A Novel Approach to Assess the Potency of Topical Corticosteroids
Abstract
:1. Introduction
Correlation of ED50 and Emax with Potency Classification
2. Materials and Methods
2.1. Materials
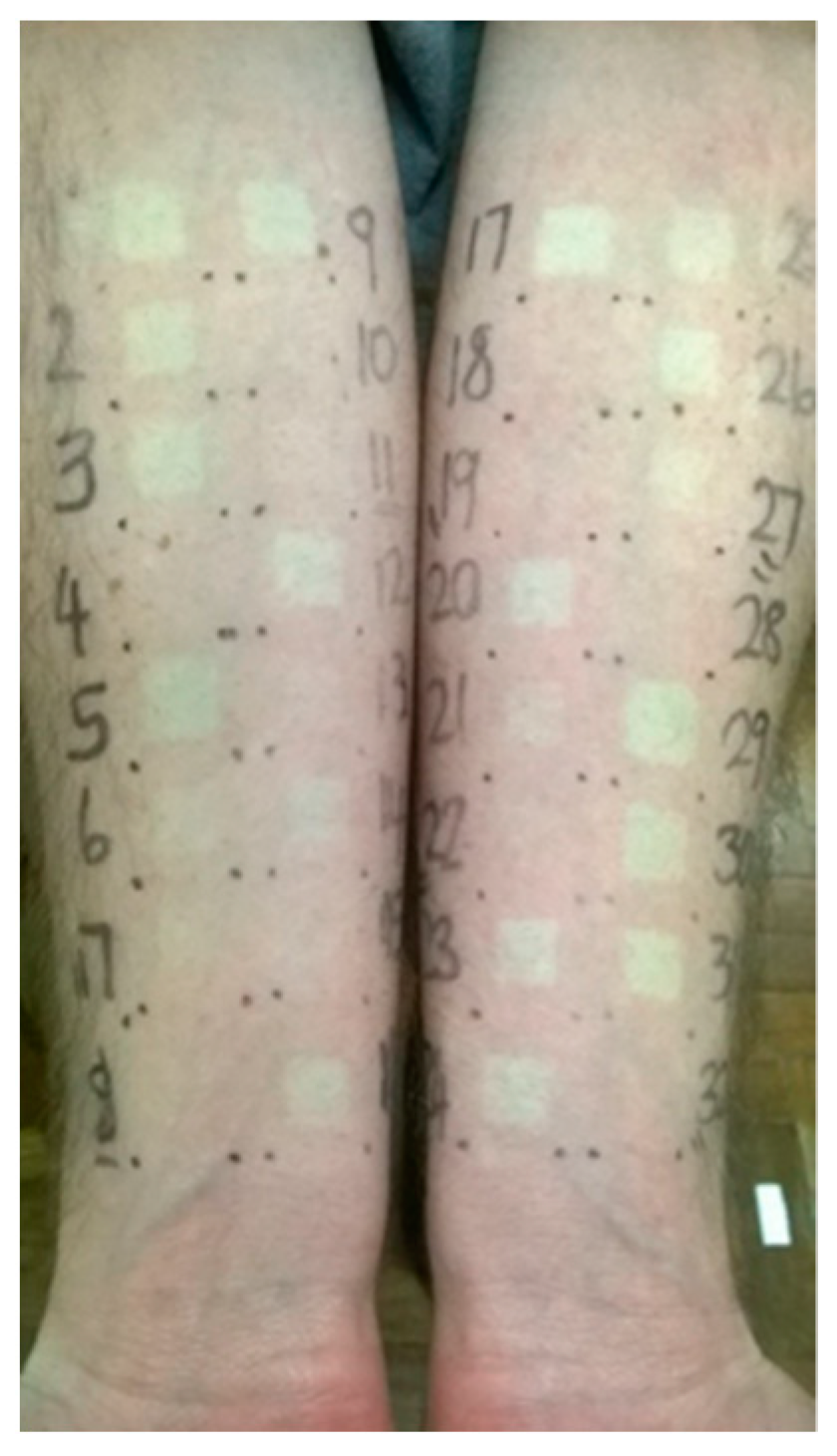
2.2. Experimental Design
2.3. Statistical Analysis
3. Results
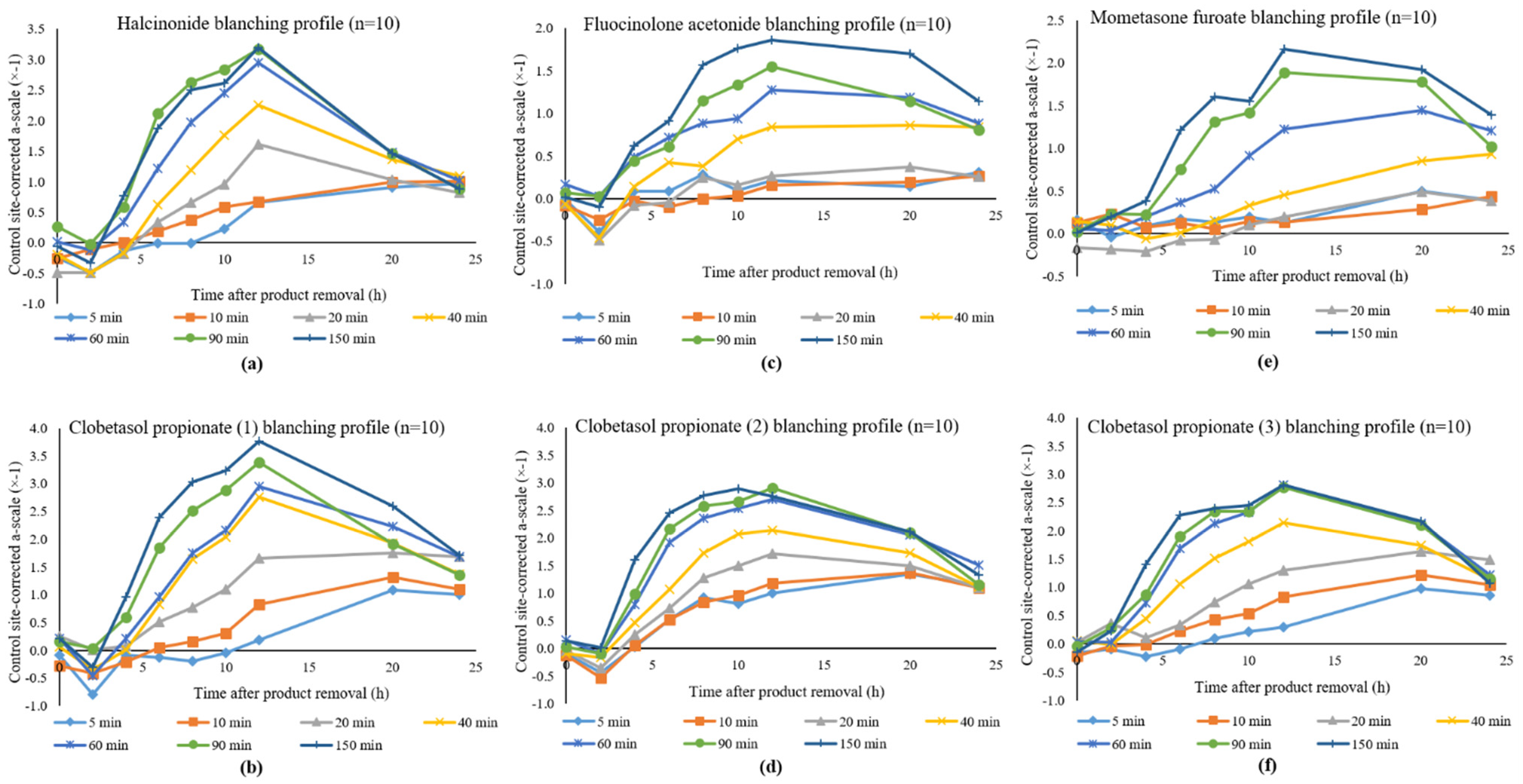
3.1. Study 1: Halcinonide vs. Clobetasol Propionate (1)
3.2. Study 2: Fluocinolone Acetonide vs. Clobetasol Propionate (2)
3.3. Study 3: Mometasone Furoate vs. Clobetasol Propionate (3)
3.4. Comparisons of Topical Corticosteroids
3.4.1. ANOVA Comparisons
3.4.2. Pairwise Comparisons
4. Discussion
Potency Rankings of Topical Corticosteroids Based on Emax Values
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Humbert, P.; Guichard, A. The topical corticosteroid classification called into question: Towards a new approach. Exp. Dermatol. 2015, 24, 393–395. [Google Scholar] [CrossRef]
- Cornell, R.C.; Stoughton, R.B. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch. Dermatol. 1985, 121, 63–67. [Google Scholar] [CrossRef]
- Topical Steroid. Available online: https://dermnetnz.org/topics/topical-steroid/ (accessed on 26 March 2021).
- Green, C.; Colquitt, J.L.; Kirby, J.; Davidson, P.; Payne, E. Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: A systematic review and economic evaluation. Health Technol. Assess. 2004, 8, 1–120. [Google Scholar] [CrossRef]
- Fusaro, R.M. Flexible classification for the clinical potency of topical corticosteroid proprietaries. Drug Intell. Clin. Pharm. 1988, 22, 412–415. [Google Scholar] [CrossRef]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15. [Google Scholar] [CrossRef]
- Wiedersberg, S.; Leopold, C.S.; Guy, R.H. Bioavailability and bioequivalence of topical glucocorticoids. Eur. J. Pharm. Biopharm. 2008, 68, 453–466. [Google Scholar] [CrossRef]
- Camisa, C. Corticosteroids. In Handbook of Psoriasis; Camisa, C., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2004; pp. 139–157. ISBN 9780470750704. [Google Scholar]
- Stoughton, R.B. Principles of topical steroid treatment in skin diseases. In Clinical Topics in Internal Medicine; Tisi, R., Ranney, H., Eds.; Williams & Wilkins: Baltimore, MD, USA, 1982; pp. 163–170. [Google Scholar]
- Cornell, R.C. Clinical trials of topical corticosteroids in psoriasis: Correlations with the vasoconstrictor assay. Int. J. Dermatol. 1992, 31, 38–40. [Google Scholar] [CrossRef]
- Hepburn, D.J.; Aeling, J.L.; Weston, W.L. A reappraisal of topical steroid potency. Pediatr. Dermatol. 1996, 13, 239–245. [Google Scholar] [CrossRef]
- Burkholder, B. Topical corticosteroids: An update. Curr. Probl. Dermatol. 2000, 12, 222–225. [Google Scholar] [CrossRef]
- Ference, J.D.; Last, A.R. Choosing topical corticosteroids. Am. Fam. Physician 2009, 79, 135–140. [Google Scholar]
- McKenzie, A.W.; Atkinson, R.M. Topical Activities of Betamethasone Esters in Man. Arch. Dermatol. 1964, 89, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.B.; Thompson, C.; McCormack, J.R.; Guin, J.D. Bioequivalence (bioavailability) of generic topical corticosteroids. J. Am. Acad. Dermatol. 1989, 20, 791–796. [Google Scholar] [CrossRef]
- Caron, D.; Queille-Roussel, C.; Shah, V.P.; Schaefer, H. Correlation between the drug penetration and the blanching effect of topically applied hydrocortisone creams in human beings. J. Am. Acad. Dermatol. 1990, 23, 458–462. [Google Scholar] [CrossRef]
- Stoughton, R.B.; Wullich, K. The same glucocorticoid in brand-name products. Does increasing the concentration result in greater topical biologic activity? Arch. Dermatol. 1989, 125, 1509–1511. [Google Scholar] [CrossRef]
- Child, K.J.; English, A.F.; Gilbert, H.G.; Hewitt, A.; Woollett, E.A. Vasoconstrictor and systemic activities of topical steroids. Arch. Dermatol. 1968, 97, 407–410. [Google Scholar] [CrossRef]
- Stoughton, R.B. Bioassay system for formulations of topically applied glucocorticosteroids. Arch. Dermatol. 1972, 106, 825–827. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, A.W. Percutaneous Absorption of Steroids. Arch. Dermatol. 1962, 86, 611–614. [Google Scholar] [CrossRef]
- Place, V.A.; Velazquez, J.G.; Burdick, K.H. Precise evaluation of topically applied corticosteroid potency. Modification of the Stoughton-McKenzie assay. Arch. Dermatol. 1970, 101, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Haigh, J.M.; Kanfer, I.; Meyer, E.; Smith, E. Relative potencies of topical corticosteroid formulations. Br. J. Dermatol. 1985, 113, 502–503. [Google Scholar] [CrossRef]
- Coldman, M.F.; Lockerbie, L.; Laws, E.A. The evaluation of several topical corticosteroid preparations in the blanching test. Br. J. Dermatol. 1971, 85, 381–387. [Google Scholar] [CrossRef]
- McKenzie, A.W.; Stoughton, R.B. Method for Comparing Percutaneous Absorption of Steroids. Arch. Dermatol. 1962, 86, 608–610. [Google Scholar] [CrossRef]
- Allenby, C.F.; Sparkes, C.G. Halogenation and topical corticosteroids: A comparison between the 17-butyrate esters of hydrocortisone and clobetasone in ointment bases. Br. J. Dermatol. 1981, 104, 179–183. [Google Scholar] [CrossRef]
- Topical Corticosteroids. In British National Formulary; British Medical Association and Royal Pharmaceutical Society of Great Britain: London, UK, 2019; pp. 1241–1242.
- Senyigit, T.; Ozer, O. Corticosteroids for skin delivery: Challenges and new formulation opportunities. In Glucocorticoids-New Recognition of Our Familiar Friend; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Kirkland, R.; Pearce, D.J.; Balkrishnan, R.; Feldman, S.R. Critical factors determining the potency of topical corticosteroids. J. Dermatolog. Treat. 2006, 17, 133–135. [Google Scholar] [CrossRef]
- Keida, T.; Hayashi, N.; Kawashima, M. Application of the Food and Drug Administration (FDA) bioequivalent guidance of topical dermatological corticosteroid in yellow-skinned Japanese population: Validation study using a chromameter. J. Dermatol. 2006, 33, 684–691. [Google Scholar] [CrossRef]
- Singh, G.J.; Adams, W.P.; Lesko, L.J.; Shah, V.P.; Molzon, J.A.; Williams, R.L.; Pershing, L.K. Development of in vivo bioequivalence methodology for dermatologic corticosteroids based on pharmacodynamic modeling. Clin. Pharmacol. Ther. 1999, 66, 346–357. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Guidance for Industry: Topical Dermatologic Corticosteroids—In Vivo Bioequivalence; FDA: Silver Spring, MD, USA, 1995. Available online: https://www.fda.gov/media/70931/download (accessed on 26 March 2021).
- Kanfer, I.; Maibach, H. The Vasoconstrictor Assay (VCA): Then and Now. In Drug Delivery Approaches: Perspectives from Pharmacokinetics and Pharmacodynamics; Berner, B., Gordi, T., Benson, H., Roberts, M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021. [Google Scholar]
- Kanfer, I.; Tettey-Amlalo, R.N.O.; Au, W.L.; Hughes-Formella, B. Assessment of topical dosage forms intended for local or regional activity. In Generic Drug Product Development: Specialty Dosage Forms; Shargel, L., Kanfer, I., Eds.; Informa Healthcare USA, Inc.: New York, NY, USA, 2016; pp. 54–103. [Google Scholar]
- Kanfer, I. Methods for the Assessment of Bioequivalence of Topical Dosage Forms: Correlations, Optimization Strategies, and Innovative Approaches. In Topical Drug Bioavailability, Bioequivalence and Penetration; Shah, V.P., Maibach, H.I., Jenner, J., Eds.; Springer: New York, NY, USA, 2014; pp. 113–151. [Google Scholar]
- Holford, N.H.; Sheiner, L.B. Understanding the dose-effect relationship: Clinical application of pharmacokinetic-pharmacodynamic models. Clin. Pharmacokinet. 1981, 6, 429–453. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. SAS/STAT 9.1: User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2004; ISBN 9781590472439. [Google Scholar]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
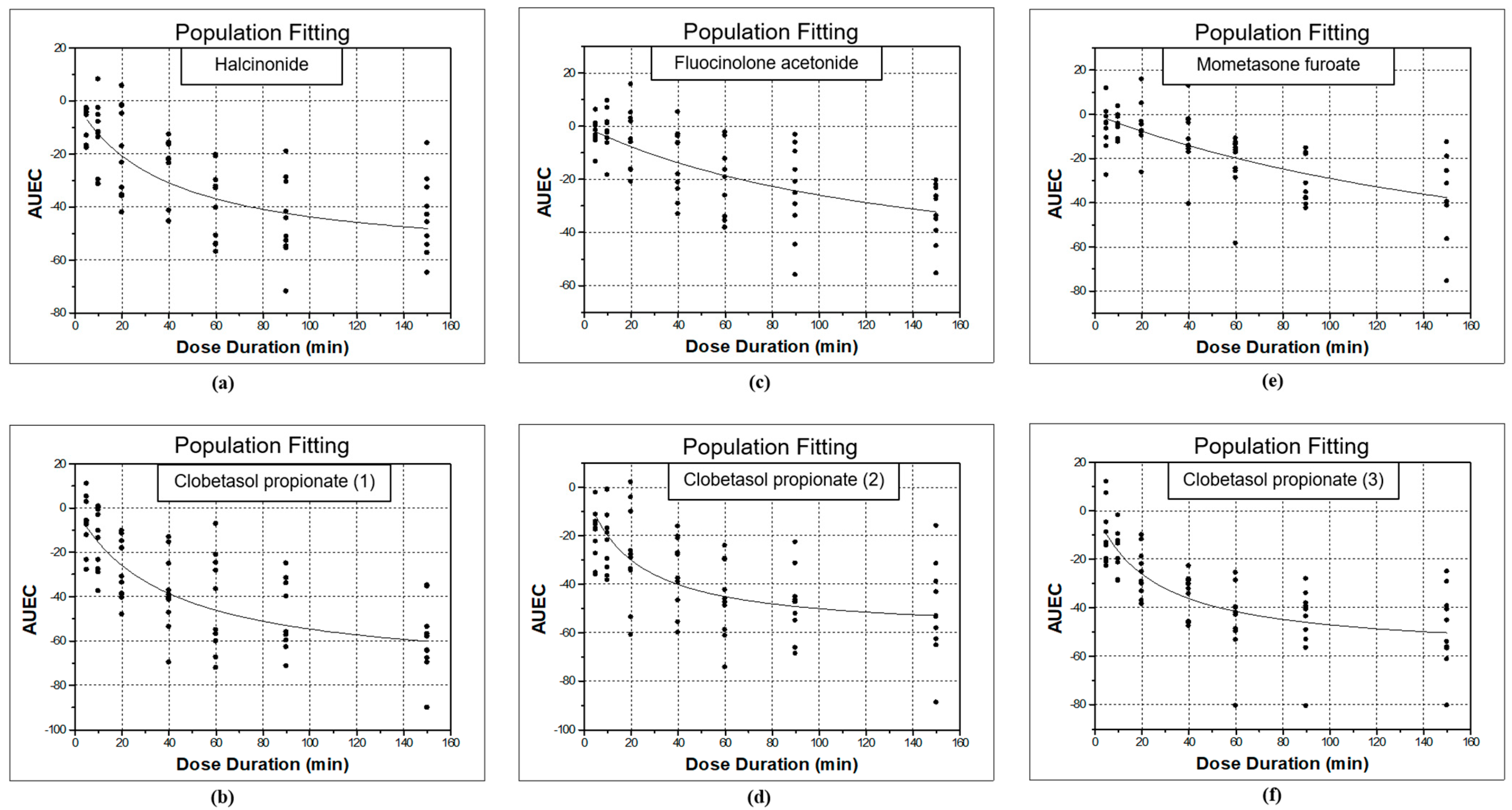
| Study | Topical Corticosteroid | ED50 | Emax | AIC |
|---|---|---|---|---|
| 1 | Clobetasol propionate (1) | 38.10 | −75.44 ± 12.89 | 3.93 |
| 2 | Clobetasol propionate (2) | 19.83 | −60.05 ± 13.31 | 3.79 |
| 3 | Clobetasol propionate (3) | 24.83 | −58.79 ± 15.65 | 3.57 |
| 1 | Halcinonide | 38.06 | −60.31 ± 3.75 | 3.84 |
| 2 | Fluocinolone acetonide | 142.46 | −62.84 ± 5.98 | 3.64 |
| 3 | Mometasone furoate | 225.77 | −94.45 ± 0.21 | 3.73 |
| Subject | Clobetasol Propionate (1) | Halcinonide |
|---|---|---|
| 1 | −80.66 | −66.45 |
| 2 | −66.29 | −57.89 |
| 3 | −91.31 | −59.61 |
| 4 | −68.67 | −56.39 |
| 5 | −74.33 | −60.27 |
| 6 | −50.49 | −56.90 |
| 7 | −93.04 | −63.16 |
| 8 | −83.35 | −55.28 |
| 9 | −66.84 | −63.06 |
| 10 | −79.41 | −64.10 |
| Subject | Observed Emax Values | Normalized Emax Values | ||
|---|---|---|---|---|
| Clobetasol Propionate (2) | Fluocinolone Acetonide | Clobetasol Propionate (2) | Fluocinolone Acetonide | |
| 1 | −56.98 | −59.20 | −72.37 | −74.59 |
| 2 | −88.12 | −66.48 | −103.51 | −81.87 |
| 3 | −39.75 | −58.76 | −55.13 | −74.15 |
| 4 | −68.04 | −63.66 | −83.43 | −79.05 |
| 5 | −62.63 | −59.23 | −78.02 | −74.62 |
| 6 | −56.50 | −55.39 | −71.89 | −70.78 |
| 7 | −68.51 | −73.88 | −83.90 | −89.27 |
| 8 | −58.31 | −59.44 | −73.70 | −74.83 |
| 9 | −56.13 | −71.39 | −71.52 | −86.78 |
| 10 | −45.52 | −60.98 | −60.91 | −76.37 |
| Subject | Observed Emax Values | Normalized Emax Values | ||
|---|---|---|---|---|
| Clobetasol Propionate (3) | Mometasone Furoate | Clobetasol Propionate (3) | Mometasone Furoate | |
| 1 | −69.72 | −94.78 | −86.36 | −111.43 |
| 2 | −61.19 | −94.50 | −77.83 | −111.15 |
| 3 | −35.84 | −94.30 | −52.48 | −110.95 |
| 4 | −54.75 | −94.23 | −71.39 | −110.87 |
| 5 | −93.29 | −94.83 | −109.93 | −111.47 |
| 6 | −49.71 | −94.27 | −66.36 | −110.91 |
| 7 | −65.43 | −94.48 | −82.07 | −111.13 |
| 8 | −44.62 | −94.25 | −61.27 | −110.89 |
| 9 | −54.50 | −94.36 | −71.15 | −111.00 |
| 10 | −58.90 | −94.48 | −75.54 | −111.12 |
| Topical Corticosteroid | N | Mean (Emax) | Tukey Grouping 1 |
|---|---|---|---|
| Halcinonide | 10 | −60.31 ± 3.75 | A |
| Clobetasol propionate | 30 | −75.44 ± 13.51 | B |
| Fluocinolone acetonide | 10 | −78.23 ± 5.98 | B |
| Mometasone furoate | 10 | −111.09 ± 0.21 | C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zvidzayi, M.; Rath, S.; Bon, C.; Abboo, S.; Kanfer, I. A Novel Approach to Assess the Potency of Topical Corticosteroids. Pharmaceutics 2021, 13, 1456. https://doi.org/10.3390/pharmaceutics13091456
Zvidzayi M, Rath S, Bon C, Abboo S, Kanfer I. A Novel Approach to Assess the Potency of Topical Corticosteroids. Pharmaceutics. 2021; 13(9):1456. https://doi.org/10.3390/pharmaceutics13091456
Chicago/Turabian StyleZvidzayi, Michael, Seeprarani Rath, Charles Bon, Sagaran Abboo, and Isadore Kanfer. 2021. "A Novel Approach to Assess the Potency of Topical Corticosteroids" Pharmaceutics 13, no. 9: 1456. https://doi.org/10.3390/pharmaceutics13091456
APA StyleZvidzayi, M., Rath, S., Bon, C., Abboo, S., & Kanfer, I. (2021). A Novel Approach to Assess the Potency of Topical Corticosteroids. Pharmaceutics, 13(9), 1456. https://doi.org/10.3390/pharmaceutics13091456








