Bioadhesive Curcumin-Mediated Photodynamic Inactivation and Its Potential to Cause Undesirable Effects on Dental and Restorative Surfaces
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Preparation of the Precursor of Liquid Crystalline System
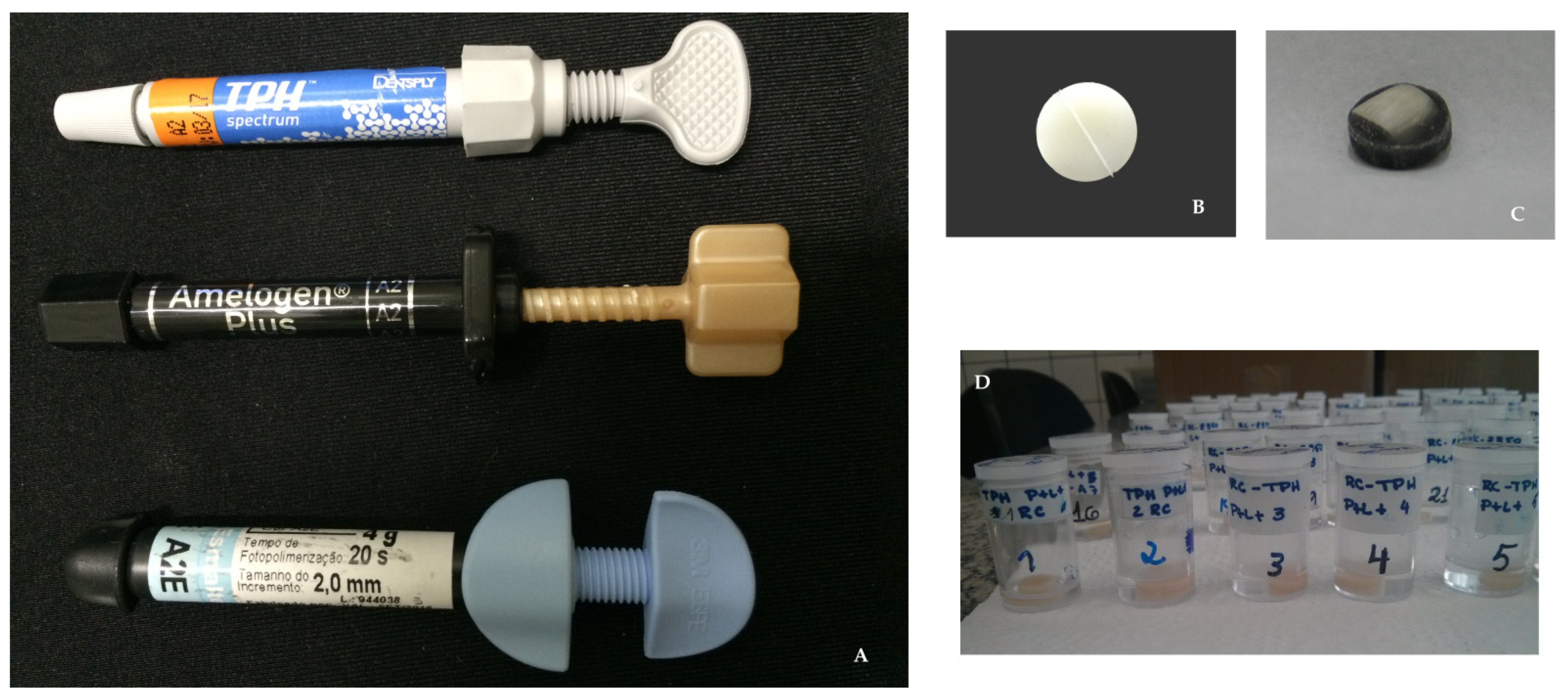
2.3. Materials Used to Create the Test Specimens
2.4. Creation of the Test Specimens
2.5. Measurement Procedures
2.6. Application of the PDI Protocol to the Test Specimens
2.7. Statistical Evaluation
3. Results
3.1. Composite Resins
3.1.1. Color Stability
3.1.2. Surface Microhardness Analysis
3.1.3. Surface Roughness Analysis
3.2. Bovine Tooth Enamel
3.2.1. Color Stability
3.2.2. Surface Microhardness Analysis
3.2.3. Surface Roughness Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dobrzański, L.A.; Dobrzański, L.B.; Dobrzańska-Danikiewicz, A.D.; Dobrzańska, J. The Concept of Sustainable Development of Modern Dentistry. Processes 2020, 8, 1605. [Google Scholar] [CrossRef]
- Haikal, D.S.; Martins, A.M.; Aguiar, P.H.; Silveira, M.F.; de Paula, A.M.; Ferreira, E.F. Access to information on oral hygiene and tooth loss due to caries among adults. Cien. Saúde Colet. 2014, 19, 287–300. [Google Scholar] [CrossRef][Green Version]
- Angker, L.; Nockolds, C.; Swain, M.V.; Kilpatrick, N. Correlating the mechanical properties to the mineral content of carious dentine—A comparative study using an ultra-micro indentation system (UMIS) and SEM-BSE signals. Arch. Oral Biol. 2004, 49, 369–378. [Google Scholar] [CrossRef]
- Verardia, G.; Cencib, M.S.; Maskeb, T.T.; Webberc, B.; Dos Santos, L.R. Antiseptics and microcosm biofilm formation on titanium surfaces. Braz. Oral Res. 2016, 30, 30. [Google Scholar] [CrossRef]
- FDI World Dental Federation. The Challenge of Oral Disease—A Call for Global Action, 2nd ed.; Myriad Editions: Brighton, UK, 2015. [Google Scholar]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of untreated caries: A systematic review and metaregression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Leites, A.C.B.R.; Pinto, M.B.; Souza, E.R. Aspectos microbiológicos da cárie dental. Salusvita 2006, 25, 135–148. [Google Scholar]
- Marsh, P.D. Dental Plaque: Biological significance of a biofilm and community life-style. J. Clin. Periodontol. 2005, 32, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Wunder, A.; Auschill, T.M.; Follo, M.; Braun, G.; Hellwig, E.; Arweiler, N.B. The in vivo dynamics of Streptococcus spp., Actinomyces naeslundii, Fusobacterium nucleatum and Veillonella spp. in dental plaque biofilm as analysed by five-colour multiplex fluorescence in situ hybridization. J. Med. Microbiol. 2007, 56, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.; Matsubara, V.H. Normal Oral Flora and the Oral Ecosystem. Dent. Clin. N. Am. 2017, 61, 199–215. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guar-nizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Watt, R.G.; Daly, B.; Allison, P.; Macpherson, L.M.D.; Venturelli, R.; Listl, S.; Weyant, R.J.; Mathur, M.R.; Guarni-zo-Herreño, C.C.; Celeste, R.K.; et al. Ending the neglect of global oral health: Time for radical action. Lancet 2019, 394, 261–272. [Google Scholar] [CrossRef]
- Fejerskov, O. Changing paradigms in concepts on dental caries: Consequences for oral health care. Caries Res. 2004, 38, 182–191. [Google Scholar] [CrossRef]
- Banerjee, A.; Doméjean, S. The contemporary approach to tooth preservation: Minimum intervention (MI) caries management in general practice. Prim. Dent. J. 2013, 2, 30–37. [Google Scholar] [CrossRef]
- Thomas, L.; Maillard, J.Y.; Lambert, R.J.; Russell, A.D. Development of resistance to chlorhexidine diacetate in Pseudomonas aeruginosa and the effect of a “residual” concentration. J. Hosp. Infect. 2000, 46, 297–303. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Kampf, G. Acquired resistance to chlorhexidine—Is it time to establish an ‘antiseptic stewardship’ initiative? J. Hosp. Infect. 2016, 94, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, 383–392. [Google Scholar] [CrossRef]
- Kellesarian, S.V.; Qayyum, F.; de Freitas, P.C.; Akram, Z.; Javed, F. Is antimicrobial photodynamic therapy a useful therapeutic protocol for oral decontamination? A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2017, 20, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Soukos, N.S.; Goodson, J.M. Photodynamic therapy in the control of oral biofilms. Periodontology 2000, 55, 143–166. [Google Scholar] [CrossRef]
- Araújo, N.C.; Fontana, C.R.; Bagnato, V.S.; Gerbi, M.E.M. Photodynamic effects of curcumin against cariogenic pathogens. Photomed. Las. Surg. 2012, 30, 393–399. [Google Scholar] [CrossRef]
- Kato, T.I.; Prates, R.A.; Sabino, C.P.; Fuchs, B.B.; Tegos, G.P.; Mylonakis, E.; Hamblin, M.R.; Ribeiro, M.S. Antimicrobial photodynamic inactivation inhibits Candida albicans virulence factors and reduces in vivo pathogenicity. Antimicrob. Agents Chemother. 2013, 57, 445–451. [Google Scholar] [CrossRef]
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef]
- Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef]
- Araújo, N.C.; Fontana, C.R.; Bagnato, V.S.; Gerbi, M.E.M. Photodynamic antimicrobial therapy of curcumin in biofilms and carious dentine. Lasers Med. Sci. 2014, 29, 629–635. [Google Scholar] [CrossRef]
- Panariello, B.H.D.; Klein, M.I.; Alves, F.; Pavarina, A.C. DNase increases the efficacy of antimicrobial photodynamic therapy on Candida albicans biofilms. Photodiagnosis Photodyn. Ther. 2019, 27, 124–131. [Google Scholar] [CrossRef]
- Baltazar, L.M.; Ray, A.; Santos, D.A.; Cisalpino, P.S.; Friedman, A.J.; Nosanchuk, J.D. Antimicrobial photodynamic therapy: An effective alternative approach to control fungal infections. Front. Microbiol. 2015, 6, 202. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, A.; Ebadian, B.; Nosouhian, S. Role of laser or photodynamic therapy in treatment of denture stomatitis: A systematic review. J. Prosthet. Dent. 2018, 120, 498–505. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, P.R.; Calixto, G.M.F.; Araújo, V.H.S.; Sato, M.R.; Rodero, C.F.; Oshiro-Junior, J.A.; Bauab, T.M.; Chorilli, M. In vivo study of hypericin-loaded poloxamer-based mucoadhesive in situ gelling liquid crystalline precursor system in a mice model of vulvovaginal candidiasis. Med. Mycol. 2021, in press. [Google Scholar] [CrossRef]
- Rodero, C.F.; Calixto, G.M.F.; Dos Santos, K.C.; Sato, M.R.; Ramos, M.A.S.; Miró, M.S.; Rodríguez, E.; Vigezzi, C.; Bauab, T.M.; Sotomayor, C.E.; et al. Curcumin-Loaded Liquid Crystalline Systems for Controlled Drug Release and Improved Treatment of Vulvovaginal Candidiasis. Mol. Pharm. 2018, 15, 4491–4504. [Google Scholar] [CrossRef] [PubMed]
- Salmazi, R.; Calixto, G.; Bernegossi, J.; Ramos, M.A.; Bauab, T.M.; Chorilli, M. A curcumin-loaded liquid crystal precursor mucoadhesive system for the treatment of vaginal candidiasis. Int. J. Nanomed. 2015, 10, 4815–4824. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Duque, C.; Aida, K.L.; Dos Santos, V.R.; Massunari, L.; Chorilli, M. Development and characterization of p1025-loaded bioadhesive liquid-crystalline system for the prevention of Streptococcus mutans biofilms. Int. J. Nanomed. 2017, 13, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.M.F.; Victorelli, F.D.; Dovigo, L.N.; Chorilli, M. Polyethyleneimine and Chitosan Polymer-Based Mucoadhesive Liquid Crystalline Systems Intended for Buccal Drug Delivery. AAPS PharmSciTech 2018, 19, 820–836. [Google Scholar] [CrossRef] [PubMed]
- Ameri, H.; Chasteen, J.E.; Ghavamnasiri, M.; Torkzadeh, M. Effect of a bleaching agent on the color stability of a microhybrid composite resin. Rev. Clín. Pesq. Odontol. 2010, 6, 215–221. [Google Scholar] [CrossRef]
- de Andrade, I.C.G.B.; Basting, R.T.; Rodrigues, J.A.; do Amaral, F.L.B.; Turssi, C.P.; França, F.M.G. Microhardness and color monitoring of nanofilled resin composite after bleaching and staining. Eur. J. Dent. 2014, 8, 160–165. [Google Scholar] [CrossRef]
- Fontes, S.T.; Fernández, M.R.; de Moura, C.M.; Meireles, S.S. Color stability of a nanofill composite: Effect of different immersion media. J. Appl. Oral Sci. 2009, 17, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Pirolo, R.; Mondelli, R.F.L.; Correr, G.M.; Gonzaga, C.C.; Furuse, A.Y. Effect of coffee and a cola-based soft drink on the color stability of bleached bovine incisors considering the time elapsed after bleaching. J. Appl. Oral Sci. 2014, 22, 534–540. [Google Scholar] [CrossRef]
- Sadyrin, E.; Swain, M.; Mitrin, B.; Rzhepakovsky, I.; Nikolaev, A.; Irkha, V.; Yogina, D.; Lyanguzov, N.; Maksyukov, S.; Aizikovich, S. Characterization of enamel and dentine about a white spot lesion: Mechanical properties, mineral density, microstructure and molecular composition. Nanomaterials 2020, 10, 1889. [Google Scholar] [CrossRef]
- Huang, T.T.; He, L.H.; Darendeliler, M.A.; Swain, M.V. Correlation of mineral density and elastic modulus of natural enamel white spot lesions using X-ray microtomography and nanoindentation. Acta Biomater. 2010, 6, 4553–4559. [Google Scholar] [CrossRef]
- Donassollo, S.H.; Fabris, C.; Gagliola, M.; Kerber, I.; Caetano, V.; Carboni, V.; Salas, M.M.S.; Donassollo, T.A.; Demarco, F.F. In situ and in vitro effects of two bleaching treatments on human enamel hardness. Braz. Dent. J. 2016, 27, 56–59. [Google Scholar] [CrossRef]
- Bernegossi, J.; Calixto, G.M.; Sanches, P.R.; Fontana, C.R.; Cilli, E.M.; Garrido, S.S.; Chorilli, M. Peptide KSL-W-Loaded Mucoadhesive Liquid Crystalline Vehicle as an Alternative Treatment for Multispecies Oral Biofilm. Molecules 2015, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.L.B.M.; Garcia, P.P.N.S.; dos Santos, P.A.; Campos, J.A.D.B. Surface roughness and hardness of a composite resin: Influence of finishing and polishing and immersion methods. Mater. Res. 2010, 13, 409–415. [Google Scholar] [CrossRef]
- Abouassi, T.; Wolkewitz, M.; Hahn, P. Effect of carbamide peroxide and hydrogen peroxide on enamel surface: An in vitro study. Clin. Oral Investig. 2011, 15, 673–680. [Google Scholar] [CrossRef]
- Santezi, C.; Reina, B.D.; de Annunzio, S.R.; Calixto, G.; Chorilli, M.; Dovigo, L.N. Photodynamic Potential of Curcumin in Bioadhesive Formulations: Optical characteristics and antimicrobial effect against biofilms. Photodiagnosis Photodyn. Ther. 2021, 35, 102416, in press. [Google Scholar] [CrossRef]
- Ribeiro, B.C.L.; Boaventura, J.M.C.; Gaião, U.; Saad, J.R.C.; Candido, M.S.M. Efeito de fontes de luz na microdureza de resinas compostas. RGO 2011, 59, 229–236. [Google Scholar]
- Spectrum TPH DENTSPLY. Available online: http://www.dentsply.com.br/bulas/diretory/T/tph-spectrum.pdf (accessed on 1 October 2016).
- Villalta, P.; Lu, H.; Okte, Z.; Garcia-Godoy, F.; Powers, J.M. Effects of staining and bleaching on color change of dental composite resins. J. Prosthet. Dent. 2006, 95, 137–142. [Google Scholar] [CrossRef]
- Mendes, A.P.K.F.; Barceleiro, M.O.; dos Reis, R.S.A.; Bonato, L.L.; Dias, K.R.H.C. Changes in surface roughness and color stability of two composites caused by different bleaching agents. Braz. Dent. J. 2012, 23, 659–666. [Google Scholar] [CrossRef]
- Gurdal, P.; Akdeniz, B.G.; Hakan Sen, B. The effects of mouthrinses on microhardness and colour stability of aesthetic restorative materials. J. Oral Rehabil. 2002, 29, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.; Tserki, V.; Papanatasiou, G. Study of water absorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials 2003, 24, 655–665. [Google Scholar] [CrossRef]
- Iazzetti, G.; Burgess, J.O.; Gardiner, D.; Ripps, A. Color stability of fluoride-containing restorative materials. Oper. Dent. 2000, 25, 520–525. [Google Scholar] [PubMed]
- AlQahtani, M.Q. The effect of a 10% carbamide peroxide bleaching agent on the microhardness of four types of direct resin based restorative materials. Oper. Dent. 2013, 38, 316–323. [Google Scholar] [CrossRef]
- Pitacas, H.M.G.; Cavalheiro, A.; Coito, C.; Silva, A.; Eira, R.; Lopes, M. Effect of external tooth bleaching on the surface of resin composites—An in vitro study. Rev. Port. Estomatol. Med. Dent. Cir. Maxilofac. 2015, 56, 149–155. [Google Scholar] [CrossRef]
- Durner, J.; Obermaier, J.; Ilie, N. Investigation of different bleaching conditions on the amount of elutable substances from nano-hybrid composites. Dent. Mater. 2014, 30, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Parreiras, S.O.; Vianna, P.; Kossatz, S.; Loguercio, A.D.; Reis, A. Effects of light activated in-office bleaching on permeability, microhardness, and mineral content of enamel. Oper. Dent. 2014, 39, 225–230. [Google Scholar] [CrossRef] [PubMed]




| Resin | Composition | Manufacturer | ||
|---|---|---|---|---|
| Organic Content | Filler Particles | Other Components | ||
| Amelogen®Plus (AML) | Bis-GMA | Non-specified particle measuring 0.7 µm in size | Not specified | Ultradent Products, Inc. South Jordan, UT, USA |
| Spectrum TPH resin (TPH) | Urethane-modified Bis-GMA | Silanized barium glass, aluminum borosilicate and silanized pyrolytic silica. Average size: 1.30 µm | Camphorquinone as a photoinitiator, EDAB, butylated hydroxytoluene, mineral dyes | DENTSPLY Ind. E Com. Ltd.a—Petrópolis, Rio de Janeiro, Brazil |
| Filtek™ Z350 Supreme Ultra Universal (Z350) | Bis-GMA, UDMA, TEGDMA, and Bis-EMA | Silica, zirconia, and zirconia/silica clusters | Not specified | 3M EPSE Dental Products, St. Paul, MN, USA |
| Composite Resin | Treatment | Evaluation Time | |
|---|---|---|---|
| Immediate | 60 Days | ||
| TPH | PDI | 0.82 ± 0.31Aa | 1.19 ± 0.37CDa |
| LCP | 0.52 ± 0.24Aa | 1.01 ± 0.37BCDb | |
| NC | 0.58 ± 0.30Aa | 0.80 ± 0.33ABCDa | |
| Z350 | PDI | 1.12 ± 0.58Ba | 0.70 ± 0.45ABCa |
| LCP | 0.54 ± 0.30Aa | 0.57 ± 0.16Aa | |
| NC | 0.45 ± 0.22Aa | 0.66 ± 0.32ABa | |
| AML | PDI | 0.88 ± 0.45Aa | 1.12 ± 0.73BCDb |
| LCP | 0.61 ± 0.27Ba | 1.22 ± 0.54Da | |
| NC | 0.52 ± 0.26Ba | 1.10 ± 0.51BCDa | |
| Composite Resin | Treatment | Evaluation Time | |
|---|---|---|---|
| Before | After | ||
| TPH | PDI | 76.37 ± 5.82Aa | 75.00 ± 9.12Aa |
| LCP | 75.97 ± 4.70Aa | 67.02 ± 8.45Aba | |
| NC | 76.91 ± 7.73Aa | 69.88 ± 6.74Aa | |
| Z350 | PDI | 85.17 ± 7.36Aa | 60.67 ± 18.34Bb |
| LCP | 83.52 ± 4.36Aa | 76.12 ± 7.34Aa | |
| NC | 83.36 ± 4.50Aa | 74.81 ± 8.18Aa | |
| AML | PDI | 54.60 ± 6.17Ba | 59.33 ± 13.36Ca |
| LCP | 54.33 ± 3.64Ba | 51.09 ± 9.85Ca | |
| NC | 53.29 ± 4.35Ba | 47.45 ± 3.46Ca | |
| Composite Resin | Treatment | Evaluation Time | |
|---|---|---|---|
| Before | After | ||
| TPH | PDI | 0.095 ± 0.02ABa | 0.093 ± 0.02ABa |
| LCP | 0.096 ± 0.01Ba | 0.094 ± 0.01Ba | |
| NC | 0.099 ± 0.02Ba | 0.093 ± 0.01Ba | |
| Z350 | PDI | 0.108 ± 0.03BCa | 0.113 ± 0.04BCa |
| LCP | 0.098 ± 0.02BCa | 0.116 ± 0.03BCa | |
| NC | 0.101 ± 0.02Ba | 0.104 ± 0.02Ba | |
| AML | PDI | 0.139 ± 0.09BCa | 0.110 ± 0.07BCa |
| LCP | 0.162 ± 0.11Ca | 0.171 ± 0.11Ca | |
| NC | 0.078 ± 0.03Aa | 0.078 ± 0.02Aa | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reina, B.D.; Santezi Neto, C.; Garcia, P.P.N.S.; Chorilli, M.; Calixto, G.M.F.; Dovigo, L.N. Bioadhesive Curcumin-Mediated Photodynamic Inactivation and Its Potential to Cause Undesirable Effects on Dental and Restorative Surfaces. Pharmaceutics 2021, 13, 1458. https://doi.org/10.3390/pharmaceutics13091458
Reina BD, Santezi Neto C, Garcia PPNS, Chorilli M, Calixto GMF, Dovigo LN. Bioadhesive Curcumin-Mediated Photodynamic Inactivation and Its Potential to Cause Undesirable Effects on Dental and Restorative Surfaces. Pharmaceutics. 2021; 13(9):1458. https://doi.org/10.3390/pharmaceutics13091458
Chicago/Turabian StyleReina, Bárbara Donadon, Carolina Santezi Neto, Patrícia Petromilli Nordi Sasso Garcia, Marlus Chorilli, Giovana Maria Fioramonti Calixto, and Lívia Nordi Dovigo. 2021. "Bioadhesive Curcumin-Mediated Photodynamic Inactivation and Its Potential to Cause Undesirable Effects on Dental and Restorative Surfaces" Pharmaceutics 13, no. 9: 1458. https://doi.org/10.3390/pharmaceutics13091458
APA StyleReina, B. D., Santezi Neto, C., Garcia, P. P. N. S., Chorilli, M., Calixto, G. M. F., & Dovigo, L. N. (2021). Bioadhesive Curcumin-Mediated Photodynamic Inactivation and Its Potential to Cause Undesirable Effects on Dental and Restorative Surfaces. Pharmaceutics, 13(9), 1458. https://doi.org/10.3390/pharmaceutics13091458








