Mesoporous Calcium-Silicate Nanoparticles Loaded with Low-Dose Triton-100+Ag+ to Achieve Both Enhanced Antibacterial Properties and Low Cytotoxicity for Dentin Disinfection of Human Teeth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MCSNs and M-AgTX
2.3. Characterization of MCSNs and M-AgTX
2.4. pH Measurement and Release Profile
2.5. Cytotoxicity Test
2.6. Minimum Inhibitory Concentration (MIC) and Bactericidal Concentration (MBC) of MCSNs and M-AgTX
2.7. Anti-Bacterial Effect against Planktonic E. faecalis
2.8. Dentinal Tubule Infiltration Ability and Anti-Bacterial Effect on Dentin Pretreated with M-AgTX
2.9. Anti-Biofilm Activities of M-AgTX against E. faecalis Mature Biofilm on Dentin
2.10. Endocytosis of Nanoparticles by E. faecalis
2.11. Microhardness Test
2.12. In Vitro Mineralization
2.13. Statistical Analysis
3. Results
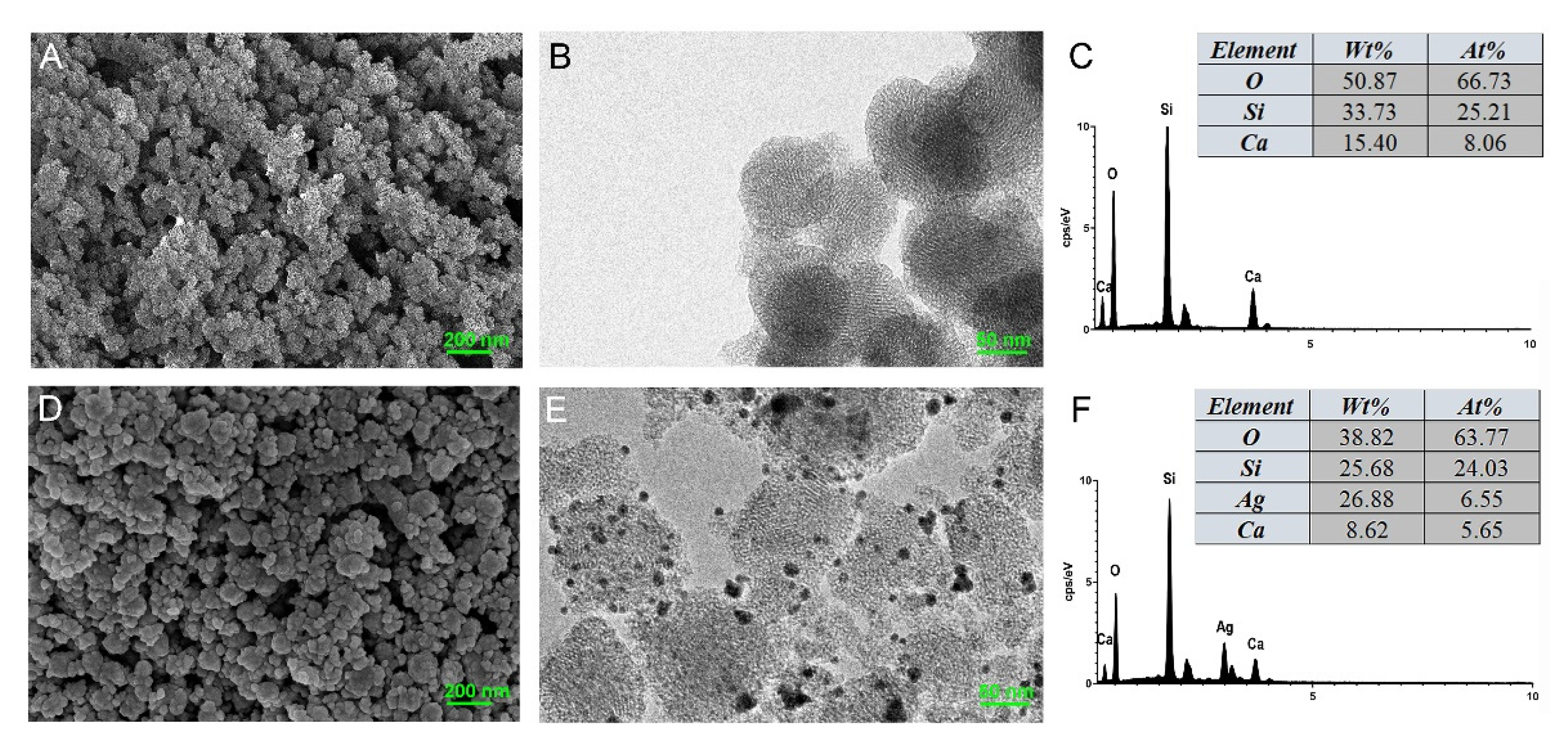
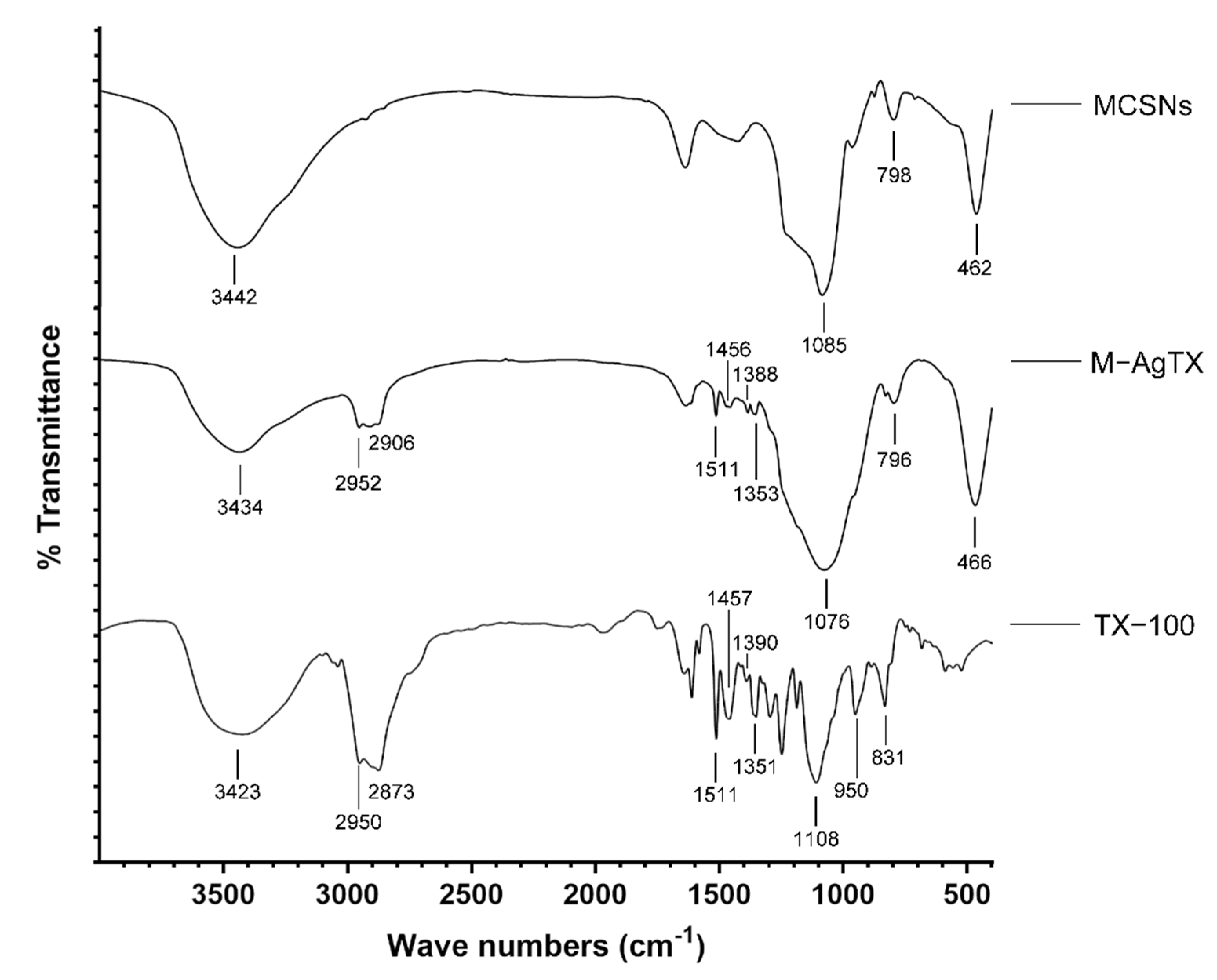
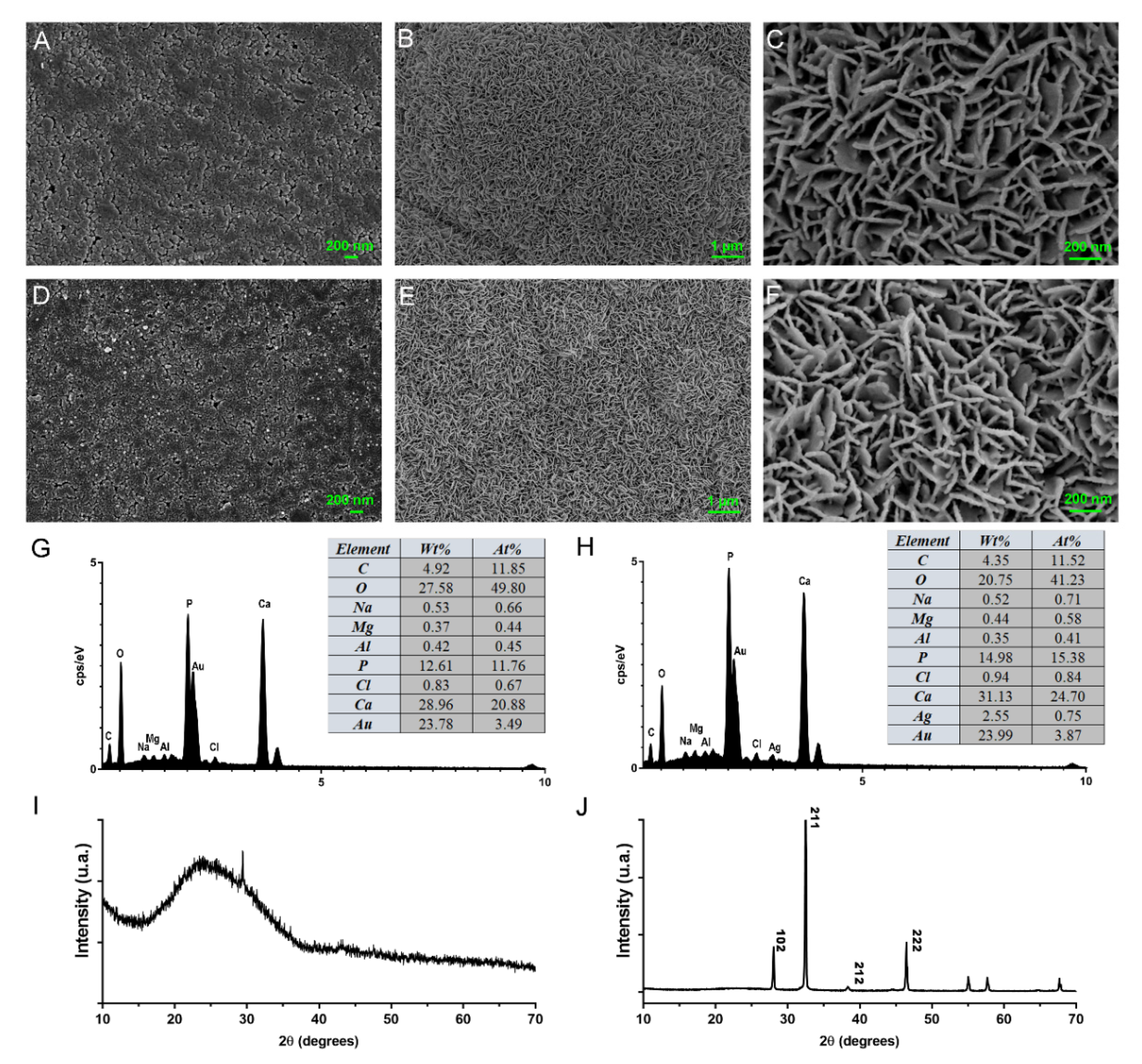
3.1. Characterization of MCSNs and M-AgTX
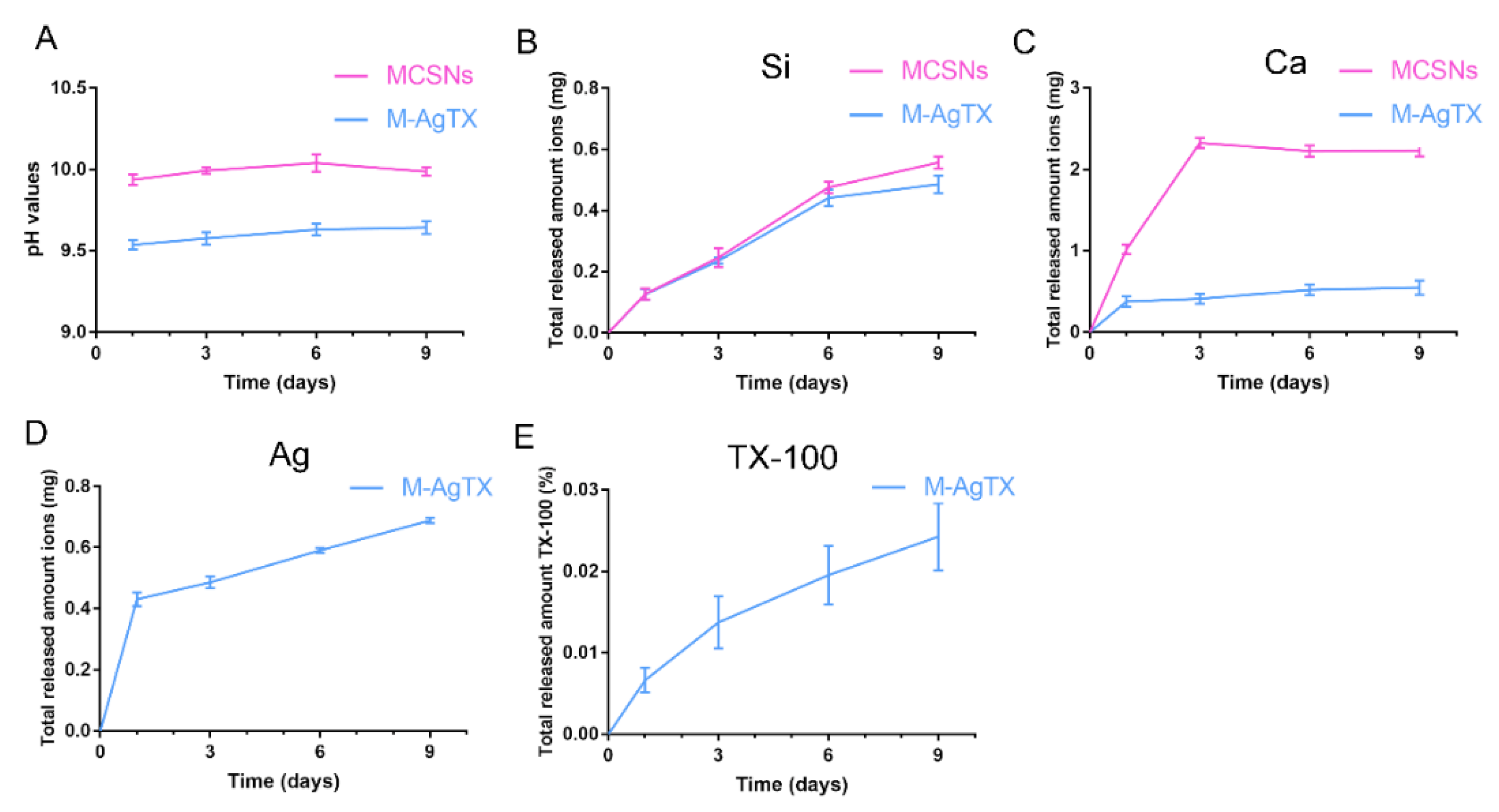
3.2. pH Measurement and Release Profile
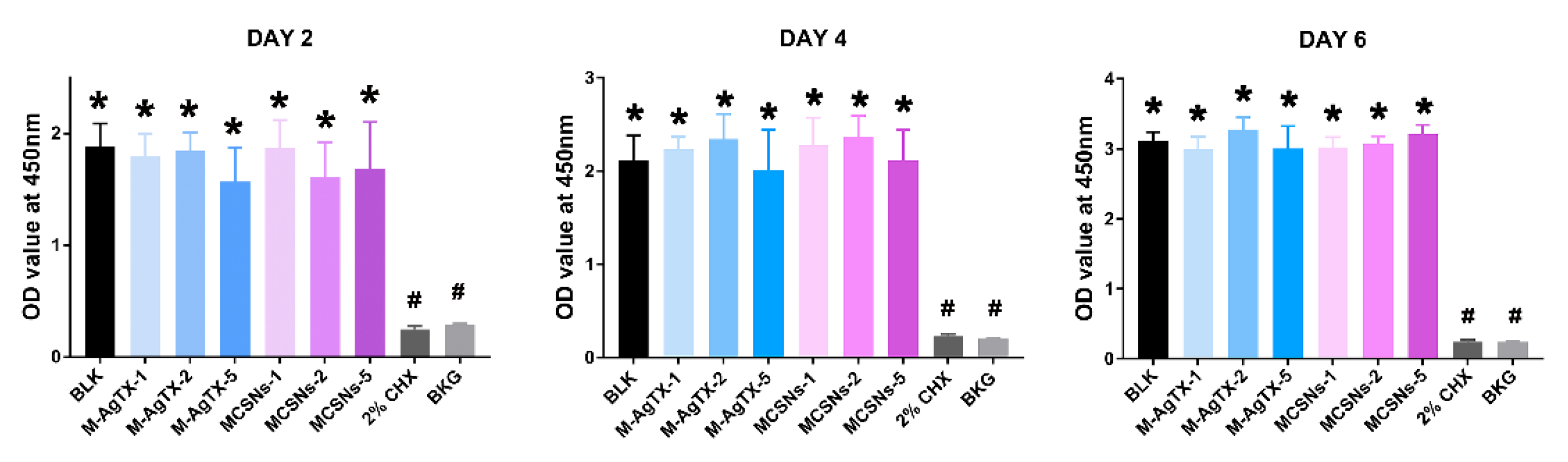
3.3. Cytotoxicity Test
3.4. MIC and MBC of MCSNs and M-AgTX
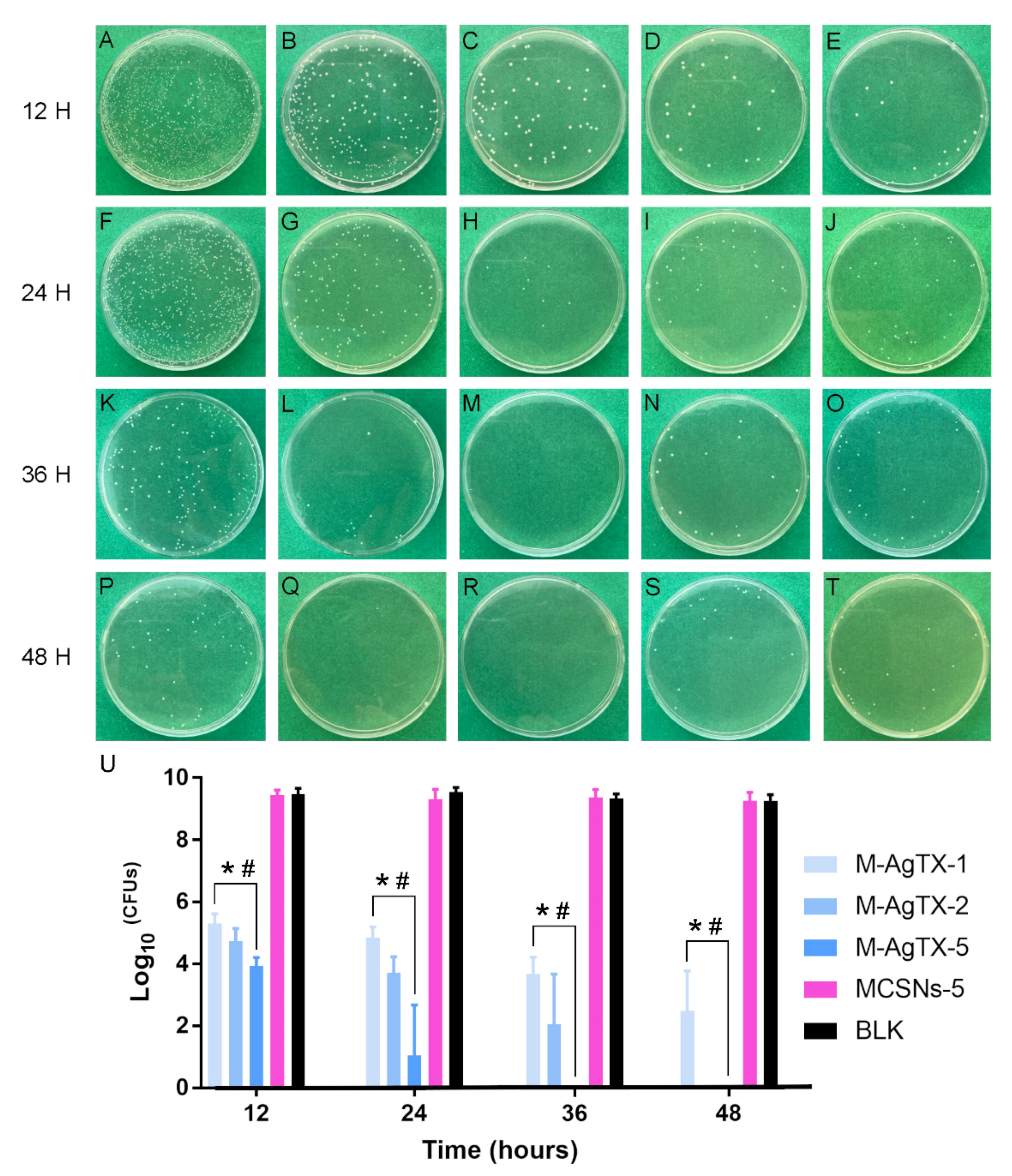
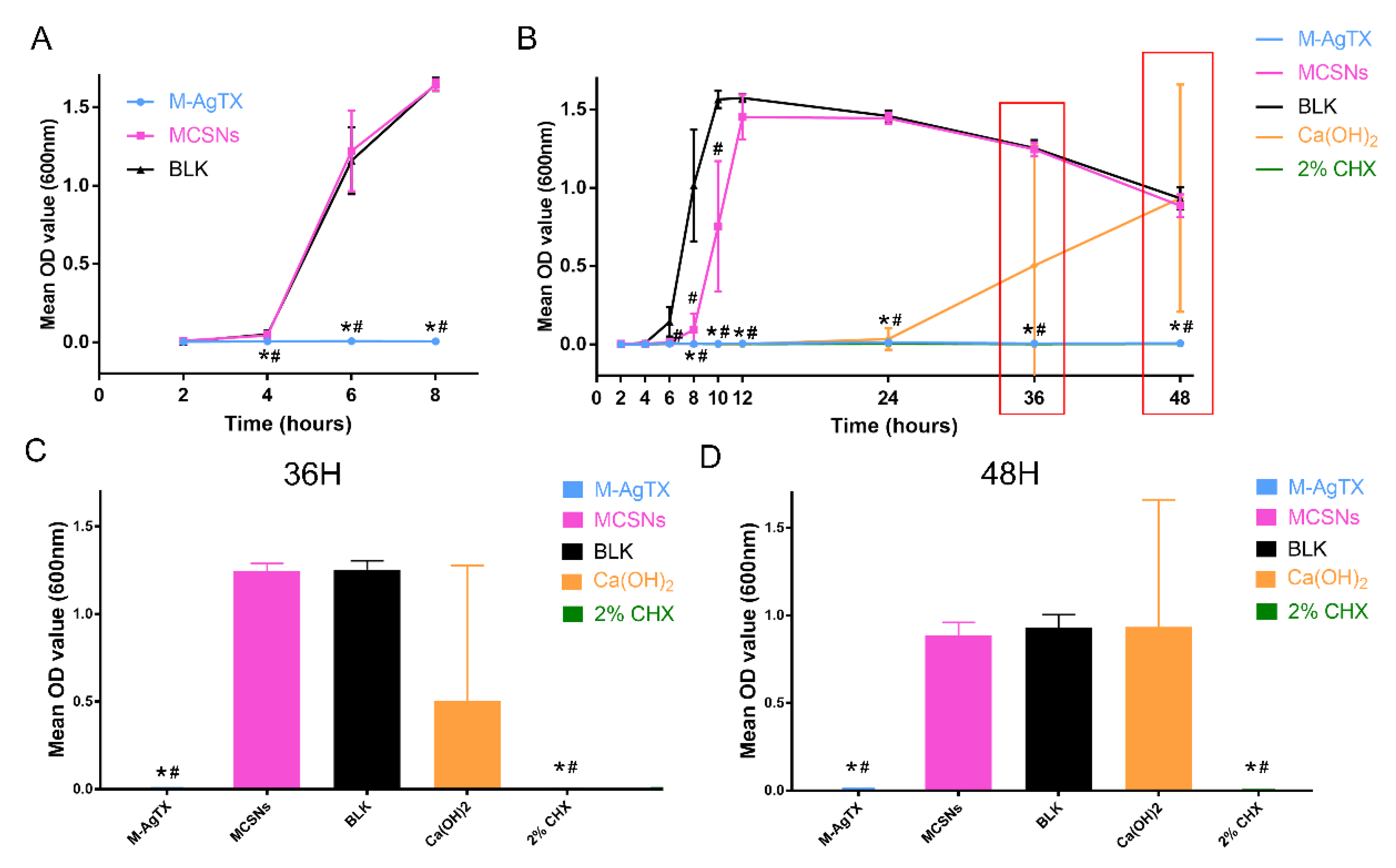
3.5. Anti-Bacterial Effect against Planktonic E. faecalis
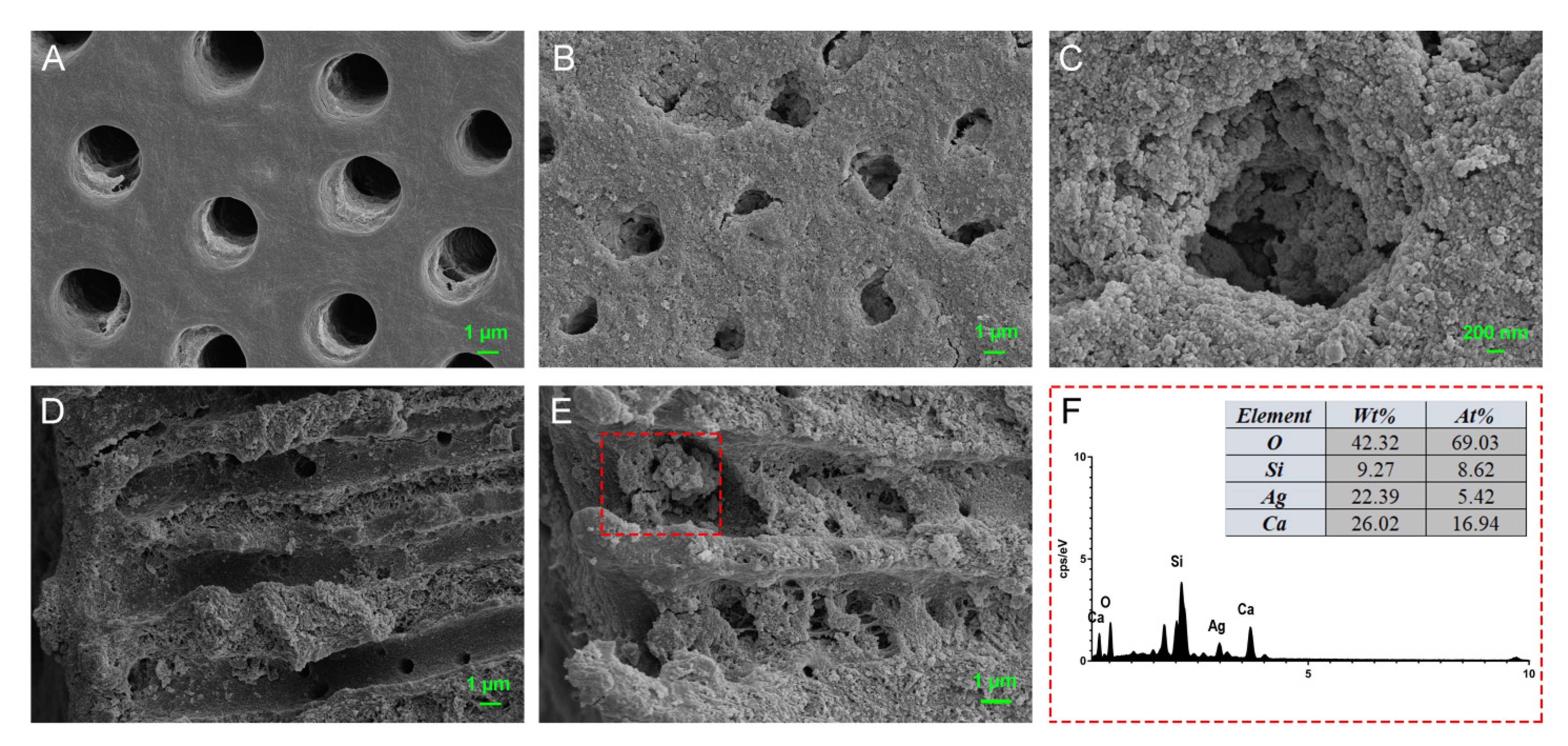
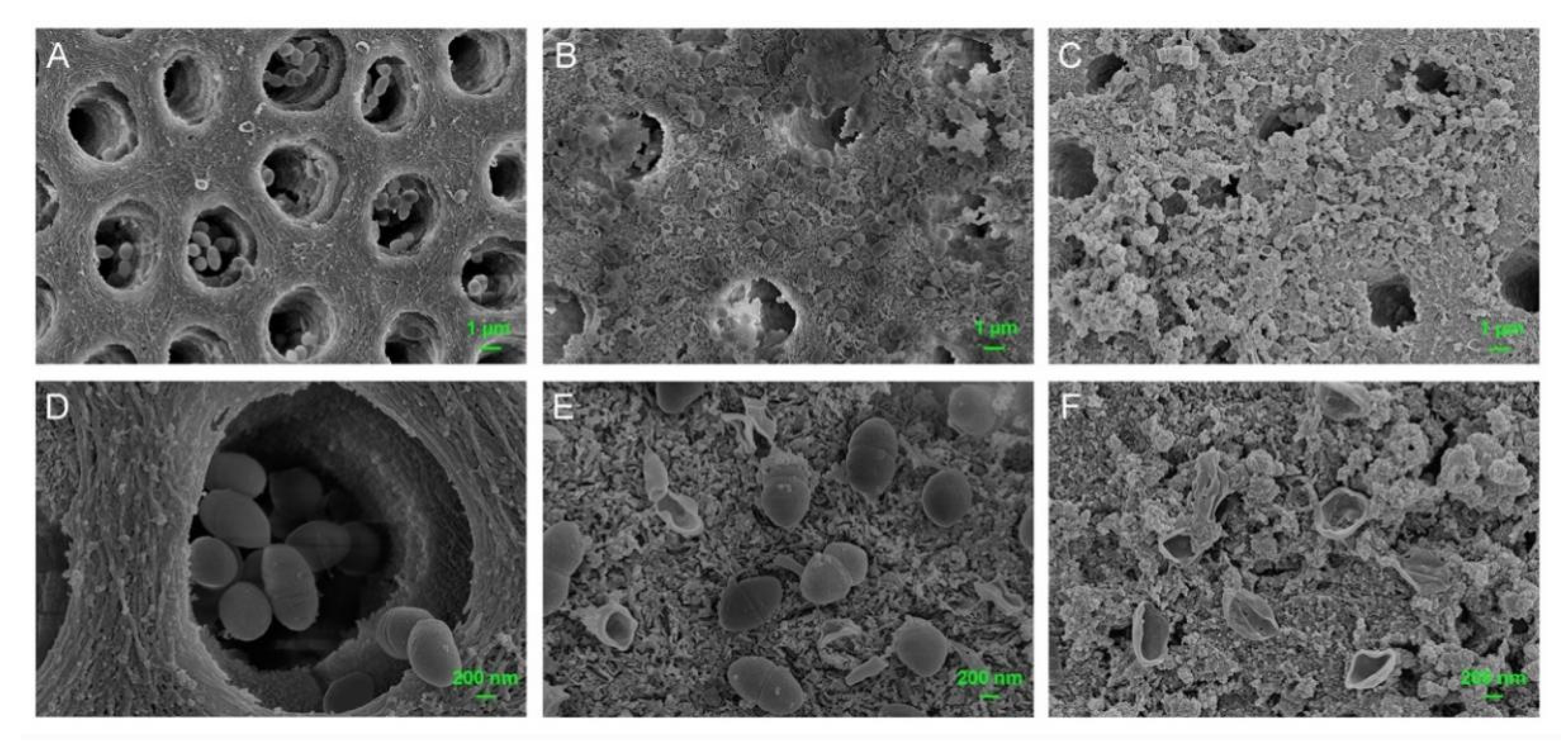
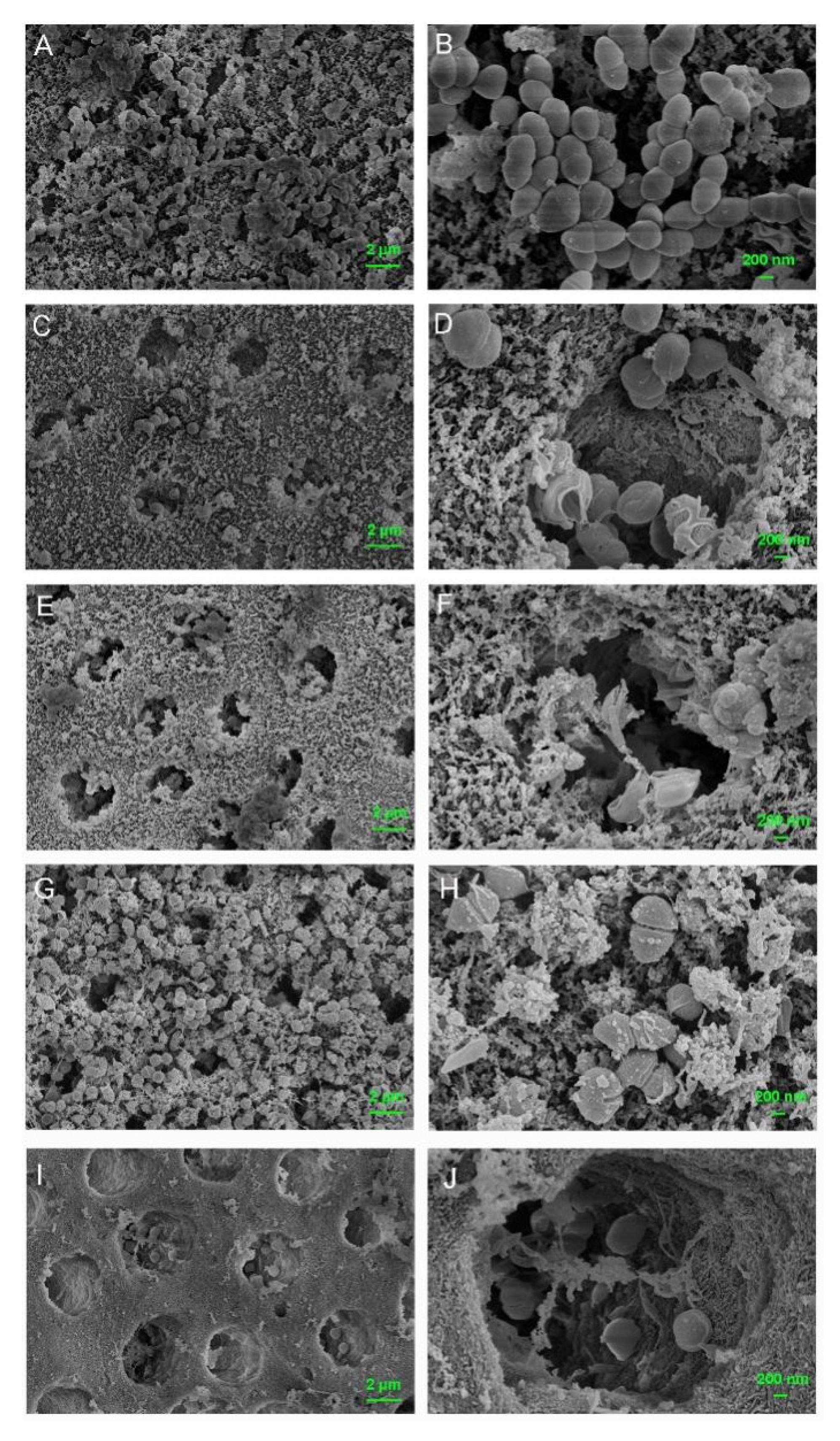
3.6. Dentinal Tubule Infiltration Ability and Anti-Bacterial Effect on Root Canal Walls Pretreated with M-AgTX
3.7. Anti-Biofilm Activities of M-AgTX against E. faecalis Mature Biofilm
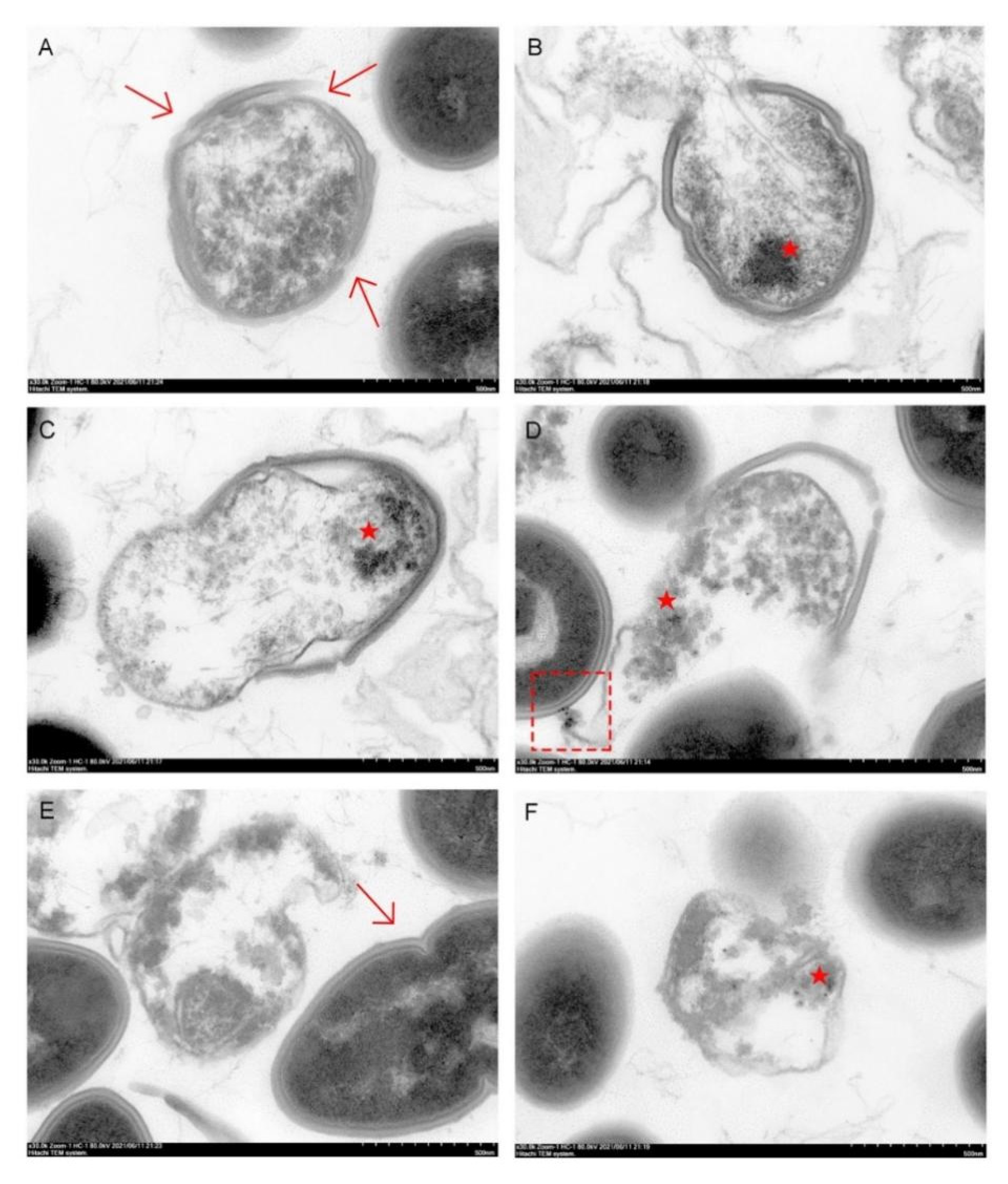
3.8. Endocytosis of Nanoparticles by E. faecalis
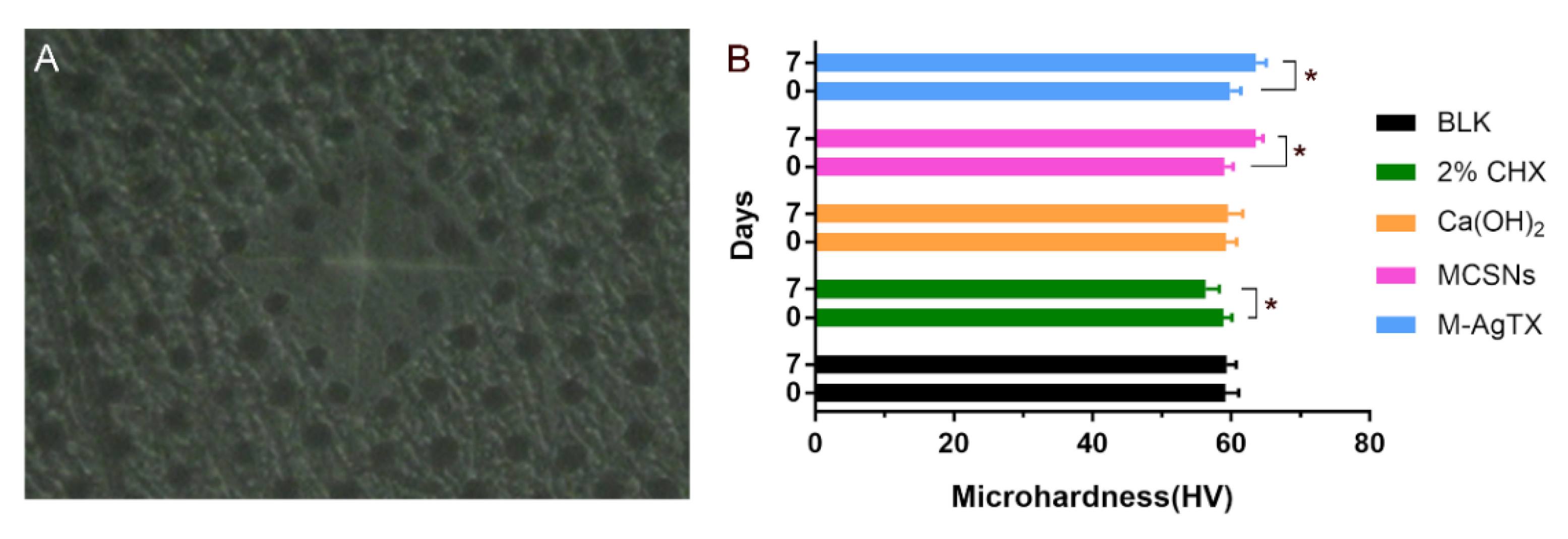
3.9. Microhardness Test
3.10. In Vitro Mineralization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Figdor, D.; Davies, J.K.; Sundqvist, G. Starvation survival, growth and recovery of Enterococcus faecalis in human serum. Oral Microbiol. Immunol. 2003, 18, 234–239. [Google Scholar] [CrossRef]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its Role in Root Canal Treatment Failure and Current Concepts in Retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef]
- Carr, G.B.; Schwartz, R.S.; Schaudinn, C.; Gorur, A.; Costerton, J.W. Ultrastructural examination of failed molar retreatment with secondary apical periodontitis: An examination of endodontic biofilms in an endodontic retreatment failure. J. Endod. 2009, 35, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.P.; Vianna, M.E.; Zaia, A.A.; Almeida, J.F.; Souza-Filho, F.J.; Ferraz, C.C. Chlorhexidine in endodontics. Braz. Dent. J. 2013, 24, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Louwakul, P.; Saelo, A.; Khemaleelakul, S. Efficacy of calcium oxide and calcium hydroxide nanoparticles on the elimination of Enterococcus faecalis in human root dentin. Clin. Oral Investig. 2017, 21, 865–871. [Google Scholar] [CrossRef]
- Marending, M.; Stark, W.J.; Brunner, T.J.; Fischer, J.; Zehnder, M. Comparative assessment of time-related bioactive glass and calcium hydroxide effects on mechanical properties of human root dentin. Dent. Traumatol. 2009, 25, 126–129. [Google Scholar] [CrossRef] [Green Version]
- Batur, Y.B.; Erdemir, U.; Sancakli, H.S. The long-term effect of calcium hydroxide application on dentin fracture strength of endodontically treated teeth. Dent. Traumatol. 2013, 29, 461–464. [Google Scholar] [CrossRef]
- Afkhami, F.; Pourhashemi, S.J.; Sadegh, M.; Salehi, Y.; Fard, M.J.K. Antibiofilm efficacy of silver nanoparticles as a vehicle for calcium hydroxide medicament against Enterococcus faecalis. J. Dent. 2015, 43, 1573–1579. [Google Scholar] [CrossRef]
- Lessa, F.C.; Aranha, A.M.; Nogueira, I.; Giro, E.M.; Hebling, J.; Costa, C.A. Toxicity of chlorhexidine on odontoblast-like cells. J. Appl. Oral Sci. 2010, 18, 50–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Yang, H.; Li, K.; Yu, J.; Huang, C. Effects of Chlorhexidine-Encapsulated Mesoporous Silica Nanoparticles on the Anti-Biofilm and Mechanical Properties of Glass Ionomer Cement. Molecules 2017, 22, 1225. [Google Scholar] [CrossRef] [Green Version]
- da Silva, R.A.B.; Leonardo, M.R.; Da Silva, L.A.B.; de Castro, L.M.S.; Rosa, A.L.; de Oliveira, P.T. Effects of the Association between a Calcium Hydroxide Paste and 0.4% Chlorhexidine on the Development of the Osteogenic Phenotype In Vitro. J. Endod. 2008, 34, 1485–1489. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Huang, T.-H.; Kao, C.-T.; Wu, Y.-H.; Chen, W.-C.; Shie, M.-Y. Mesoporous Calcium Silicate Nanoparticles with Drug Delivery and Odontogenesis Properties. J. Endod. 2017, 43, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Li, Y.; Sun, Q.; Ma, T.; Fan, B. Calcium-silicate mesoporous nanoparticles loaded with chlorhexidine for both anti- Enterococcus faecalis and mineralization properties. J. Nanobiotechnol. 2016, 14, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-Y.; Shie, M.-Y.; Lee, A.; Chou, Y.-T.; Chiang, C.; Lin, C.-P. 3D-Printed Ginsenoside Rb1-Loaded Mesoporous Calcium Silicate/Calcium Sulfate Scaffolds for Inflammation Inhibition and Bone Regeneration. Biomedicines 2021, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Duan, M.; Fan, W.; Fan, B. Ca–Si mesoporous nanoparticles with the optimal Ag–Zn ratio inhibit the Enterococcus faecalis infection of teeth through dentinal tubule infiltration: An in vitro and in vivo study. J. Mater. Chem. B 2021, 9, 2200–2211. [Google Scholar] [CrossRef]
- Peng, X.-Y.; Hu, M.; Liao, F.; Yang, F.; Ke, Q.-F.; Guo, Y.-P.; Zhu, Z.-H. La-Doped mesoporous calcium silicate/chitosan scaffolds for bone tissue engineering. Biomater. Sci. 2019, 7, 1565–1573. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.-J.; Guo, X.-X.; Sham, T.-K. Calcium silicate-based drug delivery systems. Expert Opin. Drug Deliv. 2016, 14, 215–228. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J.; Fan, W. Bioactive mesoporous calcium–silicate nanoparticles with excellent mineralization ability, osteostimulation, drug-delivery and antibacterial properties for filling apex roots of teeth. J. Mater. Chem. 2012, 22, 16801–16809. [Google Scholar] [CrossRef]
- Fan, W.; Sun, Q.; Li, Y.; Tay, F.R.; Fan, B. Synergistic mechanism of Ag+–Zn2+ in anti-bacterial activity against Enterococcus faecalis and its application against dentin infection. J. Nanobiotechnol. 2018, 16, 1–16. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Li, M.; Yu, P.; Chu, X.; Li, L.; Tan, G.; Wang, Y.; Chen, X.; Zhang, Y.; et al. The synergistic antibacterial activity and mechanism of multicomponent metal ions-containing aqueous solutions against Staphylococcus aureus. J. Inorg. Biochem. 2016, 163, 214–220. [Google Scholar] [CrossRef]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and Differences between Silver Ions and Silver in Nanoforms as Antibacterial Agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenman, K.D.; Moss, A.; Kon, S. Argyria: Clinical implications of exposure to silver nitrate and silver oxide. J. Occup. Med. 1979, 21, 430–435. [Google Scholar]
- Wan, A.T.; A Conyers, R.; Coombs, C.J.; Masterton, J.P. Determination of silver in blood, urine, and tissues of volunteers and burn patients. Clin. Chem. 1991, 37, 1683–1687. [Google Scholar] [CrossRef]
- Lu, M.-M.; Wang, Q.-J.; Chang, Z.-M.; Wang, Z.; Zheng, X.; Shao, D.; Dong, W.-F.; Zhou, Y.-M. Synergistic bactericidal activity of chlorhexidine-loaded, silver-decorated mesoporous silica nanoparticles. Int. J. Nanomed. 2017, 12, 3577–3589. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Duan, M.; Sun, Q.; Fan, B. Simvastatin enhanced antimicrobial effect of Ag+ against E. faecalis infection of dentine through PLGA co-delivery submicron particles. J. Biomater. Sci. Polym. Ed. 2020, 31, 2331–2346. [Google Scholar] [CrossRef]
- Rodi, P.; Gianello, M.B.; Corregido, M.; Gennaro, A. Comparative study of the interaction of CHAPS and Triton X-100 with the erythrocyte membrane. Biochim. Biophys. Acta 2014, 1838, 859–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.; Weir, M.D.; Zhang, K.; Deng, D.; Cheng, L.; Xu, H.H. Synthesis of new antibacterial quaternary ammonium monomer for incorporation into CaP nanocomposite. Dent. Mater. 2013, 29, 859–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Yang, H.; Li, K.; Lei, J.; Zhou, L.; Huang, C. A novel application of nanohydroxyapatite/mesoporous silica biocomposite on treating dentin hypersensitivity: An in vitro study. J. Dent. 2016, 50, 21–29. [Google Scholar] [CrossRef]
- Brezoiu, A.M.; Prundeanu, M.; Berger, D.; Deaconu, M.; Matei, C.; Oprea, O.; Vasile, E.; Negreanu-Pirjol, T.; Muntean, D.; Danciu, C. Properties of Salvia officinalis L. and Thymus serpyllum L. Extracts Free and Embedded into Mesopores of Silica and Titania Nanomaterials. Nanomaterials 2020, 10, 820. [Google Scholar] [CrossRef]
- Buda, V.; Brezoiu, A.-M.; Berger, D.; Pavel, I.; Muntean, D.; Minda, D.; Dehelean, C.; Soica, C.; Diaconeasa, Z.; Folescu, R.; et al. Biological Evaluation of Black Chokeberry Extract Free and Embedded in Two Mesoporous Silica-Type Matrices. Pharmaceutics 2020, 12, 838. [Google Scholar] [CrossRef]
- Mandal, A.; Meda, V.; Zhang, W.; Dalai, A. Spectroscopic investigation of collagen scaffolds impregnated with AgNPs coated by PEG/TX-100 mixed systems. Int. J. Biol. Macromol. 2012, 50, 603–612. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Z.; Wu, J.; Yiu, Y.-M.; Hu, Y.; Zhu, Y.-J.; Sham, T.-K. Tracking Drug Loading Capacities of Calcium Silicate Hydrate Carrier: A Comparative X-ray Absorption Near Edge Structures Study. J. Phys. Chem. B 2015, 119, 10052–10059. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; DeBeer, D.; Caldwell, D.; Korber, D.; James, G. Biofilms, the customized microniche. J. Bacteriol. 1994, 176, 2137–2142. [Google Scholar] [CrossRef] [Green Version]
- Sathorn, C.; Parashos, P.; Messer, H. Antibacterial efficacy of calcium hydroxide intracanal dressing: A systematic review and meta-analysis. Int. Endod. J. 2007, 40, 2–10. [Google Scholar] [CrossRef]
- Balto, H.; Bukhary, S.; Al-Omran, O.; BaHammam, A.; Al-Mutairi, B. Combined Effect of a Mixture of Silver Nanoparticles and Calcium Hydroxide against Enterococcus faecalis Biofilm. J. Endod. 2020, 46, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.; Kwon, J.; Soh, S.M.; Jang, H.; Mitchell, R.J. Sensitivity of predatory bacteria to different surfactants and their application to check bacterial predation. Appl. Microbiol. Biotechnol. 2019, 103, 8169–8178. [Google Scholar] [CrossRef]
- Colavita, F.; Quartu, S.; Lalle, E.; Bordi, L.; Lapa, D.; Meschi, S.; Vulcano, A.; Toffoletti, A.; Bordi, E.; Paglia, M.G.; et al. Evaluation of the inactivation effect of Triton X-100 on Ebola virus infectivity. J. Clin. Virol. 2017, 86, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Komatsuzawa, H.; Sugai, M.; Shirai, C.; Suzuki, J.; Hiramatsu, K.; Suginaka, H. Triton X-100 alters the resistance level of methicillin-resistantStaphylococcus aureusto oxacillin. Fems Microbiol. Lett. 1995, 134, 209–212. [Google Scholar] [CrossRef]
- Plutzer, B.; Zilm, P.; Ratnayake, J.; Cathro, P. Comparative efficacy of endodontic medicaments and sodium hypochlorite againstEnterococcus faecalisbiofilms. Aust. Dent. J. 2018, 63, 208–216. [Google Scholar] [CrossRef]
- Devaraj, S.; Jagannathan, N.; Neelakantan, P. Antibiofilm efficacy of photoactivated curcumin, triple and double antibiotic paste, 2% chlorhexidine and calcium hydroxide against Enterococcus fecalis in vitro. Sci. Rep. 2016, 6, 24797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, Z.; Shalavi, S.; Giardino, L.; Palazzi, F. Effect of Surfactants on the Efficacy of Root Canal Irrigants: A Review. New York State Dent. J. 2017, 83, 37–42. [Google Scholar]
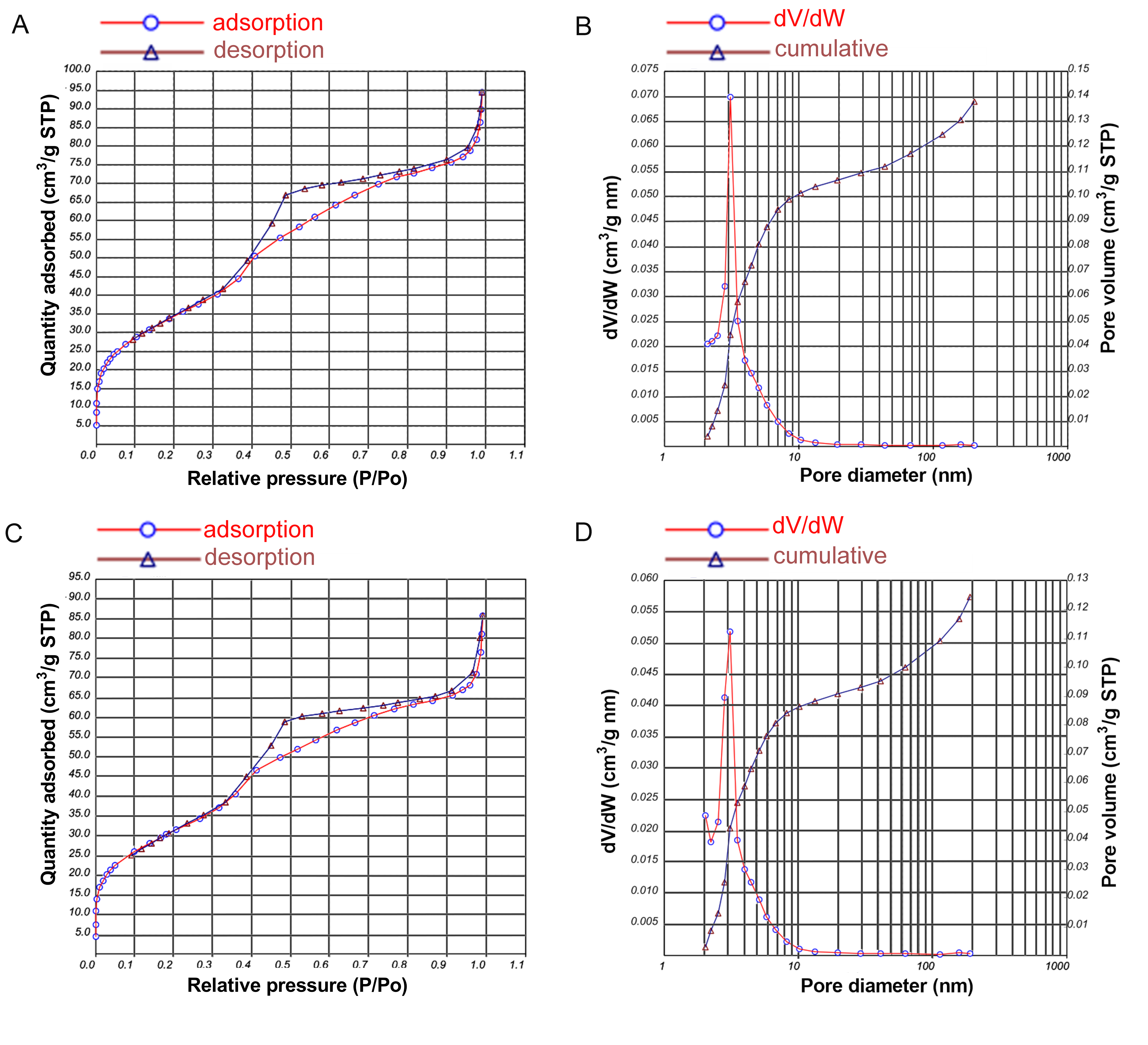
| Groups | SBET (m2 g−1) | VP (cm3 g−1) | DP (nm) |
|---|---|---|---|
| MCSNs | 120.27 | 0.15 | 4.66 |
| M-AgTX | 109.32 | 0.13 | 4.65 |
| Groups | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|
| MCSNs | - | - |
| M-AgTX | 16 | 16 |
| Times/Groups | M-AgTX-1 | M-AgTX-2 | M-AgTX-5 | MCSNs-5 | BLK |
|---|---|---|---|---|---|
| 12 h | 56.01% ± 4.74% *,# | 49.96% ± 6.18% *,# | 41.44% ± 1.90% *,# | 99.64% ± 1.24% | 1 |
| 24 h | 50.91% ± 4.68% *,# | 39.03% ± 4.30% *,# | 11.13% ± 21.54% *,# | 97.64% ± 4.32% | 1 |
| 36 h | 39.38% ± 10.83% *,# | 22.16% ± 23.80% *,# | 0.00% ± 0.00% *,# | 100.51% ± 4.61% | 1 |
| 48 h | 26.91% ± 8.80% *,# | 0.00% ± 0.00% *,# | 0.00% ± 0.00% *,# | 100.02% ± 0.96% | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, M.; Fan, W.; Fan, B. Mesoporous Calcium-Silicate Nanoparticles Loaded with Low-Dose Triton-100+Ag+ to Achieve Both Enhanced Antibacterial Properties and Low Cytotoxicity for Dentin Disinfection of Human Teeth. Pharmaceutics 2021, 13, 1518. https://doi.org/10.3390/pharmaceutics13091518
Duan M, Fan W, Fan B. Mesoporous Calcium-Silicate Nanoparticles Loaded with Low-Dose Triton-100+Ag+ to Achieve Both Enhanced Antibacterial Properties and Low Cytotoxicity for Dentin Disinfection of Human Teeth. Pharmaceutics. 2021; 13(9):1518. https://doi.org/10.3390/pharmaceutics13091518
Chicago/Turabian StyleDuan, Mengting, Wei Fan, and Bing Fan. 2021. "Mesoporous Calcium-Silicate Nanoparticles Loaded with Low-Dose Triton-100+Ag+ to Achieve Both Enhanced Antibacterial Properties and Low Cytotoxicity for Dentin Disinfection of Human Teeth" Pharmaceutics 13, no. 9: 1518. https://doi.org/10.3390/pharmaceutics13091518
APA StyleDuan, M., Fan, W., & Fan, B. (2021). Mesoporous Calcium-Silicate Nanoparticles Loaded with Low-Dose Triton-100+Ag+ to Achieve Both Enhanced Antibacterial Properties and Low Cytotoxicity for Dentin Disinfection of Human Teeth. Pharmaceutics, 13(9), 1518. https://doi.org/10.3390/pharmaceutics13091518







