Abstract
Over the past decade, ROS-sensitive formulations have been widely used in atherosclerosis applications such as ROS scavenging, drug delivery, gene delivery, and imaging. The intensified interest in ROS-sensitive formulations is attributed to their unique self-adaptive properties, involving the main molecular mechanisms of solubility switch and degradation under the pathological ROS differences in atherosclerosis. This review outlines the advances in the use of ROS-sensitive formulations in atherosclerosis applications during the past decade, especially highlighting the general design requirements in relation to biomedical functional performance.
1. Introduction
Cardiovascular diseases (CVDs) are the leading cause of death and major medical problems in the world, being responsible for more than 40% of the deaths globally from noncommunicable diseases [1,2]. Atherosclerosis is responsible for the main pathological basis of the high morbidity and mortality in patients with CVDs [3]. Atherosclerosis is a localized arterial disease caused by plaque buildup in the intimal layer of arteries, and it often occurs in the aorta, carotid, coronary, and peripheral arteries, which is characterized by lipid deposition, macrophage foaming, formation of atherosclerotic plaques, thickening and hardening of the vessel wall, and triggering of subsequent cardiovascular events [4]. Although the symptoms are usually not apparent at the early stage, atherosclerosis gives rise to various severe acute events and complications including coronary heart disease, hypertension, chronic kidney, stroke, and intermittent claudication at the advanced stage [5,6,7,8]. Furthermore, atherosclerosis is considered to be an important feature in CVD epidemiology and includes two major conditions: ischemic heart disease and stroke, which were the world’s second and third leading causes of death in 2019, respectively, causing 16% and 11% of all-cause mortality. Since 2000, the number of atherosclerosis CVDs has risen by more than 2.7 million to 9.6 million deaths with a growing burden for healthcare systems [9,10].
In particular, reactive oxygen species (ROS), oxygen-derived metabolites including hydrogen peroxide (H2O2), superoxide, and hydroxyl radicals (•OH) [11,12], play a central role in atherosclerosis from its initiation and progression to acute thrombotic complications [13]. When there is a high level of blood lipids, the passage of lipids through the walls of blood vessels cannot be effectively blocked by endothelial cells, which stimulates ROS production in neutrophils [14]. Subsequently, the accumulation of ROS promotes the oxidation of low-density lipoprotein (LDL) to form the oxidized low-density lipoprotein (Ox-LDL), which is phagocytized by macrophages to produce deteriorated foam cells [15]. Moreover, the high content of ROS in atherosclerosis endothelial injury can stimulate macrophages to produce proinflammatory cytokines and chemokines, as well as intensify the inflammatory activity of macrophages [16,17]. Excessive ROS accumulation also induces vascular cell damage, inflammatory cell recruitment, lipid peroxidation, metalloproteinase activation, and extracellular matrix deposition, which collectively lead to vascular remodeling [18,19]. Therefore, ROS have vital effects on the mediation of several physiological processes, such as cell differentiation and proliferation, cellular metabolism, survival, and immune response (Figure 1) [20,21].

Figure 1.
The determinants and consequences of oxidative stress. Oxidative stress causes an imbalance in free-radical formation through multiple pathways which can trigger programmed cell death pathways, and it has been linked to clinically relevant diseases [22,23,24].
Despite great advances in diagnostic and therapeutic technologies for AS, the overall grim statistics of atherosclerosis have not significantly improved over the last decades, and they are estimated to continuingly increase [25,26]. In clinical practice, systematical blood lipid reduction and/or local anti-inflammation are the common strategies for atherosclerosis management. However, several challenges, such as low bioavailability, slow therapeutic effect, and serious side effects should be paid great attention [6,27]. To address these drawbacks, the ideal approach for effective and safe atherosclerosis management can involve target drug delivery, which has recently been a hot research topic in biomedical nano-formulations. Nano-formulations can facilitate cargo metabolism to improve the bioavailability and ease the liver burden from the high dose of drugs [28]. In particular, stimulus-sensitive nano-formulations are capable of accelerating cargo release under specific trigger conditions, such as ROS, pH, temperature, and enzymes [29,30,31]. Responding to the pathological ROS differences, desirable multifunctional carriers are able to reveal great changes in solubility and/or cleavable bonds in the molecular structures for “on-demand” cargo release at the atherosclerosis lesion [13,32,33,34,35,36,37,38]. Thus, pharmacological targeting of ROS-sensitive nano-formulations represents a promising therapeutic avenue for safe and effective atherosclerosis management.
Based on the perspectives discussed above, recent studies have shown that such nano-formulations exhibit outstanding advantages in early diagnosis, treatment, and evaluation of therapeutic effects in atherosclerosis [39], mainly featuring the following aspects: (1) strengthening the stability during blood circulation, thus extending the half-life of cargo; (2) selectively delivering cargo to the pathological vascular lesion, even pathology-related cells; (3) enhancing the local cargo concentration at the atherosclerosis lesion to meet the demands of the effective concentration window for optimizing imaging sensitivity, resolution, accuracy, and/or therapeutic efficacy; (4) personalized and precise treatment by controlling cargo release at the favorable site and “on-demand” dosing trigged by the pathological stimuli, not only improving the bioavailability, but also reducing the undesirable toxicity for the normal cells, tissue, and organs, which is particular important for chronic atherosclerosis with long-term dosing. Herein, in this review, we systematically summarize the molecular mechanisms and focus on the advanced applications of ROS-sensitive nano-formulations for potential atherosclerosis management, including scavenging excessive ROS, controlling cargo release, and imaging. Furthermore, the future challenges in development and application are also explored.
2. ROS-Sensitive Functional Molecular Structures
Studies have shown that ROS are closely related to various pathological diseases, such as atherosclerosis, cancer, diabetes, and inflammation. Therefore, ROS accumulation can be used as specific stimuli to regulate the delivery and release of drug/gene vectors [34,40,41]. In general, ROS-sensitive functional molecular structures can be roughly divided into two categories: water solubility switch and structural degradation (Figure 2). Moreover, different ROS sensitivities are easy to construct depending on the various active bonds of structures, which is beneficial for providing flexible design strategies to meet demands in intricate pathological microenvironment applications.
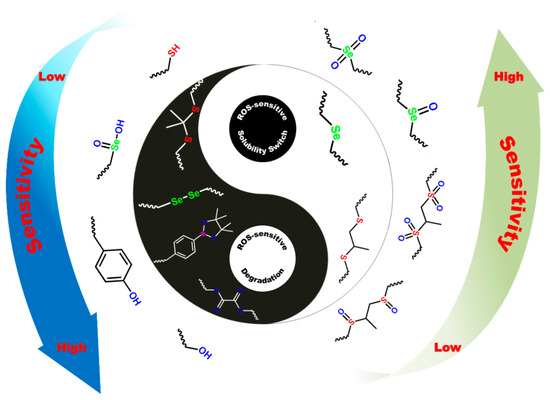
Figure 2.
Two categories of ROS-responsive structure change and corresponding sensitivity of the polymeric biomaterials under oxidation stimulus.
2.1. Water Solubility Switch
2.1.1. Polypropylene Sulfides (PPS)
Under oxidizing conditions, organic sulfur compounds change their hydrophilic or hydrophobic properties from hydrophobic sulfides to hydrophilic sulfones or sulfoxides [42,43]. In 2004, Hubbell first reported the use of polypropylene thioether as a drug delivery carrier, which was the first class of oxidization-sensitive responsive biomaterial [44]. They obtained ABA type triblock copolymers with polyethylene glycol as hydrophilic segment A and polypropylene sulfide as hydrophobic segment B via anionic ring-opening polymerization. PPS and polyethylene glycol (PEG) diblock copolymerization analogs [45] involved highly hydrophilic PEG self-assembled around the hydrophobic PPS core into nanoparticles with a particle size of about 20 nm, which could be effectively decomposed and release drugs in mouse model experiments. Because lymphocytes are able to actively utilize ROS as signaling molecules to regulate inflammation and oxidative stress, such ROS-responsive nanocarrier systems show great potential for the treatment of atherosclerosis [46].
2.1.2. Selenium-Containing Block Copolymer
Similar to the thioether groups in PPS, selenium compounds also undergo a phase change in an oxidizing environment, from their initial hydrophobic state to hydrophilic soluble selenium oxide or selenium sulfone compounds [46]. Zhang synthesized a series of selenium-containing redox-responsive nano drug carrier materials [47,48,49,50]. Furthermore, Ma developed an amphiphilic selenium-containing triblock copolymer (PEG–USE–PEG) containing hydrophobic polyurethane and hydrophilic polyethylene glycol blocks [51].
2.1.3. Polythioether Ketal
Among the multi-stimulus-responsivee materials developed today, polythioether ketal is one of the widely used ROS-sensitive structures [52,53]. Polythioether ketal polymer nanoparticles synthesized by Almutairi et al. were reported as having biological double stimulus-responsive properties and could be used as protein delivery carriers [52]. The thioether group in the material experienced a solution-change mechanism similar to PPS in response to ROS, while the ketal group had a pH response [53]. Nero red or ovalbumin were encapsulated in poly(thioether ketone) nanoparticles using a high-pressure homogenizer. When micelles enter macrophages with a high ROS concentration, the material is oxidized and dissolved, resulting in partial release of the coated material. In the presence of an acidic environment (pH = 6.5) and ROS in vitro, the polymer degrades almost completely to release the load [54].
2.2. Structural Degradation
2.2.1. Boronic Esters
Borate ester compounds are biological materials with ROS-sensitive responsiveness and degradation, which have been widely used in recent decades. It was found that borate ester is oxidized and degraded in an ROS oxidation environment [40,55,56]. For example, covalently linking borates to peptides hides the active site of matrix metalloproteinase (MMP), exposing the active part to material degradation in a highly reactive oxygen environment. Similarly, when borates are covalently conjugated to anticancer drugs and imaging agents, the active components can also be activated in specific environments with high ROS levels to enhance tracer and therapeutic effects [57].
2.2.2. Silicon
It was found that various drug molecules such as doxorubicin and dexamethasone adsorbed in porous silicon materials can be used for drug delivery therapy [58,59]. Under the stimulation of oxidation conditions, the matrix Si material is oxidized to form Si–O–Si bonds, before hydrolysis occurs to effectively release the payload. The experiment found that, in an oxidized environment simulated by peroxide nitrate (ONOO−), the fluorescence intensity expressed in the Si particles with covalently attached fluorescent dye within 24 h was 10 times that in normal saline, which effectively avoided the undesirable rapid release of the cargo [60].
2.2.3. Proline Oligomers
Since 1960, free-radical-mediated oxidation of free amino acids or polypeptides has been extensively studied [53,61]. Its importance has been repeatedly emphasized recently because of the increased ROS levels in many pathological processes that oxidize proteins [53]. It was found that amino acids such as aspartic acid, glutamic acid, and proline in polypeptides were easily cleaved when oxidized, producing protein fragmentation. Among the three amino acids mentioned above, proline is known to be susceptible to oxidative cracking in the presence of physiological ROS [62]. Notably, unlike other species which quickly respond to ROS within hours to days, the complete degradation of oligomeric proline under ROS takes several weeks. Therefore, oligomeric proline -based carriers are the favorable choice for controlling the slow cargo release of oxygen in an inflammatory response for long-term dosing [63].
2.2.4. Polythioketal
Thioketal is usually used as a protective group of carbonyl in organic synthesis and has good stability in acidic or alkaline environments [54]. It can be decomposed into ketones/aldehydes and disulfide compounds under oxidation conditions, which is a novel type of reactive oxygen chemical linkage. At first, Murthy’s group prepared thioketal nanoparticles via condensation polymerization for oral treatment of inflamed intestinal tissues with high reactive oxygen levels. Combined with siRNA, it can form load-bearing particles of about 600 nm, which can easily bind to inflammatory colon mucosa and be internalized into macrophages. These loaded particles are resistant to acids, bases, and proteases and are able to resist the harsh environments of the gastrointestinal tract for targeted delivery to inflammatory sites [64].
2.3. Other Types
In addition to the two categories of water solubility-switchable and structure-degradable structures, derivatives containing methionine [65], arylox-alate [66], vinyldithioether [67,68], and ferrocene [69] have also been explored for ROS-sensitive nano-formulation construction.
3. ROS-Sensitive Nano-Formulations for Atherosclerosis Applications
3.1. Nano-Formulations for Scavenging Excessive ROS
Introducing exogenous antioxidants into the biological system to scavenge excessive ROS is a feasible strategy to prevent oxidative stress and improve atherosclerosis. However, the clinical application of traditional antioxidants still faces numerous challenges, such as high toxicity, inefficient delivery, and short duration of drug residence. In recent years, antioxidant design strategies based on multifunctional nano-carriers have been proposed to construct ROS scavengers for the treatment of atherosclerosis. Using nano-carriers can improve the stability and bioavailability of traditional antioxidant drugs for targeted cargo delivery [31,70].
Ferulic acid is an attractive anti-atherosclerosis drug because it reduces macrophage adipogenesis and is effective in scavenging free radicals by forming a resonantly stable phenoxy radical [71]. In order to overcome the limitations of stability, dosing, and targeted delivery action of ferulic acid, Rebecca et al. designed a nanoparticle that chemically conjugates ferulic acid with poly(anhydride-ester) using an adipic acid or diglycolic acid linker [72]. This method can effectively protect ferulic acid from decarboxylation, enhance the total mass of ferulic acid in the polymer, and achieve a sustained and tunable release. After treatment with this nanoparticle, oxLDL uptake and ROS production by human monocyte-derived macrophages were significantly reduced, which is important for preventing the formation of macrophage foam cells [72]. D-α-Tocopherol, the active form of vitamin E, has multiple protective effects such as reducing oxidative lipids and scavenging ROS in cardiovascular diseases [73,74]. After combination with MnO2 microparticles, the nano-complex can inhibit progression of atherosclerosis by reducing the ROS levels and inhibiting the oxidation of LDL [75].
Recently, inorganic materials with intrinsic ROS-scavenging properties have been developed to decrease the damage induced by excessive ROS. These enzyme-like antioxidative nanoparticles defined as nanozymes exhibit high stability and biocompatibility; therefore, they have great potential for clinical application. MnO2 can catalyze the decomposition of hydrogen peroxide to produce water, oxygen, and Mn2+ ions, which has been explored for scavenging ROS in inflammatory conditions [76,77]. For example, Bizeau et al. prepared an MnO2 spherical microparticle coated with HA [75], which was biocompatible and had high scavenging capacities toward H2O2 in vitro studies [75]. CeO2 is an artificial superoxide dismutase with strong antioxidant properties as a potential free-radical scavenger [78]. Wu et al. synthesized two iron oxide (core)–cerium oxide (shell) nanoparticles (IO@CO1 and IO@CO2), which exhibited significant ROS-scavenging ability, enhanced macrophage uptake, and exhibited low cytotoxicity, showing great potential for the diagnosis and treatment of ROS-related inflammatory diseases. After treatment with 0.14 μg/mL of cerium oxide using IO@CO2 in J 774A.1 macrophages, 73% of ROS were scavenged, thereby significantly improving the pathological microenvironment [79]. Fullerenes and their derivatives also have intrinsic ROS-scavenging capabilities, which can react with free-radical species such as superoxide (O2-), •OH, and H2O2, for the treatment of neurodegenerative and inflammatory diseases [80,81]. However, their clinical application as antioxidant nanotherapies for atherosclerosis treatment is continuously improving, and the acute and long-term toxicities should be comprehensively investigated.
Some polymers have also been exploited as antioxidants, and their ROS-quenching effect is mainly attributed to their special structures and inherent catalytic properties. An amphiphilic block copolymer, poly(ethylene glycol)–poly(tyrosine-ethyl oxalyl), was synthesized and self-assembled into micelles loaded with simvastatin, a kind of statin drug with antioxidant and anti-inflammatory effects [82,83,84]. This kind of micelle can consume ROS in pathologic sites and exert a synergistic antioxidant effect with simvastatin. Intravenous administration of micelles in ApoE−/− mice can efficiently reduce the lesion area of plaque, showing great therapeutic effects on atherosclerosis [84]. Another case is represented by PBAPs that can be oxidized by H2O2 to generate phenolic compounds. The PBAP structure was employed as an H2O2-labile entity to synthesize H2O2-eliminating materials via covalent conjugation to biocompatible scaffold compound β-cyclodextrin. Importantly, the H2O2-scavenging capability was positively associated with the number of conjugated PBAP groups [85]. Hence, a broad-spectrum ROS-scavenging nanoparticle TPCD was further developed by simultaneously conjugating superoxide dismutase mimetic agent TEMPOL and PBAP to β-cyclodextrin (Figure 3) [86,87]. TPCD nanoparticles could effectively eliminate multiple reactive oxygen species after being rapidly endocytosed by macrophages and VSMCs. In vivo studies showed that TPCD nanoparticles effectively inhibited the atherosclerosis progression and stabilized plaque development by inhibiting ROS-induced inflammatory responses [86]. Furthermore, Guo et al. fabricated a novel nanoparticle with multiple pharmacological activities using luminol covalently conjugated to chemically modified β-cyclodextrin, which showed desirable therapeutic efficacy for the treatment of inflammatory diseases by inhibiting inflammatory response and oxidative stress, in addition to exhibiting a favorable safety performance in a mouse model [88].
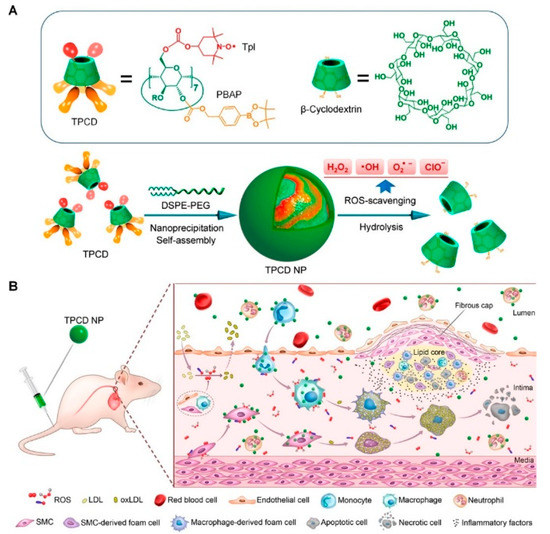
Figure 3.
(A) Chemical structure and preparation of ROS-scavenging TPCD nanoparticles. (B) Schematic of targeted treatment of atherosclerosis in mouse model using TPCD nanoparticles. Adapted from [86].
3.2. ROS-Sensitive Nano-Formulations for Controlling Cargo Release
Stimulus-responsive drug delivery systems can achieve local drug release by targeting spatiotemporal control sites, which is considered to be more reliable and effective for triggering drug release. In light of the high ROS levels in atherosclerosis lesions, ROS-sensitive materials can be designed to achieve atherosclerosis targeting and control anti-atherosclerosis drug release. Andro, a labdane diterpene, possesses remarkable anti-inflammatory and antioxidant properties by interfering with several transcription factors and signaling pathways such as AP-1, HIF-1, (NF)-κB, MAPK, and JAK/STAT [89,90]. Construction of a controlled drug carrier that releases andro in response to high levels of ROS has important implications for atherosclerosis treatment. Wu et al. designed an andro-loaded micelle that was assembled from the amphiphilic diblock copolymer PEG–PPS [14]. The hydrophobic block PPS can respond to ROS, subsequently switching into hydrophilic molecules. The changes in hydrophobic interaction can drive micellar decomposition, leading to drug release [91]. Interestingly, the ROS-sensitive switch in polymer hydrophobicity only occurs at pathological locations where the ROS concentration is much higher than that in normal tissues, thereby significantly improving the therapeutic efficacy. The micelle itself consumes ROS at the atherosclerotic lesion and acts as an ROS-responsive drug carrier for rapid andro release, thereby simultaneously alleviating inflammation and oxidative stress. After ApoE−/− mice were treated with the andro-loaded micelle, almost no lipid deposits were found, showing its prominent therapeutic effect [14]. In another study, celastrol, a hydrophobic inhibitor targeting the NF-κB signaling pathway, was encapsulated into the same carrier, i.e., ROS-responsive PEG–PPS micelles; they were found to significantly enhance the therapeutic efficacy of NF-κB inhibition and decrease the undesirable cytotoxicity for normal cells and tissues both in vitro and in vivo [92]. Dou et al. constructed an ROS-responsive nanoparticle based on chemically modified β-cyclodextrin. Compared with the nonresponsive poly(lactide-co-glycolide) nanoparticle control group, the ROS-responsive nanoparticle could serve as a superior delivery vehicle for atherosclerosis therapy by selectively releasing rapamycin, responding to the abnormally high ROS stimulus at atherosclerotic lesions. Intravenous administration of this nanoparticle in ApoE−/− mice could effectively attenuate plaque development and stabilize atheromatous lesions [93].
ROS-responsive functional groups are widely involved in nanomedicines, which have been successfully used in the research of diagnosis and treatment of atherosclerosis, ischemia reperfusion injury, and tumors [94,95]. The functional groups of thioketal linkages and ferrocene were used to design a dual ROS-sensitive nanoparticle and CD44 receptors targeting carrier material HASF. Curcumin, a traditional anti-inflammatory and antioxidant drug, was encapsulated by self-assembly to obtain HASF@Cur micelles [96,97]. Drug release profiles in vitro indicated that curcumin was effectively released from micelles trigged by the high levels of ROS, and the release rate was positively correlated with H2O2 concentration. More importantly, treatment with HASF@Cur micelles in vivo significantly inhibited the average lesion area, showing a favorable therapeutic effect compared to free curcumin [96].
Recently, cell-derived biomimetic nanoparticles have attracted extensive attention due to their long-term blood circulation, lower immunogenicity, and enhanced target capability compared to traditional nanoparticles [98]. In recent studies, biomimetic delivery systems derived from macrophage membranes were developed to encapsulate ROS-responsive nanoparticles for atherosclerosis treatment [38,99,100]. These cellular function-driven approaches can achieve targeted delivery to inflammatory tissues without resorting to additional target molecules, as well as reduce the clearance of nanoparticles by the reticuloendothelial system. After accumulation at the targeted inflammatory sites, anti-atherosclerotic drugs loaded in ROS-sensitive nanoparticles would be subsequently released responding to the high concentration level of ROS, thereby enabling effective drug therapy [99]. Moreover, in our previous study, a biomimetic and intelligent nanosystem was designed using ROS-responsive 5-aminolevulinic acid encapsulated with rapamycin, which were coated with a red blood cell membrane [101]. This biomimetic nano-formulation provided a feasible method to prevent the premature release of drugs during circulation, as well as break the shell in response to high H2O2 exposure, thus having potential therapeutic effects toward atherosclerosis. In a recent study, considering the critical role of macrophages during the development of atherosclerosis, we also employed macrophage membranes to camouflage the ROS-responsive nanoparticles loaded with rapamycin for atherosclerosis treatment. This biomimetic strategy could facilitate nanoparticle escape from macrophage clearance, thereby prolonging blood circulation time. Moreover, this biomimetic nano-formulation had a favorable biocompatibility and significantly inhibited the proliferation of macrophages in an in vitro study, showing potential application prospect for atherosclerosis treatment [38].
Shen et al. developed a system that responds to both ROS and shear stress in atheromatous plaques, consisting of red blood cells and PGED–PPS micelles [102]. These micelles can be adsorbed onto the surface of red blood cells via electrostatic incorporation, resulting in the extended circulation longevity of nanoparticles. Hydrophobic PPS is readily disassembled in response of excess ROS, thereby accelerating the loaded simvastatin release for the synergistic anti-atherosclerosis efficiency of drugs and bioactive carriers. In vivo studies showed that the application of this delivery system is capable of sustaining long-term release of simvastatin, effectively prolonging its half-life.
In addition to various kinds of ROS-responsive nano-formulations for delivering traditional anti-atherosclerosis drugs, gene delivery for atherosclerosis therapies based on ROS-responsive nano-carriers has received intensive attention. Gupta et al. developed a safe and effective vascular-targeted gene delivery tool to improve plasmid DNA transfection by synthesizing a dual ROS- and pH-responsive nanocarrier [62]. This oligo-proline peptide-derived nanocarrier with ROS-sensitive degradability performs a specific functional application only under high ROS conditions. In an in vitro study, the application of this gene delivery tool promoted the cellular uptake and expression of the luciferase reporter gene in an ROS-rich environment [62].
RNAi using ASOs or siRNA has been developed, showing promising potential for the treatment of intractable diseases including atherosclerosis [103]. Packaging ASOs or siRNAs into nanoparticles can avoid the degradation of nucleases, prolong the half-life in circulation, and improve targeted delivery and release. mTOR plays an important role in the initiation and progression of atherosclerosis by controlling autophagy and lipid metabolism, representing a promising target site for atherosclerosis treatment [104]. Gao et al. designed an H2O2-sensitive ASO delivery nanoplatform (S2P-CeO2-ASOs) to efficiently silence mTOR and rescue the impaired autophagy [105]. They used S2P to enhance plaque targeting and penetration, the PEG segment to prolong the blood circulation time in vivo, and the CeO2 core to facilitate carrier escape and ASO release in an “on-demand” manner, responding to H2O2. Following intravenous administration into ApoE−/− mice, the S2P-CeO2-ASO nano-formulation induced more than 75% of the mTOR expression knockdown in aortas, thus showing its superior therapeutic efficacy for inhibiting the progression of atherogenesis (Figure 4) [105]. Furthermore, Hou et al. developed a hyaluronan-based ROS-responsive nano-formulation that could co-deliver the anti-inflammatory drug dexamethasone and siRNAs for targeting mTOR therapy. In vitro transfection studies of this co-delivery strategy on RAW264.7 cells exhibited an excellent gene silencing effect on the mTOR gene and decreased the mRNA expression of proinflammatory cytokines, showing a synergistic anti-inflammatory effect [106].
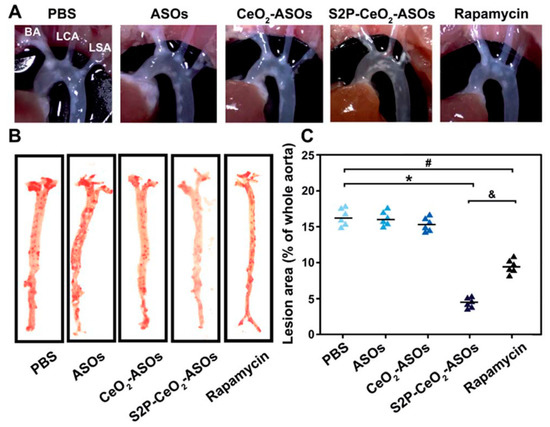
Figure 4.
(A)The atherosclerosis efficacy of indicated materials following intravenous administration into ApoE−/− mice Representative images of the in situ aortic arch lesions at the brachiocephalic artery (BA), left carotid artery (LCA), and left subclavian artery (LSA). (B) Representative images of oil red O-stained en face aorta. (C) The lesion area as a percentage of the whole aorta. * p < 0.05 for S2P-CeO2-ASOs vs. PBS, # p < 0.05 for rapamycin vs. PBS, & p < 0.05 for S2P-CeO2-ASOs vs. rapamycin. Adapted from [105].
3.3. ROS-Sensitive Nano-Formulations for Imaging
In addition to the targeted treatment of atherosclerotic plaques, the in vivo traceability of ROS-responsive nano-agents has been used for the development of novel protocols for atherosclerotic diagnosis and monitoring. Recently, Wang et al. developed an early detection method for targeting foam cells in atherosclerosis. A platelet membrane was engineered to encapsulate two naphthalimide-based fluorescent probes to detect ROS in foam cells. One of their obtained nano-detection systems, TBNG@Mp, showed low toxicity and exhibited fluorescence imaging in the thoracic aorta of early atherosclerosis model rats [107]. To further quantify ROS production in the vasculature and various tissues in atherosclerosis, Manea et al. developed an imaging nano-formulation that encapsulated a redox-sensitive fluorescent probe, ROS BriteTM700, using PEG-stabilized liposomes (Lp), which could selectively recognize the highly expressed VCAM-1 to exhibit NIRF upon ROS oxidation, prior to further analysis using a high-resolution imaging system for ROS quantification [108]. Recently, Park et al. developed an NIRF nano-sensor using HA as a ligand for the CD44 receptor, chlorin e6 as an NIRF dye, and a thioketal linker as an ROS-degradable moiety [109]. The in vivo study indicated that the nano-sensors were internalized into proinflammatory macrophages and were then cleaved selectively by the intracellular ROS stimulus, resulting in the pronounced recovery of fluorescence signals for imaging the ROS in atherosclerotic plaques (Figure 5) [109].
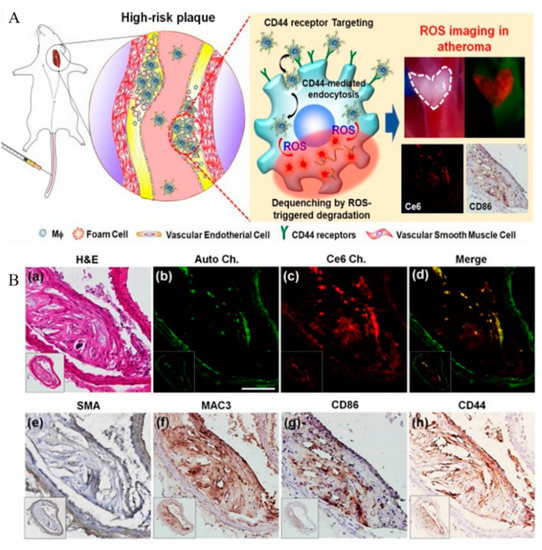
Figure 5.
(A) Schematic of ROS imaging of proinflammatory macrophages within atherosclerotic plaques. (B) Histological and immunohistochemical evaluations. (a) H&E staining. (b) Autofluorescence signals from elastin (green), (c) NIRF signals (red), and (d) merged image. Immunohistochemical staining of (e) SMA, (f) MAC3, (g) CD86, and (h) CD44. Scale bar: 25 mm. Adapted from [109].
ROS-sensitive photodynamic therapy agents can be designed as innovative theranostic platforms for target imaging and atherosclerosis therapy. Hyaluronic acid, an anionic polysaccharide, is one of the representative biomaterials used as a cleavable substrate for selective ROS detection [110,111]. Kim et al. synthesized MacTNPs by conjugating photosensitizer chlorin e6 to HA [112]. The fluorescence of MacTNPs is inhibited in the native state because of the self-quenching effect of conjugated chlorin e6. However, when MacTNPs are internalized into macrophages, the excess ROS cleaves the chemical bonds of HA, causing chlorin e6 release and resulting in fluorescence emission [112]. This ROS-activatable photosensitizing agent has a high target-to-background ratio and minimal side effects, implying its great potential in NIRF imaging and photodynamic atherosclerosis therapy.
Photoacoustic imaging is the latest imaging technique, which integrates the advantages of ultrasound and optical imaging. Its principle is to detect the ultrasonic waves generated by the energy transduction of laser through thermoelastic expansion [113]. Gao et al. constructed a photoacoustic imaging nanoprobe using a GSH/H2O2 redox couple as the primary target for the imaging of redox status changes to assess the vulnerability of atherosclerotic plaques [114]. Two types of NIRF probes, the Cy-3-NO2 response to GSH and the Mito-NIRHP response to H2O2, were introduced to functionalize BSA, harvesting a BSA-Cy-Mito nanoprobe. In vivo results demonstrated that the BSA-Cy-Mito nanoprobe showed excellent photoacoustic imaging efficacy with high specificity and sensitivity, yielding insight into the GSH/H2O2 redox-related inflammatory process (Figure 6) [114].
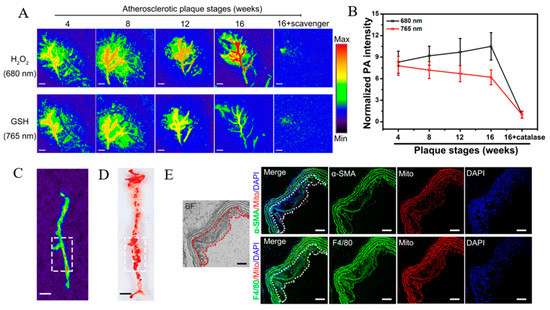
Figure 6.
(A) PA imaging of atherosclerotic plaques in ApoE−/− mice injected with BSA-Cy-Mito at the indicated stages. (B) Normalized PA intensities of plaques at different stages. (C) PA imaging at plaque lesions across the aorta of ApoE−/− mice. (D) En face aortic lesions in Oil red O-stained aorta. (E) Fluorescence microscope images of aortic plaques in ApoE−/− mice. Scale bar = 100 μm. Adapted from [114].
Fluorescent groups with TP excitation and the AIE effect would be suitable materials as nanoparticle tracers, as well as for the spatial fluorescence imaging of inflammatory tissues, because of the clearer size resolution, less auto-fluorescence interference, and stronger imaging penetration [115,116,117]. Recently, Ma et al. combined two-photon AIE bioimaging and ROS-responsive drug delivery for the diagnosis and therapy of inflammation [118]. Prednisolone, a widely used anti-inflammatory glucocorticoid [119], was linked to the TP using an ROS-responsive bond to form a diagnosis therapy compound TPP, which was then encapsulated into the amphipathic polymeric PMPC–PMEMA micelles to obtain TPP@PMM. Once TPP@PMM was accumulated at inflammatory tissues, the aggregated micelles exhibited significant two-photon fluorescence emission and subsequently triggered micelle disintegration for precise drug delivery under the concentrated ROS stimulus [118]. Moreover, an improved nano-platform with simultaneous response to the high concentration of ROS and enriched lipids at the atherosclerosis site was developed. By conjugating TP to β-cyclodextrin carrying prednisolone, the compound TPCDP was synthesized, and it was then packaged into PMM to obtained TPCDP@PMM [120]. The in vivo bioimaging of TPCDP@PMM on ApoE−/− mice showed efficient accumulation in atherosclerosis tissue and clear plaque recognition. In addition, the development of atherosclerotic plaques was effectively inhibited by the “two-pronged” anti-inflammatory and lipid removal approach, revealing their great potential for atherosclerosis theranostic applications [120].
4. Conclusions and Future Perspectives
ROS-responsive drug delivery systems have tremendous advantages in the diagnosis and treatment of many diseases, and they are even more significant in oxidative stress-related diseases. Due to the abnormal level of ROS in pathological atherosclerosis lesions, ROS-sensitive nano-formulations can be widely applied in target drug delivery and controlled cargo release for promoting bioavailability and reducing side effects, along with broad application prospects. Hydrophobic–hydrophilic phase transitions and cleavable bonds under pathological ROS stimulus are widely used to prevent premature cargo release and undesirable delivery for smart polymeric nano-formulation construction. For atherosclerosis applications, ROS-sensitive nano-formulations have been designed for scavenging excessive ROS in lesions, controlling cargo release in response to the pathological ROS stimulus, and imaging for atherosclerosis diagnosis, which provides a great platform for improving atherosclerosis applications. On the other hand, there is a long way before ROS-sensitive formulation development leads to clinical applications in atherosclerosis. ROS typically changes dynamically in terms of species and concentration as a function of location, stage, and disease. The balance between ROS sensitivity and stability is paramount for enhancing the ultimate efficacy of therapy and imaging and reducing the undesirable side effects. Further development addressing the key challenges will greatly enhance potential applications in atherosclerosis.
Author Contributions
W.W., D.S. and L.J. conceptualized this review. W.W., D.S., R.P. and H.J. analyzed the literature and wrote the manuscript. D.S., Q.Y., R.P. and H.J. generated the figures. W.W., D.S., L.J. and J.M. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (51901160, 31971301), the Natural Science Foundation of Zhejiang Province (Grant No. LQ19E010004, LQ20C200015, LQ20C020003), and the Fundamental Research Funds for Central Universities (2020CDJQY-A061, 2018CDHB1B08).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Xiangjun Kong at the University of Macau for helpful comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| OH | hydroxyl radicals |
| andro | andrographolide |
| AIE | aggregation-induced emission |
| ASOs | antisense oligonucleotides |
| BSA | bovine serum albumin |
| CeO2 | cerium oxide |
| CVDs | cardiovascular diseases |
| H2O2 | hydrogen peroxide |
| HA | hyaluronic acid |
| HASF | oligomeric hyaluronic acid-2′-[propane-2,2-diyllbls(thio)] diacetic acl-hydroxymethylferrocene |
| LDL | low-density lipoprotein |
| Lp | liposomes |
| MacTNP | macrophage-targeted theranostic nanoparticles |
| MMP | matrix metalloproteinase |
| MnO2 | manganese dioxide |
| mTOR | mammalian target of rapamycin |
| NIRF | near-infrared fluorescence |
| O2- | superoxide |
| ONOO− | peroxide nitrate |
| Ox-LDL | low-density lipoprotein |
| PBAPs | phenylboronic acid pinacol esters |
| PEG | polyethylene glycol |
| PEG–PPS | poly(ethylene glycol)–poly(propylene sulfide) |
| PGED–PPS | poly(glycidyl methacrylate)–polypropylene sulfide |
| PPS | polypropylene sulfides |
| RNAi | RNA interference |
| ROS | reactive oxygen species |
| S2P | stabilin-2-specific peptide ligand |
| siRNA | short interfering RNA |
| TEMPOL | 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl |
| TP | two-photon fluorophore |
| TPCD | superoxide dismutase mimetic agent TEMPO and PBAP in β-cyclodextrin |
| VSMCs | vascular smooth muscle cells |
References
- Tørris, C.; Småstuen, M.C.; Molin, M. Nutrients in fish and possible associations with cardiovascular disease risk factors in metabolic syndrome. Nutrients 2018, 10, 952. [Google Scholar] [CrossRef]
- Narasimhan, S.D. Beyond statins: New therapeutic frontiers for cardiovascular disease. Cell 2017, 169, 971–973. [Google Scholar] [CrossRef][Green Version]
- Lobatto, M.E.; Calcagno, C.; Millon, A.; Senders, M.L.; Fay, F.; Robson, P.M.; Ramachandran, S.; Binderup, T.; Paridaans, M.P.M.; Sensarn, S.; et al. Atherosclerotic Plaque targeting mechanism of long-circulating nanoparticles established by multimodal imaging. ACS Nano 2015, 9, 1837–1847. [Google Scholar] [CrossRef]
- Lin, J.; Phillips, E.; Riggins, T.; Sangha, G.; Chakraborty, S.; Lee, J.; Lycke, R.; Hernandez, C.; Soepriatna, A.; Thorne, B.; et al. Imaging of Small animal peripheral artery disease models: Recent advancements and translational potential. Int. J. Mol. Sci. 2015, 16, 11131–11177. [Google Scholar] [CrossRef] [PubMed]
- Malekmohammad, K.; Sewell, R.D.E.; Rafieian-Kopaei, M. Antioxidants and Atherosclerosis: Mechanistic Aspects. Biomolecules 2019, 9, 301. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Kwak, L.; Ballew, S.H.; Grams, M.E.; Selvin, E.; Folsom, A.R.; Coresh, J.; Tang, W. Chronic Kidney disease measures and the risk of abdominal aortic aneurysm. Atherosclerosis 2018, 279, 107–113. [Google Scholar] [CrossRef]
- Hess, C.N.; Norgren, L.; Ansel, G.M.; Capell, W.H.; Fletcher, J.P.; Fowkes, F.G.R.; Gottsäter, A.; Hitos, K.; Jaff, M.R.; Nordanstig, J.; et al. A structured review of antithrombotic therapy in peripheral artery disease with a focus on revascularization: A TASC (intersociety consensus for the management of peripheral artery disease) Initiative. Circulation 2017, 135, 2534–2555. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causesof-death (accessed on 1 September 2021).
- Barquera, S.; Pedroza-Tobías, A.; Medina, C.; Hernández-Barrera, L.; Bibbins-Domingo, K.; Lozano, R.; Moran, A.E. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch. Med. Res. 2015, 46, 328–338. [Google Scholar] [CrossRef]
- De Wijs-Meijler, D.P.; Duncker, D.J.; Tibboel, D.; Schermuly, R.T.; Weissmann, N.; Merkus, D.; Reiss, I.K.M. Oxidative Injury of the pulmonary circulation in the perinatal period: Short- and long-term consequences for the human cardiopulmonary system. Pulm. Circ. 2017, 7, 55–66. [Google Scholar] [CrossRef]
- Miao, L.; Clair, D.K.S. Regulation of Superoxide Dismutase Genes: Implications in Disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef]
- Tapeinos, C.; Pandit, A. Physical, chemical, and biological structures based on ros-sensitive moieties that are able to respond to oxidative microenvironments. Adv. Mater. 2016, 28, 5553–5585. [Google Scholar] [CrossRef]
- Wu, T.; Chen, X.; Wang, Y.; Xiao, H.; Peng, Y.; Lin, L.; Xia, W.; Long, M.; Tao, J.; Shuai, X. Aortic Plaque-targeted andrographolide delivery with oxidation-sensitive micelle effectively treats atherosclerosis via simultaneous ros capture and anti-inflammation. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2215–2226. [Google Scholar] [CrossRef]
- Takahashi, S. Triglyceride Rich Lipoprotein -LPL-VLDL Receptor and Lp(a)-VLDL Receptor pathways for macrophage foam cell formation. J. Atheroscler. Thromb. 2017, 24, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Sun, G.-Y.; Zhang, Y.; He, J.-J.; Zheng, S.; Lin, J.-N. Tormentic acid inhibits H2O2-Induced oxidative stress and inflammation in rat vascular smooth muscle cells via inhibition of the NF-ΚB signaling pathway. Mol. Med. Rep. 2016, 14, 3559–3564. [Google Scholar] [CrossRef]
- Velasquez, J.C.; Weiss, D.; Joseph, G.; Landazuri, N.; Taylor, W.R. Abstract 3961: Macrophage Catalase Overexpression Inhibits Atherosclerosis and Vascular Inflammation. Circulation 2008, 118, S510. [Google Scholar]
- Fruehauf, J.P.; Meyskens, F.L. Reactive Oxygen species: A breath of life or death? Clin. Cancer Res. 2007, 13, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive Oxygen species: Key regulators in vascular health and diseases: Ros in vascular diseases. Br. J. Pharm. 2018, 175, 1279–1292. [Google Scholar] [CrossRef]
- Lippert, A.R.; Van de Bittner, G.C.; Chang, C.J. Boronate Oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc. Chem. Res. 2011, 44, 793–804. [Google Scholar] [CrossRef]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The role of hydrogen peroxide in redox-dependent signaling: Homeostatic and pathological responses in mammalian cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef]
- Kim, K.S.; Song, C.G.; Kang, P.M. Targeting oxidative stress using nanoparticles as a theranostic strategy for cardiovascular diseases. Antioxid. Redox. Sign. 2019, 30, 733–746. [Google Scholar] [CrossRef]
- Nishida, K.; Otsu, K. Inflammation and metabolic cardiomyopathy. Cardiovasc. Res. 2017, 113, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Millar, L.J.; Shi, L.; Hoerder-Suabedissen, A.; Molnár, Z. Neonatal hypoxia ischaemia: Mechanisms, Models, and therapeutic challenges. Front. Cell. Neurosci. 2017, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Tomaniak, M.; Katagiri, Y.; Modolo, R.; de Silva, R.; Khamis, R.Y.; Bourantas, C.V.; Torii, R.; Wentzel, J.J.; Gijsen, F.J.H.; van Soest, G.; et al. Vulnerable plaques and patients: State-of-the-Art. Eur. Heart J. 2020, 41, 2997–3004. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Raimondo, D.; Pecoraro, R.; Arnao, V.; Pinto, A.; Licata, G. Atherosclerosis as an inflammatory disease. Curr. Pharm. Des. 2012, 18, 4266–4288. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004, 56, 1649–1659. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G.; Wang, X.; Hu, J.; Liu, S. Enzyme-responsive polymeric vesicles for bacterial-strain-selective delivery of antimicrobial agents. Angew. Chem. Int. Ed. 2016, 55, 1760–1764. [Google Scholar] [CrossRef]
- Ge, Z.; Liu, S. Functional Block copolymer assemblies responsive to tumor and intracellular microenvironments for site-specific drug delivery and enhanced imaging performance. Chem. Soc. Rev. 2013, 42, 7289. [Google Scholar] [CrossRef]
- Luo, L.; Wu, W.; Sun, D.; Dai, H.-B.; Wang, Y.; Zhong, Y.; Wang, J.-X.; Maruf, A.; Nurhidayah, D.; Zhang, X.-J.; et al. Acid-Activated Melittin for targeted and safe antitumor therapy. Bioconjug. Chem. 2018, 29, 2936–2944. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef]
- Tong, R.; Tang, L.; Ma, L.; Tu, C.; Baumgartner, R.; Cheng, J. Smart Chemistry in polymeric nanomedicine. Chem. Soc. Rev. 2014, 43, 6982–7012. [Google Scholar] [CrossRef]
- Lee, S.H.; Gupta, M.K.; Bang, J.B.; Bae, H.; Sung, H.-J. Current progress in reactive oxygen species (ROS)-responsive materials for biomedical applications. Adv. Healthc. Mater. 2013, 2, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.-L.; Chiang, W.-L.; Maiti, B.; Liao, Z.-X.; Ho, Y.-C.; Shim, M.S.; Chuang, E.-Y.; Xia, Y.; Sung, H.-W. Nanoparticles with Dual responses to oxidative stress and reduced PH for Drug release and anti-inflammatory applications. ACS Nano 2014, 8, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, J.; Liu, B. Conjugated-Polyelectrolyte-Based polyprodrug: Targeted and image-guided photodynamic and chemotherapy with on-demand drug release upon irradiation with a single light source. Angew. Chem. Int. Ed. 2014, 126, 7291–7296. [Google Scholar] [CrossRef]
- Wang, Y.; Shim, M.S.; Levinson, N.S.; Sung, H.-W.; Xia, Y. Stimuli-Responsive Materials for controlled release of theranostic agents. Adv. Funct. Mater. 2014, 24, 4206–4220. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wang, Y.; Wijaya, A.; Liu, B.; Maruf, A.; Wang, J.; Xu, J.; Liao, X.; Wu, W.; Wang, G. ROS-Responsive biomimetic nanoparticles for potential application in targeted anti-atherosclerosis. Regen. Biomater. 2021, 8, rbab033. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An Emerging treatment modality for cancer. Nat. Rev. Drug. Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Song, C.-C.; Du, F.-S.; Li, Z.-C. Oxidation-Responsive Polymers for biomedical applications. J. Mater. Chem. B 2014, 2, 3413–3426. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, Y.; Chen, K.; Zhang, X.; Xu, X.; Shi, Q.; Han, S.; Chen, X.; Gong, H.; Li, X.; et al. Biocompatible Reactive oxygen species (ros)-responsive nanoparticles as superior drug delivery vehicles. Adv. Healthc. Mater. 2015, 4, 69–76. [Google Scholar] [CrossRef]
- Deng, H.; Zhao, X.; Liu, J.; Deng, L.; Zhang, J.; Liu, J.; Dong, A. Reactive oxygen species (ROS) responsive PEG-PCL nanoparticles with pH-controlled negative-to-positive charge reversal for intracellular delivery of doxorubicin. J. Mater. Chem. B 2015, 3, 9397–9408. [Google Scholar] [CrossRef]
- Yu, L.-Y.; Su, G.-M.; Chen, C.-K.; Chiang, Y.-T.; Lo, C.-L. Specific Cancer Cytosolic Drug Delivery Triggered by Reactive Oxygen Species-Responsive Micelles. Biomacromolecules 2016, 17, 3040–3047. [Google Scholar] [CrossRef]
- Jayrajsinh, S. Montmorillonite Nanoclay as a Multifaceted Drug-Delivery Carrier: A Review. J. Drug Deliv. Sci. Technol. 2017, 39, 200–209. [Google Scholar] [CrossRef]
- Aamer, K. Rheological Studies of PLLA-PEO-PLLA triblock copolymer hydrogels. Biomaterials 2004, 25, 1087–1093. [Google Scholar] [CrossRef]
- Reddy, S.T.; Rehor, A.; Schmoekel, H.G.; Hubbell, J.A.; Swartz, M.A. In Vivo Targeting of Dendritic Cells in Lymph Nodes with Poly(Propylene Sulfide) Nanoparticles. J. Control. Release 2006, 112, 26–34. [Google Scholar] [CrossRef]
- Han, P.; Ma, N.; Ren, H.; Xu, H.; Li, Z.; Wang, Z.; Zhang, X. Oxidation-Responsive Micelles based on a selenium-containing polymeric superamphiphile. Langmuir 2010, 26, 14414–14418. [Google Scholar] [CrossRef]
- Ma, N.; Li, Y.; Xu, H.; Wang, Z.; Zhang, X. Dual Redox Responsive Assemblies Formed from Diselenide Block Copolymers. J. Am. Chem. Soc. 2010, 132, 442–443. [Google Scholar] [CrossRef]
- Tan, X.; Yu, Y.; Liu, K.; Xu, H.; Liu, D.; Wang, Z.; Zhang, X. Single-Molecule Force spectroscopy of selenium-containing amphiphilic block copolymer: Toward disassembling the polymer micelles. Langmuir 2012, 28, 9601–9605. [Google Scholar] [CrossRef]
- Ren, H.; Wu, Y.; Ma, N.; Xu, H.; Zhang, X. Side-Chain selenium-containing amphiphilic block copolymers: Redox-controlled self-assembly and disassembly. Soft Matter 2012, 8, 1460–1466. [Google Scholar] [CrossRef]
- Ma, N.; Li, Y.; Ren, H.; Xu, H.; Li, Z.; Zhang, X. Selenium-Containing Block copolymers and their oxidation-responsive aggregates. Polym. Chem. 2010, 1, 1609. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, J.; Park, S.; Khang, G.; Kang, P.M.; Lee, D. Inflammation-Responsive antioxidant nanoparticles based on a polymeric prodrug of vanillin. Biomacromolecules 2013, 14, 1618–1626. [Google Scholar] [CrossRef]
- Yang, S.C.; Bhide, M.; Crispe, I.N.; Pierce, R.H.; Murthy, N. Polyketal copolymers: A new acid-sensitive delivery vehicle for treating acute inflammatory diseases. Bioconjug. Chem. 2008, 19, 1164–1169. [Google Scholar] [CrossRef]
- Kim, J.S.; Jo, S.D.; Seah, G.L.; Kim, I.; Nam, Y.S. ROS-Induced Biodegradable polythioketal nanoparticles for intracellular delivery of anti-cancer therapeutics. J. Ind. Eng. Chem. 2015, 21, 1137–1142. [Google Scholar] [CrossRef]
- Su, Z.; Chen, M.; Xiao, Y.; Sun, M.; Zong, L.; Asghar, S.; Dong, M.; Li, H.; Ping, Q.; Zhang, C. ROS-Triggered and regenerating anticancer nanosystem: An Effective strategy to subdue tumor’s multidrug resistance. J. Control. Release 2014, 196, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-C.; Ji, R.; Du, F.-S.; Liang, D.-H.; Li, Z.-C. Oxidation-Accelerated Hydrolysis of the ortho ester-containing acid-labile polymers. ACS Macro Lett. 2013, 2, 273–277. [Google Scholar] [CrossRef]
- Jourden, J.L.M.; Daniel, K.B.; Cohen, S.M. Investigation of self-immolative linkers in the design of hydrogen peroxide activated metalloprotein inhibitors. Chem. Commun. 2011, 47, 7968. [Google Scholar] [CrossRef]
- Vaccari, L.; Canton, D.; Zaffaroni, N.; Villa, R.; Tormen, M.; di Fabrizio, E. Porous silicon as drug carrier for controlled delivery of doxorubicin anticancer agent. Microelectron. Eng. 2006, 83, 1598–1601. [Google Scholar] [CrossRef]
- Salonen, J.; Laitinen, L.; Kaukonen, A.M.; Tuura, J.; Björkqvist, M.; Heikkilä, T.; Vähä-Heikkilä, K.; Hirvonen, J.; Lehto, V.-P. Mesoporous Silicon microparticles for oral drug delivery: Loading and release of five model drugs. J. Control. Release 2005, 108, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.C.; Park, J.-H.; Park, J.; Segal, E.; Cunin, F.; Sailor, M.J. Oxidation-Triggered release of fluorescent molecules or drugs from mesoporous Si microparticles. ACS Nano 2008, 2, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Gupta, M.K.; Lee, S.H.; Crowder, S.W.; Wang, X.; Hofmeister, L.H.; Nelson, C.E.; Bellan, L.M.; Duvall, C.L.; Sung, H.-J. Oligoproline-Derived Nanocarrier for Dual Stimuli-Responsive Gene Delivery. J. Mater. Chem. B 2015, 3, 7271–7280. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.S.; Koblin, R.L.; Zachman, A.L.; Perrien, D.S.; Hofmeister, L.H.; Giorgio, T.D.; Sung, H.-J. Physiologically Relevant oxidative degradation of oligo(proline) cross-linked polymeric scaffolds. Biomacromolecules 2011, 12, 4357–4366. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Corbett, G.; Moss, A.C. Systematic review and meta-analysis: Serum Infliximab levels during maintenance therapy and outcomes in inflammatory bowel disease. J. Crohns Colitis 2016, 10, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Sanoj Rejinold, N.; Lee, D.; Jon, S.; Kim, Y.-C. Protease-Activatable Cell-penetrating peptide possessing ROS-Triggered phase transition for enhanced cancer therapy. J. Control. Release 2017, 264, 89–101. [Google Scholar] [CrossRef]
- Lee, D.; Bae, S.; Ke, Q.; Lee, J.; Song, B.; Karumanchi, S.A.; Khang, G.; Choi, H.S.; Kang, P.M. Hydrogen peroxide-responsive copolyoxalate nanoparticles for detection and therapy of ischemia–reperfusion injury. J. Control. Release 2013, 172, 1102–1110. [Google Scholar] [CrossRef]
- Saravanakumar, G.; Lee, J.; Kim, J.; Kim, W.J. Visible Light-Induced Singlet oxygen-mediated intracellular disassembly of polymeric micelles co-loaded with a photosensitizer and an anticancer drug for enhanced photodynamic therapy. Chem. Commun. 2015, 51, 9995–9998. [Google Scholar] [CrossRef]
- Baugh, S.D.P.; Yang, Z.; Leung, D.K.; Wilson, D.M.; Breslow, R. Cyclodextrin dimers as cleavable carriers of photodynamic sensitizers. J. Am. Chem. Soc. 2001, 123, 12488–12494. [Google Scholar] [CrossRef]
- Li, Q.; Chen, X.; Yue, X.; Huang, D.; Wang, X. Construction and Transformation of stimuli-responsive vesicles from the ferrocene derivative supramolecular amphiphiles. Colloids Surf. A Physicochem. Eng. Asp. 2012, 409, 98–104. [Google Scholar] [CrossRef]
- Sun, D.; Chen, J.; Wang, Y.; Ji, H.; Peng, R.; Jin, L.; Wu, W. Advances in refunctionalization of erythrocyte-based nanomedicine for enhancing cancer-targeted drug delivery. Theranostics 2019, 9, 6885–6900. [Google Scholar] [CrossRef]
- Ami, A.; Markovi, Z.; Markovi, J.M.D.; Milenkovi, D.; Stepani, V. Antioxidative potential of ferulic acid phenoxyl radical. Phytochemistry 2019, 170, 112218. [Google Scholar] [CrossRef]
- Chmielowski, R.A.; Abdelhamid, D.S.; Faig, J.J.; Petersen, L.K.; Gardner, C.R.; Uhrich, K.E.; Joseph, L.B.; Moghe, P.V. Athero-Inflammatory nanotherapeutics: Ferulic acid-based poly(anhydride-ester) nanoparticles attenuate foam cell formation by regulating macrophage lipogenesis and reactive oxygen species generation. Acta Biomater. 2017, 57, 85–94. [Google Scholar] [CrossRef]
- Ziegler, M.; Wallert, M.; Lorkowski, S.; Peter, K. Cardiovascular and Metabolic protection by vitamin E: A matter of treatment strategy? Antioxidants 2020, 9, 935. [Google Scholar] [CrossRef]
- Wallert, M.; Ziegler, M.; Wang, X.; Maluenda, A.; Peter, K. α-Tocopherol preserves cardiac function by reducing oxidative stress and inflammation in ischemia/reperfusion injury. Redox Biol. 2019, 26, 101292. [Google Scholar] [CrossRef]
- Bizeau, J.; Tapeinos, C.; Marella, C.; Larranaga, A.; Pandit, A. Synthesis and characterization of hyaluronic acid coated manganese dioxide microparticles that act as ROS scavengers. Colloids Surf. B Biointerfaces 2017, 159, 30–38. [Google Scholar] [CrossRef]
- Luo, X.L.; Xu, J.J.; Wei, Z.; Chen, H.Y. A novel glucose ENFET Based on the special reactivity of MnO2 nanoparticles. Biosens. Bioelectron. 2004, 19, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Adjei, I.M.; Brown, S.B.; Liseth, O.; Sharma, B. Manganese Dioxide nanoparticles protect cartilage from inflammation-induced oxidative stress. Biomaterials 2019, 224, 119467. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Naik, P. Synthesis and biomedical applications of cerium oxide nanoparticle—A review. Biotechnol. Rep. 2018, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Y.; Zhao, W.; Xu, Z.; Little, P.J.; Whittaker, A.K.; Zhang, R.; Ta, H.T. Novel iron oxide-cerium oxide core-shell nanoparticles as a potential theranostic material for ROS related inflammatory diseases. J. Mater. Chem. B 2018, 6, 4937–4951. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Behnam, B.; Banach, M.; Sahebkar, A. The yin and yang of carbon nanomaterials in atherosclerosis. Biotechnol. Adv. 2018, 36, 2232–2247. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef]
- Katsuki, S.; Matoba, T.; Nakashiro, S.; Sato, K.; Koga, J.-I.; Nakano, K.; Nakano, Y.; Egusa, S.; Sunagawa, K.; Egashira, K. Nanoparticle-mediated delivery of pitavastatin inhibits atherosclerotic plaque destabilization/rupture in mice by regulating the recruitment of inflammatory monocytes. Circulation 2014, 129, 896–906. [Google Scholar] [CrossRef]
- Tang, J.; Lobatto, M.E.; Hassing, L.; van deer Staay, S.; van Rijs, S.M.; Calcagno, C.; Braza, M.S.; Baxter, S.; Fay, F.; Sanchez-Gaytan, B.L.; et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci. Adv. 2015, 1, e1400223. [Google Scholar] [CrossRef]
- Mu, D.; Li, J.; Qi, Y.; Sun, X.; Liu, Y.; Shen, S.; Li, Y.; Xu, B.; Zhang, B. Hyaluronic acid-coated polymeric micelles with hydrogen peroxide scavenging to encapsulate statins for alleviating atherosclerosis. J. Nanobiotechnol. 2020, 18, 179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, F.; Chen, Y.; Dou, Y.; Tao, H.; Zhang, D.; Wang, R.; Li, X.; Zhang, J. Structure-Property correlations of reactive oxygen species-responsive and hydrogen peroxide-eliminating materials with anti-oxidant and anti-inflammatory activities. Chem. Mater. 2017, 29, 8221–8238. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Zhao, W.; Dou, Y.; An, H.; Tao, H.; Xu, X.; Jia, Y.; Lu, S.; Zhang, J.; et al. Targeted therapy of atherosclerosis by a broad-spectrum reactive oxygen species scavenging nanoparticle with intrinsic anti-inflammatory activity. ACS Nano 2018, 12, 8943–8960. [Google Scholar] [CrossRef]
- Li, L.; Guo, J.; Wang, Y.; Xiong, X.; Zhang, J. A Broad-Spectrum ROS-eliminating material for prevention of inflammation and drug-induced organ toxicity. Adv. Sci. 2018, 5, 1800781. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, D.; Tao, H.; Li, G.; Liu, R.; Dou, Y.; Jin, T.; Li, L.; Huang, J.; Hu, H.; et al. Cyclodextrin-Derived intrinsically bioactive nanoparticles for treatment of acute and chronic inflammatory diseases. Adv. Mater. 2019, 31, 1904607. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Guan, S.; Chang, C.; Wu, S.; Wong, W. A novel antiinflammatory Role for andrographolide in asthma via inhibition of the nuclear factor-kB pathway. Am. J. Respir. Crit. Care Med. 2012, 179, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Burgos, R.A.; Alarcon, N.P.; Quiroga, J.; Manosalva, C.; Hancke, J. Andrographolide, an anti-inflammatory multitarget drug: All roads lead to cellular metabolism. Molecules 2020, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Napoli, A.; Valentini, M.; Tirelli, N.; Müller, M.; Hubbell, J.A. Oxidation-Responsive polymeric vesicles. Nat. Mater. 2004, 3, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.D.; Liu, Y.-G.; Kim, T.; Bobbala, S.; Yi, S.; Zhang, X.; Choi, J.; Scott, E.A. Celastrol-Loaded PEG-b-PPS Nanocarriers as an anti-inflammatory treatment for atherosclerosis. Biomater. Sci. 2019, 7, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Chen, Y.; Zhang, X.; Xu, X.; Chen, Y.; Guo, J.; Zhang, D.; Wang, R.; Li, X.; Zhang, J. Non-Proinflammatory and responsive nanoplatforms for targeted treatment of atherosclerosis. Biomaterials 2017, 143, 93. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, Y.; Zhao, M.; Gao, W.; Zhang, H.; Li, S.; He, B.; Pu, Y. A reactive oxygen species–responsive prodrug micelle with efficient cellular uptake and excellent bioavailability. J. Mater. Chem. B 2018, 6, 1076–1084. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.; Xia, Y.; Liu, S.; Lu, Y. Preparation of ROS-Responsive core crosslinked polycarbonate micelles with thioketal linkage. Colloid Surf. B 2020, 195, 111276. [Google Scholar] [CrossRef]
- Hou, X.; Lin, H.; Zhou, X.; Cheng, Z.; Li, Y.; Liu, X.; Zhao, F.; Zhu, Y.; Zhang, P.; Chen, D. Novel dual ROS-Sensitive and CD44 Receptor targeting nanomicelles based on oligomeric hyaluronic acid for the efficient therapy of atherosclerosis. Carbohyd. Polym. 2020, 232, 115787. [Google Scholar] [CrossRef]
- Hong, L.; Sureda, A.; Devkota, H.P.; Pittalà, V.; Nabavi, S.M. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol. Adv. 2019, 38, 107343. [Google Scholar] [CrossRef]
- Yang, L.; Zang, G.; Li, J.; Li, X.; Li, Y.; Zhao, Y. Cell-Derived Biomimetic nanoparticles as a novel drug delivery system for atherosclerosis: Predecessors and perspectives. Regen. Biomater. 2020, 7, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.T.; Yue, L.; Wan, J.-B.; Lee, S.M.Y.; Wang, R. Treatment of Atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 2020, 11, 2622. [Google Scholar] [CrossRef]
- Peng, R.; Ji, H.; Jin, L.; Lin, S.; Huang, Y.; Xu, K.; Yang, Q.; Sun, D.; Wu, W. Macrophage-Based therapies for atherosclerosis management. J. Immunol. Res. 2020, 2020, 8131754. [Google Scholar] [CrossRef]
- Maruf, A.; Wang, Y.; Luo, L.; Zhong, Y.; Nurhidayah, D.; Liu, B.; Tang, D.; Rouf, M.A.; Zhang, H.; Yin, Y.; et al. Nanoerythrocyte membrane-enveloped ROS-responsive 5-aminolevulinic Acid prodrug nanostructures with robust atheroprotection. Part. Part. Syst. Charact. 2020, 37, 2000021. [Google Scholar] [CrossRef]
- Shen, M.; Li, H.; Yao, S.; Wu, X.; Liu, S.; Yang, Q.; Zhang, Y.; Du, J.; Qi, S.; Li, Y. Shear stress and ROS-Responsive biomimetic micelles for atherosclerosis via ROS consumption. Mater. Sci. Eng. C 2021, 126, 112164. [Google Scholar] [CrossRef]
- Mkinen, P.; Ruotsalainen, A.K.; Yl-Herttuala, S. Nucleic acid-based therapies for atherosclerosis. Curr. Atheroscler. Rep. 2020, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Niu, X.; Dang, X.; Pan, L. mTOR enhances foam cell formation by suppressing the autophagy pathway. DNA Cell Biol. 2014, 33, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhao, Y.; Li, X.; Sun, Y.; Cai, M.; Cao, W.; Liu, Z.; Tong, L.; Cui, G.; Tang, B. H2O2-responsive and plaque-penetrating nanoplatform for mTOR Gene silencing with robust anti-atherosclerosis efficacy. Chem. Sci. 2018, 9, 439–445. [Google Scholar] [CrossRef]
- Hou, C.; Bai, H.; Wang, Z.; Qiu, Y.; Kong, L.-L.; Sun, F.; Wang, D.; Yin, H.; Zhang, X.; Mu, H.; et al. A Hyaluronan-Based Nanosystem enables combined anti-inflammation of mTOR gene silencing and pharmacotherapy. Carbohyd. Polym. 2018, 195, 339–348. [Google Scholar] [CrossRef]
- Wang, Q.; Lou, R.; Yin, Q.; Yang, R.; Li, S.; Zhou, J. A nano-detection system based on a chemical probe for early diagnosis of atherosclerosis in situ. Analyst 2021, 146, 4674–4682. [Google Scholar] [CrossRef]
- Manea, S.-A.; Vlad, M.-L.; Rebleanu, D.; Lazar, A.-G.; Fenyo, I.M.; Calin, M.; Simionescu, M.; Manea, A. Detection of vascular reactive oxygen species in experimental atherosclerosis by high-resolution near-infrared fluorescence imaging using VCAM-1-targeted liposomes entrapping a fluorogenic redox-sensitive probe. Oxid. Med. Cell. Longev. 2021, 2021, 6685612. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Song, J.W.; Kim, H.J.; Kim, C.-S.; Song, Y.J.; Yang, D.H.; Yoo, H.; Kim, J.W.; Park, K. In vivo Imaging of reactive oxygen species (ROS)-Producing pro-inflammatory macrophages in murine carotid atheromas using a CD44-targetable and ROS-responsive nanosensor. J. Ind. Eng. Chem. 2020, 92, 158–166. [Google Scholar] [CrossRef]
- Rao, N.V.; Rho, J.G.; Um, W.; Pramod Kumar, E.K.; Nguyen, V.Q.; Oh, B.H.; Kim, W.; Park, J.H. Hyaluronic acid nanoparticles as nanomedicine for treatment of inflammatory diseases. Pharmaceutics 2020, 12, 931. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Kim, I.; Park, T.G. Fluorescent gold nanoprobe sensitive to intracellular reactive oxygen species. Adv. Funct. Mater. 2009, 19, 1884–1890. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Kim, I.-H.; Kim, K.; Choi, Y. ROS-Responsive activatable photosensitizing agent for imaging and photodynamic therapy of activated macrophages. Theranostics 2014, 4, 1–11. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kang, M.S.; Lee, H.; Lee, J.H.; Kim, J.; Han, D.W.; Kim, K.S. Recent Trends in photoacoustic imaging techniques for 2D nanomaterial-based phototherapy. Biomedicines 2021, 9, 80. [Google Scholar] [CrossRef]
- Gao, W.; Li, X.; Liu, Z.; Fu, W.; Sun, Y.; Cao, W.; Tong, L.; Tang, B. A redox-responsive self-assembled nanoprobe for photoacoustic inflammation imaging to assess atherosclerotic plaque vulnerability. Anal. Chem. 2019, 91, 1150–1156. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhuang, W.; He, H.; Su, X.; Wang, Y. Two-Photon AIE probe conjugated theranostic nanoparticles for tumor bioimaging and PH-sensitive drug delivery. Nano Res. 2019, 12, 1703–1712. [Google Scholar] [CrossRef]
- Situ, B.; Gao, M.; He, X.; Li, S.; He, B.; Guo, F.; Kang, C.; Liu, S.; Yang, L.; Jiang, M. A Two-Photon AIEgen for Simultaneous Dual-Color Imaging of Atherosclerotic Plaques. Mater. Horiz. 2019, 6, 546–553. [Google Scholar] [CrossRef]
- Ma, B.; Xu, H.; Zhuang, W.; Wang, Y.; Li, G.; Wang, Y. Reactive oxygen species responsive theranostic nanoplatform for two-photon aggregation-induced emission imaging and therapy of acute and chronic inflammation. ACS Nano 2020, 14, 5862–5873. [Google Scholar] [CrossRef]
- Tenório-Neto, E.T.; Lima, D.; Guilherme, M.R.; Lima-Tenório, M.K.; Scariot, D.B.; Nakamura, C.V.; Kunita, M.H.; Rubira, A.F. Synthesis and drug release profile of a dual-responsive poly(ethylene glycol) hydrogel nanocomposite. RSC Adv. 2017, 7, 27637–27644. [Google Scholar] [CrossRef]
- Ma, B.; Xu, H.; Zhuang, W.; Wang, Y.; Li, G.; Wang, Y. ROS responsive nanoplatform with two-photon AIE Imaging for atherosclerosis diagnosis and “two-pronged” therapy. Nano Micro Small 2020, 16, 2003253. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).