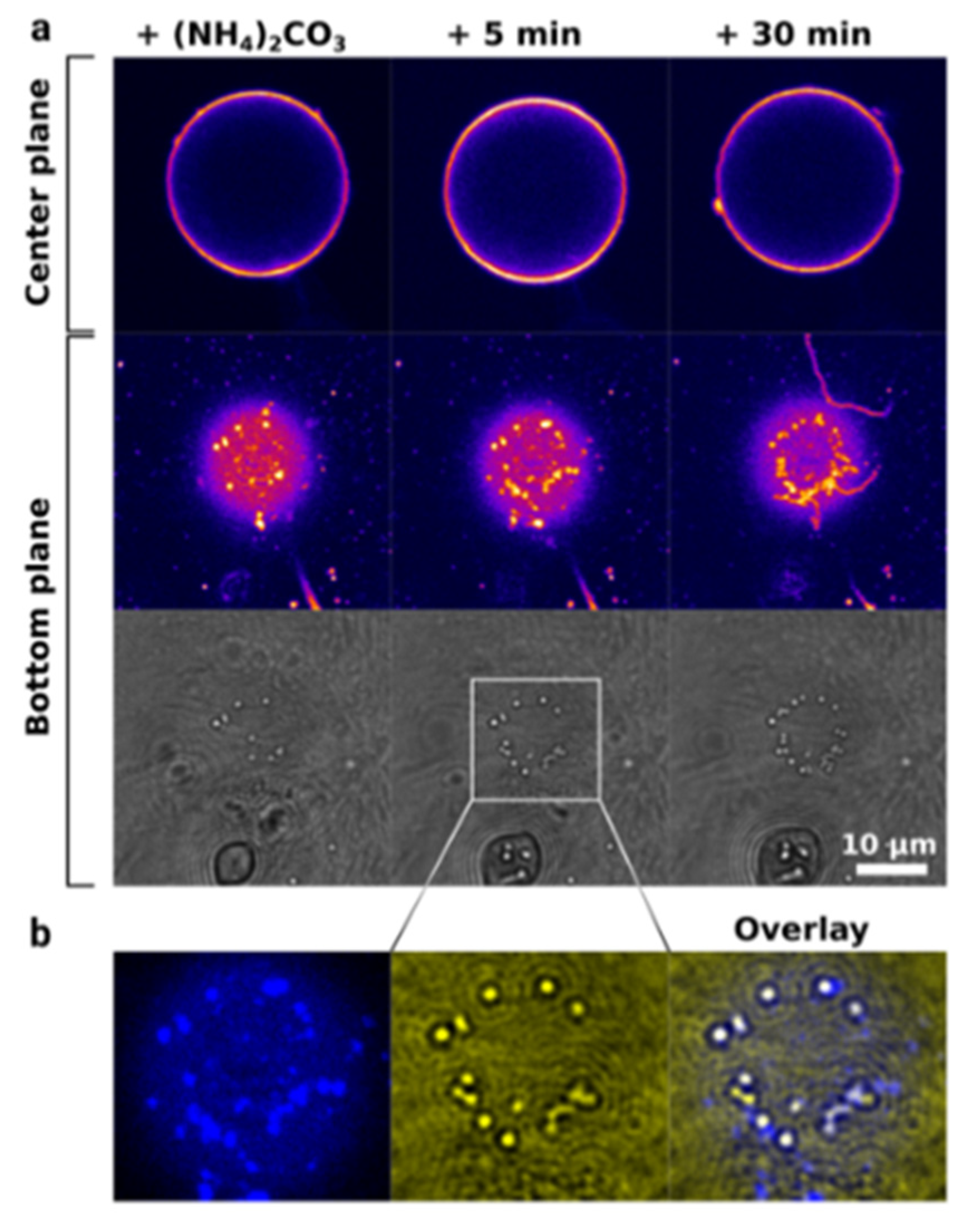
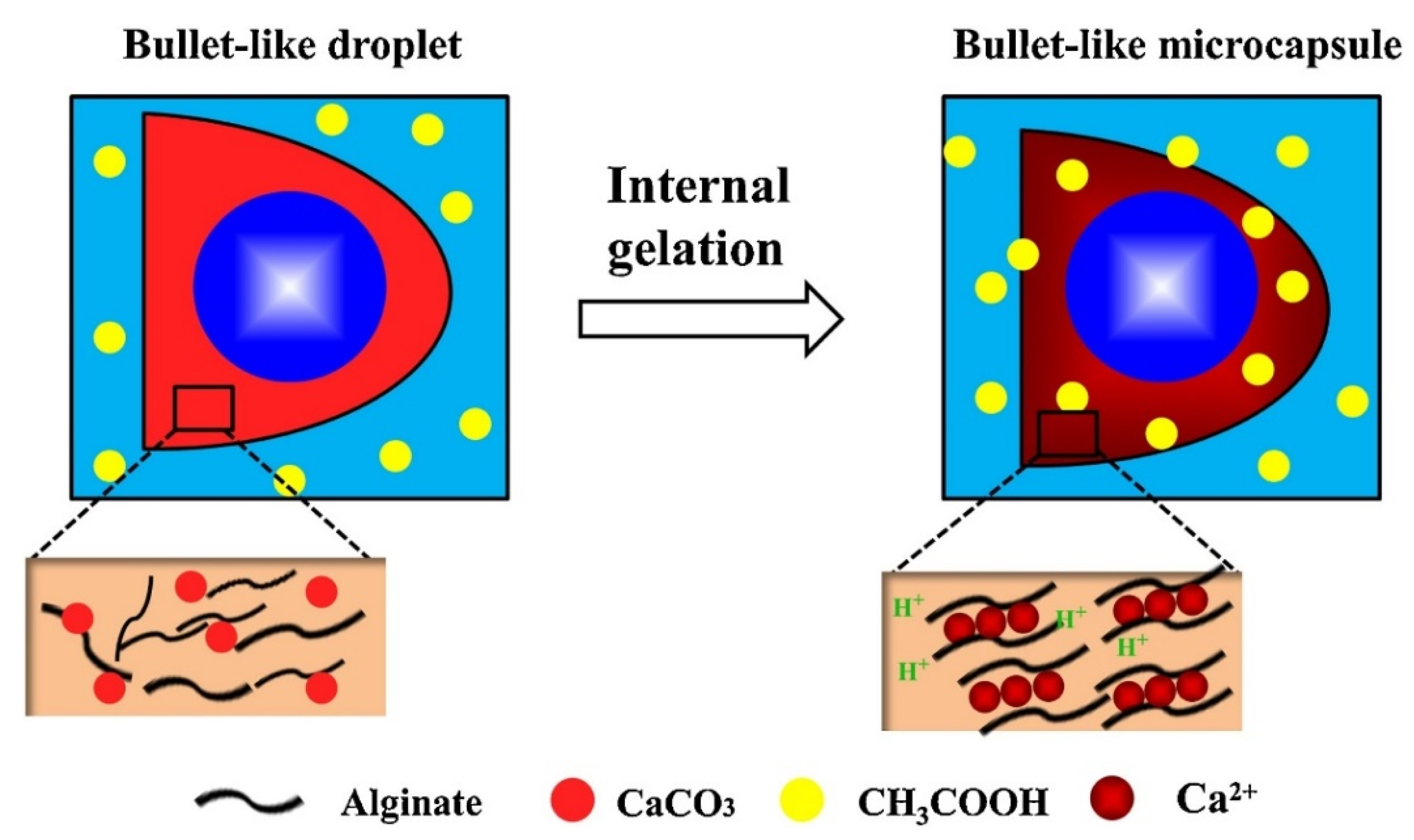
3. CaCO3-Based Core-Shell Structures
4. Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Zhang, L.; Ge, X.; Xu, B.; Zhang, W.; Qu, L.; Choi, C.-H.; Xu, J.; Zhang, A.; Lee, H.; et al. Microfluidic fabrication of microparticles for biomedical applications. Chem. Soc. Rev. 2018, 47, 5646–5683. [Google Scholar] [CrossRef]
- Chiesa, E.; Dorati, R.; Pisani, S.; Conti, B.; Bergamini, G.; Modena, T.; Genta, I. The Microfluidic Technique and the Manufacturing of Polysaccharide Nanoparticles. Pharmaceutics 2018, 10, 267. [Google Scholar] [CrossRef]
- Campbell, Z.S.; Abolhasani, M. Facile synthesis of anhydrous microparticles using plug-and-play microfluidic reactors. React. Chem. Eng. 2020, 5, 1198–1211. [Google Scholar] [CrossRef]
- Desai, D.; Guerrero, Y.A.; Balachandran, V.; Morton, A.; Lyon, L.; Larkin, B.; Solomon, D.E. Towards a microfluidics platform for the continuous manufacture of organic and inorganic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2021, 35, 102402. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Haase, A.; Antolini, R. Sub-Micrometer Vaterite Containers: Synthesis, Substance Loading, and Release. Angew. Chem. 2012, 124, 1221–1223. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K.; Lotfipour, F. Calcium carbonate nanoparticles as cancer drug delivery system. Expert Opin. Drug Deliv. 2015, 12, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, F.C. Calcium carbonate in biomineralisation and biomimetic chemistry. Int. Mater. Rev. 2003, 48, 187–224. [Google Scholar] [CrossRef]
- Addadi, L.; Raz, S.; Weiner, S. Taking Advantage of Disorder: Amorphous Calcium Carbonate and Its Roles in Biomineralization. Adv. Mater. 2003, 15, 959–970. [Google Scholar] [CrossRef]
- Weiner, S.; Addadi, L. Crystallization Pathways in Biomineralization. Annu. Rev. Mater. Res. 2011, 41, 21–40. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. 2013, 4, 314. [Google Scholar] [CrossRef]
- Mass, T.; Giuffre, A.J.; Sun, C.-Y.; Stifler, C.A.; Frazier, M.J.; Neder, M.; Tamura, N.; Stan, C.V.; Marcus, M.A.; Gilbert, P.U.P.A. Amorphous calcium carbonate particles form coral skeletons. Proc. Natl. Acad. Sci. USA 2017, 114, E7670–E7678. [Google Scholar] [CrossRef]
- Jantschke, A.; Pinkas, I.; Schertel, A.; Addadi, L.; Weiner, S. Biomineralization pathways in calcifying dinoflagellates: Uptake, storage in MgCaP-rich bodies and formation of the shell. Acta Biomater. 2020, 102, 427–439. [Google Scholar] [CrossRef]
- Dupont, L.; Portemer, F.; Figlarz, M. Synthesis and study of a well crystallized CaCO3 vaterite showing a new habitus. J. Mater. Chem. 1997, 7, 797–800. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Volodkin, D.V.; Günther, A.M.; Petrov, A.I.; Shenoy, D.B.; Möhwald, H. Porous calcium carbonate microparticles as templates for encapsulation of bioactive compounds. J. Mater. Chem. 2004, 14, 2073–2081. [Google Scholar] [CrossRef]
- Lengert, E.V.; Averkhovskii, R.; Genina, E.A.; Terentyuk, G.S.; Svenskaya, Y.I. Mesoporous particles for transdermal delivery of the antifungal drug griseofulvin. J. Phys. Conf. Ser. 2020, 1461, 012083. [Google Scholar] [CrossRef]
- Wang, Y.; Price, A.D.; Caruso, F. Nanoporous colloids: Building blocks for a new generation of structured materials. J. Mater. Chem. 2009, 19, 6451. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, Q.; Gao, C. Sustained delivery of doxorubicin by porous CaCO3 and chitosan/alginate multilayers-coated CaCO3 microparticles. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 132–139. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Möhwald, H. Self-Repairing Coatings Containing Active Nanoreservoirs. Small 2007, 3, 926–943. [Google Scholar] [CrossRef]
- Sokolsky-Papkov, M.; Agashi, K.; Olaye, A.; Shakesheff, K.; Domb, A.J. Polymer carriers for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 187–206. [Google Scholar] [CrossRef]
- Feoktistova, N.; Rose, J.; Prokopović, V.Z.; Vikulina, A.S.; Skirtach, A.; Volodkin, D. Controlling the Vaterite CaCO3 Crystal Pores. Design of Tailor-Made Polymer Based Microcapsules by Hard Templating. Langmuir 2016, 32, 4229–4238. [Google Scholar] [CrossRef] [PubMed]
- Parakhonskiy, B.V.; Abalymov, A.; Ivanova, A.; Khalenkow, D.; Skirtach, A.G. Magnetic and silver nanoparticle functionalized calcium carbonate particles—Dual functionality of versatile, movable delivery carriers which can surface-enhance Raman signals. J. Appl. Phys. 2019, 126, 203102. [Google Scholar] [CrossRef]
- Lengert, E.; Verkhovskii, R.; Yurasov, N.; Genina, E.; Svenskaya, Y. Mesoporous carriers for transdermal delivery of antifungal drug. Mater. Lett. 2019, 248, 211–213. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Vikulina, A.S.; Volodkin, D. CaCO3 crystals as versatile carriers for controlled delivery of antimicrobials. J. Control. Release 2020, 328, 470–489. [Google Scholar] [CrossRef]
- Kiryukhin, M.V.; Lim, S.H.; Lau, H.H.; Antipina, M.; Khin, Y.W.; Chia, C.Y.; Harris, P.; Weeks, M.; Berry, C.; Hurford, D.; et al. Surface-reacted calcium carbonate microparticles as templates for lactoferrin encapsulation. J. Colloid Interface Sci. 2021, 594, 362–371. [Google Scholar] [CrossRef]
- Vikulina, A.S.; Feoktistova, N.A.; Balabushevich, N.G.; Skirtach, A.G.; Volodkin, D. The mechanism of catalase loading into porous vaterite CaCO3 crystals by co-synthesis. Phys. Chem. Chem. Phys. 2018, 20, 8822–8831. [Google Scholar] [CrossRef] [PubMed]
- Jenjob, R.; Phakkeeree, T.; Crespy, D. Core–shell particles for drug-delivery, bioimaging, sensing, and tissue engineering. Biomater. Sci. 2020, 8, 2756–2770. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.G.; De Koker, S.; Sukhorukov, G.B.; Kreft, O.; Parak, W.J.; Skirtach, A.G.; Demeester, J.; De Smedt, S.C.; Hennink, W.E. Polyelectrolyte microcapsules for biomedical applications. Soft Matter 2009, 5, 282–291. [Google Scholar] [CrossRef]
- Lengert, E.V.; Savkina, A.A.; Ermakov, A.V.; Saveleva, M.S.; Lagutina, D.D.; Stepanova, T.V.; Ivanov, A.N. Influence of the new formulation based on silver alginate microcapsules loaded with tannic acid on the microcirculation of the experimental periodontitis in rats. Mater. Sci. Eng. C 2021, 126, 112144. [Google Scholar] [CrossRef]
- Ermakov, A.V.; Verkhovskii, R.A.; Babushkina, I.V.; Trushina, D.B.; Inozemtseva, O.A.; Lukyanets, E.A.; Ulyanov, V.J.; Gorin, D.A.; Belyakov, S.; Antipina, M.N. In Vitro Bioeffects of Polyelectrolyte Multilayer Microcapsules Post-Loaded with Water-Soluble Cationic Photosensitizer. Pharmaceutics 2020, 12, 610. [Google Scholar] [CrossRef]
- Yin, H.; Ji, B.; Dobson, P.S.; Mosbahi, K.; Glidle, A.; Gadegaard, N.; Freer, A.; Cooper, J.M.; Cusack, M. Screening of Biomineralization Using Microfluidics. Anal. Chem. 2009, 81, 473–478. [Google Scholar] [CrossRef]
- Ji, B.; Cusack, M.; Freer, A.; Dobson, P.S.; Gadegaard, N.; Yin, H. Control of crystal polymorph in microfluidics using molluscan 28 kDa Ca2+-binding protein. Integr. Biol. 2010, 2, 528. [Google Scholar] [CrossRef]
- Zeng, Y.; Cao, J.; Wang, Z.; Guo, J.; Lu, J. Formation of Amorphous Calcium Carbonate and Its Transformation Mechanism to Crystalline CaCO3 in Laminar Microfluidics. Cryst. Growth Des. 2018, 18, 1710–1721. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Kudłacz, K.; Cizer, Ö.; Ruiz-Agudo, E. Formation of amorphous calcium carbonate and its transformation into mesostructured calcite. CrystEngComm 2015, 17, 58–72. [Google Scholar] [CrossRef]
- Gower, L.B. Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and Its Role in Biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar] [CrossRef]
- Ko, K.-Y.; Kim, I.-H. Aragonite crystallization in a Christmas-tree model microfluidic device with the addition of magnesium ions. Biotechnol. Bioprocess Eng. 2016, 21, 453–462. [Google Scholar] [CrossRef]
- Zeng, Y.; Cao, J.; Wang, Z.; Guo, J.; Zhou, Q.; Lu, J. Insights into the Confined Crystallization in Microfluidics of Amorphous Calcium Carbonate. Cryst. Growth Des. 2018, 18, 6538–6546. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Bots, P.; Roncal-Herrero, T.; Benning, L.G. The role of pH and Mg on the stability and crystallization of amorphous calcium carbonate. J. Alloys Compd. 2012, 536, S477–S479. [Google Scholar] [CrossRef]
- Raiteri, P.; Gale, J.D. Water Is the Key to Nonclassical Nucleation of Amorphous Calcium Carbonate. J. Am. Chem. Soc. 2010, 132, 17623–17634. [Google Scholar] [CrossRef]
- Demichelis, R.; Raiteri, P.; Gale, J.D.; Quigley, D.; Gebauer, D. Stable prenucleation mineral clusters are liquid-like ionic polymers. Nat. Commun. 2011, 2, 590. [Google Scholar] [CrossRef]
- Yoshino, T.; Maruyama, K.; Kagi, H.; Nara, M.; Kim, J.C. Pressure-Induced Crystallization from Amorphous Calcium Carbonate. Cryst. Growth Des. 2012, 12, 3357–3361. [Google Scholar] [CrossRef]
- Du, H.; Steinacher, M.; Borca, C.; Huthwelker, T.; Murello, A.; Stellacci, F.; Amstad, E. Amorphous CaCO3: Influence of the Formation Time on Its Degree of Hydration and Stability. J. Am. Chem. Soc. 2018, 140, 14289–14299. [Google Scholar] [CrossRef]
- Kim, D.; Mahabadi, N.; Jang, J.; Paassen, L.A. Assessing the Kinetics and Pore-Scale Characteristics of Biological Calcium Carbonate Precipitation in Porous Media using a Microfluidic Chip Experiment. Water Resour. Res. 2020, 56. [Google Scholar] [CrossRef]
- Wang, Y.; Soga, K.; Dejong, J.T.; Kabla, A.J. A microfluidic chip and its use in characterising the particle-scale behaviour of microbial-induced calcium carbonate precipitation (MICP). Géotechnique 2019, 69, 1086–1094. [Google Scholar] [CrossRef]
- Wang, Y.; Soga, K.; DeJong, J.T.; Kabla, A.J. Microscale Visualization of Microbial-Induced Calcium Carbonate Precipitation Processes. J. Geotech. Geoenviron. Eng. 2019, 145, 04019045. [Google Scholar] [CrossRef]
- Donatan, S.; Yashchenok, A.; Khan, N.; Parakhonskiy, B.; Cocquyt, M.; Pinchasik, B.E.; Khalenkow, D.; Möhwald, H.; Konrad, M.; Skirtach, A. Loading Capacity versus Enzyme Activity in Anisotropic and Spherical Calcium Carbonate Microparticles. ACS Appl. Mater. Interfaces 2016, 8, 14284–14292. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Yashchenok, A.M.; Donatan, S.; Volodkin, D.V.; Tessarolo, F.; Antolini, R.; Möhwald, H.; Skirtach, A.G. Macromolecule Loading into Spherical, Elliptical, Star-Like and Cubic Calcium Carbonate Carriers. ChemPhysChem 2014, 15, 2817–2822. [Google Scholar] [CrossRef]
- Sviben, S.; Gal, A.; Hood, M.A.; Bertinetti, L.; Politi, Y.; Bennet, M.; Krishnamoorthy, P.; Schertel, A.; Wirth, R.; Sorrentino, A.; et al. A vacuole-like compartment concentrates a disordered calcium phase in a key coccolithophorid alga. Nat. Commun. 2016, 7, 11228. [Google Scholar] [CrossRef]
- Stephens, C.J.; Kim, Y.-Y.; Evans, S.D.; Meldrum, F.C.; Christenson, H.K. Early Stages of Crystallization of Calcium Carbonate Revealed in Picoliter Droplets. J. Am. Chem. Soc. 2011, 133, 5210–5213. [Google Scholar] [CrossRef]
- Schenk, A.S.; Albarracin, E.J.; Kim, Y.-Y.; Ihli, J.; Meldrum, F.C. Confinement stabilises single crystal vaterite rods. Chem. Commun. 2014, 50, 4729–4732. [Google Scholar] [CrossRef]
- Picker, A.; Nuss, H.; Guenoun, P.; Chevallard, C. Polymer Vesicles as Microreactors for Bioinspired Calcium Carbonate Precipitation. Langmuir 2011, 27, 3213–3218. [Google Scholar] [CrossRef]
- Witt, H.; Yandrapalli, N.; Sari, M.; Turco, L.; Robinson, T.; Steinem, C. Precipitation of Calcium Carbonate Inside Giant Unilamellar Vesicles Composed of Fluid-Phase Lipids. Langmuir 2020, 36, 13244–13250. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.; Whittaker, M.L.; Joester, D. Crystallization kinetics of amorphous calcium carbonate in confinement. Chem. Sci. 2019, 10, 5039–5043. [Google Scholar] [CrossRef] [PubMed]
- Yashina, A.; Meldrum, F.; DeMello, A. Calcium carbonate polymorph control using droplet-based microfluidics. Biomicrofluidics 2012, 6, 022001. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Y.; Meldrum, F.C.; Shui, L.; Wang, Z.; Li, S.; Li, G. Investigating the Nucleation Kinetics of Calcium Carbonate Using a Zero-Water-Loss Microfluidic Chip. Cryst. Growth Des. 2020, 20, 2787–2795. [Google Scholar] [CrossRef]
- Levenstein, M.A.; Anduix-Canto, C.; Kim, Y.; Holden, M.A.; González Niño, C.; Green, D.C.; Foster, S.E.; Kulak, A.N.; Govada, L.; Chayen, N.E.; et al. Droplet Microfluidics XRD Identifies Effective Nucleating Agents for Calcium Carbonate. Adv. Funct. Mater. 2019, 29, 1808172. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, H.; Gupta, A.; Chang, S.; Doyle, P.S. Site-Selective In Situ Grown Calcium Carbonate Micromodels with Tunable Geometry, Porosity, and Wettability. Adv. Funct. Mater. 2016, 26, 4896–4905. [Google Scholar] [CrossRef]
- Le-Anh, D.; Rao, A.; Ayirala, S.C.; Alotaibi, M.B.; Duits, M.H.G.; Gardeniers, H.; Yousef, A.A.; Mugele, F. Optical measurements of oil release from calcite packed beds in microfluidic channels. Microfluid. Nanofluidics 2020, 24, 47. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Raichur, A.M. Biologically triggered exploding protein based microcapsules for drug delivery. Chem. Commun. 2012, 48, 2307. [Google Scholar] [CrossRef][Green Version]
- Schmidt, S.; Volodkin, D. Microparticulate biomolecules by mild CaCO3 templating. J. Mater. Chem. B 2013, 1, 1210–1218. [Google Scholar] [CrossRef]
- Bukreeva, T.V.; Parakhonsky, B.V.; Skirtach, A.G.; Susha, A.S.; Sukhorukov, G.B. Preparation of polyelectrolyte microcapsules with silver and gold nanoparticles in a shell and the remote destruction of microcapsules under laser irradiation. Crystallogr. Rep. 2006, 51, 863–869. [Google Scholar] [CrossRef]
- Vergaro, V.; Scarlino, F.; Bellomo, C.; Rinaldi, R.; Vergara, D.; Maffia, M.; Baldassarre, F.; Giannelli, G.; Zhang, X.; Lvov, Y.M.; et al. Drug-loaded polyelectrolyte microcapsules for sustained targeting of cancer cells. Adv. Drug Deliv. Rev. 2011, 63, 847–864. [Google Scholar] [CrossRef]
- Yan, X.; Li, J.; Möhwald, H. Templating assembly of multifunctional hybrid colloidal spheres. Adv. Mater. 2012, 24, 2663–2667. [Google Scholar] [CrossRef]
- Xu, P.; Jiang, F.; Zhang, H.; Yin, R.; Cen, L.; Zhang, W. Calcium Carbonate/Gelatin Methacrylate Microspheres for 3D Cell Culture in Bone Tissue Engineering. Tissue Eng. Part C Methods 2020, 26, 418–432. [Google Scholar] [CrossRef] [PubMed]
- Drotleff, S.; Lungwitz, U.; Breunig, M.; Dennis, A.; Blunk, T.; Tessmar, J.; Göpferich, A. Biomimetic polymers in pharmaceutical and biomedical sciences. Eur. J. Pharm. Biopharm. 2004, 58, 385–407. [Google Scholar] [CrossRef] [PubMed]
- Leong, J.-Y.; Lam, W.-H.; Ho, K.-W.; Voo, W.-P.; Lee, M.F.-X.; Lim, H.-P.; Lim, S.-L.; Tey, B.-T.; Poncelet, D.; Chan, E.-S. Advances in fabricating spherical alginate hydrogels with controlled particle designs by ionotropic gelation as encapsulation systems. Particuology 2016, 24, 44–60. [Google Scholar] [CrossRef]
- Caetano, L.; Almeida, A.; Gonçalves, L. Effect of Experimental Parameters on Alginate/Chitosan Microparticles for BCG Encapsulation. Mar. Drugs 2016, 14, 90. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, S.; Wang, H.; Zhu, J.; Yang, Y. Alginate droplets pre-crosslinked in microchannels to prepare monodispersed spherical microgels. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 371–377. [Google Scholar] [CrossRef]
- Huang, L.; Wu, K.; He, X.; Yang, Z.; Ji, H. One-Step microfluidic synthesis of spherical and bullet-like alginate microcapsules with a core–shell structure. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125612. [Google Scholar] [CrossRef]
- Huang, L.; Wu, K.; Zhang, R.; Ji, H. Fabrication of Multicore Milli- and Microcapsules for Controlling Hydrophobic Drugs Release Using a Facile Approach. Ind. Eng. Chem. Res. 2019, 58, 17017–17026. [Google Scholar] [CrossRef]
- Shimoni, O.; Yan, Y.; Wang, Y.; Caruso, F. Shape-Dependent Cellular Processing of Polyelectrolyte Capsules. ACS Nano 2013, 7, 522–530. [Google Scholar] [CrossRef]
- Ejima, H.; Yanai, N.; Best, J.P.; Sindoro, M.; Granick, S.; Caruso, F. Near-Incompressible Faceted Polymer Microcapsules from Metal-Organic Framework Templates. Adv. Mater. 2013, 25, 5767–5771. [Google Scholar] [CrossRef] [PubMed]
- Volodkin, D. CaCO3 templated micro-beads and -capsules for bioapplications. Adv. Colloid Interface Sci. 2014, 207, 306–324. [Google Scholar] [CrossRef] [PubMed]
- She, S.; Li, Q.; Shan, B.; Tong, W.; Gao, C. Fabrication of Red-Blood-Cell-Like Polyelectrolyte Microcapsules and Their Deformation and Recovery Behavior Through a Microcapillary. Adv. Mater. 2013, 25, 5814–5818. [Google Scholar] [CrossRef] [PubMed]
- Doshi, N.; Mitragotri, S. Macrophages Recognize Size and Shape of Their Targets. PLoS ONE 2010, 5, e10051. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kozlovskaya, V.; Goins, A.; Campos-Gomez, J.; Saeed, M.; Kharlampieva, E. Biocompatible Shaped Particles from Dried Multilayer Polymer Capsules. Biomacromolecules 2013, 14, 3830–3841. [Google Scholar] [CrossRef]
- Yu, L.; Sun, Q.; Hui, Y.; Seth, A.; Petrovsky, N.; Zhao, C.-X. Microfluidic formation of core-shell alginate microparticles for protein encapsulation and controlled release. J. Colloid Interface Sci. 2019, 539, 497–503. [Google Scholar] [CrossRef]
- Damiati, S. In Situ Microfluidic Preparation and Solidification of Alginate Microgels. Macromol. Res. 2020, 28, 1046–1053. [Google Scholar] [CrossRef]
- Soldatov, M.A.; Butova, V.V.; Pashkov, D.; Butakova, M.A.; Medvedev, P.V.; Chernov, A.V.; Soldatov, A.V. Self-Driving Laboratories for Development of New Functional Materials and Optimizing Known Reactions. Nanomaterials 2021, 11, 619. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lengert, E.V.; Trushina, D.B.; Soldatov, M.; Ermakov, A.V. Microfluidic Synthesis and Analysis of Bioinspired Structures Based on CaCO3 for Potential Applications as Drug Delivery Carriers. Pharmaceutics 2022, 14, 139. https://doi.org/10.3390/pharmaceutics14010139
Lengert EV, Trushina DB, Soldatov M, Ermakov AV. Microfluidic Synthesis and Analysis of Bioinspired Structures Based on CaCO3 for Potential Applications as Drug Delivery Carriers. Pharmaceutics. 2022; 14(1):139. https://doi.org/10.3390/pharmaceutics14010139
Chicago/Turabian StyleLengert, Ekaterina V., Daria B. Trushina, Mikhail Soldatov, and Alexey V. Ermakov. 2022. "Microfluidic Synthesis and Analysis of Bioinspired Structures Based on CaCO3 for Potential Applications as Drug Delivery Carriers" Pharmaceutics 14, no. 1: 139. https://doi.org/10.3390/pharmaceutics14010139
APA StyleLengert, E. V., Trushina, D. B., Soldatov, M., & Ermakov, A. V. (2022). Microfluidic Synthesis and Analysis of Bioinspired Structures Based on CaCO3 for Potential Applications as Drug Delivery Carriers. Pharmaceutics, 14(1), 139. https://doi.org/10.3390/pharmaceutics14010139







