Synthetic Antiferromagnetic Gold Nanoparticles as Bimodal Contrast Agents in MRI and CT—An Experimental In Vitro and In Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticle Synthesis and Functionalization
2.2. Cell Culture
2.3. Confirmation of Cell Labeling In Vitro
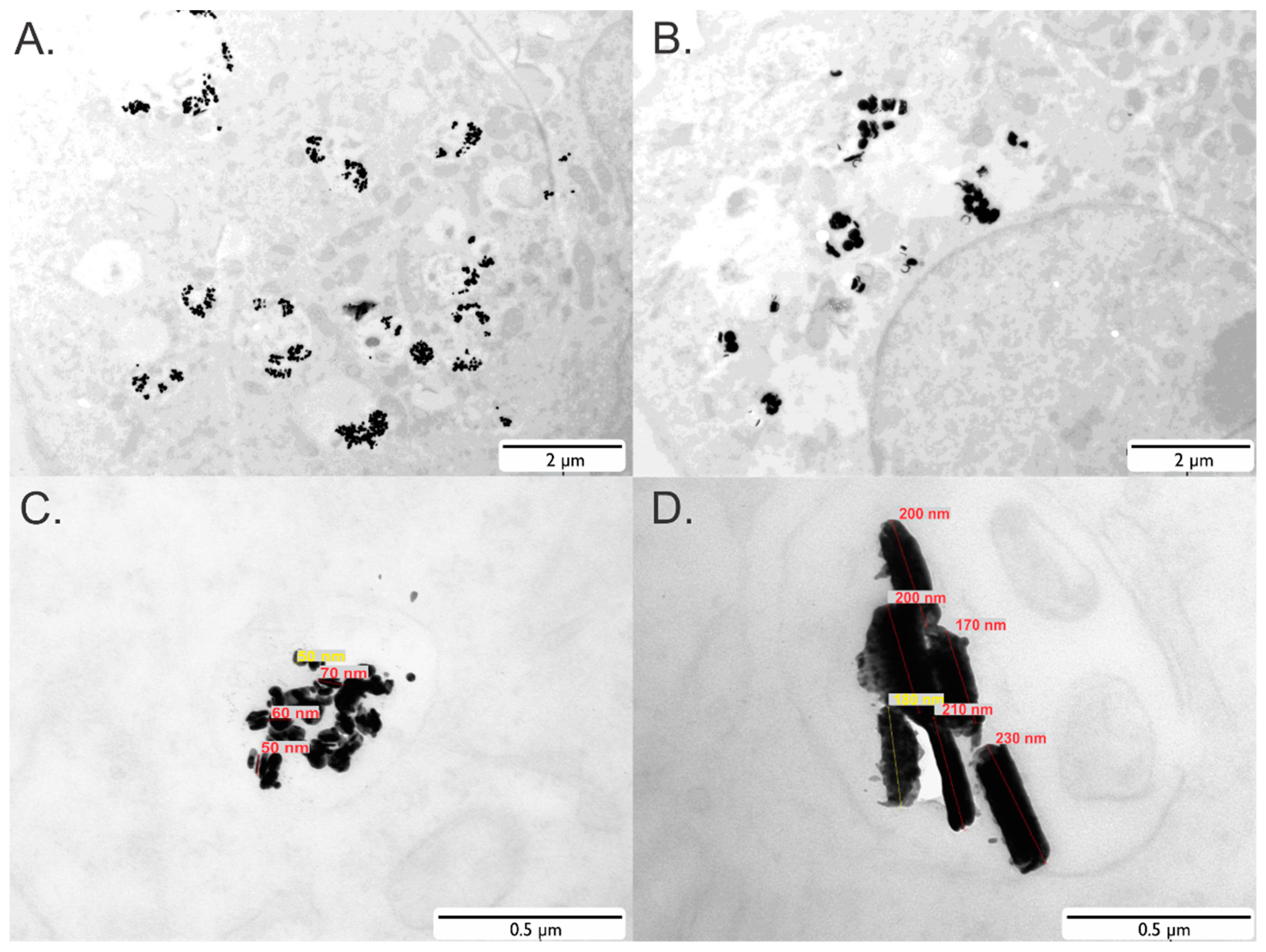
2.4. Transmission Electron Microscopy (TEM)
2.5. Animal Model
2.6. In Vivo Bioluminescence Imaging (BLI)
2.7. Computed Tomography (CT)
2.8. Magnetic Resonance Imaging (MRI)
2.9. Ex Vivo Dark-Field Microscopy
2.10. Ex Vivo TEM
2.11. Statistical Analysis
3. Results
3.1. Nanoparticles and Cell Labeling
3.2. In Vitro Imaging Experiments
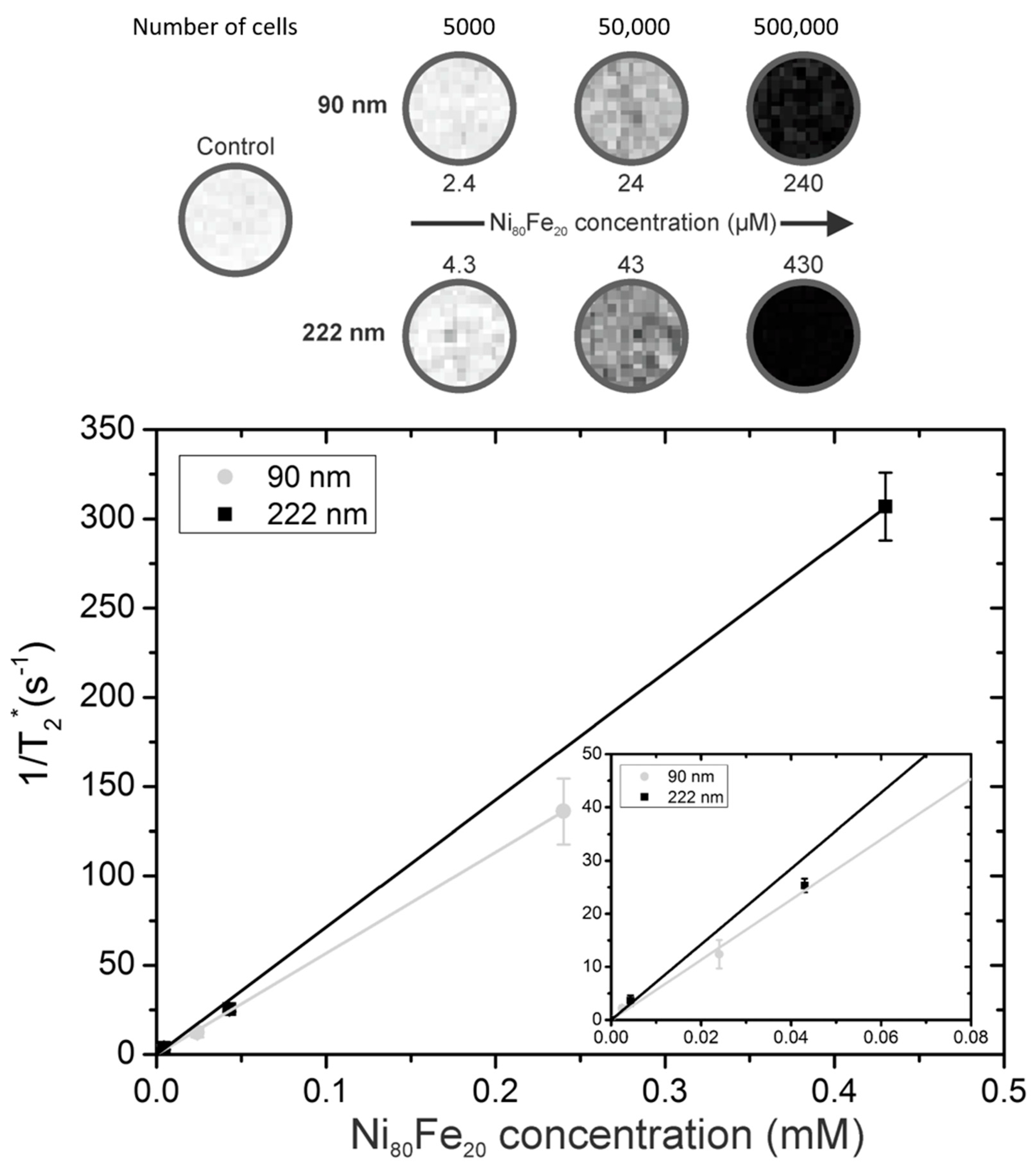
3.2.1. Magnetic Resonance Imaging (MRI)
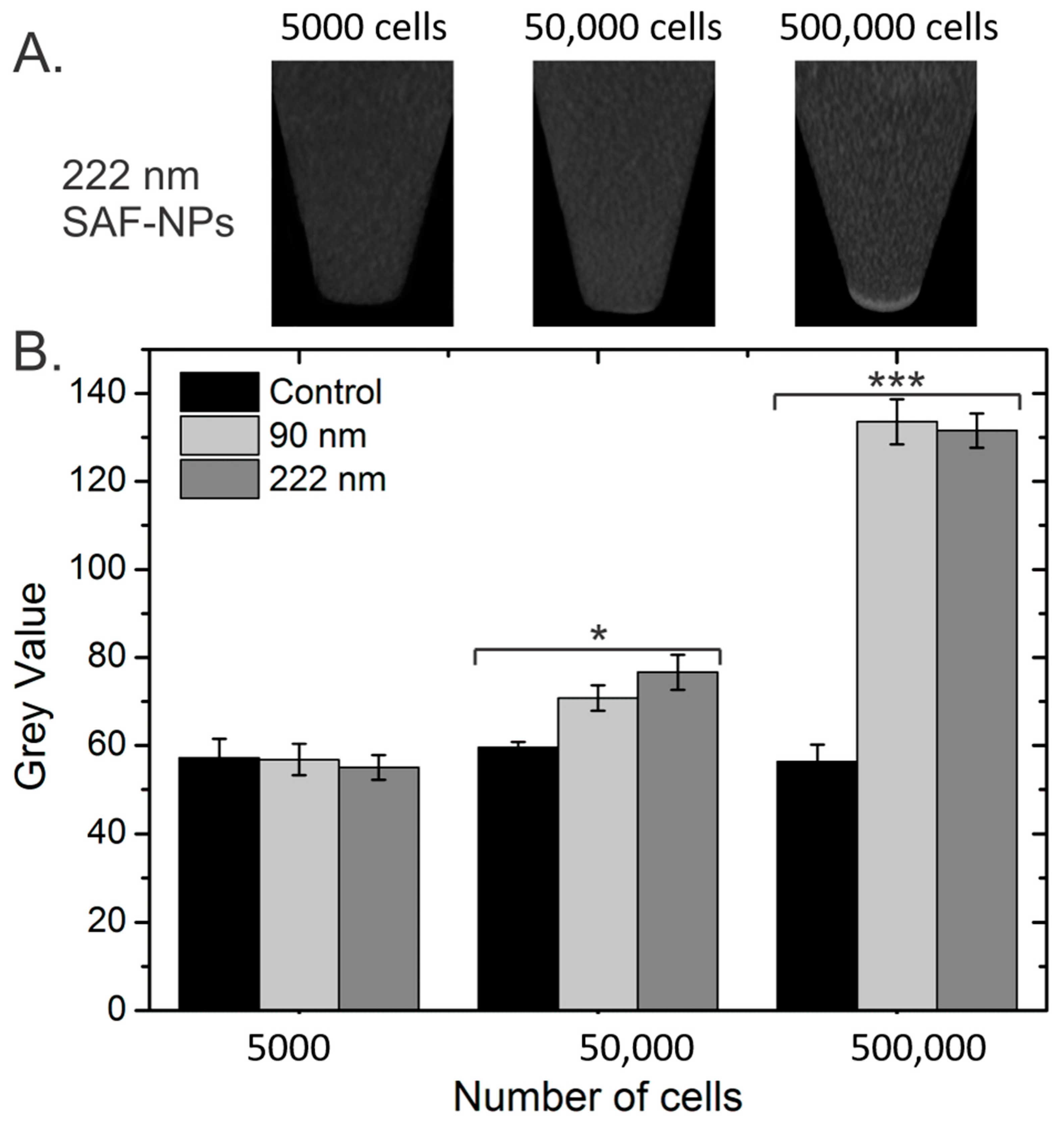
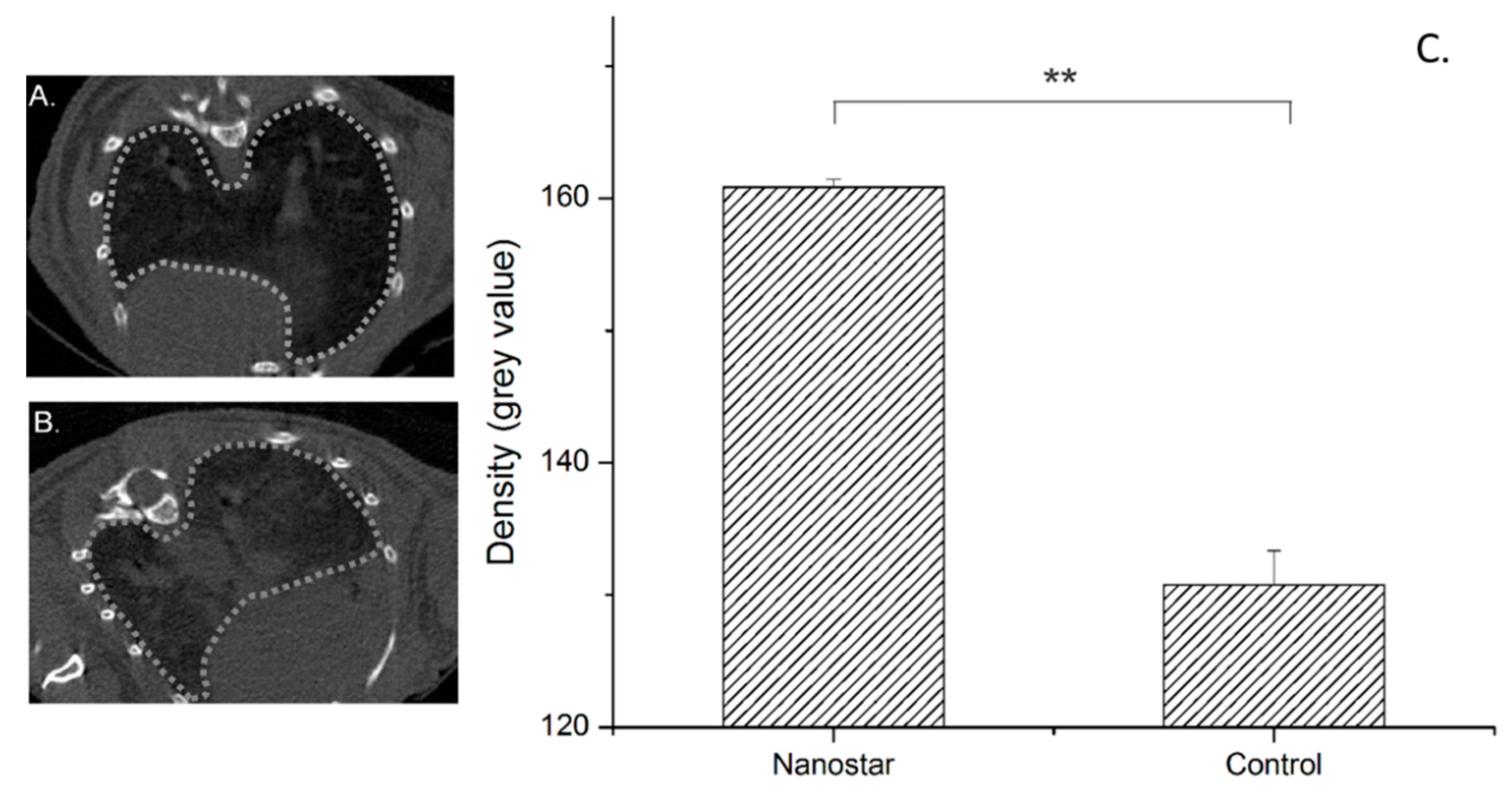
3.2.2. Computed Tomography (CT)
3.3. In Vivo Imaging
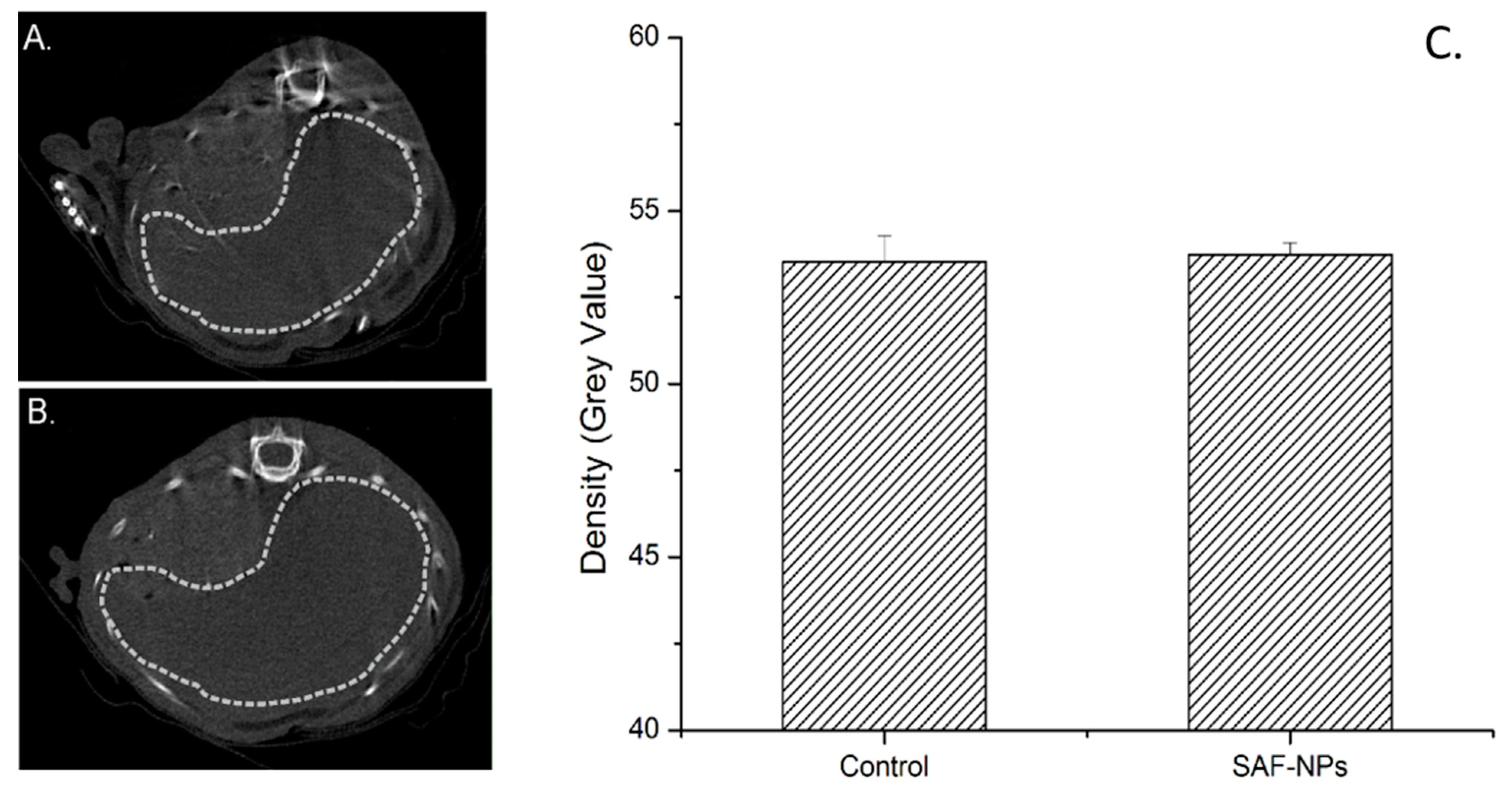
3.3.1. Intravenous Injection of SAF-NPs
3.3.2. Intravenous Injection of SAF-NP Labeled Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thakor, A.; Gambhir, S.S. Nanooncology: The Future of Cancer Diagnosis and Therapy. CA-A Cancer J. Clin. 2013, 63, 395–418. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia-a promising tumour therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knuechel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.B.; Pure, E. Imaging of T-cell Responses in the Context of Cancer Immunotherapy. Cancer Immunol. Res. 2021, 9, 490–502. [Google Scholar] [CrossRef]
- Kustermann, E.; Roell, W.; Breitbach, M.; Wecker, S.; Wiedermann, D.; Buehrle, C.; Welz, A.; Hescheler, J.; Fleischmann, B.K.; Hoehn, M. Stem cell implantation in ischemic mouse heart: A high-resolution magnetic resonance imaging investigation. NMR Biomed. 2005, 18, 362–370. [Google Scholar] [CrossRef]
- Leten, C.; Trekker, J.; Struys, T.; Dresselaers, T.; Gijsbers, R.; Vande Velde, G.; Lambrichts, I.; Van der Linden, A.; Verfaillie, C.M.; Himmelreich, U. Assessment of bystander killing-mediated therapy of malignant brain tumors using a multimodal imaging approach. Stem Cell Res. Ther. 2015, 6, 163. [Google Scholar] [CrossRef] [Green Version]
- Hoehn, M.; Kustermann, E.; Blunk, J.; Wiedermann, D.; Trapp, T.; Wecker, S.; Focking, M.; Arnold, H.; Hescheler, J.; Fleischmann, B.K.; et al. Monitoring of implanted stem cell migration in vivo: A highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc. Natl. Acad. Sci. USA 2002, 99, 16267–16272. [Google Scholar] [CrossRef] [Green Version]
- Janowski, M.; Bulte, J.W.M.; Walczak, P. Personalized nanomedicine advancements for stem cell tracking. Adv. Drug Deliv. Rev. 2012, 64, 1488–1507. [Google Scholar] [CrossRef] [Green Version]
- Geppert, M.; Himly, M. Iron Oxide Nanoparticles in Bioimaging—An Immune Perspective. Front. Immunol. 2021, 12, 688927. [Google Scholar] [CrossRef]
- Wolfs, E.; Verfaillie, C.M.; Van Laere, K.; Deroose, C.M. Radiolabeling Strategies for Radionuclide Imaging of Stem Cells. Stem Cell Rev. Rep. 2015, 11, 254–274. [Google Scholar] [CrossRef]
- Iafrate, M.; Fruhwirth, G.O. How Non-invasive in vivo Cell Tracking Supports the Development and Translation of Cancer Immunotherapies. Front. Physiol. 2021, 11, 154. [Google Scholar] [CrossRef]
- Krekorian, M.; Fruhwirth, G.O.; Srinivas, M.; Figdor, C.G.; Heskamp, S.; Witney, T.H.; Aarntzen, E.H.J.G. Imaging of T-cells and their responses during anti-cancer immunotherapy. Theranostics 2019, 9, 7924–7947. [Google Scholar] [CrossRef]
- Vande Velde, G.; Himmelreich, U.; Neeman, M. Reporter gene approaches for mapping cell fate decisions by MRI: Promises and pitfalls. Contrast Media Mol. Imag. 2013, 8, 424–431. [Google Scholar] [CrossRef]
- Himmelreich, U.; Hoehn, M. Stem cell labeling for Magnetic Resonance Imaging. Minim. Invasive Ther. Allied. Technol. 2008, 17, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Van Schooneveld, M.; Cormode, D.P.; Koole, R.; van Wijngaarden, J.T.; Calcagno, C.; Skajaa, T.; Hilhorst, J.; ’t Hart, D.C.; Fayad, Z.A.; Mulder, W.J.M.; et al. A fluorescent, paramagnetic and PEGylated gold/silica nanoparticle for MRI, CT and fluorescence imaging. Contrast Media Mol. Imag. 2010, 5, 231–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.; Louchami, K.; Holvoet, B.; Verbeke, R.; Deroose, C.M.; Manshian, B.; Soenen, S.J.; Lentacker, I.; Himmelreich, U. Tri-modal in vivo imaging of pancreatic islets transplanted subcutaneously in mice. Mol. Imaging Biol. 2018, 20, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Park, J.C.; Nah, H.; Woo, S.; Oh, J.; Kim, K.M.; Cheon, G.J.; Chang, Y.; Yoo, J.; Cheon, J. A hybrid nanoparticle probe for dual-modality positron emission tomography and magnetic resonance imaging. Angew. Chem. Int. Ed. Engl. 2008, 47, 6259–6262. [Google Scholar] [CrossRef] [PubMed]
- Lahooti, A.; Sarkar, S.; Laurent, S.; Shanehsazzadeh, S. Dual nano-sized contrast agents in PET/MRI: A systematic review. Contrast Media Mol. Imaging 2016, 11, 428–447. [Google Scholar] [CrossRef] [Green Version]
- Belderbos, S.; González-Gómez, M.A.; Cleeren, F.; Wouters, J.; Piñeiro, Y.; Deroose, C.M.; Coosemans, A.; Gsell, W.; Bormans, G.; Rivas, J.; et al. Simultaneous in vivo PET/MRI using fluorine-18 labeled Fe3O4@Al(OH)3 nanoparticles: Comparison of nanoparticle and nanoparticle-labeled stem cell distribution. Eur. J. Nucl. Med. Mol. Imaging Res. 2020, 10, 73. [Google Scholar] [CrossRef]
- Yang, H.W.; Liu, H.L.; Li, M.L.; Hsi, I.W.; Fan, C.T.; Huang, C.Y.; Lu, Y.J.; Hua, M.Y.; Chou, H.Y.; Liaw, J.W.; et al. Magnetic gold-nanorod/ PNIPAAmMA nanoparticles for dual magnetic resonance and photoacoustic imaging and targeted photothermal therapy. Biomaterials 2013, 34, 5651–5660. [Google Scholar] [CrossRef]
- D’Hollander, A.; Vande Velde, G.; Jans, H.; Vanspauwen, B.; Vermeersch, E.; Jose, J.; Struys, T.; Stakenborg, T.; Lagae, L.; Himmelreich, U. Assessment of the theranostic potential of gold nanostars—A multimodal imaging and photothermal treatment study. Nanomaterials 2020, 10, 2112. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.W.; Shau, Y.H.; Wu, P.C.; Yang, Y.S.; Shieh, D.B.; Chen, C.C. In Vitro and in Vivo Studies of FePt Nanoparticles for Dual Modal CT/MRI Molecular Imaging. J. Am. Chem. Soc. 2010, 132, 13270–13278. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Bulte, J.W.M. Seeing stem cells at work in vivo. Stem Cell Rev. Rep. 2014, 10, 127–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argibay, B.; Trekker, J.; Himmelreich, U.; Beiras, A.; Topete, A.; Taboada, P.; Pérez-Mato, M.; Vieites-Prado, A.; Iglesias-Rey, R.; Rivas, J.; et al. Intraarterial route increases the risk of cerebral lesions after mesenchymal cell administration in animal model of ischemia. Sci. Rep. 2017, 7, 40758. [Google Scholar] [CrossRef]
- Lusic, H.M.; Grinstaff, M.W. X-ray-Computed Tomography Contrast Agents. Chem. Rev. 2013, 113, 1641–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.; Choi, S.H.; Hyeon, T. Nano-Sized CT Contrast Agents. Adv. Mater. 2013, 25, 2641–2660. [Google Scholar] [CrossRef]
- von Maltzahn, G.; Park, J.H.; Agrawal, A.; Bandaru, N.K.; Das, S.K.; Sailor, M.J.; Bhatia, S.N. Computationally Guided Photothermal Tumor Therapy Using Long-Circulating Gold Nanorod Antennas. Cancer Res. 2009, 69, 3892–3900. [Google Scholar] [CrossRef] [Green Version]
- Hainfeld, J.F.; Slatkin, D.N.; Focella, T.M.; Smilowitz, H.M. Gold nanoparticles: A new X-ray contrast agent. Br. J. Radiol. 2006, 79, 248–253. [Google Scholar] [CrossRef]
- Popovtzer, R.; Agrawal, A.; Kotov, N.A.; Popovtzer, A.; Balter, J.; Carey, T.E.; Kopelman, R. Targeted Gold Nanoparticles Enable Molecular CT Imaging of Cancer. Nano Lett. 2008, 8, 4593–4596. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Wu, B.Y.; Xing, D. Bio-modified Fe3O4 core/Au shell nanoparticles for targeting and multimodal imaging of cancer cells. J. Mater. Chem. 2012, 22, 470–477. [Google Scholar] [CrossRef]
- Wang, X.D.; Liu, H.Y.; Chen, D.; Meng, X.W.; Liu, T.L.; Fu, C.H.; Hao, N.J.; Zhang, Y.Q.; Wu, X.L.; Ren, J.; et al. Multifunctional Fe3O4@P(St/MAA)@Chitosan@Au Core/Shell Nanoparticles for Dual Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2013, 5, 4966–4971. [Google Scholar] [CrossRef]
- González Gómez, M.A.; Belderbos, S.; Yáñez Vilar, S.; Piñeiro Redondo, Y.; Cleeren, F.; Bormans, G.; Deroose, C.M.; Gsell, W.; Himmelreich, U.; Rivas, J. Development of superparamagnetic nanoparticles coated with polyacrylic acid and aluminum hydroxide as an efficient contrast agent for multimode imaging. Nanomaterials 2019, 9, 1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirui, D.K.; Rey, D.A.; Batt, C.A. Gold hybrid nanoparticles for targeted phototherapy and cancer imaging. Nanotechnology 2010, 21, 105105. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Sathy, B.N.; Mony, U.; Koyakutty, M.; Nair, S.V.; Menon, D. Biocompatible Magnetite/Gold Nanohybrid Contrast Agents via Green Chemistry for MRI and CT Bioimaging. ACS Appl. Mater. Interfaces 2012, 4, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Zheng, L.F.; Cai, H.D.; Sun, W.J.; Shen, M.W.; Zhang, G.X.; Shi, X.Y. Facile One-Pot Synthesis of Fe3O4@Au Composite Nanoparticles for Dual-Mode MR/CT Imaging Applications. ACS Appl. Mater. Interfaces 2013, 5, 10357–10366. [Google Scholar] [CrossRef]
- Zhang, S.; Qi, Y.Y.; Yang, H.; Gong, M.F.; Zhang, D.; Zou, L.G. Optimization of the composition of bimetallic core/shell Fe2O3/Au nanoparticles for MRI/CT dual-mode imaging. J. Nanoparticle Res. 2013, 15, 2023. [Google Scholar] [CrossRef]
- Van Roosbroeck, R.; Van Roy, W.; Stakenborg, T.; Trekker, J.; D’Hollander, A.; Dresselaers, T.; Himmelreich, U.; Lammertyn, J.; Lagae, L. Synthetic Antiferromagnetic Nanoparticles as Potential Contrast Agents in MRI. ACS Nano 2014, 8, 2269–2278. [Google Scholar] [CrossRef]
- Gijsbers, R.; Ronen, K.; Vets, S.; Malani, N.; De Rijck, J.; McNeely, M.; Bushman, F.D.; Debyser, Z. LEDGF Hybrids Efficiently Retarget Lentiviral Integration Into Heterochromatin. Mol. Ther. 2010, 18, 552–560. [Google Scholar] [CrossRef]
- Trekker, J.; Leten, C.; Struys, T.; Lazenka, V.V.; Argibay, B.; Micholt, L.; Lambrichts, I.; Van Roy, W.; Lagae, L.; Himmelreich, U. Sensitive in vivo cell detection using size-optimized superparamagnetic nanoparticles. Biomaterials 2014, 35, 1627–1635. [Google Scholar] [CrossRef]
- Schrepfer, S.; Deuse, T.; Reichenspurner, H.; Fischbein, M.P.; Robbins, R.C.; Pelletier, M.P. Stem cell transplantation: The lung barrier. Transplant. Proc. 2007, 39, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xie, J.; Tong, Y.W.; Wang, C.H. Effect of PEG conformation and particle size on the cellular uptake efficiency of nanoparticles with the HepG2 cells. J. Control. Release 2007, 118, 7–17. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Gratton, S.E.A.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.; Hou, S.; Zheng, Z.; Zhou, J.; Bao, G. Coating optimization of superparamagnetic iron oxide nanoparticles for high T-2 relaxivity. Nano Lett. 2010, 10, 4607–4613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cormode, D.P.; Skajaa, T.; Fayad, Z.A.; Mulder, W.J.M. Nanotechnology in Medical Imaging Probe Design and Applications. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 992–1000. [Google Scholar] [CrossRef] [Green Version]
- Hachani, R.; Birchall, M.A.; Lowdell, M.W.; Kasparis, G.; Tung, L.D.; Manshian, B.B.; Soenen, S.J.; Gsell, W.; Himmelreich, U.; Gharagouzloo, C.A.; et al. Assessing cell-nanoparticle interactions by high content imaging of biocompatible iron oxide nanoparticles as potential contrast agents for magnetic resonance imaging. Sci. Rep. 2017, 7, 7850. [Google Scholar] [CrossRef] [PubMed]
- Debbage, P.; Jaschke, W. Molecular imaging with nanoparticles: Giant roles for dwarf actors. Histochem. Cell Biol. 2008, 130, 845–875. [Google Scholar] [CrossRef] [Green Version]
- Terreno, E.; Castelli, D.D.; Viale, A.; Aime, S. Challenges for molecular magnetic resonance imaging. Chem. Rev. 2010, 110, 3019–3042. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 2010, 188, 759–768. [Google Scholar] [CrossRef] [Green Version]
- Jain, T.K.; Reddy, M.K.; Morales, M.A.; Leslie-Pelecky, D.L.; Labhasetwar, V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol. Pharm. 2008, 5, 316–327. [Google Scholar] [CrossRef]
- Kunzmann, A.; Andersson, B.; Thurnherr, T.; Krug, H.; Scheynius, A.; Fadeel, B. Toxicology of engineered nanomaterials: Focus on biocompatibility, biodistribution and biodegradation. Biochim. Biophys. Acta 2011, 1810, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.C.; Geissler, E.K.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.H.; Hoogduijn, M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012, 3, 297. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Fu, C.; Song, L.; Yao, Y.; Zhang, X.; Chen, Z.; Li, Y.; Ma, G.; Shen, C. Exposure to supernatants of macrophages that phagocytized dead mesenchymal stem cells improves hypoxic cardiomyocytes survival. Int. J. Cardiol. 2013, 165, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Eggenhofer, E.; Luk, F.; Dahlke, M.H.; Hoogduijn, M.J. The life and fate of mesenchymal stem cells. Front. Immunol. 2014, 5, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- D’Hollander, A.; Jans, H.; Vande Velde, G.; Verstraete, C.; Massa, S.; Devoogdt, N.; Stakenborg, T.; Muyldermans, S.; Lagae, L.; Himmelreich, U. Limiting the protein corona: A successful strategy for in vivo active targeting of anti-HER2 nanobody-functionalized nanostars. Biomaterials 2017, 123, 15–23. [Google Scholar] [CrossRef]
- Harris, M.; Ceulemans, M.; Verstraete, C.; Bloemen, M.; Manshian, B.B.; Soenen, S.J.; Himmelreich, U.; Verbiest, T.; De Borggraeve, W.M.; Parac-Vogt, T.N. Ultrasmall iron oxide nanoparticles functionalized with BODIPY derivatives as potential bimodal probes for MRI and optical imaging. Nano Select 2021, 2, 406–416. [Google Scholar] [CrossRef]
- Garcia Ribeiro, R.S.; Belderbos, S.; Danhier, P.; Gallo, J.; Manshian, B.B.; Gallez, B.; Bañobre, M.; de Cuyper, M.; Soenen, S.J.; Gsell, W.; et al. Targeting tumor cells and neovascularization using RGD-functionalized magnetoliposomes. Int. J. Nanomed. 2019, 14, 5911–5924. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Hollander, A.; Van Roosbroeck, R.; Trekker, J.; Stakenborg, T.; Dresselaers, T.; Vande Velde, G.; Struys, T.; Lambrichts, I.; Lammertyn, J.; Lagae, L.; et al. Synthetic Antiferromagnetic Gold Nanoparticles as Bimodal Contrast Agents in MRI and CT—An Experimental In Vitro and In Vivo Study. Pharmaceutics 2021, 13, 1494. https://doi.org/10.3390/pharmaceutics13091494
D’Hollander A, Van Roosbroeck R, Trekker J, Stakenborg T, Dresselaers T, Vande Velde G, Struys T, Lambrichts I, Lammertyn J, Lagae L, et al. Synthetic Antiferromagnetic Gold Nanoparticles as Bimodal Contrast Agents in MRI and CT—An Experimental In Vitro and In Vivo Study. Pharmaceutics. 2021; 13(9):1494. https://doi.org/10.3390/pharmaceutics13091494
Chicago/Turabian StyleD’Hollander, Antoine, Ruben Van Roosbroeck, Jesse Trekker, Tim Stakenborg, Tom Dresselaers, Greetje Vande Velde, Tom Struys, Ivo Lambrichts, Jeroen Lammertyn, Liesbet Lagae, and et al. 2021. "Synthetic Antiferromagnetic Gold Nanoparticles as Bimodal Contrast Agents in MRI and CT—An Experimental In Vitro and In Vivo Study" Pharmaceutics 13, no. 9: 1494. https://doi.org/10.3390/pharmaceutics13091494
APA StyleD’Hollander, A., Van Roosbroeck, R., Trekker, J., Stakenborg, T., Dresselaers, T., Vande Velde, G., Struys, T., Lambrichts, I., Lammertyn, J., Lagae, L., & Himmelreich, U. (2021). Synthetic Antiferromagnetic Gold Nanoparticles as Bimodal Contrast Agents in MRI and CT—An Experimental In Vitro and In Vivo Study. Pharmaceutics, 13(9), 1494. https://doi.org/10.3390/pharmaceutics13091494








