Combining Nanocarrier-Assisted Delivery of Molecules and Radiotherapy
Abstract
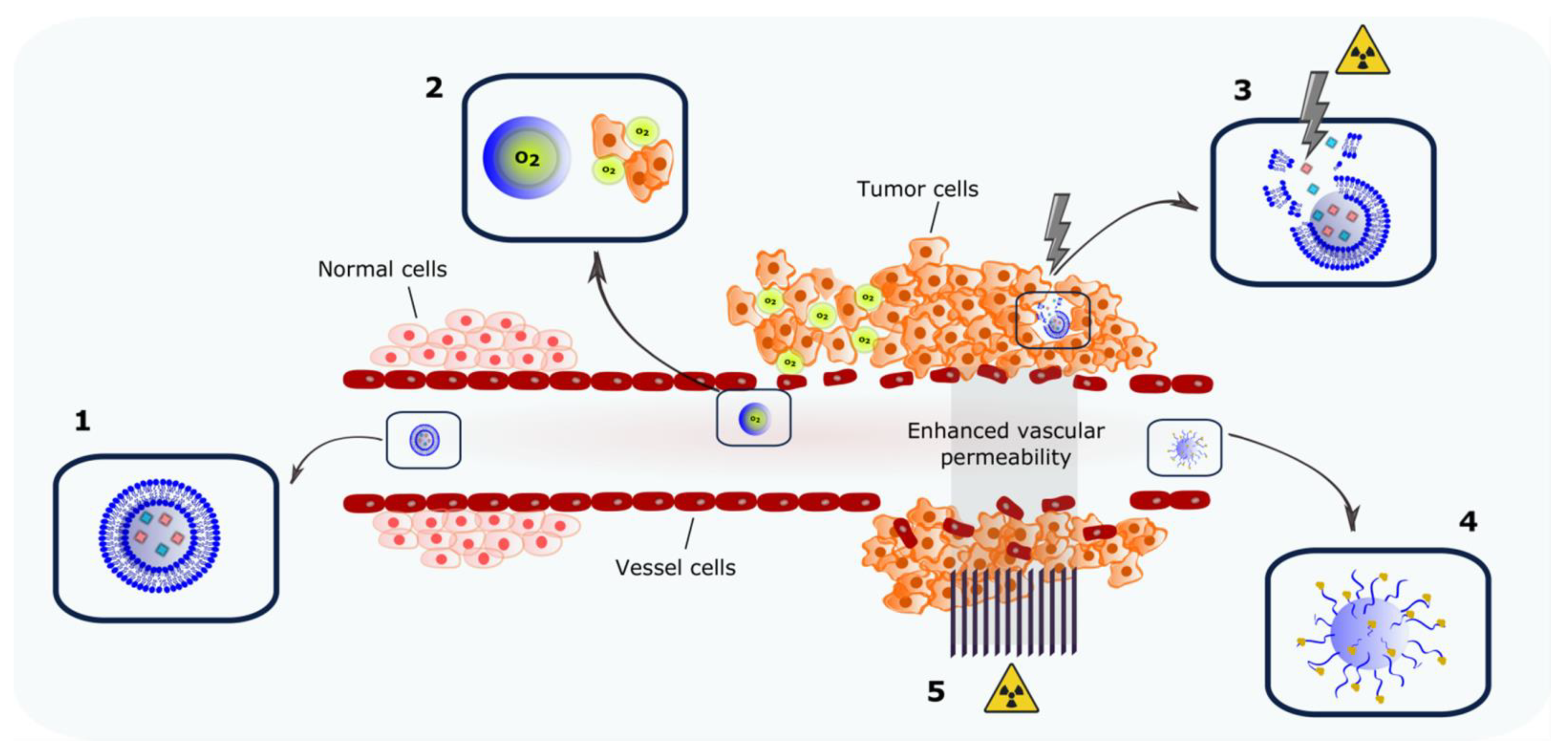
1. Introduction
2. Nanocarriers Encapsulating Radiosensitizers
2.1. Chemotherapeutic Drugs That Act as Radiosensitizers
2.1.1. Cisplatin
| Formulation | Composition | Encapsulated Agent | Mean Diameter | Irradiation Dose (Gy) * | Reference |
|---|---|---|---|---|---|
| Nanocarriers Encapsulating Radiosensitizers | |||||
| Liposome | HSPC:CHOL:DSPE-PEG2000 | Cisplatin | ~100 nm | 6 Gy | [35] |
| Liposome | (DPPC):CHOL:ganglioside:DCP:DPPE) (35:40:15:5:5 molar ratio) and anti-EGFR antibodies | Cisplatin | 247.9 nm | 5 Gy | [36] |
| Liposome (Promitil®) | HSPC:CHOL:DSPE-PEG2000:MLP (60:30:5:5 molar ratio) HSPC:CHOL:DSPE-PEG2000:MLP (55:30:5:10 molar ratio) | Mitomycin C | 98.61 nm | 5 Gy | [38,39,40,41] |
| Liposome (Myocet®) | EPC:CHOL (55:34 molar ratio) | Doxorubicin | ~160 nm | 2 Gy | [42,43] |
| Liposome | DSPE-PEG2000:MDH:CHOL | Doxorubicin | 169.4 nm | 2 Gy | [44] |
| Micelles | PEG-PCL/P105 | Doxorubicin | ~20 nm | 6 Gy | [45] |
| Nanoparticle | Precirol ATO, Pluronic F68, dimethyldioctadecyl-ammonium bromide | Curcumin | ~300 nm | 2 Gy to 9 Gy | [46] |
| Liposome | lecithin:CHOL:CUR (18:1:1 weight ratio) | Curcumin | 114.9 nm | 5 Gy | [47] |
| Liposome | DOPC:CHOL:DSPE-PEG2000 | Cupric tirapazamine complex | 160–180 nm | 7 Gy or 10 Gy | [48] |
| Liposome | DPPC:MSPC:DSPE-PEG2000 (86:10:4 molar ratio) | Pimonidazole | ~100 nm | 4 Gy | [49] |
| Nanoparticle | H1 nanopolymer:Dbait | Dbait | 170 nm | 9 Gy | [50] |
| Co-delivery of Molecules: The Search for Synergism | |||||
| Nanoparticle | PLGA-PEG | Cisplatin and Paclitaxel | 82.9 nm | 5 Gy | [51] |
| Nanoparticle | PLGA-PEG | Wortmannin and Cisplatin | 80–200 nm | 5 Gy | [52] |
| Nanoparticle | PLGA-PEG | Cisplatin and Etoposide | 100 nm | 5 Gy | [53] |
| Nanoparticle | PLGA-PEG:transferrin at a molar ratio of 1:3 | Tetrahydrocurcumin and Doxorubicin | 255.8 nm | 3 Gy | [54] |
| Nanoparticle | angiopep-2:DSPE-PEG2000:DOTAP:PLGA | Temozolomide and Dbait | 99.9 nm | 3 Gy | [55] |
| Nanoparticle | H1 nanopolymer:Docetaxel:Dbait | Docetaxel and Dbait | 117 nm | 3 Gy | [56] |
| Nanoparticle | magnetic graphene oxide:FePt nanoparticles | Metronidazol and 5-fluorouracil | 243 nm | 2 Gy | [57] |
| Nanoparticle | (Poly-metronidazole)n:DSPE-PEG2000: lecitina:angiopep-2-DSPE-PEG-2000 | Metronidazol and Doxorubicin | ~80 nm | 2 Gy | [58] |
| Liposome | DSPE-PEG2000: MDH: CHOL | Metronidazole and Dbait | 127 nm | 2 Gy | [59] |
| Nanoparticle | 1,4-dicarboxybenzene (BDC): Hafnium (Hf):PEG | Talazoparib and Buparlisib | 112 nm | 4 Gy or 8 Gy | [60] |
| Nanocarriers Encapsulating Oxygen: Targeting Hypoxia | |||||
| Nanoparticle | perfluorotributylamine (PFTBA)@albumin | Oxygen | 150 nm | 5 Gy | [61] |
| Nanodroplets | perfluoro-15-crown-5-ether (PFCE)@cisPt(IV)-Lip cisPt(IV)-Lip is prepared by mixing 2.5 mg cisPt(IV)-DSPE, 5 mg DPPC, 1.5 mg cholesterol and 4 mg DSPE-mPEG5k | Oxygen Cisplatin | ~200 nm | 6 Gy | [62] |
| Nanoparticle | PEG-Bi2Se3 @perfluorohexane | Oxygen | ~35 nm | 6 Gy | [63] |
| Nanoparticle decorated nanodroplets | TaOx@PFC-PEG | Oxygen | ~150 | 6 Gy | [64] |
| Liposome | PFH@DSPE-PEG2000:CHOL:lecithin (3.79:4.28:24.65 weight ratio) | Oxygen | ~100 nm | 10 Gy | [65] |
| Nanocarriers Designed to Boost the Abscopal Effect | |||||
| Nanoparticle | PLGA based NP coated with either amine polyethylene glycol; DOTAP or PEG-maleimide | - | <200 nm | - | [66] |
| Nanoparticle | Mesoporous silica nanoparticles functionalized with APTES | - | ~100 nm | 8 Gy | [67] |
| Nanoparticle | PEG-maleimide-mPEG-functionalized hollow mesoporous titanium dioxide (HTiO2) | IDOi (Indole-amine-2,3-dioxygenase inhibitor) | ~50 nm | 4 Gy | [68] |
| Radiation-Triggered Delivery Systems | |||||
| Nanoparticle | DNA:AuNP | Doxorubicin | NA | 5 Gy | [69] |
| Nanoparticle | bismuth nanoparticles functionalized with S-nitrosothiol | - | 36 nm | 5 Gy | [70] |
| Nanoparticle | Pegylated thioether-hybridized hollow mesoporous organosilica nanoparticles | tert-butyl hydroperoxide (TBHP) and iron pentacarbonyl (Fe(CO)5) | ~50 nm | 8 Gy | [71] |
| Liposome | DOTAP:DOPC (~1:1 weight ratio) | Doxorubicin | NA | 4 Gy | [72] |
| Liposome | egg lecithin-80: DSPE-PEG2000 (60:9 w/w) | Hemoglobin and Doxorubicin | ~140 nm | 8 Gy | [73] |
2.1.2. Mitomycin
2.1.3. Doxorubicin
2.2. Natural Products as Radiosensitizers
Curcumin
2.3. Hypoxic Cell Radiosensitizers
2.3.1. Tirapazamine
2.3.2. Nitroimidazoles
2.4. DNA Repair Inhibitors
Dbait
3. Co-Delivery of Molecules: The Search for Synergism
4. Nanocarriers Encapsulating Oxygen: Targeting Hypoxia
5. Nanocarriers Designed to Boost the Abscopal Effect
6. Radiation-Triggered Delivery Systems
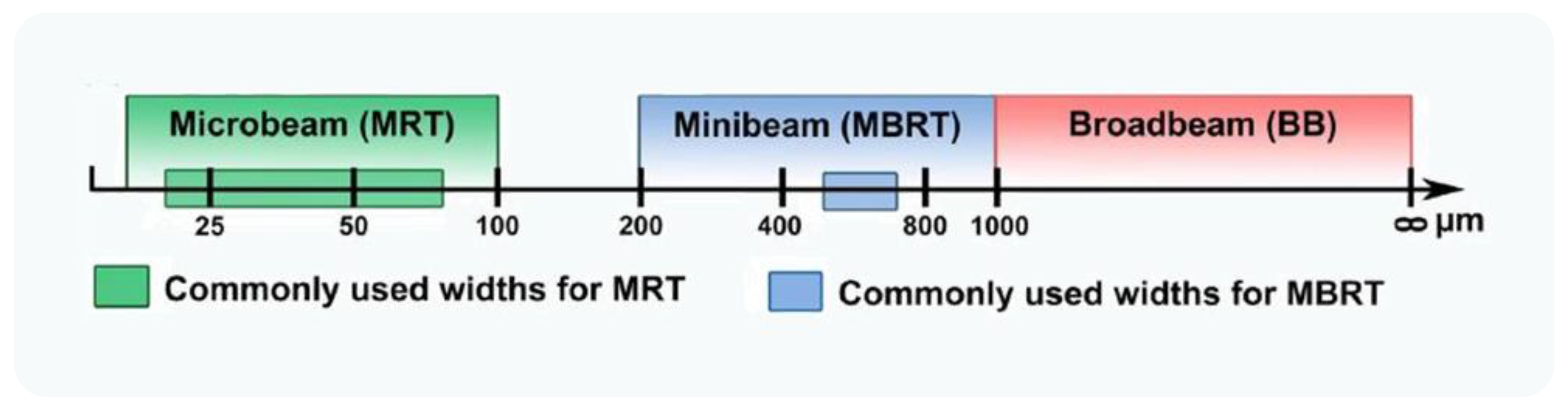
7. Microbeam and Minibeam Radiation Therapy to Modulate the EPR Effect
7.1. Microbeam Radiation Therapy
7.2. Minibeam Radiation Therapy
8. Limitations and Future Directions
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A2780 | platinum-sensitive murine ovarian cancer |
| A549 | human non-small-cell lung cancer cell |
| C4-2B | human prostatic carcinoma cell |
| C6 | rat glioma cell line |
| CDDP | cisplatin |
| CT26 | colon carcinoma cell line |
| CUR | curcumin |
| DTX | docetaxel |
| DXR | doxorubicin |
| ET | etoposide |
| FaDu | human hypopharyngeal carcinoma cell |
| HCT 116 | human colorectal carcinoma cell line |
| HT-29 | human colorectal adenocarcinoma cell |
| H1975 | human non-small-cell lung cancer cell |
| H460 | human non-small-cell lung cancer cell |
| K562/ADR | human myelogenous leukemia |
| LL2 | murine Lewis lung carcinoma |
| MCF-7 | human breast cancer cell line with estrogen, progesterone and glucocorticoid receptors |
| MDA-MB-231 | triple-negative breast cancer cell |
| MMC | mitomycin C |
| PC3 | human prostate adenocarcinoma cell |
| PMZ | pimonidazole |
| PTX | paclitaxel |
| TCG4 | human glioma cell line |
| U14 | murine cervical carcinoma |
| THC | tetahydrocurcumin |
| TMZ | temozolomide |
| TPZ | tirapazamine |
| U87 | human primary glioblastoma cell |
| WTMN | wortmannin |
| SW480 | human colon adenocarcinoma cell |
| 22Rv1 | human prostate carcinoma cell |
| 344SQ | human lung cancer cell |
| 4T1 | murine mammary carcinoma cell line |
| 5-FU | 5-fluorouracil |
References
- Arul, N.; Balachandar, V. Recent advances in radiotherapy and its associated side effects in cancer—A review. J. Basic Appl. Zoöl. 2019, 80, 14. [Google Scholar] [CrossRef]
- Kim, K.; Khang, D. Past, Present, and Future of Anticancer Nanomedicine. Int. J. Nanomed. 2020, 15, 5719–5743. [Google Scholar] [CrossRef]
- Islam, R.; Maeda, H.; Fang, J. Factors affecting the dynamics and heterogeneity of the EPR effect: Pathophysiological and pathoanatomic features, drug formulations and physicochemical factors. Expert Opin. Drug Deliv. 2021, 1–14. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Officer, H.; Refaat, T.; Medicine, N.; Lurie, R.H. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.S.; Gomes, E.R.; Roque, M.C.; Oliveira, M.C. Triggered Drug Release from Liposomes: Exploiting the Outer and Inner Tumor Environment. Front. Oncol. 2021, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 2020, 11, 940. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.-S.; Wang, R.; Xu, H.-X.; Miao, M.-Y.; Yao, Z.-Z. Nanotherapy Targeting the Tumor Microenvironment. Curr. Cancer Drug Targets 2019, 19, 525–533. [Google Scholar] [CrossRef]
- Fernandes, C.; Suares, D.; Yergeri, M.C. Tumor Microenvironment Targeted Nanotherapy. Front. Pharmacol. 2018, 9, 1230. [Google Scholar] [CrossRef]
- Muthu, M.S.; Leong, D.; Mei, L.; Feng, S.-S. Nanotheranostics—Application and Further Development of Nanomedicine Strategies for Advanced Theranostics. Theranostics 2014, 4, 660–677. [Google Scholar] [CrossRef]
- Jo, S.D.; Ku, S.H.; Won, Y.-Y.; Kim, S.H.; Kwon, I.C. Targeted Nanotheranostics for Future Personalized Medicine: Recent Progress in Cancer Therapy. Theranostics 2016, 6, 1362–1377. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-S.; Sun, J.-H.; Yu, H.-H.; Yu, S.-Q. Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Deliv. 2017, 24, 1909–1926. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.S.; Oliveira, M.C. Liposomes Co- encapsulating Anticancer Drugs in Synergistic Ratios as an Approach to Promote Increased Efficacy and Greater Safety. Anti-Cancer Agents Med. Chem. 2019, 19, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.S.; Oliveira, M.C. Ratiometric drug delivery using non-liposomal nanocarriers as an approach to increase efficacy and safety of combination chemotherapy. Biomed. Pharmacother. 2017, 96, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Caponigro, F.; Cornelia, P.; Budillon, A.; Bryce, J.; Avallone, A.; De Rosa, V.; Ionna, F.; Cornelia, G. Phase I study of Caelyx (doxorubicin HCL, pegylated liposomal) in recurrent or metastatic head and neck cancer. Ann. Oncol. 2000, 11, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Shabason, J.E.; Chen, J.; Apisarnthanarax, S.; Damjanov, N.; Giantonio, B.; Loaiza-Bonilla, A.; O’Dwyer, P.J.; O’Hara, M.; Reiss, K.A.; Teitelbaum, U.; et al. A phase I dose escalation trial of nab-paclitaxel and fixed dose radiation in patients with unresectable or borderline resectable pancreatic cancer. Cancer Chemother. Pharmacol. 2018, 81, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Lammers, P.E.; Lu, B.; Horn, L.; Shyr, Y.; Keedy, V. nab—Paclitaxel in Combination with Weekly Carboplatin with Concurrent Radiotherapy in Stage III Non-Small Cell Lung Cancer. Oncologist 2015, 20, 491–492. [Google Scholar] [CrossRef]
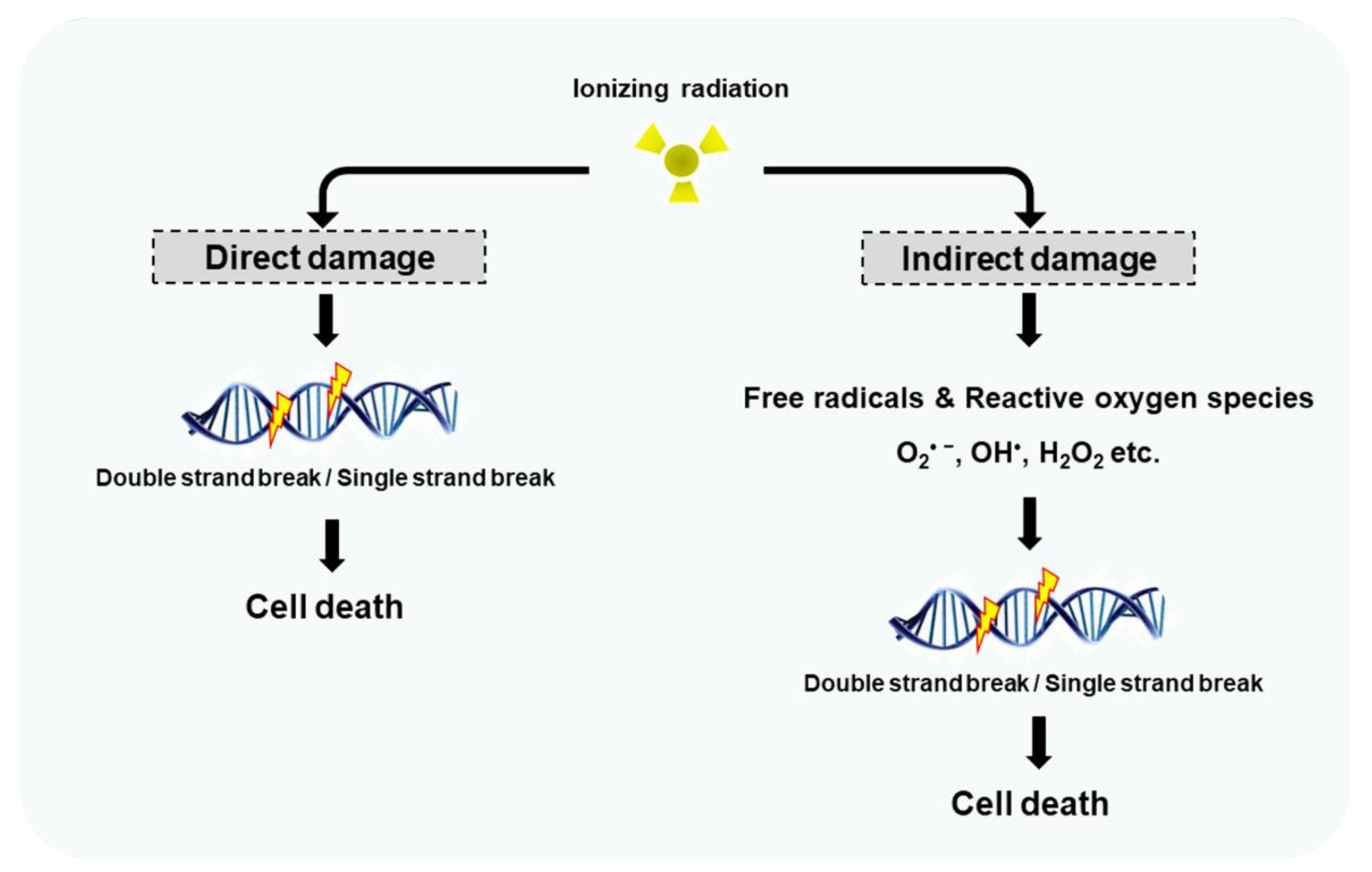
- Sevilla, M.; Bernhard, W. Mechanisms of direct radiation damage to DNA. In Radiation Chemistry; EDP Sciences: Les Ulis, France, 2020; pp. 191–202. [Google Scholar]
- Desouky, O.; Ding, N.; Zhou, G. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar] [CrossRef]
- Dayal, R.; Singh, A.; Pandey, A.; Mishra, K.P. Reactive oxygen species as mediator of tumor radiosensitivity. J. Cancer Res. Ther. 2014, 10, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Qi, F.; Kobayashi, J. Potential relationship between the biological effects of low-dose irradiation and mitochondrial ROS production. J. Radiat. Res. 2018, 59, ii91–ii97. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Lyu, X.; Yuan, S.; Wang, S.; Li, W.; Chen, Z.; Yu, H.; Li, F.; Jiang, Q. Oxidative stress: A critical hint in ionizing radiation induced pyroptosis. Radiat. Med. Prot. 2020, 1, 179–185. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.; Seo, D.; Kang, J.; Seong, K.M.; Youn, H. Cellular Stress Responses in Radiotherapy. Cells 2019, 8, 1105. [Google Scholar] [CrossRef]
- Hur, W.; Yoon, S.K. Molecular Pathogenesis of Radiation-Induced Cell Toxicity in Stem Cells. Int. J. Mol. Sci. 2017, 18, 2749. [Google Scholar] [CrossRef] [PubMed]
- Garibaldi, C.; Jereczek-Fossa, B.A.; Marvaso, G.; Dicuonzo, S.; Rojas, D.P.; Cattani, F.; Starzyńska, A.; Ciardo, D.; Surgo, A.; Leonardi, M.C.; et al. Recent advances in radiation oncology. Ecancermedicalscience 2017, 11, 785. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Krause, M.; Overgaard, J.; Debus, J.; Bentzen, S.M.; Daartz, J.; Richter, C.; Zips, D.; Bortfeld, T. Radiation oncology in the era of precision medicine. Nat. Cancer 2016, 16, 234–249. [Google Scholar] [CrossRef]
- Chen, H.H.W.; Kuo, M.T. Improving radiotherapy in cancer treatment: Promises and challenges. Oncotarget 2017, 8, 62742–62758. [Google Scholar] [CrossRef]
- Begg, A.C.; Stewart, F.A.; Vens, C. Strategies to improve radiotherapy with targeted drugs. Nat. Cancer 2011, 11, 239–253. [Google Scholar] [CrossRef]
- Xie, Y.; Han, Y.; Zhang, X.; Ma, H.; Li, L.; Yu, R.; Liu, H. Application of New Radiosensitizer Based on Nano-Biotechnology in the Treatment of Glioma. Front. Oncol. 2021, 11, 855. [Google Scholar] [CrossRef]
- Lawrence, T.S.; Blackstock, A.; McGinn, C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Semin. Radiat. Oncol. 2003, 13, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.; Bentzen, S.M.; Harari, P.M. Biologic Basis for Combining Drugs with Radiation. Semin. Radiat. Oncol. 2006, 16, 2–9. [Google Scholar] [CrossRef]
- Wainford, R.D.; Weaver, R.J.; Stewart, K.N.; Brown, P.; Hawksworth, G.M. Cisplatin nephrotoxicity is mediated by gamma glutamyltranspeptidase, not via a C-S lyase governed biotransformation pathway. Toxicology 2008, 249, 184–193. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Gu, K.; Chen, J.; Rui, M.; Jiang, G.-L. In vitro and in vivo study of a nanoliposomal cisplatin as a radiosensitizer. Int. J. Nanomed. 2011, 6, 437–444. [Google Scholar] [CrossRef]
- Jung, J.; Jeong, S.-Y.; Park, S.S.; Shin, S.H.; Ju, E.J.; Choi, J.; Park, J.; Lee, J.H.; Kim, I.; Suh, Y.-A.; et al. A cisplatin-incorporated liposome that targets the epidermal growth factor receptor enhances radiotherapeutic efficacy without nephrotoxicity. Int. J. Oncol. 2014, 46, 1268–1274. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Tian, X.; Warner, S.B.; Wagner, K.T.; Caster, J.M.; Zhang, T.; Ohana, P.; Gabizon, A.A.; Wang, A.Z. Preclinical Evaluation of Promitil, a Radiation-Responsive Liposomal Formulation of Mitomycin C Prodrug, in Chemoradiotherapy. Int. J. Radiat. Oncol. 2016, 96, 547–555. [Google Scholar] [CrossRef]
- Tahover, E.; Bar-Shalom, R.; Sapir, E.; Pfeffer, R.; Nemirovsky, I.; Turner, Y.; Gips, M.; Ohana, P.; Corn, B.W.; Wang, A.Z.; et al. Chemo-Radiotherapy of Oligometastases of Colorectal Cancer with Pegylated Liposomal Mitomycin-C Prodrug (Promitil): Mechanistic Basis and Preliminary Clinical Experience. Front. Oncol. 2018, 8, 544. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Tahover, E.; Kornev, G.; Patil, Y.; Amitay, Y.; Ohana, P.; Sapir, E.; Zalipsky, S. Development of Promitil®, a lipidic prodrug of mitomycin c in PEGylated liposomes: From bench to bedside. Adv. Drug Deliv. Rev. 2020, 154-155, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Amitay, Y.; Tzemach, D.; Gorin, J.; Shmeeda, H.; Zalipsky, S. Therapeutic efficacy of a lipid-based prodrug of mitomycin C in pegylated liposomes: Studies with human gastro-entero-pancreatic ectopic tumor models. J. Control. Release 2012, 160, 245–253. [Google Scholar] [CrossRef]
- Chastagner, P.; Sudour, H.; Mriouah, J.; Barberi-Heyob, M.; Bernier-Chastagner, V.; Pinel, S. Preclinical Studies of Pegylated- and Non-Pegylated Liposomal Forms of Doxorubicin as Radiosensitizer on Orthotopic High-Grade Glioma Xenografts. Pharm. Res. 2014, 32, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Labussière, M.; Aarnink, A.; Pinel, S.; Taillandier, L.; Escanyé, J.-M.; Barberi-Heyob, M.; Bernier-Chastagner, V.; Plénat, F.; Chastagner, P. Interest of liposomal doxorubicin as a radiosensitizer in malignant glioma xenografts. Anti-Cancer Drugs 2008, 19, 991–998. [Google Scholar] [CrossRef]
- Liu, H.; Xie, Y.; Zhang, Y.; Cai, Y.; Li, B.; Mao, H.; Liu, Y.-G.; Lu, J.; Zhang, L.; Yu, R. Development of a hypoxia-triggered and hypoxic radiosensitized liposome as a doxorubicin carrier to promote synergetic chemo-/radio-therapy for glioma. Biomaterials 2017, 121, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.-C.; Xu, W.-H.; Han, M.; Dong, Q.; Fu, Z.-X.; Diao, Y.-Y.; Liu, H.; Xu, J.; Jiang, H.-L.; Zheng, S.; et al. Doxorubicin-mediated radiosensitivity in multicellular spheroids from a lung cancer cell line is enhanced by composite micelle encapsulation. Int. J. Nanomed. 2012, 7, 2661–2671. [Google Scholar] [CrossRef] [PubMed]
- Minafra, L.; Porcino, N.; Bravatà, V.; Gaglio, D.; Bonanomi, M.; Amore, E.; Cammarata, F.P.; Russo, G.; Militello, C.; Savoca, G.; et al. Radiosensitizing effect of curcumin-loaded lipid nanoparticles in breast cancer cells. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Gao, X.X.; Shi, H.-S.; Li, D.; Zhang, Q.-W.; Wang, Y.-S.; Zheng, Y.; Cai, L.-L.; Zhong, R.-M.; Rui, A.; Li, Z.-Y.; et al. A systemic administration of liposomal curcumin inhibits radiation pneumonitis and sensitizes lung carcinoma to radiation. Int. J. Nanomed. 2012, 7, 2601–2611. [Google Scholar] [CrossRef][Green Version]
- Silva, V.L.; Ruiz, A.; Ali, A.; Pereira, S.; Seitsonen, J.; Ruokolainen, J.; Furlong, F.; Coulter, J.; Al-Jamal, W.T. Hypoxia-targeted cupric-tirapazamine liposomes potentiate radiotherapy in prostate cancer spheroids. Int. J. Pharm. 2021, 607, 121018. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, N.; Kok, R.J.; Bos, C.; Zandvliet, M.; Geerts, W.J.C.; Storm, G.; Moonen, C.T.W.; Lammers, T.; Deckers, R. Hyperthermia-triggered release of hypoxic cell radiosensitizers from temperature-sensitive liposomes improves radiotherapy efficacy in vitro. Nanotechnology 2019, 30, 264001. [Google Scholar] [CrossRef]
- Yao, H.; Qiu, H.; Shao, Z.; Wang, G.; Wang, J.; Yao, Y.; Xin, Y.; Zhou, M.; Wang, A.Z.; Zhang, L. Nanoparticle formulation of small DNA molecules, Dbait, improves the sensitivity of hormone-independent prostate cancer to radiotherapy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2261–2271. [Google Scholar] [CrossRef]
- Tian, J.; Min, Y.; Rodgers, Z.; Au, K.M.; Hagan, C.T.; Zhang, M.; Roche, K.; Yang, F.; Wagner, K.; Wang, A. Co-delivery of paclitaxel and cisplatin with biocompatible PLGA–PEG nanoparticles enhances chemoradiotherapy in non-small cell lung cancer models. J. Mater. Chem. B 2017, 5, 6049–6057. [Google Scholar] [CrossRef]
- Zhang, M.; Hagan, C.T.; Min, Y.; Foley, H.; Tian, X.; Yang, F.; Mi, Y.; Au, K.M.; Medik, Y.; Roche, K.; et al. Nanoparticle co-delivery of wortmannin and cisplatin synergistically enhances chemoradiotherapy and reverses platinum resistance in ovarian cancer models. Biomaterials 2018, 169, 1–10. [Google Scholar] [CrossRef]
- Zhang, M.; Hagan, C.T.; Foley, H.; Tian, X.; Yang, F.; Au, K.M.; Mi, Y.; Medik, Y.; Roche, K.; Wagner, K.; et al. Co-delivery of etoposide and cisplatin in dual-drug loaded nanoparticles synergistically improves chemoradiotherapy in non-small cell lung cancer models. Acta Biomater. 2021, 124, 327–335. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, L.; Zhai, G.; Ji, J.; Liu, A. Multifunctional Polyethylene Glycol (PEG)-Poly (Lactic-Co-Glycolic Acid) (PLGA)-Based Nanoparticles Loading Doxorubicin and Tetrahydrocurcumin for Combined Chemoradiotherapy of Glioma. Med. Sci. Monit. 2019, 25, 9737–9751. [Google Scholar] [CrossRef]
- Li, S.; Xu, Q.; Zhao, L.; Ye, C.; Hua, L.; Liang, J.; Yu, R.; Liu, H. Angiopep-2 Modified Cationic Lipid-Poly-Lactic-Co-Glycolic Acid Delivery Temozolomide and DNA Repair Inhibitor Dbait to Achieve Synergetic Chemo-Radiotherapy Against Glioma. J. Nanosci. Nanotechnol. 2019, 19, 7539–7545. [Google Scholar] [CrossRef]
- Liu, N.; Ji, J.; Qiu, H.; Shao, Z.; Wen, X.; Chen, A.; Yao, S.; Zhang, X.; Yao, H.; Zhang, L. Improving radio-chemotherapy efficacy of prostate cancer by co-deliverying docetaxel and dbait with biodegradable nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 48, 305–314. [Google Scholar] [CrossRef]
- Yang, C.; Peng, S.; Sun, Y.; Miao, H.; Lyu, M.; Ma, S.; Luo, Y.; Xiong, R.; Xie, C.; Quan, H. Development of a hypoxic nanocomposite containing high-Z element as 5-fluorouracil carrier activated self-amplified chemoradiotherapy co-enhancement. R. Soc. Open Sci. 2019, 6, 181790. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Wang, Z.; Zhao, L.; Mao, H.; Wang, G.; Zhang, K.; Liu, X.; Wu, D.; Zheng, Y.; Lu, J.; et al. Hypoxia-responsive lipid-poly-(hypoxic radiosensitized polyprodrug) nanoparticles for glioma chemo- and radiotherapy. Theranostics 2018, 8, 5088–5105. [Google Scholar] [CrossRef]
- Liu, H.; Cai, Y.; Zhang, Y.; Xie, Y.; Qiu, H.; Hua, L.; Liu, X.; Li, Y.; Lu, J.; Zhang, L.; et al. Development of a Hypoxic Radiosensitizer-Prodrug Liposome Delivery DNA Repair Inhibitor Dbait Combination with Radiotherapy for Glioma Therapy. Adv. Heal. Mater. 2017, 6, 1601377. [Google Scholar] [CrossRef]
- Neufeld, M.J.; DuRoss, A.N.; Landry, M.R.; Winter, H.; Goforth, A.M.; Sun, C. Co-delivery of PARP and PI3K inhibitors by nanoscale metal–organic frameworks for enhanced tumor chemoradiation. Nano Res. 2019, 12, 3003–3017. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, B.; Wang, H.; Yuan, A.; Hu, Y.; Wu, J. Two-stage oxygen delivery for enhanced radiotherapy by perfluorocarbon nanoparticles. Theranostics 2018, 8, 4898–4911. [Google Scholar] [CrossRef]
- Yao, L.; Feng, L.; Tao, D.; Tao, H.; Zhong, X.; Liang, C.; Zhu, Y.; Hu, B.; Liu, Z.; Zheng, Y. Perfluorocarbon nanodroplets stabilized with cisplatin-prodrug-constructed lipids enable efficient tumor oxygenation and chemo-radiotherapy of cancer. Nanoscale 2020, 12, 14764–14774. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Liang, C.; Yi, X.; Zhao, Q.; Cheng, L.; Yang, K.; Liu, Z. Perfluorocarbon-Loaded Hollow Bi2Se3Nanoparticles for Timely Supply of Oxygen under Near-Infrared Light to Enhance the Radiotherapy of Cancer. Adv. Mater. 2016, 28, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Ji, C.; Liang, C.; Song, X.; Yi, X.; Dong, Z.; Yang, K.; Liu, Z. TaOx decorated perfluorocarbon nanodroplets as oxygen reservoirs to overcome tumor hypoxia and enhance cancer radiotherapy. Biomaterials 2016, 112, 257–263. [Google Scholar] [CrossRef]
- Xu, L.; Qiu, X.; Zhang, Y.; Cao, K.; Zhao, X.; Wu, J.; Hu, Y.; Guo, H. Liposome encapsulated perfluorohexane enhances radiotherapy in mice without additional oxygen supply. J. Transl. Med. 2016, 14, 268. [Google Scholar] [CrossRef]
- Min, Y.; Roche, K.C.; Tian, S.; Eblan, M.J.; McKinnon, K.P.; Caster, J.; Chai, S.; Herring, L.E.; Zhang, L.; Zhang, T.; et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol. 2017, 12, 877–882. [Google Scholar] [CrossRef]
- Yang, K.; Choi, C.; Cho, H.; Ahn, W.; Kim, S.; Shin, S. Antigen-Capturing Mesoporous Silica Nanoparticles Enhance the Radiation-Induced Abscopal Effect in Murine Hepatocellular Carcinoma Hepa1-6 Models. Pharmaceutics 2021, 13, 1811. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, W.; Gao, P.; Shi, M.; Wu, T.; Li, N.; Tang, B. Boosting the abscopal effect of radiotherapy: A smart antigen-capturing radiosensitizer to eradicate metastatic breast tumors. Chem. Commun. 2020, 56, 10353–10356. [Google Scholar] [CrossRef]
- Starkewolf, Z.B.; Miyachi, L.; Wong, J.; Guo, T. X-ray triggered release of doxorubicin from nanoparticle drug carriers for cancer therapy. Chem. Commun. 2013, 49, 2545–2547. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, S.; Zhang, N.; Kuang, Y.; Li, W.; Gai, S.; He, F.; Gulzar, A.; Yang, P. X-ray-triggered NO-released Bi–SNO nanoparticles: All-in-one nano-radiosensitizer with photothermal/gas therapy for enhanced radiotherapy. Nanoscale 2020, 12, 19293–19307. [Google Scholar] [CrossRef]
- Fan, W.; Lu, N.; Shen, Z.; Tang, W.; Shen, B.; Cui, Z.; Shan, L.; Yang, Z.; Wang, Z.; Jacobson, O.; et al. Generic synthesis of small-sized hollow mesoporous organosilica nanoparticles for oxygen-independent X-ray-activated synergistic therapy. Nat. Commun. 2019, 10, 1241. [Google Scholar] [CrossRef]
- Deng, W.; Chen, W.; Clement, S.; Guller, A.; Zhao, Z.; Engel, A.; Goldys, E.M. Controlled gene and drug release from a liposomal delivery platform triggered by X-ray radiation. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Guo, X.; Luo, L.; Wu, Z.; Luo, Z.; Jiang, M.; Zhang, J.; Qin, B.; Shi, Y.; Lou, Y.; et al. Extremely Effective Chemoradiotherapy by Inducing Immunogenic Cell Death and Radio-Triggered Drug Release under Hypoxia Alleviation. ACS Appl. Mater. Interfaces 2019, 11, 46536–46547. [Google Scholar] [CrossRef]
- Shafei, A.; El-Bakly, W.; Sobhy, A.; Wagdy, O.; Reda, A.; Aboelenin, O.; Marzouk, A.; El Habak, K.; Mostafa, R.; Ali, M.A.; et al. A review on the efficacy and toxicity of different doxorubicin nanoparticles for targeted therapy in metastatic breast cancer. Biomed. Pharmacother. 2017, 95, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Diao, Y.-Y.; Jiang, H.-L.; Ying, X.-Y.; Chen, D.-W.; Liang, W.-Q.; Gao, J.-Q. Molecular mechanism study of chemosensitization of doxorubicin-resistant human myelogenous leukemia cells induced by a composite polymer micelle. Int. J. Pharm. 2011, 420, 404–411. [Google Scholar] [CrossRef]
- Kim, N.; Kim, H.K.; Lee, K.; Hong, Y.; Cho, J.H.; Choi, J.W.; Lee, J.-I.; Suh, Y.-L.; Ku, B.M.; Eum, H.H.; et al. Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Yi, J.J.; Zhu, J.-Q.; Zhao, C.C.; Kang, Q.; Zhang, X.; Suo, K.; Cao, N.; Hao, L.; Lu, J. Potential of natural products as radioprotectors and radiosensitizers: Opportunities and challenges. Food Funct. 2021, 12, 5204–5218. [Google Scholar] [CrossRef]
- Hosseinimehr, S.J. Beneficial effects of natural products on cells during ionizing radiation. Rev. Environ. Health 2014, 29, 341–353. [Google Scholar] [CrossRef]
- Javed, R.; Ghonaim, R.; Shathili, A.; Khalifa, S.A.; El-Seedi, H.R. Phytonanotechnology: A greener approach for biomedical applications. In Biogenic Nanoparticles for Cancer Theranostics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 43–86. [Google Scholar] [CrossRef]
- Sørensen, B.S.; Horsman, M.R. Tumor Hypoxia: Impact on Radiation Therapy and Molecular Pathways. Front. Oncol. 2020, 10, 562. [Google Scholar] [CrossRef]
- Grimes, D.R.; Partridge, M. A mechanistic investigation of the oxygen fixation hypothesis and oxygen enhancement ratio. Biomed. Phys. Eng. Express. 2016, 1, 1–22. [Google Scholar] [CrossRef]
- Chang, D.S.; Lasley, F.D.; Das, I.J.; Mendonca, M.S.; Dynlacht, J.R. Basic Radiotherapy Physics and Biology; Springer: Cham, Switzerland, 2014. [Google Scholar] [CrossRef]
- Telarovic, I.; Wenger, R.H.; Pruschy, M. Interfering with Tumor Hypoxia for Radiotherapy Optimization. J. Exp. Clin. Cancer Res. 2021, 40, 1–26. [Google Scholar] [CrossRef]
- Graham, K.; Unger, E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int. J. Nanomed. 2018, 13, 6049–6058. [Google Scholar] [CrossRef]
- Arthur-Baidoo, E.; Ameixa, J.; Ončák, M.; Denifl, S. Ring-Selective Fragmentation in the Tirapazamine Molecule upon Low-Energy Electron Attachment. Int. J. Mol. Sci. 2021, 22, 3159. [Google Scholar] [CrossRef]
- Romero, J.; Maihom, T.; Limão-Vieira, P.; Probst, M. Electronic structure and reactivity of tirapazamine as a radiosensitizer. J. Mol. Model. 2021, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.L.; Kaassis, A.; Dehsorkhi, A.; Koffi, C.-R.; Severic, M.; Abdelhamid, M.; Nyimanu, D.; Morris, C.; Al-Jamal, W.T. Enhanced selectivity, cellular uptake, and in vitro activity of an intrinsically fluorescent copper–tirapazamine nanocomplex for hypoxia targeted therapy in prostate cancer. Biomater. Sci. 2020, 8, 2420–2433. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Hong, C.R.; Wong, W.W.; Liew, L.P.; Shome, A.; Wang, J.; Gu, Y.; Stevenson, R.J.; Qi, W.; Anderson, R.F.; et al. Next-Generation Hypoxic Cell Radiosensitizers: Nitroimidazole Alkylsulfonamides. J. Med. Chem. 2018, 61, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Nitroimidazoles as hypoxic cell radiosensitizers and hypoxia probes: Misonidazole, myths and mistakes. Br. J. Radiol. 2018, 92, 20170915. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, J.A.; Taylor, L.; Sharma, N.; Kato, T.A. Exploiting DNA repair pathways for tumor sensitization, mitigation of resistance, and normal tissue protection in radiotherapy. Cancer Drug Resist. 2021, 4, 244–263. [Google Scholar] [CrossRef]
- Biau, J.; Chautard, E.; Berthault, N.; De Koning, L.; Court, F.; Pereira, B.; Verrelle, P.; Dutreix, M. Combining the DNA Repair Inhibitor Dbait With Radiotherapy for the Treatment of High Grade Glioma: Efficacy and Protein Biomarkers of Resistance in Preclinical Models. Front. Oncol. 2019, 9, 549. [Google Scholar] [CrossRef]
- Biau, J.; Devun, F.; Verrelle, P.; Dutreix, M. Dbait: Un concept innovant pour inhiber la réparation de l’ADN et contribuer aux traitements des cancers. Bull Cancer 2016, 103, 227–235. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y. Combination therapy based on nano codelivery for overcoming cancer drug resistance. Med. Drug Discov. 2020, 6, 100024. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Kemp, J.A.; Shim, M.S.; Heo, C.Y.; Kwon, Y.J. “Combo” nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv. Drug Deliv. Rev. 2015, 98, 3–18. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2015, 98, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Marupudi, N.; E Han, J.; Li, K.W.; Renard, V.M.; Tyler, B.M.; Brem, H. Paclitaxel: A review of adverse toxicities and novel delivery strategies. Expert Opin. Drug Saf. 2007, 6, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Pandey, M.; Chin, P.X.; Phang, Y.L.; Cheah, J.Y.; Ooi, S.C.; Mak, K.-K.; Pichika, M.R.; Kesharwani, P.; Hussain, Z.; et al. Transferrin receptors-targeting nanocarriers for efficient targeted delivery and transcytosis of drugs into the brain tumors: A review of recent advancements and emerging trends. Drug Deliv. Transl. Res. 2018, 8, 1545–1563. [Google Scholar] [CrossRef]
- Ho, M.Y.; Mackey, J. Presentation and management of docetaxel-related adverse effects in patients with breast cancer. Cancer Manag. Res. 2014, 6, 253–259. [Google Scholar] [CrossRef]
- Cordier, P.-Y.; Nau, A.; Ciccolini, J.; Oliver, M.; Mercier, C.; Lacarelle, B.; Peytel, E. 5-FU-induced neurotoxicity in cancer patients with profound DPD deficiency syndrome: A report of two cases. Cancer Chemother. Pharmacol. 2011, 68, 823–826. [Google Scholar] [CrossRef]
- Rabus, H.; Gargioni, E.; Li, W.B.; Nettelbeck, H.; Villagrasa, C. Erratum: Determining dose enhancement factors of high-Z nanoparticles from simulations where lateral secondary particle disequilibrium exists. Phys. Med. Biol. 2019, 64, 155016. [Google Scholar] [CrossRef]
- Chen, M.-H.; Hanagata, N.; Ikoma, T.; Huang, J.-Y.; Li, K.-Y.; Lin, C.-P.; Lin, F.-H. Hafnium-doped hydroxyapatite nanoparticles with ionizing radiation for lung cancer treatment. Acta Biomater. 2016, 37, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold Nanoparticles as Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2020, 15, 9407–9430. [Google Scholar] [CrossRef]
- Cunningham, C.; de Kock, M.; Engelbrecht, M.; Miles, X.; Slabbert, J.; Vandevoorde, C. Radiosensitization Effect of Gold Nanoparticles in Proton Therapy. Front. Public Health 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.J.; Nanavaty, N.S.; Devanaboyina, M.; Stanbery, L.; Hamouda, D.; Edelman, G.; Dworkin, L.; Nemunaitis, J.J. The abscopal effect of radiation therapy. Futur. Oncol. 2021, 17, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Daguenet, E.; Louati, S.; Wozny, A.-S.; Vial, N.; Gras, M.; Guy, J.-B.; Vallard, A.; Rodriguez-Lafrasse, C.; Magné, N. Radiation-induced bystander and abscopal effects: Important lessons from preclinical models. Br. J. Cancer 2020, 123, 339–348. [Google Scholar] [CrossRef]
- Morales-Orue, I.; Chicas-Sett, R.; Lara, P.C. Nanoparticles as a promising method to enhance the abscopal effect in the era of new targeted therapies. Rep. Pr. Oncol. Radiother. 2019, 24, 86–91. [Google Scholar] [CrossRef]
- Thanekar, A.M.; Sankaranarayanan, S.A.; Rengan, A.K. Role of nano-sensitizers in radiation therapy of metastatic tumors. Cancer Treat. Res. Commun. 2021, 26, 100303. [Google Scholar] [CrossRef]
- Murugan, B.; Sagadevan, S.; Fatimah, I.; Oh, W.C.; Hossain, M.A.M.; Johan, M.R. Smart stimuli-responsive nanocarriers for the cancer therapy—Nanomedicine. Nanotechnol. Rev. 2021, 10, 933–953. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef]
- Guo, T.; Starkewolfe, Z.B. Methods and Compositions for Targeted Release of Molecules from Nanoscale Carriers. U.S. Patent No. 9,993,553, 14 March 2014. [Google Scholar]
- Fologea, D.; Henry, R.; Salamo, G.; Mazur, Y.; Borelli, M.J. Methods and Compositions for X-ray Induced Release from Ph Sensitive Liposomes. U.S. Patent No. 9,849,087, 8 November 2012. [Google Scholar]
- Fologea, D.; Salamo, G.; Henry, R.; Borelli, M.J. Corry, P.M. Method of Controlled Drug Release from a Liposome Carrier. U.S. Patent No. 8,808,733, 31 March 2010. [Google Scholar]
- Bondurant, K.A. Mcgovern, R.M. Sutherland, Radiation Sensitive Liposomes. U.S. Patent No. 2009/0304589 A1, 26 September 2008. [Google Scholar]
- Brönnimann, D.; Bouchet, A.; Schneider, C.; Potez, M.; Serduc, R.; Bräuer-Krisch, E.; Graber, W.; Von Gunten, S.; Laissue, J.A.; Djonov, V. Synchrotron microbeam irradiation induces neutrophil infiltration, thrombocyte attachment and selective vascular damage in vivo. Sci. Rep. 2016, 6, 33601. [Google Scholar] [CrossRef] [PubMed]
- Slatkin, D.N.; Spanne, P.; Dilmanian, F.A.; Sandborg, M. Microbeam radiation therapy. Med Phys. 1992, 19, 1395–1400. [Google Scholar] [CrossRef]
- Smyth, L.; Donoghue, J.; Ventura, J.A.; Livingstone, J.; Bailey, T.; Day, L.R.J.; Crosbie, J.C.; Rogers, P.A.W. Comparative toxicity of synchrotron and conventional radiation therapy based on total and partial body irradiation in a murine model. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Bartzsch, S.H.; Corde, S.; Crosbie, J.C.; Day, L.R.J.; Donzelli, M.; Krisch, M.; Lerch, M.L.F.; Pellicioli, P.; Smyth, L.M.L.; Tehei, M. Technical advances in X-ray microbeam radiation therapy. Phys. Med. Biol. 2019, 65, 02TR01. [Google Scholar] [CrossRef]
- Bouchet, A.; Lemasson, B.; Christen, T.; Potez, M.; Rome, C.; Coquery, N.; Le Clec’H, C.; Moisan, A.; Brӓuer-Krisch, E.; Leduc, G.; et al. Synchrotron microbeam radiation therapy induces hypoxia in intracerebral gliosarcoma but not in the normal brain. Radiother. Oncol. 2013, 108, 143–148. [Google Scholar] [CrossRef]
- Bouchet, A.; Sakakini, N.; El Atifi, M.; Le Clec’H, C.; Brauer, E.; Moisan, A.; Deman, P.; Rihet, P.; Le Duc, G.; Pelletier, L. Early Gene Expression Analysis in 9L Orthotopic Tumor-Bearing Rats Identifies Immune Modulation in Molecular Response to Synchrotron Microbeam Radiation Therapy. PLoS ONE 2013, 8, e81874. [Google Scholar] [CrossRef]
- Bouchet, A.; Lemasson, B.; Le Duc, G.; Maisin, C.; Brӓuer-Krisch, E.; Siegbahn, A.; Renaud, L.; Khalil, E.; Rémy, C.; Poillot, C.; et al. Preferential Effect of Synchrotron Microbeam Radiation Therapy on Intracerebral 9L Gliosarcoma Vascular Networks. Int. J. Radiat. Oncol. 2010, 78, 1503–1512. [Google Scholar] [CrossRef]
- Shi, Y.; Van Der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef] [PubMed]
- Sabatasso, S.; Fernandez-Palomo, C.; Hlushchuk, R.; Fazzari, J.; Tschanz, S.; Pellicioli, P.; Krisch, M.; Laissue, J.; Djonov, V. Transient and Efficient Vascular Permeability Window for Adjuvant Drug Delivery Triggered by Microbeam Radiation. Cancers 2021, 13, 2103. [Google Scholar] [CrossRef]
- Bazyar, S.; Inscoe, C.R.; O’Brian, E.T.; Zhou, O.Z.; Lee, Y.Z.; Iii, E.T.O. Minibeam radiotherapy with small animal irradiators; in vitro and in vivo feasibility studies. Phys. Med. Biol. 2017, 62, 8924–8942. [Google Scholar] [CrossRef] [PubMed]
- Bartzsch, S.; Cummings, C.; Eismann, S.; Oelfke, U. A preclinical microbeam facility with a conventional X-ray tube. Med. Phys. 2016, 43, 6301–6308. [Google Scholar] [CrossRef] [PubMed]
- González, W.; dos Santos, M.; Guardiola, C.; Delorme, R.; Lamirault, C.; Juchaux, M.; Le Dudal, M.; Jouvion, G.; Prezado, Y. Minibeam radiation therapy at a conventional irradiator: Dose-calculation engine and first tumor-bearing animals irradiation. Phys. Med. 2020, 69, 256–261. [Google Scholar] [CrossRef]
- Price, L.S.; Rivera, J.N.; Madden, A.J.; Herity, L.B.; Piscitelli, J.A.; Mageau, S.; Santos, C.M.; Roques, J.R.; Midkiff, B.; Feinberg, N.N.; et al. Minibeam radiation therapy enhanced tumor delivery of PEGylated liposomal doxorubicin in a triple-negative breast cancer mouse model. Ther. Adv. Med. Oncol. 2021, 13. [Google Scholar] [CrossRef]
- Ali, I. Nano Anti-Cancer Drugs: Pros and Cons and Future Perspectives. Curr. Cancer Drug Targets 2011, 11, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef]
- Ali, E.S.; Sharker, S.M.; Islam, M.T.; Khan, I.N.; Shaw, S.; Rahman, A.; Uddin, S.J.; Shill, M.C.; Rehman, S.; Das, N.; et al. Targeting cancer cells with nanotherapeutics and nanodiagnostics: Current status and future perspectives. Semin. Cancer Biol. 2020, 69, 52–68. [Google Scholar] [CrossRef]
- Yan, S.; Zhao, P.; Yu, T.; Gu, N. Current applications and future prospects of nanotechnology in cancer immunotherapy. Cancer Biol. Med. 2019, 16, 486–497. [Google Scholar]
- A Chandra, R.; Keane, F.K.; Voncken, F.E.M.; Thomas, C.R. Contemporary radiotherapy: Present and future. Lancet 2021, 398, 171–184. [Google Scholar] [CrossRef]
- Durante, M.; Orecchia, R.; Loeffler, J.S. Charged-particle therapy in cancer: Clinical uses and future perspectives. Nat. Rev. Clin. Oncol. 2017, 14, 483–495. [Google Scholar] [CrossRef]
- Baumann, M.; Ebert, N.; Kurth, I.; Bacchus, C.; Overgaard, J. What will radiation oncology look like in 2050? A look at a changing professional landscape in Europe and beyond. Mol. Oncol. 2020, 14, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Gomathi, H.; Ayisha Hamna, T.P.; Jijo, A.J.; Saradha Devi, K.M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, E.R.; Franco, M.S. Combining Nanocarrier-Assisted Delivery of Molecules and Radiotherapy. Pharmaceutics 2022, 14, 105. https://doi.org/10.3390/pharmaceutics14010105
Gomes ER, Franco MS. Combining Nanocarrier-Assisted Delivery of Molecules and Radiotherapy. Pharmaceutics. 2022; 14(1):105. https://doi.org/10.3390/pharmaceutics14010105
Chicago/Turabian StyleGomes, Eliza Rocha, and Marina Santiago Franco. 2022. "Combining Nanocarrier-Assisted Delivery of Molecules and Radiotherapy" Pharmaceutics 14, no. 1: 105. https://doi.org/10.3390/pharmaceutics14010105
APA StyleGomes, E. R., & Franco, M. S. (2022). Combining Nanocarrier-Assisted Delivery of Molecules and Radiotherapy. Pharmaceutics, 14(1), 105. https://doi.org/10.3390/pharmaceutics14010105







