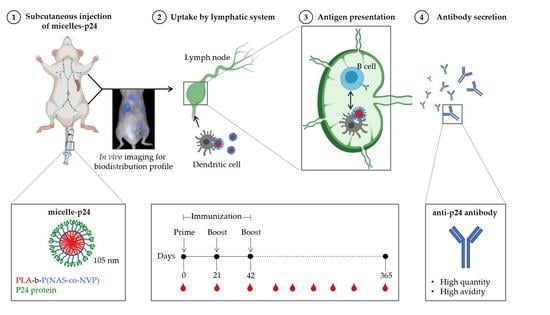
A Polylactide-Based Micellar Adjuvant Improves the Intensity and Quality of Immune Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLA-Based Micelles
2.3. Physico-Chemical Characterization
2.4. Cell Culture Protocol
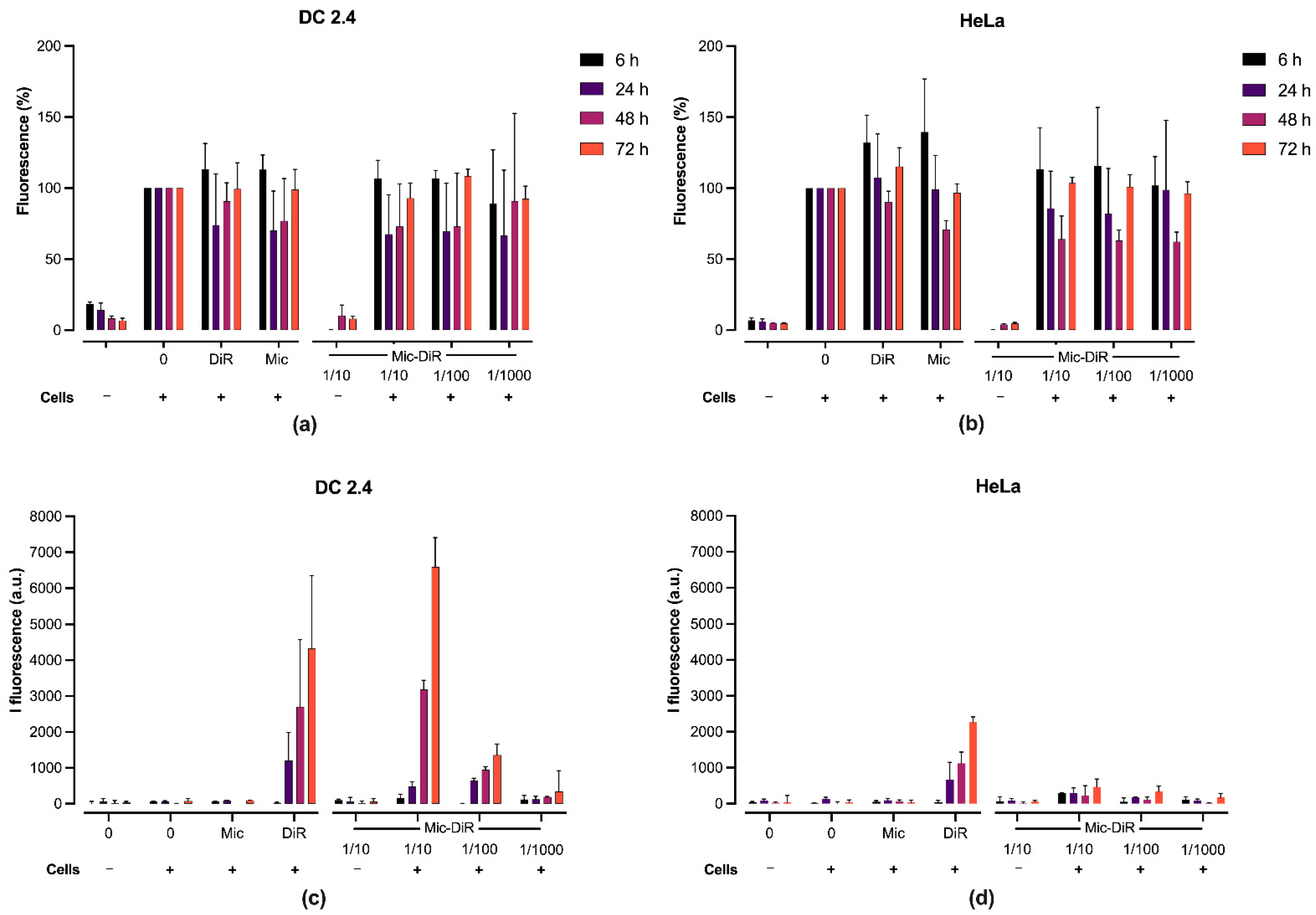
2.5. In Vitro Fluorescence and Cytotoxicity Studies
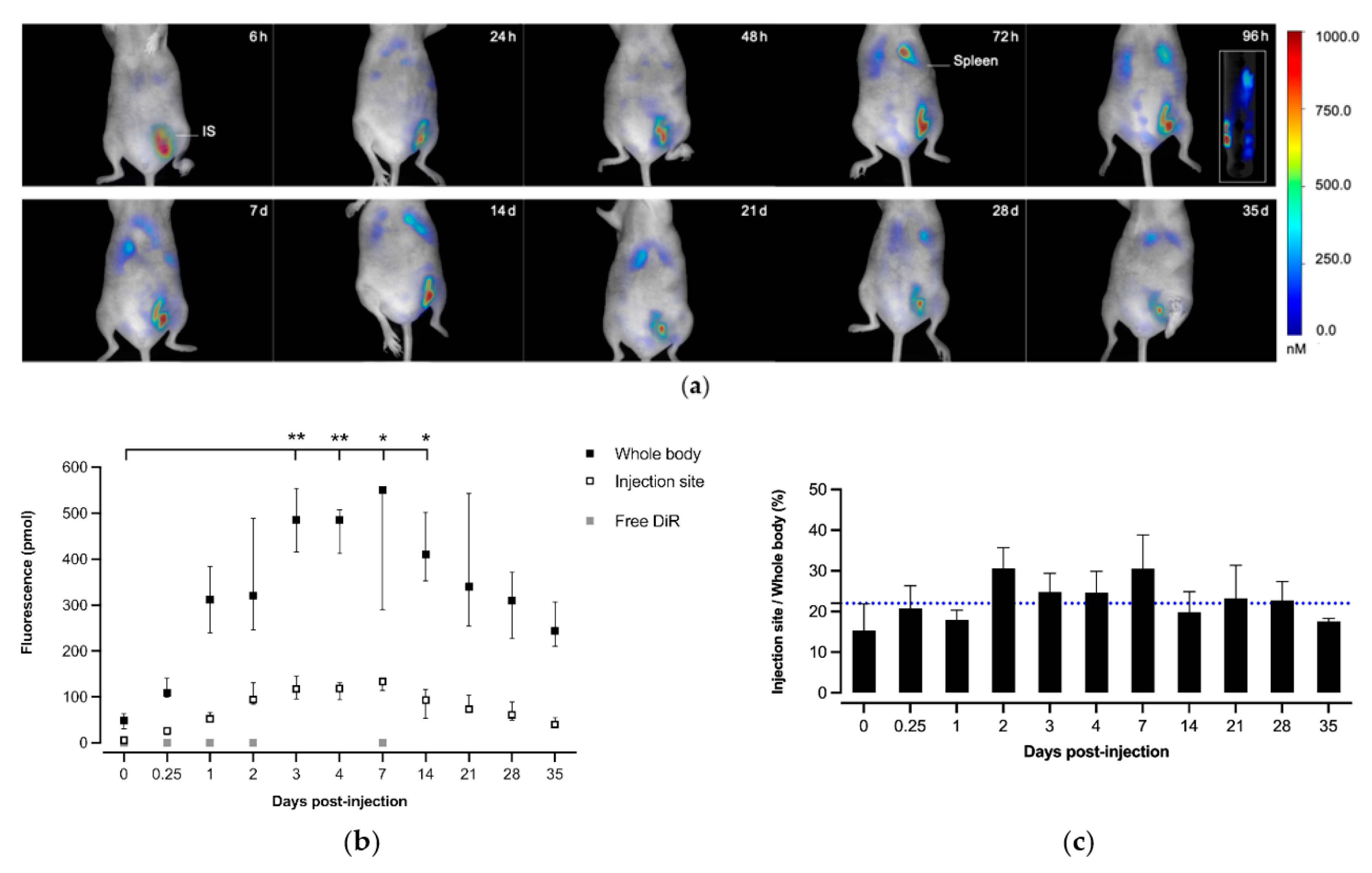
2.6. In Vivo Biodistribution Studies
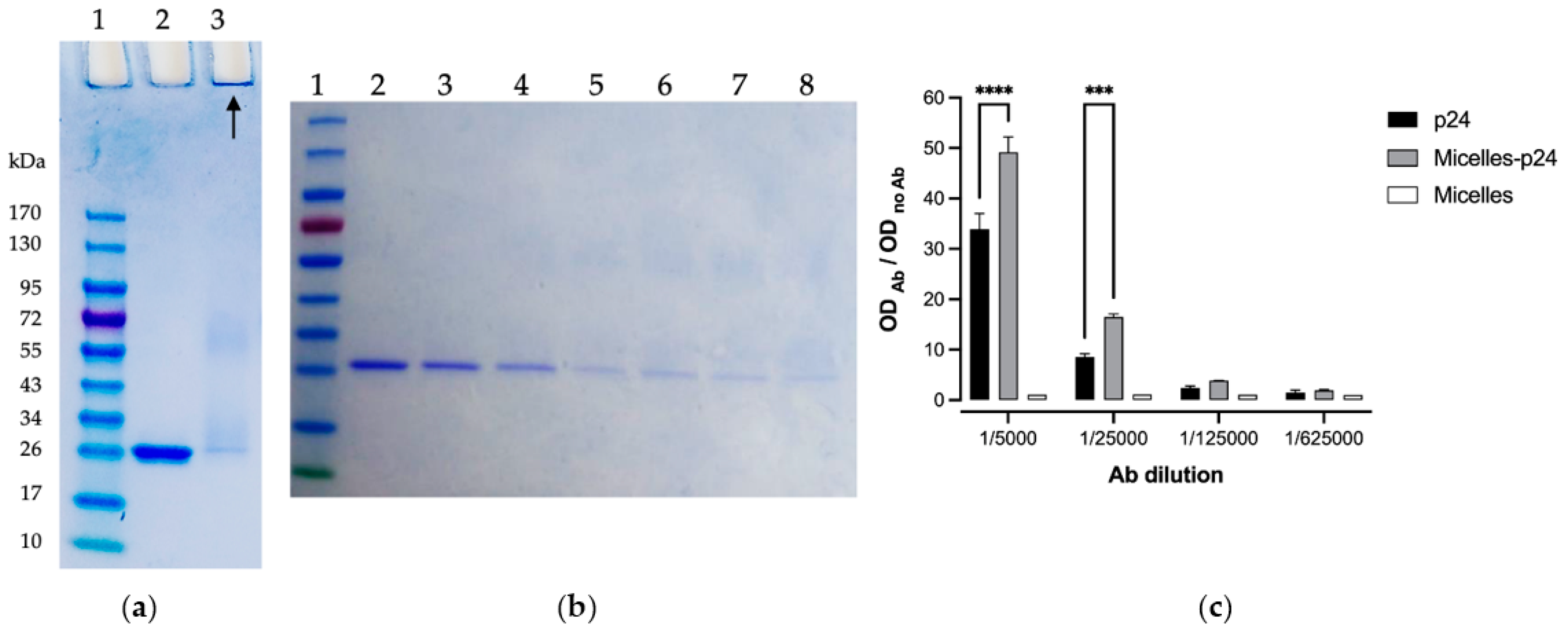
2.7. p24 Antigen Immobilization
2.8. Antigenicity of Micelle-Immobilized p24
2.9. Immunization Protocol
2.10. Antibody Titers
2.11. Antibody Avidity
2.12. Statistical Analysis
3. Results
3.1. Micelle Preparation and Fluorophore Loading
3.2. In Vitro Cytotoxicity and DiR Fluorescence Studies
3.3. In Vivo Biodistribution Studies
3.4. p24 Immobilization on Micelles
3.5. Antigenicity of Micelle-Immobilized p24
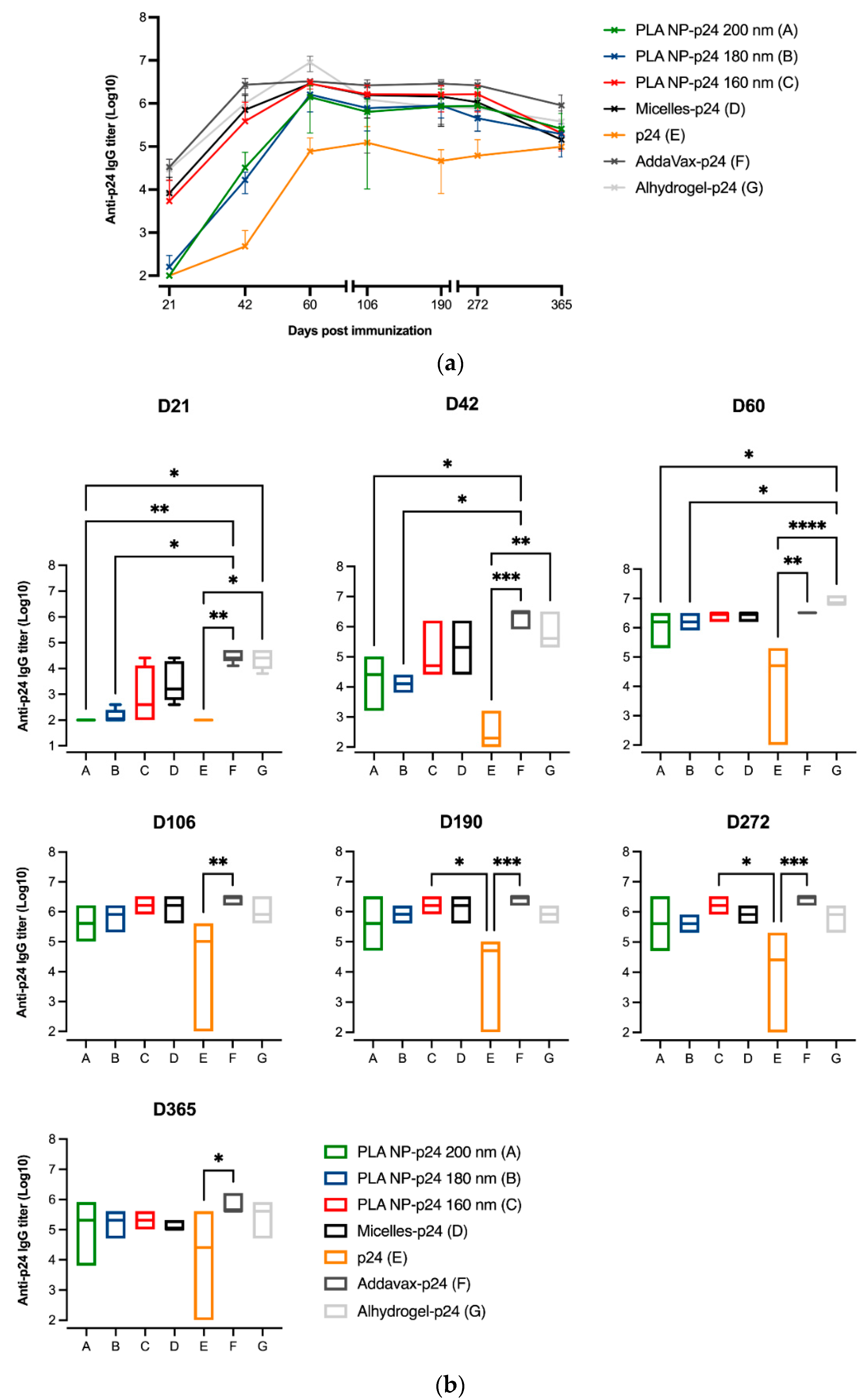
3.6. Immune Responses
3.7. Avidity Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 Vaccine BNT162b1 Elicits Human Antibody and TH1 T Cell Responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the MRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef]
- Ren, J.; Cao, Y.; Li, L.; Wang, X.; Lu, H.; Yang, J.; Wang, S. Self-Assembled Polymeric Micelle as a Novel MRNA Delivery Carrier. J. Controlled Release 2021, 338, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key Roles of Adjuvants in Modern Vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Tomljenovic, L.; Shaw, C.A. Aluminum Vaccine Adjuvants: Are They Safe? Curr. Med. Chem. 2011, 18, 2630–2637. [Google Scholar] [CrossRef] [PubMed]
- Sahly, H.E. MF59TM as a Vaccine Adjuvant: A Review of Safety and Immunogenicity. Expert Rev. Vaccines 2010, 9, 1135–1141. [Google Scholar] [CrossRef]
- Alving, C.R.; Beck, Z.; Matyas, G.R.; Rao, M. Liposomal Adjuvants for Human Vaccines. Expert Opin. Drug Deliv. 2016, 1–10. [Google Scholar] [CrossRef]
- Galliher-Beckley, A.; Pappan, L.K.; Madera, R.; Burakova, Y.; Waters, A.; Nickles, M.; Li, X.; Nietfeld, J.; Schlup, J.R.; Zhong, Q.; et al. Characterization of a Novel Oil-in-Water Emulsion Adjuvant for Swine Influenza Virus and Mycoplasma Hyopneumoniae Vaccines. Vaccine 2015, 33, 2903–2908. [Google Scholar] [CrossRef] [Green Version]
- Daftarian, P.; Kaifer, A.E.; Li, W.; Blomberg, B.B.; Frasca, D.; Roth, F.; Chowdhury, R.; Berg, E.A.; Fishman, J.B.; Al Sayegh, H.A.; et al. Peptide-Conjugated PAMAM Dendrimer as a Universal DNA Vaccine Platform to Target Antigen-Presenting Cells. Cancer Res. 2011, 71, 7452–7462. [Google Scholar] [CrossRef] [Green Version]
- Oyewumi, M.O.; Kumar, A.; Cui, Z. Nano-Microparticles as Immune Adjuvants: Correlating Particle Sizes and the Resultant Immune Responses. Expert Rev. Vaccines 2010, 9, 1095–1107. [Google Scholar] [CrossRef] [Green Version]
- Manish, M.; Rahi, A.; Kaur, M.; Bhatnagar, R.; Singh, S. A Single-Dose PLGA Encapsulated Protective Antigen Domain 4 Nanoformulation Protects Mice against Bacillus Anthracis Spore Challenge. PLoS ONE 2013, 8, e61885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayari-Riabi, S.; Trimaille, T.; Mabrouk, K.; Bertin, D.; Gigmes, D.; Benlasfar, Z.; Zaghmi, A.; Bouhaouala-Zahar, B.; Elayeb, M. Venom Conjugated Polylactide Applied as Biocompatible Material for Passive and Active Immunotherapy against Scorpion Envenomation. Vaccine 2016, 34, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- Lamalle-Bernard, D.; Munier, S.; Compagnon, C.; Charles, M.-H.; Kalyanaraman, V.S.; Delair, T.; Verrier, B.; Ataman-Önal, Y. Coadsorption of HIV-1 P24 and Gp120 Proteins to Surfactant-Free Anionic PLA Nanoparticles Preserves Antigenicity and Immunogenicity. J. Controlled Release 2006, 115, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kunduru, K.R.; Basu, A.; Mizrahi, B.; Domb, A.J.; Khan, W. Injectable Formulations of Poly(Lactic Acid) and Its Copolymers in Clinical Use. Adv. Drug Deliv. Rev. 2016, 107, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Chan, G.; Hu, Y.; Hu, H.; Ouyang, D. A Comprehensive Map of FDA-Approved Pharmaceutical Products. Pharmaceutics 2018, 10, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; He, X.; Zhang, Y.; Zhao, Y.; Lu, S.; Peng, Y.; Lu, L.; Hu, X.; Zhan, M. Native Mitochondria-Targeting Polymeric Nanoparticles for Mild Photothermal Therapy Rationally Potentiated with Immune Checkpoints Blockade to Inhibit Tumor Recurrence and Metastasis. Chem. Eng. J. 2021, 424, 130171. [Google Scholar] [CrossRef]
- Trimaille, T.; Lacroix, C.; Verrier, B. Self-Assembled Amphiphilic Copolymers as Dual Delivery System for Immunotherapy. Eur. J. Pharm. Biopharm. 2019, 142, 232–239. [Google Scholar] [CrossRef]
- Trimaille, T.; Verrier, B. Micelle-Based Adjuvants for Subunit Vaccine Delivery. Vaccines 2015, 3, 803–813. [Google Scholar] [CrossRef]
- Sevimli, S.; Knight, F.C.; Gilchuk, P.; Joyce, S.; Wilson, J.T. Fatty Acid-Mimetic Micelles for Dual Delivery of Antigens and Imidazoquinoline Adjuvants. ACS Biomater. Sci. Eng. 2017, 3, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Brubaker, C.E.; Panagiotou, V.; Demurtas, D.; Bonner, D.K.; Swartz, M.A.; Hubbell, J.A. A Cationic Micelle Complex Improves CD8+ T Cell Responses in Vaccination Against Unmodified Protein Antigen. ACS Biomater. Sci. Eng. 2016, 2, 231–240. [Google Scholar] [CrossRef]
- Li, P.; Song, H.; Zhang, H.; Yang, P.; Zhang, C.; Huang, P.; Kong, D.; Wang, W. Engineering Biodegradable Guanidyl-Decorated PEG-PCL Nanoparticles as Robust Exogenous Activators of DCs and Antigen Cross-Presentation. Nanoscale 2017, 9, 13413–13418. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, C.; Qin, Y.; Wang, Z.; Wang, L.; Wei, X.; Zhou, Y.; Li, Q.; Zhou, H.; Wang, W.; et al. Coordinating Antigen Cytosolic Delivery and Danger Signaling to Program Potent Cross-Priming by Micelle-Based Nanovaccine. Cell Discov. 2017, 3, 17007. [Google Scholar] [CrossRef]
- Nuhn, L.; Bolli, E.; Massa, S.; Vandenberghe, I.; Movahedi, K.; Devreese, B.; Van Ginderachter, J.A.; De Geest, B.G. Targeting Protumoral Tumor-Associated Macrophages with Nanobody-Functionalized Nanogels through Strain Promoted Azide Alkyne Cycloaddition Ligation. Bioconjug. Chem. 2018, 29, 2394–2405. [Google Scholar] [CrossRef]
- Li, P.; Shi, G.; Zhang, X.; Song, H.; Zhang, C.; Wang, W.; Li, C.; Song, B.; Wang, C.; Kong, D. Guanidinylated Cationic Nanoparticles as Robust Protein Antigen Delivery Systems and Adjuvants for Promoting Antigen-Specific Immune Responses in Vivo. J. Mater. Chem. B 2016, 4, 5608–5620. [Google Scholar] [CrossRef]
- Van Herck, S.; Van Hoecke, L.; Louage, B.; Lybaert, L.; De Coen, R.; Kasmi, S.; Esser-Kahn, A.P.; David, S.A.; Nuhn, L.; Schepens, B.; et al. Transiently Thermoresponsive Acetal Polymers for Safe and Effective Administration of Amphotericin B as a Vaccine Adjuvant. Bioconjug. Chem. 2018, 29, 748–760. [Google Scholar] [CrossRef]
- Zeng, Q.; Jiang, H.; Wang, T.; Zhang, Z.; Gong, T.; Sun, X. Cationic Micelle Delivery of Trp2 Peptide for Efficient Lymphatic Draining and Enhanced Cytotoxic T-Lymphocyte Responses. J. Controlled Release 2015, 200, 1–12. [Google Scholar] [CrossRef]
- Adams, J.R.; Haughney, S.L.; Mallapragada, S.K. Effective Polymer Adjuvants for Sustained Delivery of Protein Subunit Vaccines. Acta Biomater. 2015, 14, 104–114. [Google Scholar] [CrossRef]
- Liu, L.; Yi, H.; Wang, C.; He, H.; Li, P.; Pan, H.; Sheng, N.; Ji, M.; Cai, L.; Ma, Y. Integrated Nanovaccine with MicroRNA-148a Inhibition Reprograms Tumor-Associated Dendritic Cells by Modulating MiR-148a/DNMT1/SOCS1 Axis. J. Immunol. 2016, 197, 1231–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.; Hanson, S.; Han, W.; Zhang, X.; Yao, N.; Li, H.; Zhang, L.; Wang, C. Well-Defined Star Polymers for Co-Delivery of Plasmid DNA and Imiquimod to Dendritic Cells. Acta Biomater. 2017, 48, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Z.; Qin, Y.; Liang, W. Delivered Antigen Peptides to Resident CD8α+ DCs in Lymph Node by Micelle-Based Vaccine Augment Antigen-Specific CD8+ Effector T Cell Response. Eur. J. Pharm. Biopharm. 2020, 147, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.L.Z.; Yang, C.; Gao, S.; Hedrick, J.L.; Yang, Y.Y. Subcutaneous Vaccination Using Injectable Biodegradable Hydrogels for Long-Term Immune Response. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102056. [Google Scholar] [CrossRef]
- Jiang, D.; Mu, W.; Pang, X.; Liu, Y.; Zhang, N.; Song, Y.; Garg, S. Cascade Cytosol Delivery of Dual-Sensitive Micelle-Tailored Vaccine for Enhancing Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2018, 10, 37797–37811. [Google Scholar] [CrossRef]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG Antibodies: Properties, Formation, Testing and Role in Adverse Immune Reactions to PEGylated Nano-Biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(Ethylene Glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Handké, N.; Lahaye, V.; Bertin, D.; Delair, T.; Verrier, B.; Gigmes, D.; Trimaille, T. Elaboration of Glycopolymer-Functionalized Micelles from an N-Vinylpyrrolidone/Lactide-Based Reactive Copolymer Platform. Macromol. Biosci. 2013, 13, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, G.; Terrat, C.; Verrier, B.; Gigmes, D.; Trimaille, T. Improving Bioassay Sensitivity through Immobilization of Bio-Probes onto Reactive Micelles. Chem. Commun. 2017, 53, 8062–8065. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, G.; Pavot, V.; Chane-Haong, C.; Handké, N.; Terrat, C.; Gigmes, D.; Trimaille, T.; Verrier, B. Preparation and In Vitro Evaluation of Imiquimod Loaded Polylactide-Based Micelles as Potential Vaccine Adjuvants. Pharm. Res. 2015, 32, 311–320. [Google Scholar] [CrossRef]
- Ataman-Önal, Y.; Munier, S.; Ganée, A.; Terrat, C.; Durand, P.-Y.; Battail, N.; Martinon, F.; Le Grand, R.; Charles, M.-H.; Delair, T.; et al. Surfactant-Free Anionic PLA Nanoparticles Coated with HIV-1 P24 Protein Induced Enhanced Cellular and Humoral Immune Responses in Various Animal Models. J. Controlled Release 2006, 112, 175–185. [Google Scholar] [CrossRef]
- Handké, N.; Trimaille, T.; Luciani, E.; Rollet, M.; Delair, T.; Verrier, B.; Bertin, D.; Gigmes, D. Elaboration of Densely Functionalized Polylactide Nanoparticles from N-Acryloxysuccinimide-Based Block Copolymers. J. Polym. Sci. Part Polym. Chem. 2011, 49, 1341–1350. [Google Scholar] [CrossRef]
- Aline, F.; Brand, D.; Pierre, J.; Roingeard, P.; Séverine, M.; Verrier, B.; Dimier-Poisson, I. Dendritic Cells Loaded with HIV-1 P24 Proteins Adsorbed on Surfactant-Free Anionic PLA Nanoparticles Induce Enhanced Cellular Immune Responses against HIV-1 after Vaccination. Vaccine 2009, 27, 5284–5291. [Google Scholar] [CrossRef]
- Pavot, V.; Rochereau, N.; Primard, C.; Genin, C.; Perouzel, E.; Lioux, T.; Paul, S.; Verrier, B. Encapsulation of Nod1 and Nod2 Receptor Ligands into Poly(Lactic Acid) Nanoparticles Potentiates Their Immune Properties. J. Controlled Release 2013, 167, 60–67. [Google Scholar] [CrossRef]
- Kontio, M.; Jokinen, S.; Paunio, M.; Peltola, H.; Davidkin, I. Waning Antibody Levels and Avidity: Implications for MMR Vaccine-Induced Protection. J. Infect. Dis. 2012, 206, 1542–1548. [Google Scholar] [CrossRef]
- López-Sagaseta, J.; Malito, E.; Rappuoli, R.; Bottomley, M.J. Self-Assembling Protein Nanoparticles in the Design of Vaccines. Comput. Struct. Biotechnol. J. 2016, 14, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilinskaya, A.N.; Dobrovolskaia, M.A. Understanding the Immunogenicity and Antigenicity of Nanomaterials: Past, Present and Future. Toxicol. Appl. Pharmacol. 2016, 299, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Gause, K.T.; Wheatley, A.K.; Cui, J.; Yan, Y.; Kent, S.J.; Caruso, F. Immunological Principles Guiding the Rational Design of Particles for Vaccine Delivery. Adv. Drug Deliv. Rev. 2017, 11, 54–68. [Google Scholar] [CrossRef]
- Lacroix, C.; Humanes, A.; Coiffier, C.; Gigmes, D.; Verrier, B.; Trimaille, T. Polylactide-Based Reactive Micelles as a Robust Platform for MRNA Delivery. Pharm. Res. 2020, 37. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Lin, S.-Y.; Hsu, T.-A.; Hsieh, H.-P.; Huang, M.-H. Colloidal Assemblies Composed of Polymeric Micellar/Emulsified Systems Integrate Cancer Therapy Combining a Tumor-Associated Antigen Vaccine and Chemotherapeutic Regimens. Nanomaterials 2021, 11, 1844. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Darling, R.J.; Ross, K.A.; Wannemeuhler, M.J.; Narasimhan, B.; Mallapragada, S.K. Self-Assembling Synthetic Nanoadjuvant Scaffolds Cross-Link B Cell Receptors and Represent New Platform Technology for Therapeutic Antibody Production. Sci. Adv. 2021, 7, eabj1691. [Google Scholar] [CrossRef]
- Havenar-Daughton, C.; Carnathan, D.G.; Boopathy, A.V.; Upadhyay, A.A.; Murrell, B.; Reiss, S.M.; Enemuo, C.A.; Gebru, E.H.; Choe, Y.; Dhadvai, P.; et al. Rapid Germinal Center and Antibody Responses in Non-Human Primates after a Single Nanoparticle Vaccine Immunization. Cell Rep. 2019, 29, 1756–1766. [Google Scholar] [CrossRef] [Green Version]
- Irvine, D.J.; Aung, A.; Silva, M. Controlling Timing and Location in Vaccines. Adv. Drug Deliv. Rev. 2020, 158, 91–115. [Google Scholar] [CrossRef]
- Thalhauser, S.; Peterhoff, D.; Wagner, R.; Breunig, M. Critical Design Criteria for Engineering a Nanoparticulate HIV-1 Vaccine. J. Controlled Release 2020, 317, 322–335. [Google Scholar] [CrossRef]
- Benne, N.; van Duijn, J.; Kuiper, J.; Jiskoot, W.; Slütter, B. Orchestrating Immune Responses: How Size, Shape and Rigidity Affect the Immunogenicity of Particulate Vaccines. J. Controlled Release 2016, 234, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Schudel, A.; Francis, D.M.; Thomas, S.N. Material Design for Lymph Node Drug Delivery. Nat. Rev. Mater. 2019, 4, 415–428. [Google Scholar] [CrossRef]
- Zhang, R.; Smith, J.D.; Allen, B.N.; Kramer, J.S.; Schauflinger, M.; Ulery, B.D. Peptide Amphiphile Micelle Vaccine Size and Charge Influence the Host Antibody Response. ACS Biomater. Sci. Eng. 2018, 4, 2463–2472. [Google Scholar] [CrossRef]
- Bale, S.; Goebrecht, G.; Stano, A.; Wilson, R.; Ota, T.; Tran, K.; Ingale, J.; Zwick, M.B.; Wyatt, R.T. Covalent Linkage of HIV-1 Trimers to Synthetic Liposomes Elicits Improved B Cell and Antibody Responses. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [Green Version]


| Formulation | Day Post Synthesis | Mean Size (nm) | PdI | Surface Charge (mV) |
|---|---|---|---|---|
| Micelles | 0 | 102.0 (±0.9) | 0.172 (±0.013) | −29.9 (±1.7) |
| Micelles | 7 | 107.8 (±1.1) | 0.091 (±0.042) | −35.5 (±0.5) |
| Micelles-DiR | 0 | 107.0 (±1.7) | 0.119 (±0.024) | −31.8 (±1.0) |
| Micelles-DiR | 7 | 113.9 (±1.9) | 0.162 (±0.006) | −36.5 (±0.5) |
| Formulation | p24 Adsorption Yield (%) | Mean Size (nm) | PdI | Surface Charge (mV) |
|---|---|---|---|---|
| PLA NP 160 nm | - | 158.7 (±2.9) | 0.068 (±0.005) | −54.4 (±0.5) |
| PLA NP-p24 160 nm | 99.8 | 160.6 (±1.1) | 0.073 (±0.023) | −59.2 (±2.1) |
| PLA NP 180 nm | - | 181.7 (±6.3) | 0.029 (±0.002) | −53.8 (±1.2) |
| PLA NP-p24 180 nm | 89.2 | 184.8 (±2.0) | 0.034 (±0.013) | −58.3 (±3.4) |
| PLA NP 200 nm | - | 198.7 (±8.1) | 0.044 (±0.027) | −59.2 (±2.3) |
| PLA NP-p24 200 nm | ~100 | 197.9 (±1.1) | 0.035 (±0.009) | −60.5(±0.9) |
| PLA-micelles | - | 105.0 (±2.3) | 0.063 (±0.021) | −29.9 (±5.8) |
| PLA-micelles-p24 | ~100 | 114.5 (±6.0) | 0.040 (±0.026) | −37.2 (±0.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamrayah, M.; Phelip, C.; Coiffier, C.; Lacroix, C.; Willemin, T.; Trimaille, T.; Verrier, B. A Polylactide-Based Micellar Adjuvant Improves the Intensity and Quality of Immune Response. Pharmaceutics 2022, 14, 107. https://doi.org/10.3390/pharmaceutics14010107
Lamrayah M, Phelip C, Coiffier C, Lacroix C, Willemin T, Trimaille T, Verrier B. A Polylactide-Based Micellar Adjuvant Improves the Intensity and Quality of Immune Response. Pharmaceutics. 2022; 14(1):107. https://doi.org/10.3390/pharmaceutics14010107
Chicago/Turabian StyleLamrayah, Myriam, Capucine Phelip, Céline Coiffier, Céline Lacroix, Thibaut Willemin, Thomas Trimaille, and Bernard Verrier. 2022. "A Polylactide-Based Micellar Adjuvant Improves the Intensity and Quality of Immune Response" Pharmaceutics 14, no. 1: 107. https://doi.org/10.3390/pharmaceutics14010107
APA StyleLamrayah, M., Phelip, C., Coiffier, C., Lacroix, C., Willemin, T., Trimaille, T., & Verrier, B. (2022). A Polylactide-Based Micellar Adjuvant Improves the Intensity and Quality of Immune Response. Pharmaceutics, 14(1), 107. https://doi.org/10.3390/pharmaceutics14010107