A Physiologically Based Pharmacokinetic Model for Predicting Diazepam Pharmacokinetics after Intravenous, Oral, Intranasal, and Rectal Applications
Abstract
1. Introduction
2. Methods
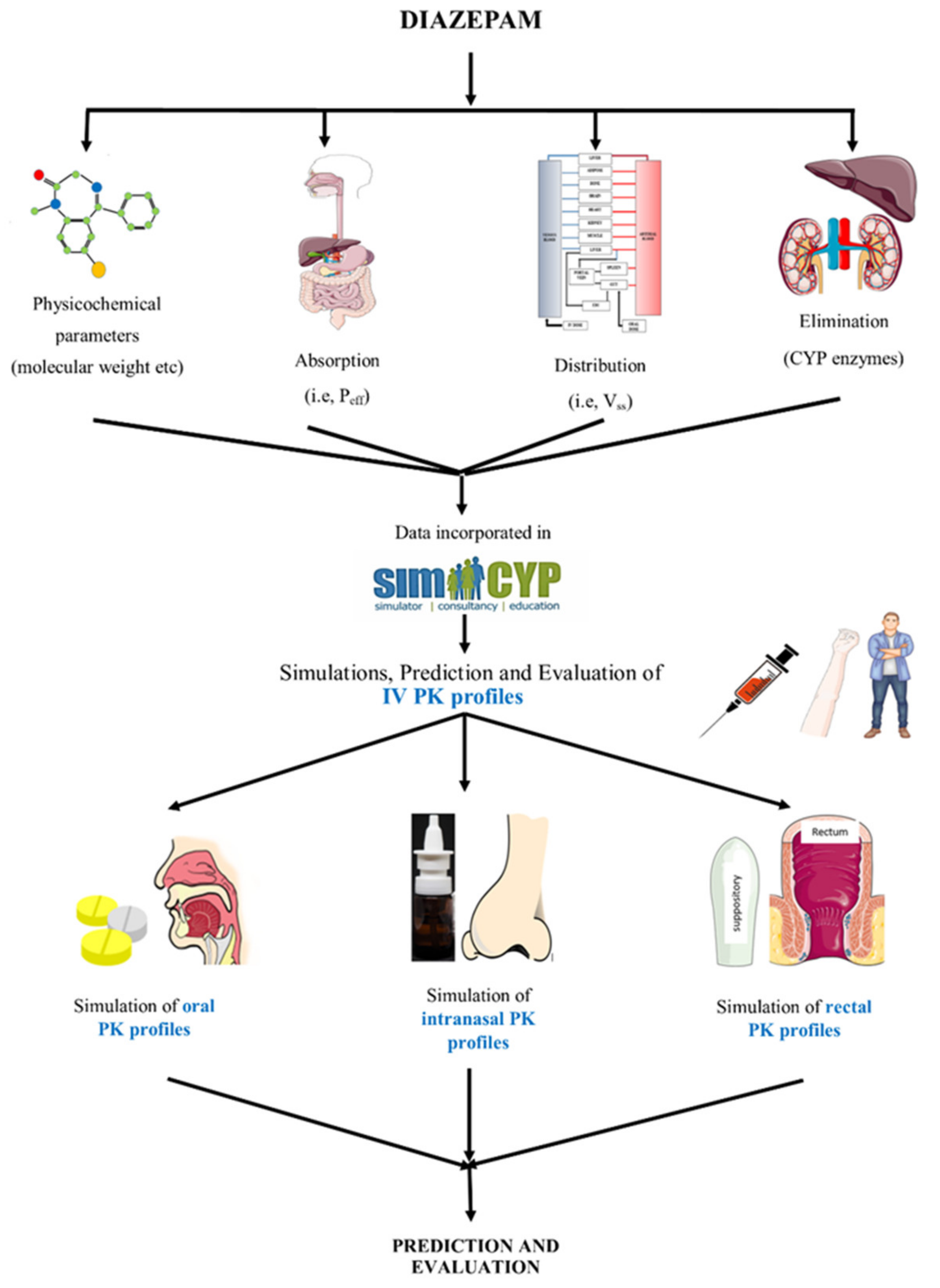
2.1. Modeling Software
2.2. Modeling Approach
2.3. Model Parameters
2.4. Pharmacokinetic Data
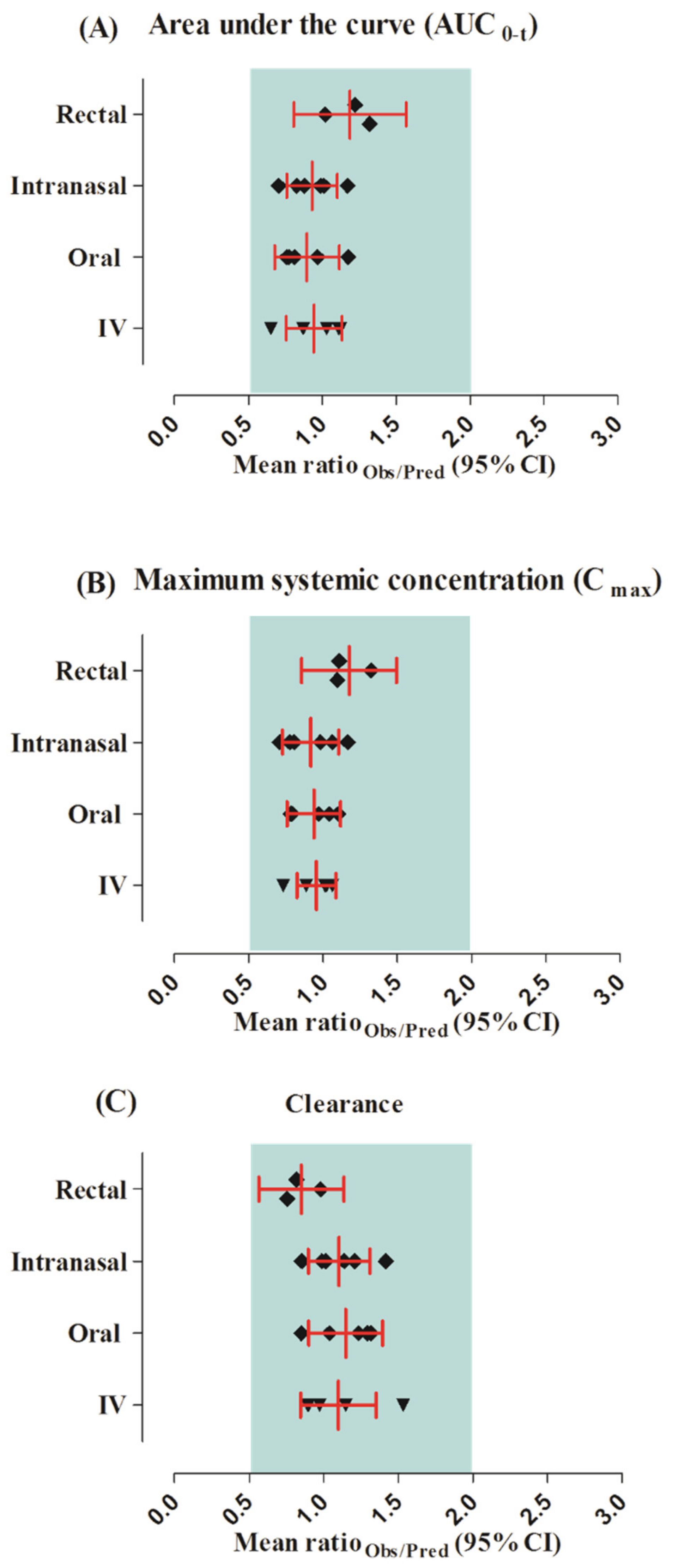
2.5. Model Evaluation
3. Results
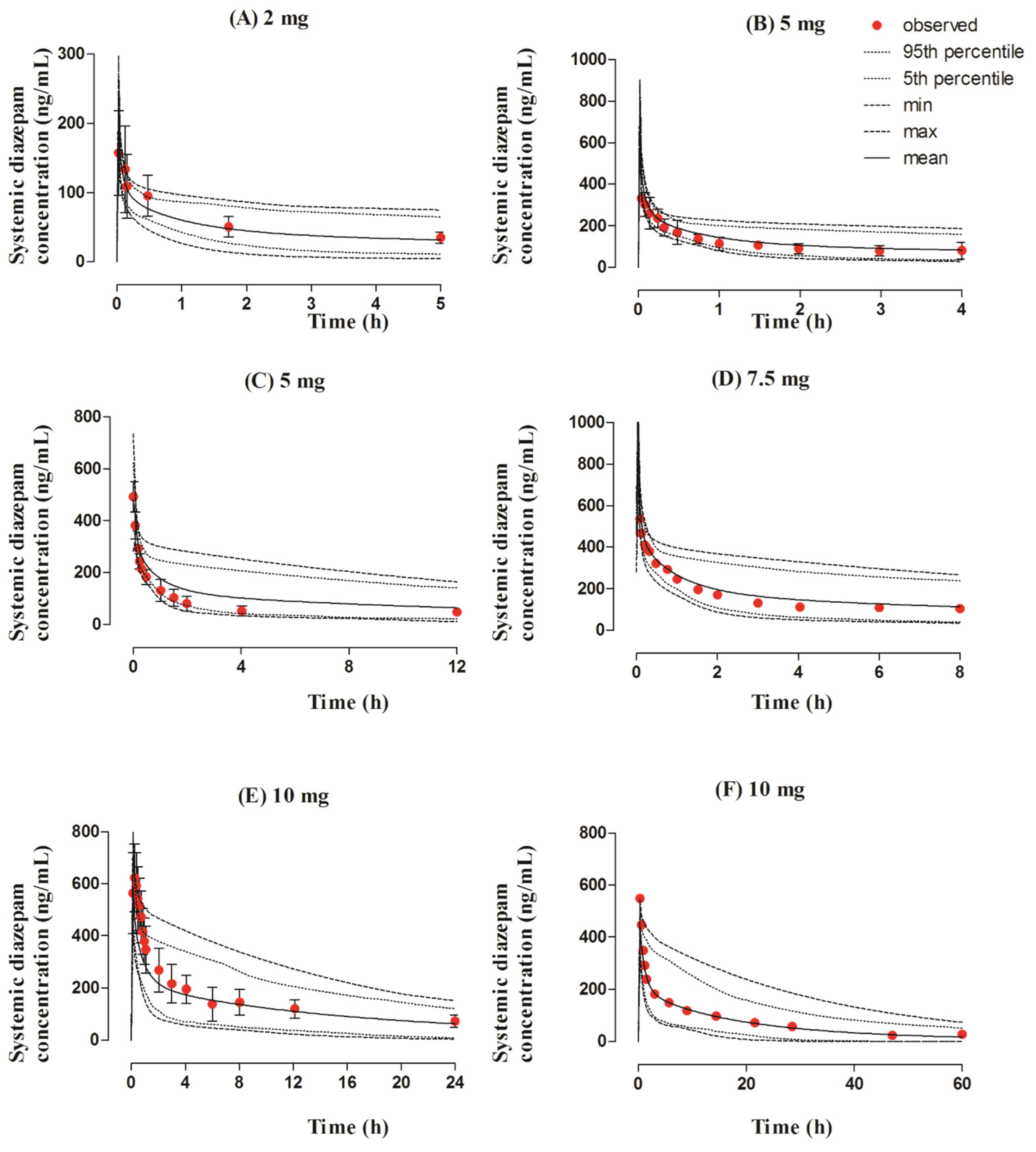
3.1. Intravenous Dose Administration in the Adult Population
3.2. Oral Dose Administration in the Adult Population
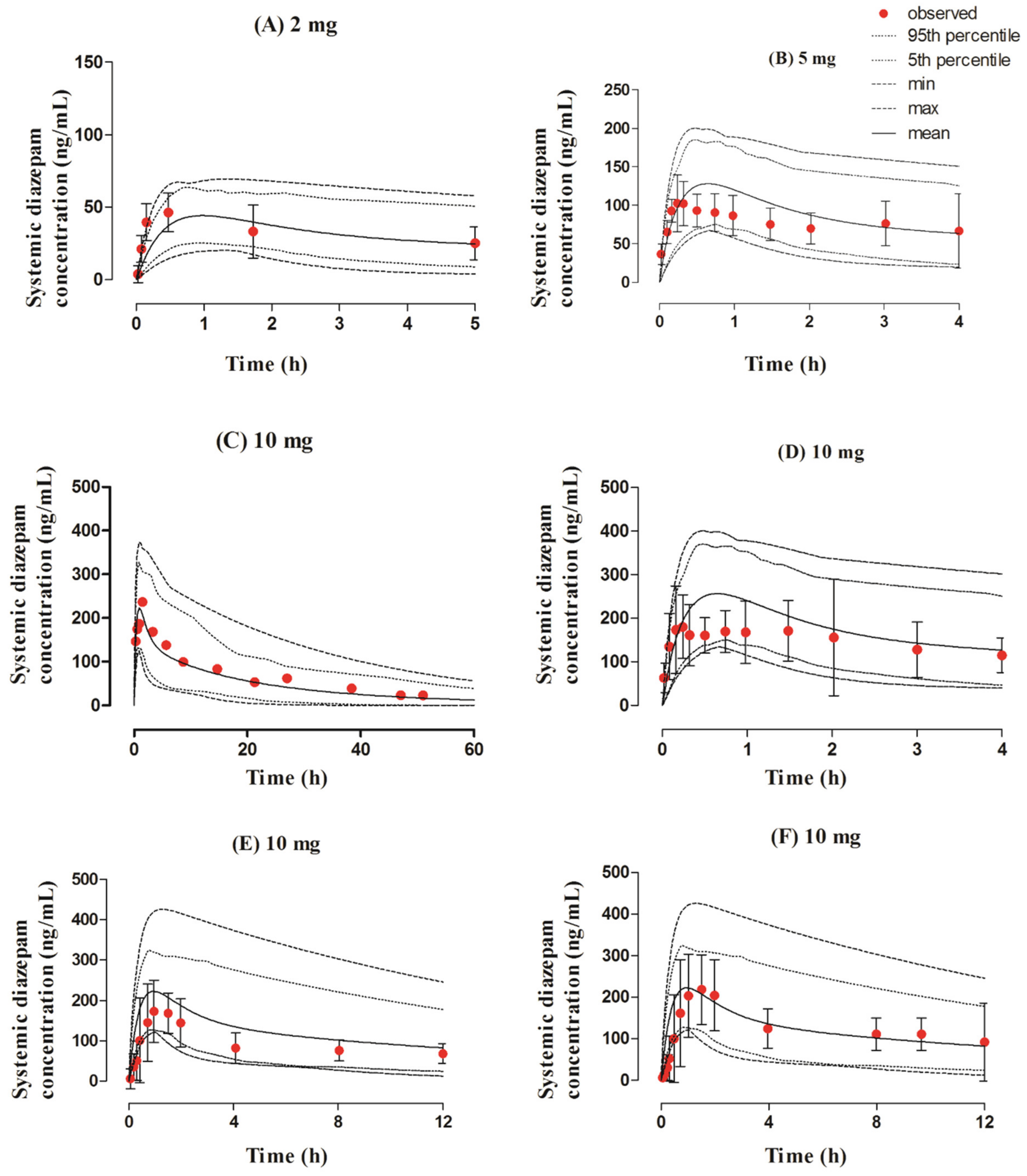
3.3. Intranasal Dose Administered in the Adult Population
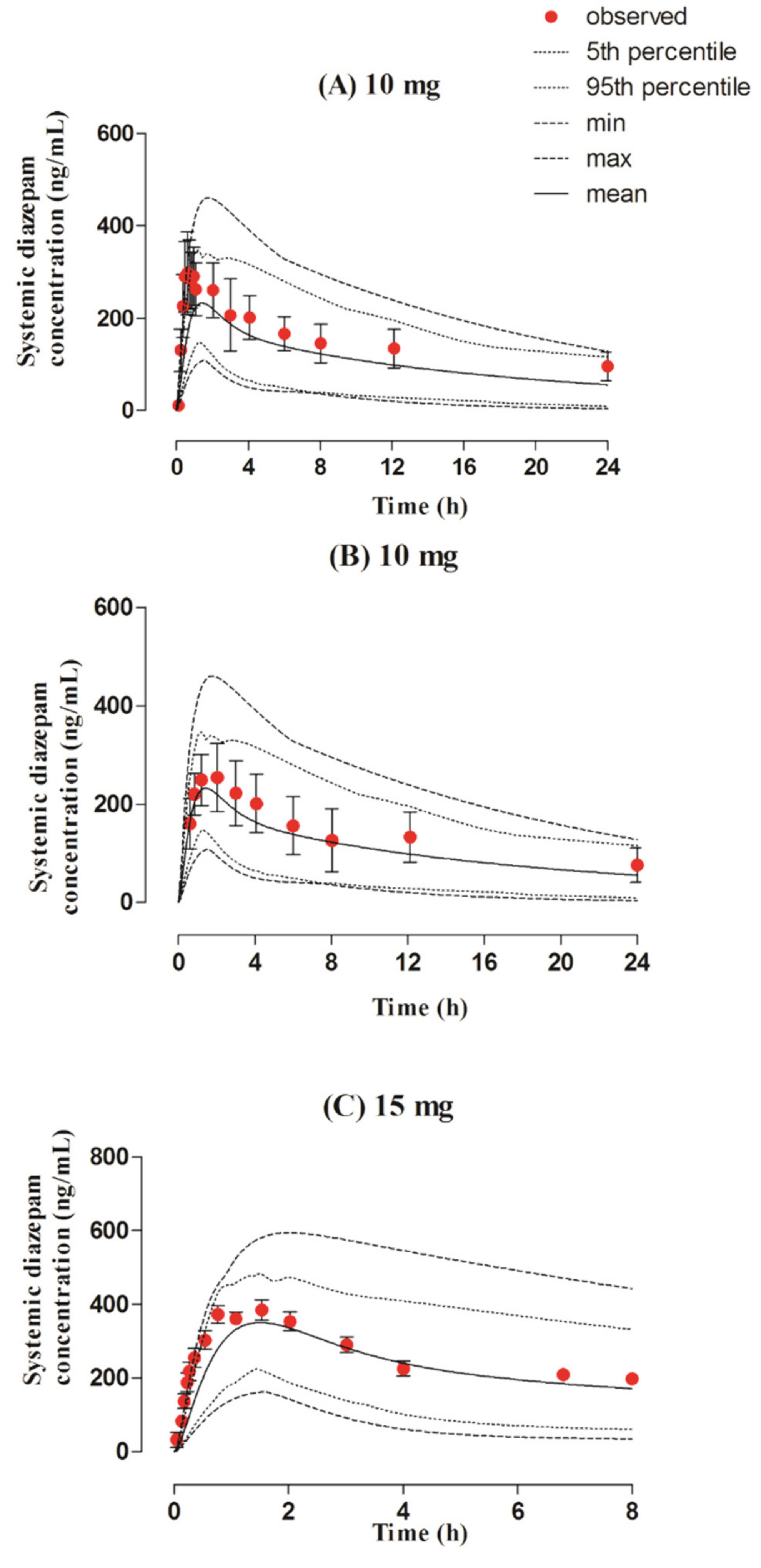
3.4. Rectal Dose Administered in the Adult Population
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Crestani, F.; Löw, K.; Keist, R.; Mandelli, M.-J.; Möhler, H.; Rudolph, U. Molecular targets for the myorelaxant action of diazepam. Mol. Pharmacol. 2001, 59, 442–445. [Google Scholar] [CrossRef]
- Griffin, C.E.; Kaye, A.M.; Bueno, F.R.; Kaye, A.D. Benzodiazepine pharmacology and central nervous system–mediated effects. Ochsner J. 2013, 13, 214–223. [Google Scholar] [PubMed]
- Mandelli, M.; Tognoni, G.; Garattini, S. Clinical pharmacokinetics of diazepam. Clin. Pharmacokinet. 1978, 3, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Manchikanti, L.; Christo, P.J.; Trescot, A.; Falco, F.J.E. Pain Medicine & Interventional Pain Management: A Comprehensive Review: Clinical Aspects; ASIPP Publishing: Paducah, KY, USA, 2011. [Google Scholar]
- Andersson, T.; Miners, J.; Veronese, M.; Birkett, D. Diazepam metabolism by human liver microsomes is mediated by both S-mephenytoin hydroxylase and CYP3A isoforms. Br. J. Clin. Pharmacol. 1994, 38, 131–137. [Google Scholar] [CrossRef]
- Kaplan, S.; Jack, M.; Alexander, K.; Weinfeld, R. Pharmacokinetic profile of diazepam in man following single intravenous and oral and chronic oral administrations. J. Pharm. Sci. 1973, 62, 1789–1796. [Google Scholar] [CrossRef]
- Fukasawa, T.; Suzuki, A.; Otani, K. Effects of genetic polymorphism of cytochrome P450 enzymes on the pharmacokinetics of benzodiazepines. J. Clin. Pharm. Ther. 2007, 32, 333–341. [Google Scholar] [CrossRef]
- Qin, X.P.; Xie, H.G.; Wang, W.; He, N.; Huang, S.L.; Xu, Z.H.; Ou-Yang, D.S.; Wang, Y.J.; Zhou, H.H. Effect of the gene dosage of CYP2C19 on diazepam metabolism in Chinese subjects. Clin. Pharmacol. Ther. 1999, 66, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Bertilsson, L.; Henthorn, T.K.; Sanz, E.; Tybring, G.; Säwe, J.; Villén, T. Importance of genetic factors in the regulation of diazepam metabolism: Relationship to S-mephenytoin, but not debrisoquin, hydroxylation phenotype. Clin. Pharmacol. Ther. 1989, 45, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Skryabin, V.Y.; Zastrozhin, M.S.; Torrado, M.V.; Grishina, E.A.; Ryzhikova, K.A.; Shipitsyn, V.V.; Galaktionova, T.E.; Sorokin, A.S.; Bryun, E.A.; Sychev, D.A. How do CYP2C19* 2 and CYP2C19* 17 genetic polymorphisms affect the efficacy and safety of diazepam in patients with alcohol withdrawal syndrome? Drug Metab. Pers. Ther. 2020, 35, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Boddu, S.H.; Kumari, S. A Short Review on the Intranasal Delivery of Diazepam for Treating Acute Repetitive Seizures. Pharmaceutics 2020, 12, 1167. [Google Scholar] [CrossRef] [PubMed]
- Rogawski, M.A.; Heller, A.H. Diazepam buccal film for the treatment of acute seizures. Epilepsy Behav. 2019, 101, 106537. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, D.; Arendt, R.; Abernethy, D.R.; Giles, H.; Sellers, E.; Shader, R. In vitro quantitation of benzodiazepine lipophilicity: Relation to in vivo distribution. BJA Br. J. Anaesth. 1983, 55, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.D.; Saneto, R.P. Current oral and non-oral routes of antiepileptic drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 911–918. [Google Scholar] [CrossRef]
- Moolenaar, F.; Bakker, S.; Visser, J.; Huizinga, T. Biopharmaceutics of rectal administration of drugs in man IX. Comparative biopharmaceutics of diazepam after single rectal, oral, intramuscular and intravenous administration in man. Int. J. Pharm. 1980, 5, 127–137. [Google Scholar] [CrossRef]
- Maglalang, P.D.; Rautiola, D.; Siegel, R.A.; Fine, J.M.; Hanson, L.R.; Coles, L.D.; Cloyd, J.C. Rescue therapies for seizure emergencies: New modes of administration. Epilepsia 2018, 59, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Wermeling, D.P.H.; Miller, J.L.; Archer, S.M.; Manaligod, J.M.; Rudy, A.C. Bioavailability and pharmacokinetics of lorazepam after intranasal, intravenous, and intramuscular administration. J. Clin. Pharmacol. 2001, 41, 1225–1231. [Google Scholar] [CrossRef]
- Vyas, T.K.; Shahiwala, A.; Marathe, S.; Misra, A. Intranasal drug delivery for brain targeting. Curr. Drug Deliv. 2005, 2, 165–175. [Google Scholar] [CrossRef]
- Schwarz, J.S.; Weisspapir, M.R.; Friedman, D.I. Enhanced transdermal delivery of diazepam by submicron emulsion (SME) creams. Pharm. Res. 1995, 12, 687–692. [Google Scholar] [CrossRef]
- Mehmood, Y. Preparation of diazipam delayed release patch, for anxiolytic treatment. Int. J. 2014, 3, 19–23. [Google Scholar]
- Xu, Q.; Zan, Y.; Gao, C. Preparation of diazepam transdermal gel and its bioavailability. Zhejiang Da Xue Xue Bao Yi Xue Ban = J. Zhejiang Univ. Med Sci. 2012, 41, 441–444. [Google Scholar]
- Cottura, N.; Howarth, A.; Rajoli, R.K.; Siccardi, M. The Current Landscape of Novel Formulations and the Role of Mathematical Modeling in Their Development. J. Clin. Pharmacol. 2020, 60, S77–S97. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.; Chen, Y.; Gibson, C.; Heimbach, T.; Parrott, N.; Peters, S.; Snoeys, J.; Upreti, V.; Zheng, M.; Hall, S. Physiologically based pharmacokinetic modeling in drug discovery and development: A pharmaceutical industry perspective. Clin. Pharmacol. Ther. 2015, 97, 247–262. [Google Scholar] [CrossRef]
- Teorell, T. Kinetics of distribution of substances administered to the body, I: The extravascular modes of administration. Arch. Int. De Pharmacodyn. Et De Ther. 1937, 57, 205–225. [Google Scholar]
- Poulin, P.; Theil, F.-P. Prediction of pharmacokinetics prior to in vivo studies. II. Generic physiologically based pharmacokinetic models of drug disposition. J. Pharm. Sci. 2002, 91, 1358–1370. [Google Scholar] [CrossRef]
- Theil, F.-P.; Guentert, T.W.; Haddad, S.; Poulin, P. Utility of physiologically based pharmacokinetic models to drug development and rational drug discovery candidate selection. Toxicol. Lett. 2003, 138, 29–49. [Google Scholar] [CrossRef]
- Edginton, A.N.; Theil, F.-P.; Schmitt, W.; Willmann, S. Whole body physiologically-based pharmacokinetic models: Their use in clinical drug development. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Rowland, M.; Peck, C.; Tucker, G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 45–73. [Google Scholar] [CrossRef] [PubMed]
- De Buck, S.S.; Mackie, C.E. Physiologically based approaches towards the prediction of pharmacokinetics: In vitro–in vivo extrapolation. Expert Opin. Drug Metab. Toxicol. 2007, 3, 865–878. [Google Scholar] [CrossRef]
- Rasool, M.F.; Läer, S. Development and evaluation of a physiologically based pharmacokinetic model to predict carvedilol-paroxetine metabolic drug–drug interaction in healthy adults and its extrapolation to virtual chronic heart failure patients for dose optimization. Expert Opin. Drug Metab. Toxicol. 2021, 17, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Jamei, M.; Marciniak, S.; Feng, K.; Barnett, A.; Tucker, G.; Rostami-Hodjegan, A. The Simcyp® population-based ADME simulator. Expert Opin. Drug Metab. Toxicol. 2009, 5, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Lu, C. PBPK modeling and simulation in drug research and development. Acta Pharm. Sin. B 2016, 6, 430–440. [Google Scholar] [CrossRef]
- Jamei, M.; Turner, D.; Yang, J.; Neuhoff, S.; Polak, S.; Rostami-Hodjegan, A.; Tucker, G. Population-based mechanistic prediction of oral drug absorption. AAPS J. 2009, 11, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Tsiros, P.; Bois, F.Y.; Dokoumetzidis, A.; Tsiliki, G.; Sarimveis, H. Population pharmacokinetic reanalysis of a Diazepam PBPK model: A comparison of Stan and GNU MCSim. J. Pharmacokinet. Pharmacodyn. 2019, 46, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Liu, S.; Xue, Y.; He, X.; Man, V.H.; Xie, X.-Q.; Wang, J. Prediction of drug–drug interactions between opioids and overdosed benzodiazepines using physiologically based pharmacokinetic (PBPK) modeling and simulation. Drugs RD 2019, 19, 297–305. [Google Scholar] [CrossRef]
- Gueorguieva, I.I.; Nestorov, I.A.; Rowland, M. Fuzzy simulation of pharmacokinetic models: Case study of whole body physiologically based model of diazepam. J. Pharmacokinet. Pharmacodyn. 2004, 31, 185–213. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.F.; Khalid, S.; Majeed, A.; Saeed, H.; Imran, I.; Mohany, M.; Al-Rejaie, S.S.; Alqahtani, F. Development and Evaluation of Physiologically Based Pharmacokinetic Drug–Disease Models for Predicting Rifampicin Exposure in Tuberculosis and Cirrhosis Populations. Pharmaceutics 2019, 11, 578. [Google Scholar] [CrossRef]
- Rasool, M.F.; Khalid, R.; Imran, I.; Majeed, A.; Saeed, H.; Alasmari, F.; Alanazi, M.M.; Alqahtani, F. Investigating the role of altered systemic albumin concentration on the disposition of theophylline in adult and pediatric patients with asthma by using the physiologically based pharmacokinetic approach. Drug Metab. Dispos. 2020, 48, 570–579. [Google Scholar] [CrossRef]
- Drugbank (Diazepam Compound Summary). Available online: https://go.drugbank.com/drugs/DB00829 (accessed on 14 December 2020).
- Pubchem (Diazepam Compound Summary). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Diazepam#section=Computed-Properties (accessed on 6 January 2021).
- Ye, M.; Nagar, S.; Korzekwa, K. A physiologically based pharmacokinetic model to predict the pharmacokinetics of highly protein-bound drugs and the impact of errors in plasma protein binding. Biopharm. Drug Dispos. 2016, 37, 123–141. [Google Scholar] [CrossRef]
- Gonzalez, F.; Coughtrie, M.; Tukey, R. Goodman and Gilman’s The Pharmacological Basis of Therapeutics; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2006; pp. 71–92. [Google Scholar]
- FDA (Valium Label). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/013263s094lbl.pdf (accessed on 15 January 2021).
- Onof, S.; Hatanaka, T.; Miyazawa, S.; Tsutsui, M.; Aoyama, T.; Gonzalez, F.; Satoh, T. Human liver microsomal diazepam metabolism using cDNA-expressed cytochrome P450s: Role of CYP2B6, 2C19 and the 3A subfamily. Xenobiotica 1996, 26, 1155–1166. [Google Scholar] [CrossRef]
- GDG Digitizer. GetData Graph Digitizer (2.26); GDG Digitizer: 2013. Available online: http://getdata-graph-digitizer.com, (accessed on 12 September 2021).
- Gizurarson, S.; Gudbrandsson, F.K.; Jonsson, H.; BECHGAARD, E. Intranasal administration of diazepam aiming at the treatment of acute seizures: Clinical trials in healthy volunteers. Biol. Pharm. Bull. 1999, 22, 425–427. [Google Scholar] [CrossRef][Green Version]
- Ivaturi, V.D.; Riss, J.R.; Kriel, R.L.; Siegel, R.A.; Cloyd, J.C. Bioavailability and tolerability of intranasal diazepam in healthy adult volunteers. Epilepsy Res. 2009, 84, 120–126. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Kriel, R.L.; Brundage, R.C.; Ivaturi, V.D.; Cloyd, J.C. A pilot study assessing the bioavailability and pharmacokinetics of diazepam after intranasal and intravenous administration in healthy volunteers. Epilepsy Res. 2013, 105, 362–367. [Google Scholar] [CrossRef]
- Cloyd, J.C.; Lalonde, R.L.; Beniak, T.E.; Novack, G.D. A single-blind, crossover comparison of the pharmacokinetics and cognitive effects of a new diazepam rectal gel with intravenous diazepam. Epilepsia 1998, 39, 520–526. [Google Scholar] [CrossRef]
- Lau, S.; Slattery, J.T. Absorption of diazepam and lorazepam following intranasal administration. Int. J. Pharm. 1989, 54, 171–174. [Google Scholar] [CrossRef]
- Friedman, H.; Greenblatt, D.J.; Peters, G.R.; Metzler, C.M.; Charlton, M.D.; Harmatz, J.S.; Antal, E.J.; Sanborn, E.C.; Francom, S.F. Pharmacokinetics and pharmacodynamics of oral diazepam: Effect of dose, plasma concentration, and time. Clin. Pharmacol. Ther. 1992, 52, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, D.J.; Harmatz, J.S.; Friedman, H.; Locniskar, A.; Shader, R.I. A large-sample study of diazepam pharmacokinetics. Ther. Drug Monit. 1989, 11, 652–657. [Google Scholar] [CrossRef]
- Rasool, M.F.; Ali, S.; Khalid, S.; Khalid, R.; Majeed, A.; Imran, I.; Saeed, H.; Usman, M.; Ali, M.; Alali, A.S. Development and evaluation of physiologically based pharmacokinetic drug-disease models for predicting captopril pharmacokinetics in chronic diseases. Sci. Rep. 2021, 11, 8589. [Google Scholar] [CrossRef] [PubMed]
- Divoll, M.; Greenblatt, D.J.; Ochs, H.R.; Shader, R.I. Absolute bioavailability of oral and intramuscular diazepam: Effects of age and sex. Anesth. Analg. 1983, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Ali, M.; Baboota, S.; Kaur Sahni, J.; Ramassamy, C.; Dao, L. Potential of nanoparticulate drug delivery systems by intranasal administration. Curr. Pharm. Des. 2010, 16, 1644–1653. [Google Scholar] [CrossRef]
- Choi, H.-G.; Jung, J.-H.; Ryu, J.-M.; Yoon, S.-J.; Oh, Y.-K.; Kim, C.-K. Development of in situ-gelling and mucoadhesive acetaminophen liquid suppository. Int. J. Pharm. 1998, 165, 33–44. [Google Scholar] [CrossRef]
- Ugwoke, M.I.; Verbeke, N.; Kinget, R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J. Pharm. Pharmacol. 2001, 53, 3–22. [Google Scholar] [CrossRef]
- Arora, P.; Sharma, S.; Garg, S. Permeability issues in nasal drug delivery. Drug Discov. Today 2002, 7, 967–975. [Google Scholar] [CrossRef]
- Schrier, L.; Zuiker, R.; Merkus, F.W.; Klaassen, E.S.; Guan, Z.; Tuk, B.; van Gerven, J.M.; van der Geest, R.; Groeneveld, G.J. Pharmacokinetics and pharmacodynamics of a new highly concentrated intranasal midazolam formulation for conscious sedation. Br. J. Clin. Pharmacol. 2017, 83, 721–731. [Google Scholar] [CrossRef]
- Ivaturi, V.D. Intranasal and Rectal Diazepam for Rescue Therapy: Assessment of Pharmacokinetics and Tolerability; University of Minnesota: Minneapolis, MN, USA, 2010. [Google Scholar]
- Hou, H.; Siegel, R.A. Enhanced permeation of diazepam through artificial membranes from supersaturated solutions. J. Pharm. Sci. 2006, 95, 896–905. [Google Scholar] [CrossRef]
- FDA. Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products—Chemistry, Manufacturing and Controls Documentation; FDA: New Hampshire, MD, USA, 2002. [Google Scholar]
- Hogan, R.E.; Tarquinio, D.; Sperling, M.R.; Klein, P.; Miller, I.; Segal, E.B.; Rabinowicz, A.L.; Carrazana, E. Pharmacokinetics and safety of VALTOCO (NRL-1; diazepam nasal spray) in patients with epilepsy during seizure (ictal/peri-ictal) and nonseizure (interictal) conditions: A phase 1, open-label study. Epilepsia 2020, 61, 935–943. [Google Scholar] [CrossRef]
- Detyniecki, K.; Van Ess, P.J.; Sequeira, D.J.; Wheless, J.W.; Meng, T.C.; Pullman, W.E. Safety and efficacy of midazolam nasal spray in the outpatient treatment of patients with seizure clusters—A randomized, double-blind, placebo-controlled trial. Epilepsia 2019, 60, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M. Buccal midazolam for pediatric convulsive seizures: Efficacy, safety, and patient acceptability. Patient Prefer. Adherence 2013, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Sarma, A.K.; Khandker, N.; Kurczewski, L.; Brophy, G.M. Medical management of epileptic seizures: Challenges and solutions. Neuropsychiatr. Dis. Treat. 2016, 12, 467. [Google Scholar] [PubMed]
- Pellock, J.M. Safety of Diastat®, a rectal gel formulation of diazepam for acute seizure treatment. Drug Saf. 2004, 27, 383–392. [Google Scholar] [CrossRef]
- Chetty, M.; Rose, R.H.; Abduljalil, K.; Patel, N.; Lu, G.; Cain, T.; Jamei, M.; Rostami-Hodjegan, A. Applications of linking PBPK and PD models to predict the impact of genotypic variability, formulation differences, differences in target binding capacity and target site drug concentrations on drug responses and variability. Front. Pharmacol. 2014, 5, 258. [Google Scholar] [CrossRef]

| Parameters | Values | Ref. | |
|---|---|---|---|
| Physicochemical Properties | |||
| Molecular Weight (g/mol) | 284.74 | [40] | |
| LogPo:w | 2.82 | [35] | |
| pKa | 3.4 | [41] | |
| Absorption | |||
| Model | |||
| Peff,man (cm/s) | 12.434 × 10−4 a,b | [35] | |
| PSA (Ao) | 32.67 | [39] | |
| HBD | 0 | [39] | |
| Distribution | |||
| Model Prediction Method | The Rodger and Rowland Method + Ion Membrane Permeability | ||
| B/P ratio | 0.58 | [41] | |
| fu | 0.03 | [3,42] | |
| Vss (L/kg) | 0.66 a,c | [35,43] | |
| For Intranasal Administration | |||
| Lung fa | 0.7 d | ||
| Lung ka | 1.6 d,e | ||
| Elimination | |||
| fumic | 0.59 a | ||
| N-demethylation | Vmax (pmol/min/pmol) | Km (μM) | |
| CYP2B6 | 3.6 | 113 | [44] |
| CYP2C19 | 2.3 | 32 | |
| CYP3A4 | 14.8 | 1828 | |
| CYP3A5 | 1.8 | 293 | |
| 3-hydroxylation | |||
| CYP2B6 | 0.1 | 150 | [44] |
| CYP2C19 | 20.2 | 846 | |
| CYP3A4 | 151.3 | 2235 | |
| CYP3A5 | 48.4 | 316 | |
| No. | Population | No. of Subjects | Dose (mg) | Dosage Form | Age (Years) | Weight (kg) | Female Proportion | Ref. |
|---|---|---|---|---|---|---|---|---|
| IV | ||||||||
| 1 | Healthy | 9 | 2 | IV solution | 20−30 | 58−80 | 0 | [46] |
| 2 | Healthy | 8 | 5 | IV solution | Mean 28.3 | - | 0.33 | [47] |
| 3 | Healthy | 24 | 5 | IV solution | 18−45 | Mean 71.8 | 0.2 | [48] |
| 4 | Healthy | 20 | 7.5 | IV solution | Mean 28.8 | Mean 72.6 | 0 | [49] |
| 5 | Healthy | 9 | 1 | IV solution | 18−25 | 58−70 | 0.33 | [15] |
| 6 | Healthy | 1 | 10 | IV solution | 26−37 | 60−85 | 0.2 | [50] |
| Oral | ||||||||
| 7 | Healthy | 11 | 2 | - | 19−35 | - | 0.27 | [51] |
| 8 | Healthy | 11 | 5 | - | 19−35 | - | 0.27 | [51] |
| 9 | Healthy | 48 | 10 | Tablets | 18−44 | 59.1−95 | 0 | [52] |
| 10 | Healthy | 11 | 10 | - | 19−35 | - | 0.27 | [51] |
| 11 | Healthy | 9 | 10 | Tablet | 18−25 | 58−70 | 0.33 | [15] |
| Intranasal | ||||||||
| 12 | Healthy | 9 | 2 | Solution | 20−30 | 58–80 | 0 | [46] |
| 13 | Healthy | 8 | 5 | Supersaturated solution | Mean 28.3 | - | 0.33 | [47] |
| 14 | Healthy | 24 | 10 | Solution | 18−45 | Mean 71.8 | 0.2 | [48] |
| 15 | Healthy | 24 | 10 | Suspension | 18−45 | Mean 71.8 | 0.2 | [48] |
| 16 | Healthy | 8 | 10 | Supersaturated solution | Mean 28.3 | - | 0.33 | [47] |
| 17 | Healthy | 1 | 10 | Solution | 26−37 | 60−85 | 0.2 | [50] |
| Rectal | ||||||||
| 18 | Healthy | 9 | 10 | Solution | 18−25 | 58−70 | 0.33 | [15] |
| 19 | Healthy | 9 | 10 | Suppository | 18−25 | 58−70 | 0.33 | [15] |
| 19 | Healthy | 20 | 15 | Gel | Mean 28.8 | Mean 173.9 | 0 | [49] |
| Dose (mg) | Cmax (ng/mL) | Ratio | AUC0–t (ng.h/mL) | Ratio | CL (L/h) | Ratio | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Predicted | Observed | Predicted | Observed | Predicted | ||||||
| IV | |||||||||||
| 1 | 2 | 157.06 | 154.39 | 1.01 | 285.45 | 256.60 | 1.11 | 7.00 | 7.79 | 0.89 | [46] |
| 2 | 5 | 332.28 | 452.92 | 0.73 | 455.11 | 522.20 | 0.87 | 10.98 | 9.57 | 1.14 | [47] |
| 3 | 5 | 492.1 | 479.77 | 1.02 | 851.74 | 1305.30 | 0.65 | 5.87 | 3.83 | 1.53 | [48] |
| 4 | 7.5 | 536.44 | 605.14 | 0.88 | 1244 | 1428.08 | 0.87 | 6.27 | 5.46 | 1.14 | [49] |
| 5 | 10 | 623 | 586.11 | 1.06 | 3505.37 | 3117.92 | 1.12 | 2.85 | 3.2 | 0.89 | [15] |
| 6 | 10 | 642.95 | 634.13 | 1.01 | 4491.90 | 4364.79 | 1.02 | 2.22 | 2.29 | 0.97 | [50] |
| Oral | |||||||||||
| 1 | 2 | 62.07 | 63.97 | 0.97 | 330 | 406.55 | 0.81 | 6.06 | 4.91 | 1.23 | [51] |
| 2 | 5 | 125.24 | 159.95 | 0.78 | 784.19 | 1012.40 | 0.77 | 6.37 | 4.93 | 1.29 | [51] |
| 3 | 10 | 325 | 311.87 | 1.04 | 3487 | 2965.75 | 1.17 | 2.86 | 3.37 | 0.80 | [15] |
| 4 | 10 | 255.53 | 322.62 | 0.80 | 1540.28 | 2026.76 | 0.75 | 6.49 | 4.93 | 1.31 | [51] |
| 5 | 10 | 352 | 318.99 | 1.10 | 1445.96 | 1493.59 | 0.96 | 6.91 | 6.66 | 1.03 | [52] |
| Intranasal | |||||||||||
| 1 | 2 | 46.25 | 39.66 | 1.16 | 161.73 | 159.94 | 1.01 | 12.36 | 12.5 | 0.98 | [46] |
| 2 | 5 | 102.66 | 127.34 | 0.80 | 309.82 | 352.62 | 0.87 | 16.13 | 14.18 | 1.13 | [47] |
| 3 | 10 | 218.15 | 222.08 | 0.98 | 1513.78 | 1532.92 | 0.98 | 6.60 | 6.52 | 1.01 | [48] |
| 4 | 10 | 172.99 | 222.48 | 0.77 | 1084 | 1523.59 | 0.70 | 9.22 | 6.56 | 1.41 | [48] |
| 5 | 10 | 180.51 | 254.69 | 0.70 | 588.64 | 710.80 | 0.82 | 16.98 | 14.06 | 1.20 | [47] |
| 6 | 10 | 236.82 | 222.70 | 1.06 | 3558.44 | 3033.84 | 1.17 | 2.81 | 3.29 | 0.85 | [50] |
| Rectal | |||||||||||
| 1 | 10 | 297 | 224.17 | 1.32 | 3566.35 | 2699.95 | 1.32 | 2.80 | 3.70 | 0.75 | [15] |
| 2 | 10 | 254 | 229.40 | 1.10 | 3258.12 | 2666.01 | 1.22 | 3.06 | 3.75 | 0.81 | [15] |
| 3 | 15 | 384.86 | 350.74 | 1.09 | 2070.02 | 2028.11 | 1.02 | 7.24 | 7.39 | 0.97 | [49] |
| Parameters | Robs/pred | AFE |
|---|---|---|
| IV | ||
| AUC0–t | 0.94 | 0.93 |
| CL | 1.10 | 1.08 |
| Cmax | 0.96 | 0.95 |
| Oral | ||
| AUC0–t | 0.90 | 0.89 |
| CL | 1.15 | 1.13 |
| Cmax | 0.94 | 0.93 |
| Intranasal | ||
| AUC0–t | 0.93 | 0.92 |
| CL | 1.10 | 1.09 |
| Cmax | 0.92 | 0.90 |
| Rectal | ||
| AUC0–t | 1.19 | 1.18 |
| CL | 0.85 | 0.85 |
| Cmax | 1.18 | 1.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, S.; Rasool, M.F.; Imran, I.; Majeed, A.; Saeed, H.; Rehman, A.u.; Ashraf, W.; Ahmad, T.; Bin Jardan, Y.A.; Alqahtani, F. A Physiologically Based Pharmacokinetic Model for Predicting Diazepam Pharmacokinetics after Intravenous, Oral, Intranasal, and Rectal Applications. Pharmaceutics 2021, 13, 1480. https://doi.org/10.3390/pharmaceutics13091480
Khalid S, Rasool MF, Imran I, Majeed A, Saeed H, Rehman Au, Ashraf W, Ahmad T, Bin Jardan YA, Alqahtani F. A Physiologically Based Pharmacokinetic Model for Predicting Diazepam Pharmacokinetics after Intravenous, Oral, Intranasal, and Rectal Applications. Pharmaceutics. 2021; 13(9):1480. https://doi.org/10.3390/pharmaceutics13091480
Chicago/Turabian StyleKhalid, Sundus, Muhammad Fawad Rasool, Imran Imran, Abdul Majeed, Hamid Saeed, Anees ur Rehman, Waseem Ashraf, Tanveer Ahmad, Yousef A. Bin Jardan, and Faleh Alqahtani. 2021. "A Physiologically Based Pharmacokinetic Model for Predicting Diazepam Pharmacokinetics after Intravenous, Oral, Intranasal, and Rectal Applications" Pharmaceutics 13, no. 9: 1480. https://doi.org/10.3390/pharmaceutics13091480
APA StyleKhalid, S., Rasool, M. F., Imran, I., Majeed, A., Saeed, H., Rehman, A. u., Ashraf, W., Ahmad, T., Bin Jardan, Y. A., & Alqahtani, F. (2021). A Physiologically Based Pharmacokinetic Model for Predicting Diazepam Pharmacokinetics after Intravenous, Oral, Intranasal, and Rectal Applications. Pharmaceutics, 13(9), 1480. https://doi.org/10.3390/pharmaceutics13091480