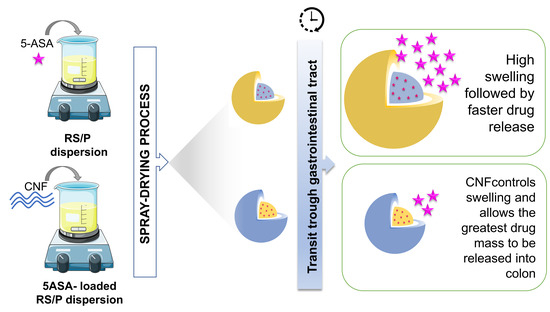
Cellulose Nanofibers Improve the Performance of Retrograded Starch/Pectin Microparticles for Colon-Specific Delivery of 5-ASA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch Retrogradation
2.3. Evaluation of CNF Influence on the Retrogradation Process
2.3.1. Quantification of RS Content after Retrogradation
2.3.2. X-ray Diffraction Pattern (XRD)
2.3.3. Simultaneous Thermogravimetry and Differential Scanning Calorimetry (TG-DSC)
2.3.4. Differential Scanning Calorimetry (DSC)
2.4. Preparation of RS/P Microparticles by Spray Drying
2.4.1. Set-Up of Factorial Design
2.4.2. Process Yield
2.4.3. Powder Moisture
2.4.4. Powder Packing
2.4.5. Powder Flowability
2.4.6. Field Emission Gun-Scanning Electron Microscopy (FEG-SEM)
2.5. Performance Studies
2.5.1. Preparation of 5-ASA-Loaded RS/P Microparticles
2.5.2. Encapsulation Efficiency (EE%) and Drug Loading (DL)
2.5.3. FEG/SEM
2.6. Preparation of 5-ASA-Loaded RS/P/CNF Microparticles
2.6.1. In Vitro Drug Release
2.6.2. Release Kinetics Models
3. Results and Discussion
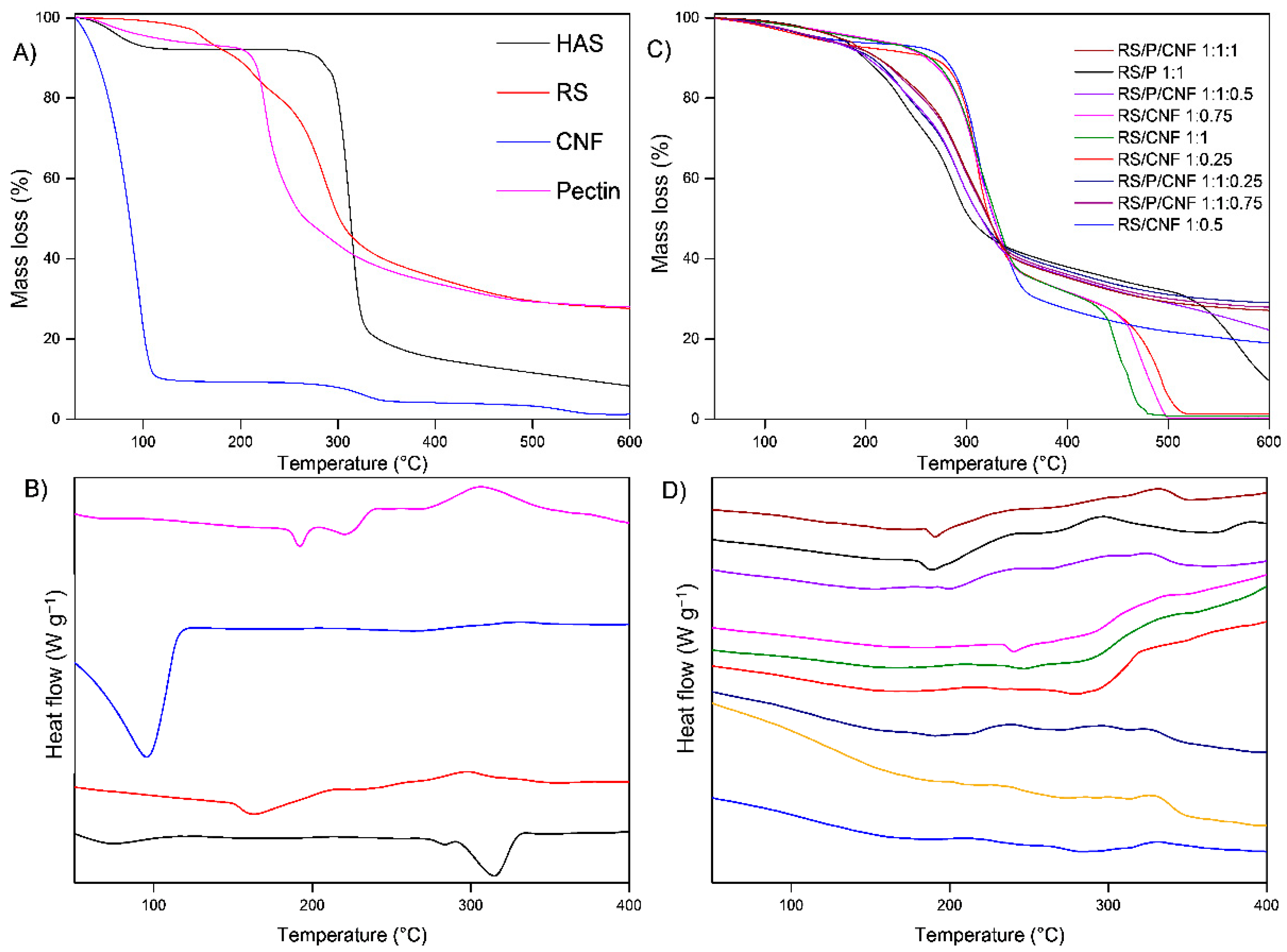
3.1. Evaluation of CNF Influence on the Retrogradation Process
3.1.1. Quantification of RS
3.1.2. XRD
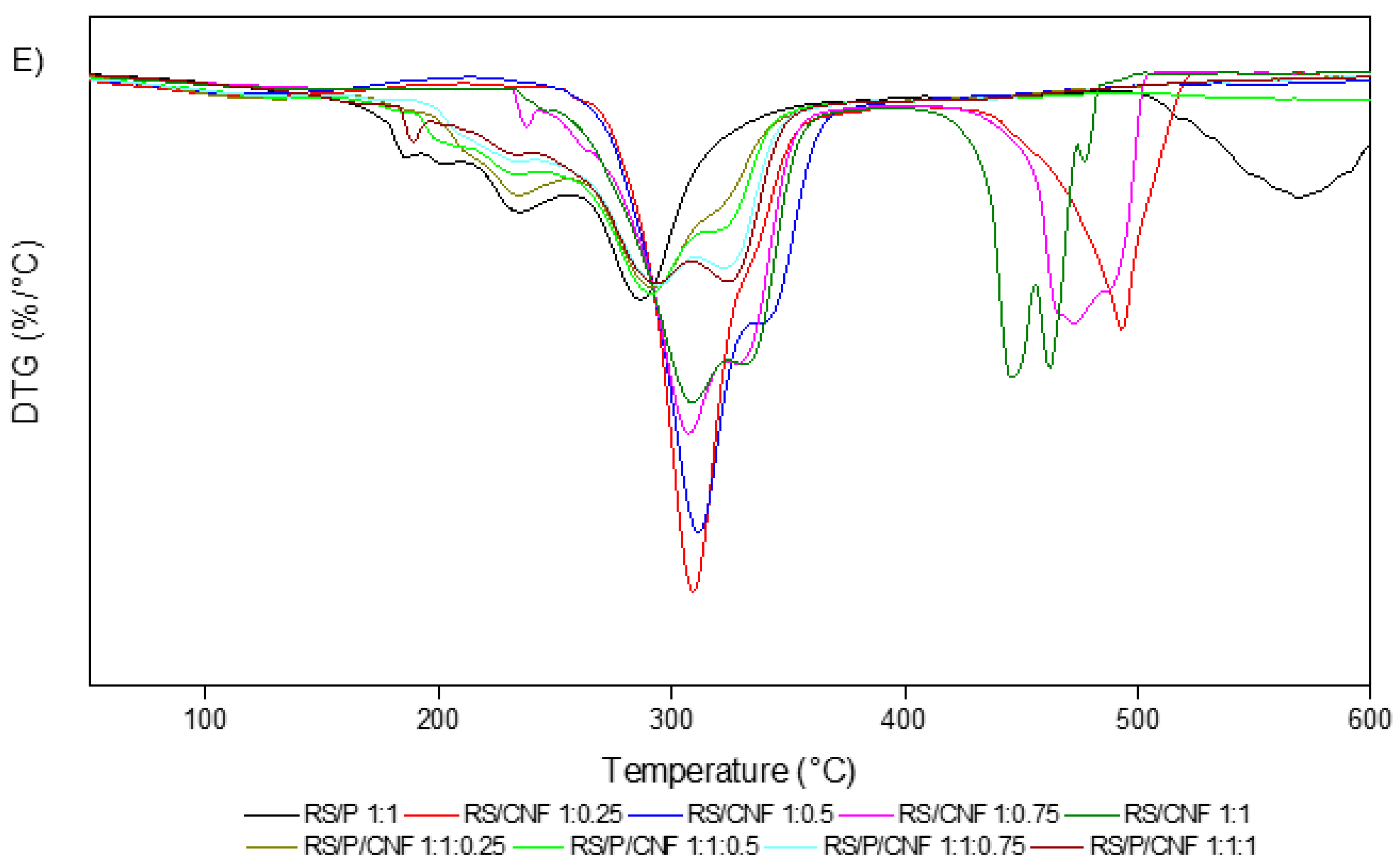
3.1.3. TG/DSC
3.2. Factorial Design
3.3. Powder Packing and Flowability
3.4. FEG/SEM
3.5. Spray-Drying Process of 5-ASA-Loaded RS/P
3.5.1. Encapsulation Efficiency (EE%) and Drug Loading (DL)
3.5.2. FEG/SEM
3.6. Spray-Drying Process of 5-ASA-Loaded RS/P/CNF
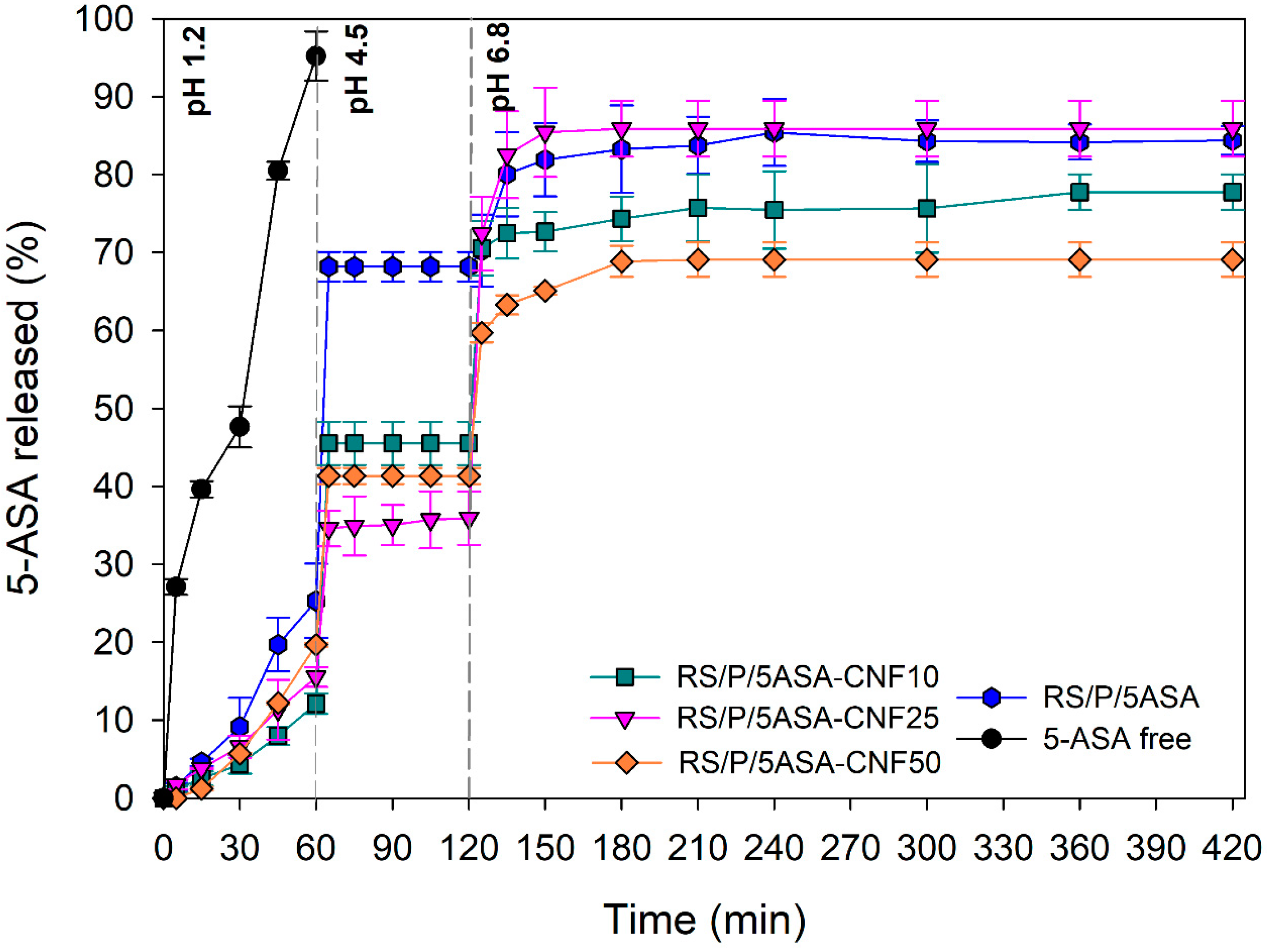
3.6.1. In Vitro 5-ASA Release
3.6.2. Release Kinetic
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, C.; Panaccione, R.; Ghosh, S.; Rioux, K. Optimizing clinical use of mesalazine (5-aminosalicylic acid) in inflammatory bowel disease. Ther. Adv. Gastroenterol. 2011, 4, 237–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lautenschläger, C.; Schmidt, C.; Fischer, D.; Stallmach, A. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2014, 71, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Nunthanid, J.; Huanbutta, K.; Luangtana-Anan, M.; Sriamornsak, P.; Limmatvapirat, S.; Puttipipatkhachorn, S. Development of time-, pH-, and enzyme-controlled colonic drug delivery using spray-dried chitosan acetate and hydroxypropyl methylcellulose. Eur. J. Pharm. Biopharm. 2008, 68, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.N.; Bondesen, S.; Hvidberg, E.F.; Hansen, S.H.; Binder, V.; Halskov, S.; Flachs, H. 5-Aminosalicylic Acid in a Slow-Release Preparation: Bioavailability, Plasma Level, and Excretion in Humans. Gastroenterology 1982, 83, 1062–1070. [Google Scholar] [CrossRef]
- Ye, B. Mesalazine preparations for the treatment of ulcerative colitis: Are all created equal? World J. Gastrointest. Pharmacol. Ther. 2015, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Hatton, G.B.; Merchant, H.A.; Basit, A.W. Gastrointestinal release behaviour of modified-release drug products: Dynamic dissolution testing of mesalazine formulations. Int. J. Pharm. 2015, 484, 103–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotla, N.G.; Rana, S.; Sivaraman, G.; Sunnapu, O.; Vemula, P.K.; Pandit, A.; Rochev, Y. Bioresponsive drug delivery systems in intestinal inflammation: State-of-the-art and future perspectives. Adv. Drug Deliv. Rev. 2019, 146, 248–266. [Google Scholar] [CrossRef]
- Shahdadi Sardo, H.; Saremnejad, F.; Bagheri, S.; Akhgari, A.; Afrasiabi Garekani, H.; Sadeghi, F. A review on 5-aminosalicylic acid colon-targeted oral drug delivery systems. Int. J. Pharm. 2019, 558, 367–379. [Google Scholar] [CrossRef]
- Patel, M.M. Cutting-edge technologies in colon-targeted drug delivery systems. Expert Opin. Drug Deliv. 2011, 8, 1247–1258. [Google Scholar] [CrossRef]
- Rubinstein, A. Colonic drug delivery. Drug Discov. Today Technol. 2005, 2, 33–37. [Google Scholar] [CrossRef]
- Elyagoby, A.; Layas, N.; Wong, T.W. Colon-Specific Delivery of 5-Fluorouracil from Zinc Pectinate Pellets through In Situ Intracapsular Ethylcellulose–Pectin Plug Formation. J. Pharm. Sci. 2013, 102, 604–616. [Google Scholar] [CrossRef]
- Bhavsar, D.; Patel, V.; Sawant, K. Systematic investigation of In Vitro and In Vivo safety, toxicity and degradation of mesoporous silica nanoparticles synthesized using commercial sodium silicate. Microporous Mesoporous Mater. 2019, 284, 343–352. [Google Scholar] [CrossRef]
- Wang, K.; Wen, H.F.; Yu, D.G.; Yang, Y.; Zhang, D.F. Electrosprayed hydrophilic nanocomposites coated with shellac for colon-specific delayed drug delivery. Mater. Des. 2018, 143, 248–255. [Google Scholar] [CrossRef]
- Ding, Y.; Dou, C.; Chang, S.; Xie, Z.; Yu, D.-G.; Liu, Y.; Shao, J. Core–Shell Eudragit S100 Nanofibers Prepared via Triaxial Electrospinning to Provide a Colon-Targeted Extended Drug Release. Polymers 2020, 12, 2034. [Google Scholar] [CrossRef]
- Yibin, W.; Liang, T.; Tianhao, Z.; Jing, M.; Zezhong, C.; Deng-Guang, Y. Electrospun Aspirin/Eudragit/Lipid Hybrid Nanofibers for Colon-targeted Delivery Using an Energy-saving Process. Chem. Res. Chin. Univ. 2021, 37, 443–449. [Google Scholar] [CrossRef]
- Meneguin, A.B.; Ferreira Cury, B.S.; Dos Santos, A.M.; Franco, D.F.; Barud, H.S.; Da Silva Filho, E.C. Resistant starch/pectin free-standing films reinforced with nanocellulose intended for colonic methotrexate release. Carbohydr. Polym. 2017, 157, 1013–1023. [Google Scholar] [CrossRef]
- Meneguin, A.B.; Beyssac, E.; Garrait, G.; Hsein, H.; Cury, B.S.F. Retrograded starch/pectin coated gellan gum-microparticles for oral administration of insulin: A technological platform for protection against enzymatic degradation and improvement of intestinal permeability. Eur. J. Pharm. Biopharm. 2018, 123, 84–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saadatzadeh, A.; Atyabi, F.; Fazeli, M.R.; Dinarvand, R.; Jamalifar, H.; Abdolghaffari, A.H.; Mahdaviani, P.; Mahbod, M.; Baeeri, M.; Baghaei, A.; et al. Biochemical and pathological evidences on the benefit of a new biodegradable nanoparticles of probiotic extract in murine colitis. Fundam. Clin. Pharmacol. 2012, 26, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Meneguin, A.B.; Cury, B.S.F.; Evangelista, R.C. Films from resistant starch-pectin dispersions intended for colonic drug delivery. Carbohydr. Polym. 2014, 99, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Sawarkar, S.P.; Deshpande, S.G.; Bajaj, A.N.; Nikam, V.S. In Vivo Evaluation of 5-ASA Colon-Specific Tablets Using Experimental-Induced Colitis Rat Animal Model. Aaps Pharmscitech 2015, 16, 1445–1454. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, C.; Wen, L.; Han, M.K.; Xiao, B.; Zhou, J.; Zhang, Y.; Zhang, Z.; Viennois, E.; Merlin, D. A hyaluronidase-responsive nanoparticle-based drug delivery system for targeting colon cancer cells. Cancer Res. 2016, 76, 7208–7218. [Google Scholar] [CrossRef] [Green Version]
- Samak, Y.O.; El Massik, M.; Coombes, A.G.A. A Comparison of Aerosolization and Homogenization Techniques for Production of Alginate Microparticles for Delivery of Corticosteroids to the Colon. J. Pharm. Sci. 2017, 106, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Hu, X.; Xu, X.; Jin, Z.; Tian, Y. Slowly digestible starch prepared from rice starches by temperature-cycled retrogradation. Carbohydr. Polym. 2011, 84, 970–974. [Google Scholar] [CrossRef]
- Dona, A.C.; Pages, G.; Gilbert, R.G.; Kuchel, P.W. Digestion of starch: In Vivo and In Vitro kinetic models used to characterise oligosaccharide or glucose release. Carbohydr. Polym. 2010, 80, 599–617. [Google Scholar] [CrossRef]
- Liu, H.; Yu, L.; Chen, L.; Li, L. Retrogradation of corn starch after thermal treatment at different temperatures. Carbohydr. Polym. 2007, 69, 756–762. [Google Scholar] [CrossRef]
- Haralampu, S.G. Resistant starch—A review of the physical properties and biological impact of RS3. Carbohydr. Polym. 2000, 41, 285–292. [Google Scholar] [CrossRef]
- United States Pharmacopeial Convention. The United States Pharmacopeia & National Formulary, 30th ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2007. [Google Scholar]
- Mandal, A.; Chakrabarty, D. Characterization of nanocellulose reinforced semi-interpenetrating polymer network of poly(vinyl alcohol) & polyacrylamide composite films. Carbohydr. Polym. 2015, 134, 240–250. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Bras, J. Microfibrillated cellulose coatings as new release systems for active packaging. Carbohydr. Polym. 2014, 103, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Meneguin, A.B.; Da Silva Barud, H.; Sábio, R.M.; De Sousa, P.Z.; Manieri, K.F.; De Freitas, L.A.P.; Pacheco, G.; Alonso, J.D.; Chorilli, M. Spray-dried bacterial cellulose nanofibers: A new generation of pharmaceutical excipient intended for intestinal drug delivery. Carbohydr. Polym. 2020, 249, 116838. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.; Kingman, S.; Cummings, J. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [PubMed]
- Zhang, J.; Wang, Z.W. Optimization of reaction conditions for resistant Canna edulis Ker starch phosphorylation and its structural characterization. Ind. Crops Prod. 2009, 30, 105–113. [Google Scholar] [CrossRef]
- Langenbucher, F. Linearization of dissolution rate curves by the Weibull distribution. J. Pharm. Pharmacol. 1972, 24, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Gibaldi, M.; Feldman, S. Establishment of sink conditions in dissolution rate determinations. Theoretical considerations and application to nondisintegrating dosage forms. J. Pharm. Sci. 1967, 56, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Hoover, R.; Zhou, Y. In Vitro and In Vivo hydrolysis of legume starches by α-amylase and resistant starch formation in legumes-a review. Carbohydr. Polym. 2003, 4, 401–417. [Google Scholar] [CrossRef]
- Perera, A.; Meda, V.; Tyler, R.T. Resistant starch: A review of analytical protocols for determining resistant starch and of factors affecting the resistant starch content of foods. Food Res. Int. 2010, 43, 1959–1974. [Google Scholar] [CrossRef]
- Cristina Freire, A.; Fertig, C.C.; Podczeck, F.; Veiga, F.; Sousa, J. Starch-based coatings for colon-specific drug delivery. Part I: The influence of heat treatment on the physico-chemical properties of high amylose maize starches. Eur. J. Pharm. Biopharm. 2009, 72, 574–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimento, M.L.F.; Zanotto, E.D. Does viscosity describe the kinetic barrier for crystal growth from the liquidus to the glass transition? J. Chem. Phys. 2010, 133, 174701. [Google Scholar] [CrossRef] [Green Version]
- Karim, A.A.; Norziah, M.H.; Seow, C.C. Methods for the study of starch retrogradation. Food Chem. 2000, 71, 9–36. [Google Scholar] [CrossRef]
- Thanki, K.; Gangwal, R.P.; Sangamwar, A.T.; Jain, S. Oral delivery of anticancer drugs: Challenges and opportunities. J. Control. Release 2013, 170, 15–40. [Google Scholar] [CrossRef]
- Freire, C.; Podczeck, F.; Veiga, F.; Sousa, J. Starch-based coatings for colon-specific delivery. Part II: Physicochemical properties and In Vitro drug release from high amylose maize starch films. Eur. J. Pharm. Biopharm. 2009, 72, 587–594. [Google Scholar] [CrossRef]
- Carbinatto, F.M.; De Castro, A.D.; Cury, B.S.F.; Magalhães, A.; Evangelista, R.C. Physical properties of pectin–high amylose starch mixtures cross-linked with sodium trimetaphosphate. Int. J. Pharm. 2012, 423, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Li, Y.; Xu, X.; Jin, Z. Starch retrogradation studied by thermogravimetric analysis (TGA). Carbohydr. Polym. 2011, 84, 1165–1168. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Kunzek, H.; Dongowski, G. Thermal analysis of chemically and mechanically modified pectins. Food Hydrocoll. 2007, 21, 1101–1112. [Google Scholar] [CrossRef]
- Mahendra, I.P.; Wirjosentono, B.; Tamrin, I.H.; Mendez, J.A. Thermal and Morphology Properties of Cellulose Nanofiber from TEMPO-oxidized Lower part of Empty Fruit Bunches (LEFB). Open Chem. 2019, 17, 526–536. [Google Scholar] [CrossRef]
- Couto, R.O.; Martins, F.S.; Chaul, L.T.; Conceição, E.C.; Freitas, L.A.P.; Bara, M.T.F.; Paula, J.R. Spray drying of Eugenia dysenterica extract: Effects of in-process parameters on product quality. Rev. Bras. Farmacogn. 2013, 23, 115–123. [Google Scholar] [CrossRef]
- Adhikari, B.; Howes, T.; Lecomte, D.; Bhandari, B.R. A glass transition temperature approach for the prediction of the surface stickiness of a drying droplet during spray drying. Powder Technol. 2005, 149, 168–179. [Google Scholar] [CrossRef] [Green Version]
- Cal, K.; Sollohub, K. Spray drying technique. I: Hardware and process parameters. J. Pharm. Sci. 2010, 99, 575–586. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, L.A.P.; Teixeira, C.C.C.; Zamarioli, C.M. Recent Developments in Phytomedicine Technology; Nova Science Publishers Inc.: New York, NY, USA, 2017. [Google Scholar]
- Ameri, M.; Maa, Y.-F. Spray Drying of Biopharmaceuticals: Stability and Process Considerations. Dry. Technol. 2006, 24, 763–768. [Google Scholar] [CrossRef]
- Martins, R.M.; Siqueira, S.; Tacon, L.A.; Freitas, L.A.P. Microstructured ternary solid dispersions to improve carbamazepine solubility. Powder Technol. 2012, 215–216, 156–165. [Google Scholar] [CrossRef]
- Sommerfeld, J.T. Principles of Unit Operations, 2nd ed.; Foust, A.S., Wenzel, L.A., Clump, C.W., Maus, L., Anderson, L.B., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1980; 768p. [Google Scholar] [CrossRef]
- Van Den Mooter, G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov. Today Technol. 2012, 9, e79–e85. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical Particle Engineering via Spray Drying. Pharm. Res. 2008, 25, 999. [Google Scholar] [CrossRef] [Green Version]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Maury, M.; Murphy, K.; Kumar, S.; Shi, L.; Lee, G. Effects of process variables on the powder yield of spray-dried trehalose on a laboratory spray-dryer. Eur. J. Pharm. Biopharm. 2005, 59, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Jüptner, A.; Scherließ, R. Spray Dried Formulations for Inhalation—Meaningful Characterisation of Powder Properties. Pharmaceutics 2020, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Focaroli, S.; Mah, P.T.; Hastedt, J.E.; Gitlin, I.; Oscarson, S.; Fahy, J.V.; Healy, A.M. A Design of Experiment (DoE) approach to optimise spray drying process conditions for the production of trehalose/leucine formulations with application in pulmonary delivery. Int. J. Pharm. 2019, 562, 228–240. [Google Scholar] [CrossRef]
- Recife, A.C.D.; Meneguin, A.B.; Cury, B.S.F.; Evangelista, R.C. Evaluation of retrograded starch as excipient for controlled release matrix tablets. J. Drug Deliv. Sci. Technol. 2017, 40, 83–94. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Cardoso, V.M.; Evangelista, R.C.; Daflon Gremião, M.P.; Stringhetti Ferreira Cury, B. Insights into the impact of cross-linking processes on physicochemical characteristics and mucoadhesive potential of gellan gum/retrograded starch microparticles as a platform for colonic drug release. J. Drug Deliv. Sci. Technol. 2020, 55, 101445. [Google Scholar] [CrossRef]
- Dos Santos, A.M.; Meneguin, A.B.; Akhter, D.T.; Fletcher, N.; Houston, Z.H.; Bell, C.; Thurecht, K.J.; Gremião, M.P.D. Understanding the role of colon-specific microparticles based on retrograded starch/pectin in the delivery of chitosan nanoparticles along the gastrointestinal tract. Eur. J. Pharm. Biopharm. 2021, 158, 371–378. [Google Scholar] [CrossRef]
- Schnieders, J.; Gbureck, U.; Vorndran, E.; Schossig, M.; Kissel, T. The effect of porosity on drug release kinetics from vancomycin microsphere/calcium phosphate cement composites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 99, 391–398. [Google Scholar] [CrossRef]
- Kolakovic, R.; Laaksonen, T.; Peltonen, L.; Laukkanen, A.; Hirvonen, J. Spray-dried nanofibrillar cellulose microparticles for sustained drug release. Int. J. Pharm. 2012, 430, 47–55. [Google Scholar] [CrossRef] [PubMed]

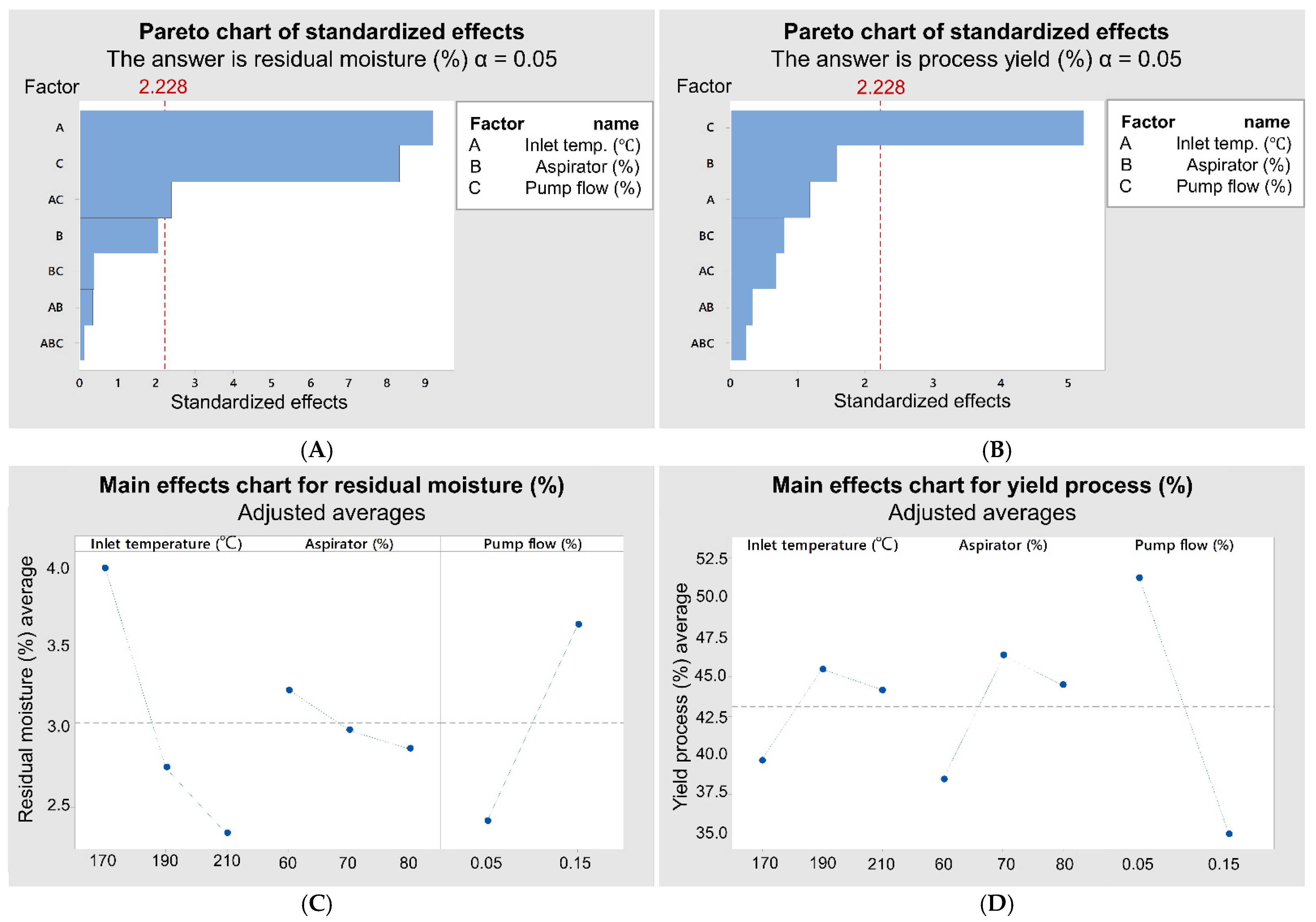
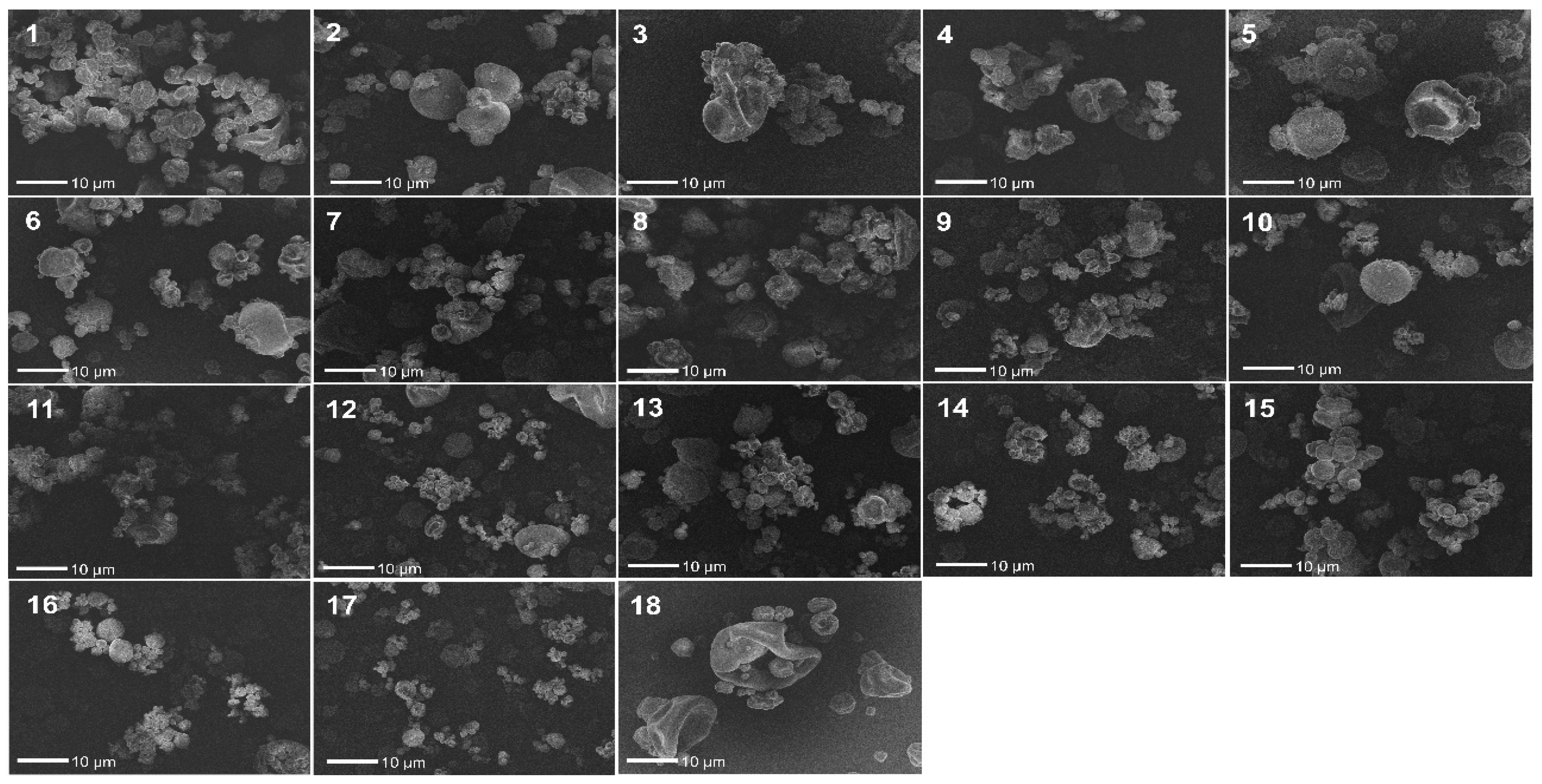
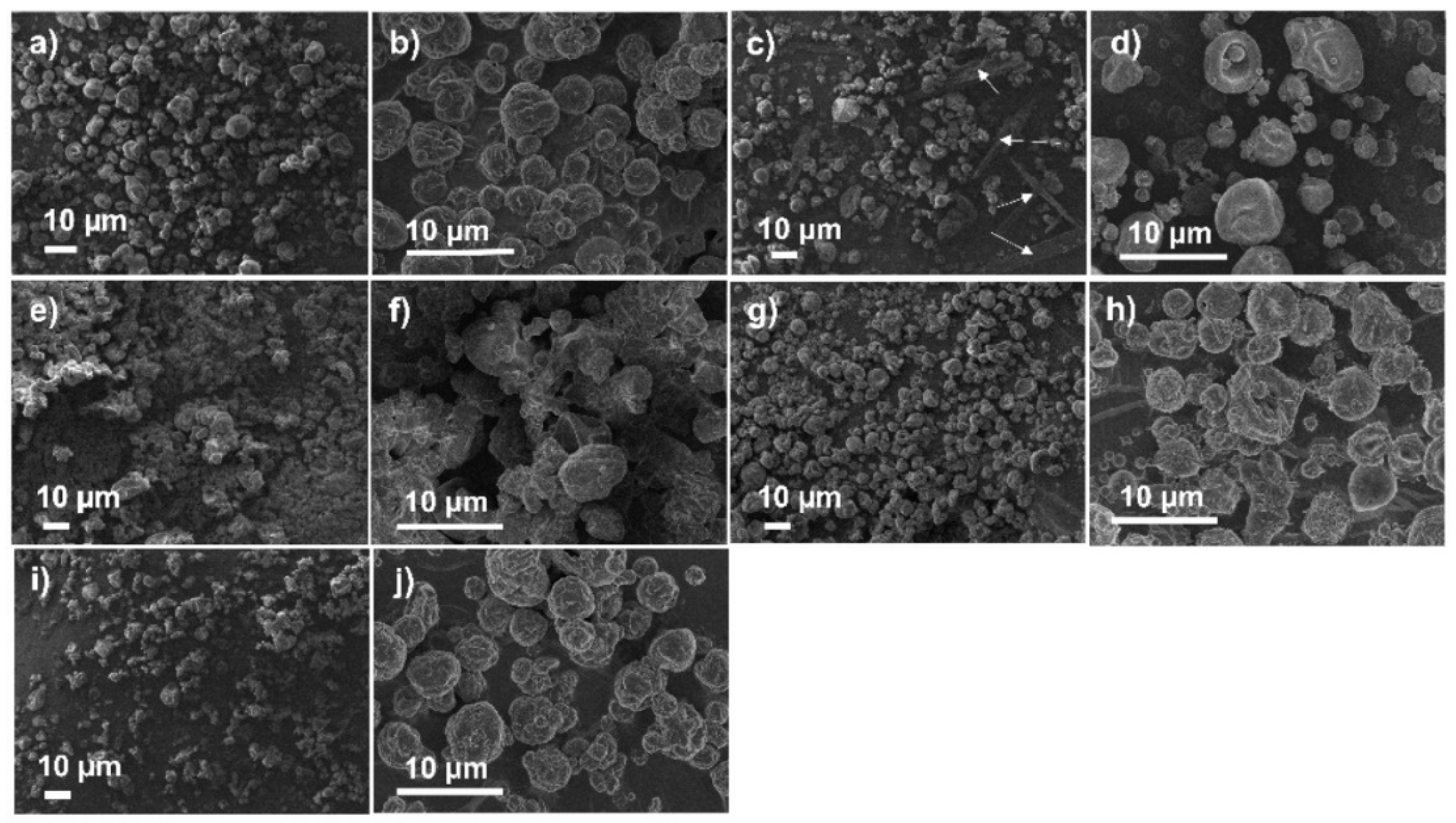
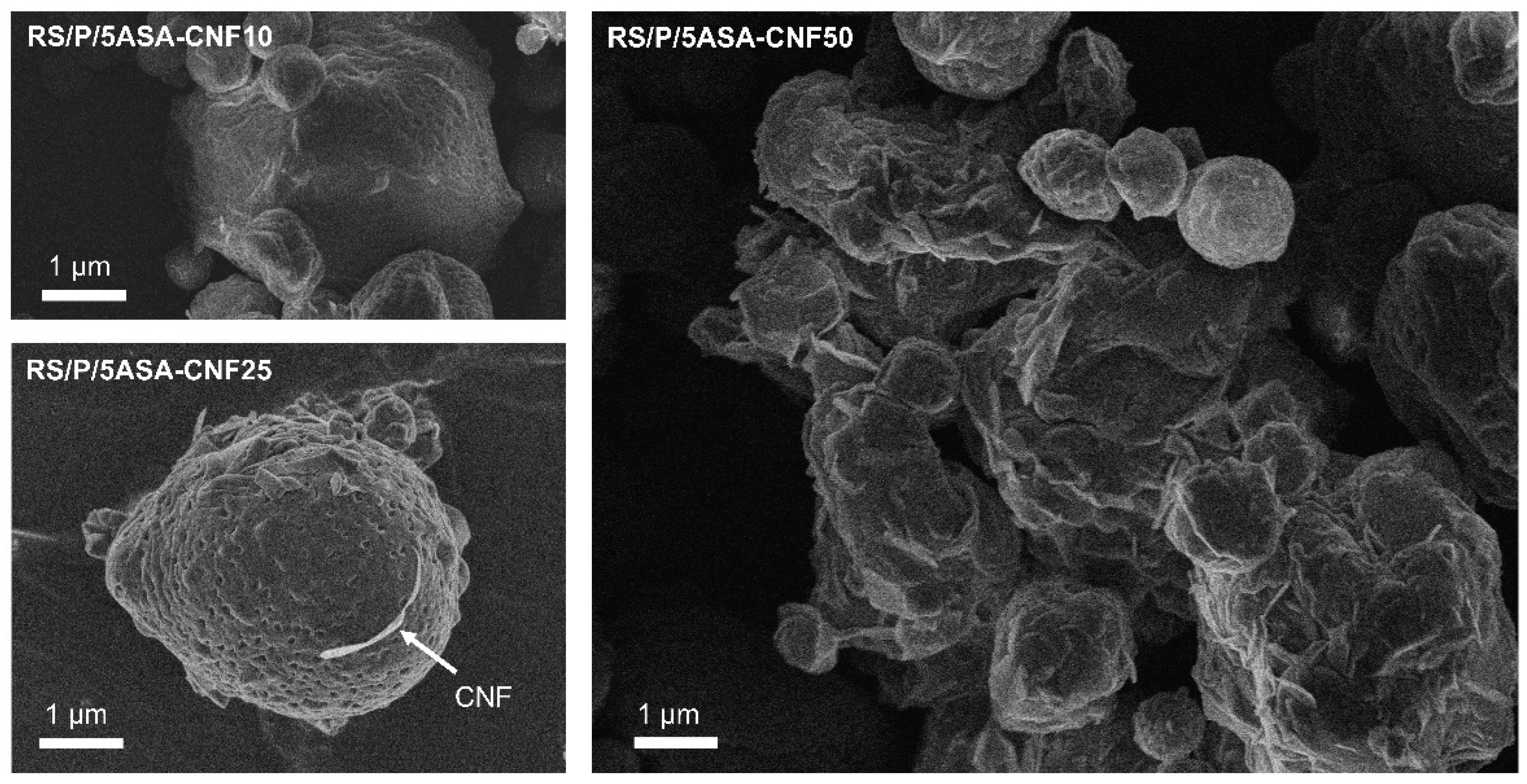
| Experiments | Inlet Air Temperature (°C) | Aspirator (%) | Pump Flow (%) | Process Yield (%) | Residual Moisture (%) |
|---|---|---|---|---|---|
| 1 | 210 | 70 | 0.05 | 57.1 ± 0.8 | 1.2 ± 0.2 |
| 2 | 210 | 80 | 0.05 | 52.5 ± 1.1 | 1.3 ± 0.2 |
| 3 | 210 | 60 | 0.05 | 48.9 ± 0.7 | 1.8 ± 0.1 |
| 4 | 190 | 70 | 0.05 | 55.1 ± 0.5 | 2.2 ± 0.1 |
| 5 | 190 | 80 | 0.05 | 54.4 ± 0.5 | 2.1 ± 0.3 |
| 6 | 190 | 60 | 0.05 | 40.0 ± 0.9 | 2.5 ± 0.5 |
| 7 | 170 | 70 | 0.05 | 57.6 ± 0.4 | 3.6 ± 0.1 |
| 8 | 170 | 80 | 0.05 | 52.0 ± 0.6 | 3.3 ± 0.4 |
| 9 | 170 | 60 | 0.05 | 43.2 ± 1.3 | 3.7 ± 0.2 |
| 10 | 210 | 70 | 0.15 | 40.8 ± 1.0 | 3.2 ± 0.3 |
| 11 | 210 | 80 | 0.15 | 36.0 ± 0.8 | 3.1 ± 0.1 |
| 12 | 210 | 60 | 0.15 | 29.6 ± 0.3 | 3.4 ± 0.5 |
| 13 | 190 | 70 | 0.15 | 38.4 ± 1.4 | 3.2 ± 0.3 |
| 14 | 190 | 80 | 0.15 | 40.0 ± 0.7 | 3.0 ± 0.2 |
| 15 | 190 | 60 | 0.15 | 44.8 ± 0.9 | 3.5 ± 0.7 |
| 16 | 170 | 70 | 0.15 | 28.9 ± 1.0 | 4.5 ± 0.6 |
| 17 | 170 | 80 | 0.15 | 31.8 ± 0.5 | 4.4 ± 0.3 |
| 18 | 170 | 60 | 0.15 | 24.5 ± 1.5 | 4.5 ± 0.4 |
| Sample | ρbulk (g mL−1) | ρtapped (g mL−1) | Hr | Ci (%) |
|---|---|---|---|---|
| 1 | 0.202 ± 0.001 | 0.504 ± 0.005 | 2.50 ± 0.03 | 60 ± 1.5 |
| 2 | 0.267 ± 0.001 | 0.593 ± 0.003 | 2.22 ± 0.05 | 55 ± 0.9 |
| 3 | 0.236 ± 0.004 | 0.535 ± 0.001 | 2.27 ± 0.01 | 56 ± 0.4 |
| 4 | 0.261 ± 0.002 | 0.544 ± 0.001 | 2.08 ± 0.12 | 52 ± 1.0 |
| 5 | 0.147 ± 0.006 | 0.293 ± 0.001 | 2.00 ± 0.06 | 50 ± 1.0 |
| 6 | 0.126 ± 0.006 | 0.279 ± 0.007 | 2.22 ± 0.03 | 55 ± 1.6 |
| 7 | 0.128 ± 0.001 | 0.285 ± 0.005 | 2.22 ± 0.05 | 55 ± 0.3 |
| 8 | 0.180 ± 0.009 | 0.328 ± 0.010 | 1.81 ± 0.01 | 45 ± 0.6 |
| 9 | 0.156 ± 0.008 | 0.301 ± 0.008 | 1.92 ± 0.01 | 48 ± 1.1 |
| 10 | 0.132 ± 0.003 | 0.274 ± 0.002 | 2.08 ± 0.07 | 52 ± 0.9 |
| 11 | 0.145 ± 0.004 | 0.291 ± 0.002 | 2.00 ± 0.02 | 50 ± 0.8 |
| 12 | 0.146 ± 0.004 | 0.292 ± 0.013 | 2.00 ± 0.02 | 50 ± 0.2 |
| 13 | 0.136 ± 0.012 | 0.273 ± 0.004 | 2.00 ± 0.11 | 50 ± 0.3 |
| 14 | 0.284 ± 0.002 | 0.558 ± 0.005 | 1.96 ± 0.08 | 49 ± 1.2 |
| 15 | 0.230 ± 0.006 | 0.524 ± 0.001 | 2.27 ± 0.01 | 56 ± 1.1 |
| 16 | 0.293 ± 0.007 | 0.606 ± 0.006 | 2.06 ± 0.05 | 51 ± 1.4 |
| 17 | 0.299 ± 0.002 | 0.612 ± 0.003 | 2.04 ± 0.04 | 51 ± 0.9 |
| 18 | 0.261 ± 0.005 | 0.575 ± 0.004 | 2.19 ± 0.10 | 54 ± 0.5 |
| Sample | EE% | DL (mass5-ASA (mg)/100 mgmicroparticles) |
|---|---|---|
| RS/P/5ASA0.5% | 93.72 ± 8.2 | 15.61 ± 1.36 |
| RS/P/5ASA1.0% | 93.23 ± 6.21 | 26.63 ± 1.77 |
| RS/P/5ASA-HCl | 98.73 ± 9.97 | 16.45 ± 1.66 |
| RS/P/5ASA-NaOH | 90.71 ± 2.48 | 15.11 ± 0.41 |
| RS/P/5ASA-polysorbate | 47.19 ± 1.23 | 7.86 ± 0.20 |
| RS/P/5ASA-CNF10 | 95.81 ± 2.29 | 14.75 ± 0.35 |
| RS/P/5ASA-CNF25 | 95.93 ± 3.37 | 13.28 ± 0.46 |
| RS/P/5ASA-CNF50 | 16.76 ± 0.85 | 1.97 ± 0.10 |
| Release Models | Samples | ||||
|---|---|---|---|---|---|
| RS/P/5ASA | RS/P/5ASA-CNF10 | RS/P/5ASA-CNF25 | RS/P/5ASA-CNF50 | ||
| Baker–Lonsdale | K (% min−1) | 0.0009 | 0.0005 | 0.0006 | 0.0004 |
| r2 | 0.8119 | 0.7933 | 0.7519 | 0.8309 | |
| Higuchi | K (% min−1/2) | 5.4173 | 4.5807 | 4.8873 | 4.1571 |
| r2 | 0.7526 | 0.7982 | 0.7824 | 0.8270 | |
| Korsmeyer–Peppas | K (% min−n) | 0.1317 | 0.2660 | 0.4667 | 0.2807 |
| r2 | 0.9887 | 0.7945 | 0.8649 | 0.9033 | |
| n | 1.2800 | 1.1112 | 0.9387 | 1.0960 | |
| First order | K (1 min−1) | 0.0098 | 0.0063 | 0.0068 | 0.0053 |
| r2 | 0.8824 | 0.8721 | 0.8635 | 0.8708 | |
| Hixson–Crowell | K (%1/3 min−1) | 0.0028 | 0.0018 | 0.0020 | 0.0015 |
| r2 | 0.8671 | 0.8471 | 0.8727 | 0.8185 | |
| r2 | 0.9793 | 0.9916 | 0.9716 | 0.9213 | |
| b | 1.3550 | 0.7636 | 0.0070 | 0.8246 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meneguin, A.B.; Sábio, R.M.; de Souza, M.P.C.; Fernandes, R.P.; de Oliveira, A.G.; Chorilli, M. Cellulose Nanofibers Improve the Performance of Retrograded Starch/Pectin Microparticles for Colon-Specific Delivery of 5-ASA. Pharmaceutics 2021, 13, 1515. https://doi.org/10.3390/pharmaceutics13091515
Meneguin AB, Sábio RM, de Souza MPC, Fernandes RP, de Oliveira AG, Chorilli M. Cellulose Nanofibers Improve the Performance of Retrograded Starch/Pectin Microparticles for Colon-Specific Delivery of 5-ASA. Pharmaceutics. 2021; 13(9):1515. https://doi.org/10.3390/pharmaceutics13091515
Chicago/Turabian StyleMeneguin, Andréia Bagliotti, Rafael Miguel Sábio, Maurício Palmeira Chaves de Souza, Richard Perosa Fernandes, Anselmo Gomes de Oliveira, and Marlus Chorilli. 2021. "Cellulose Nanofibers Improve the Performance of Retrograded Starch/Pectin Microparticles for Colon-Specific Delivery of 5-ASA" Pharmaceutics 13, no. 9: 1515. https://doi.org/10.3390/pharmaceutics13091515
APA StyleMeneguin, A. B., Sábio, R. M., de Souza, M. P. C., Fernandes, R. P., de Oliveira, A. G., & Chorilli, M. (2021). Cellulose Nanofibers Improve the Performance of Retrograded Starch/Pectin Microparticles for Colon-Specific Delivery of 5-ASA. Pharmaceutics, 13(9), 1515. https://doi.org/10.3390/pharmaceutics13091515