Machine Learning for Process Monitoring and Control of Hot-Melt Extrusion: Current State of the Art and Future Directions
Abstract
1. Introduction
2. Machine Learning
2.1. Supervised Machine Learning
2.2. Unsupervised Machine Learning
2.3. Reinforcement Learning
3. Pre-Processing Techniques for In-Process Spectral Data
4. Application of PCA for In-Process Monitoring of Critical Quality Attributes (CQAs)
5. Application of PLS for In-Process Monitoring of Critical Quality Attributes (CQAs)
5.1. In-Process Monitoring of the Drug Content
5.2. In-Process Monitoring of Cocrystal Concentration
5.3. In-Process Monitoring of the Polymer Blend Concentration and Filler Content
5.4. In-Process Monitoring of Polymer Degradation
5.5. In-Process Monitoring of the Mechanical Properties of Polymer Product
5.6. In-Process Monitoring of Filler Particle Size
6. Application of PCA and PLS for Process Fault Detection and Statistical Process Control
7. Application of Non-Linear ML Algorithms for HME Process
7.1. Non-Linear ML Algorithms to Monitor CPP
7.2. Application of Non-Linear ML Algorithms for On/In-Line Monitoring of Product Quality
8. Discussion
8.1. Improvement of Conventional Linear Methods
8.2. The Role of Sensor Integrity and Location
8.3. Potential for Non-Linear ML Methods
8.4. Transferability Challenges for ML Models
8.5. Validation of ML Models
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tiwari, R.V.; Patil, H.; Repka, M.A. Contribution of hot-melt extrusion technology to advance drug delivery in the 21st century. Expert Opin. Drug Deliv. 2016, 13, 451–464. [Google Scholar] [CrossRef]
- Saerens, L.; Ghanam, D.; Raemdonck, C.; Francois, K.; Manz, J.; Krüger, R.; Krüger, S.; Vervaet, C.; Remon, J.P.; De Beer, T. In-line solid state prediction during pharmaceutical hot-melt extrusion in a 12 mm twin screw extruder using Raman spectroscopy. Eur. J. Pharm. Biopharm. 2014, 87, 606–615. [Google Scholar] [CrossRef]
- Van Renterghem, J.; Kumar, A.; Vervaet, C.; Remon, J.P.; Nopens, I.; Vander Heyden, Y.; De Beer, T. Elucidation and visualization of solid-state transformation and mixing in a pharmaceutical mini hot melt extrusion process using in-line Raman spectroscopy. Int. J. Pharm. 2017, 517, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Psimadas, D.; Georgoulias, P.; Valotassiou, V.; Loudos, G. Molecular Nanomedicine towards Cancer. J. Pharm. Sci. 2012, 101, 2271–2280. [Google Scholar] [CrossRef]
- Thiry, J.; Lebrun, P.; Vinassa, C.; Adam, M.; Netchacovitch, L.; Ziemons, E.; Hubert, P.; Krier, F.; Evrard, B. Continuous production of itraconazole-based solid dispersions by hot melt extrusion: Preformulation, optimization and design space determination. Int. J. Pharm. 2016, 515, 114–124. [Google Scholar] [CrossRef]
- Huang, S.; O’Donnell, K.P.; Delpon de Vaux, S.M.; O’Brien, J.; Stutzman, J.; Williams, R.O. Processing thermally labile drugs by hot-melt extrusion: The lesson with gliclazide. Eur. J. Pharm. Biopharm. 2017, 119, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pang, H.; Guo, Z.; Lin, L.; Dong, Y.; Li, G.; Lu, M.; Wu, C. Interactions between drugs and polymers influencing hot melt extrusion. J. Pharm. Pharmacol. 2014, 66, 148–166. [Google Scholar] [CrossRef]
- Crowley, M.M.; Zhang, F.; Koleng, J.J.; McGinity, J.W. Stability of polyethylene oxide in matrix tablets prepared by hot-melt extrusion. Biomaterials 2002, 23, 4241–4248. [Google Scholar] [CrossRef]
- Backes, E.H.; Pires, L.D.N.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Analysis of the Degradation During Melt Processing of PLA/Biosilicate® Composites. J. Compos. Sci. 2019, 3, 52. [Google Scholar] [CrossRef]
- Guo, Z.; Lu, M.; Li, Y.; Pang, H.; Lin, L.; Liu, X.; Wu, C. The utilization of drug-polymer interactions for improving the chemical stability of hot-melt extruded solid dispersions. J. Pharm. Pharmacol. 2014, 66, 285–296. [Google Scholar] [CrossRef]
- Huang, S.; O’Donnell, K.P.; Keen, J.M.; Rickard, M.A.; McGinity, J.W.; Williams, R.O. A New Extrudable Form of Hypromellose: AFFINISOLTM HPMC HME. AAPS PharmSciTech 2016, 17, 106–119. [Google Scholar] [CrossRef]
- Haser, A.; Huang, S.; Listro, T.; White, D.; Zhang, F. An Approach for Chemical Stability during Melt Extrusion of a Drug Substance with a High Melting Point; Elsevier: Amsterdam, The Netherlands, 2017; Volume 524, ISBN 5124710942. [Google Scholar]
- Hengsawas Surasarang, S.; Keen, J.M.; Huang, S.; Zhang, F.; McGinity, J.W.; Williams, R.O. Hot melt extrusion versus spray drying: Hot melt extrusion degrades albendazole. Drug Dev. Ind. Pharm. 2017, 43, 797–811. [Google Scholar] [CrossRef]
- Liu, X.; Lu, M.; Guo, Z.; Huang, L.; Feng, X.; Wu, C. Improving the chemical stability of amorphous solid dispersion with cocrystal technique by hot melt extrusion. Pharm. Res. 2012, 29, 806–817. [Google Scholar] [CrossRef]
- Haser, A.; Cao, T.; Lubach, J.; Listro, T.; Acquarulo, L.; Zhang, F. Melt Extrusion vs. Spray Drying: The Effect of Processing Methods on Crystalline Content of Naproxen-Povidone Formulations; Elsevier: Amsterdam, The Netherlands, 2017; Volume 102, ISBN 5124710942. [Google Scholar]
- Repka, M.A.; McGinity, J.W. Influence of Vitamin E TPGS on the properties of hydrophilic films produced by hot-melt extrusion. Int. J. Pharm. 2000, 202, 63–70. [Google Scholar] [CrossRef]
- Saerens, L.; Dierickx, L.; Lenain, B.; Vervaet, C.; Remon, J.P.; Beer, T. De Raman spectroscopy for the in-line polymer-drug quantification and solid state characterization during a pharmaceutical hot-melt extrusion process. Eur. J. Pharm. Biopharm. 2011, 77, 158–163. [Google Scholar] [CrossRef]
- Saerens, L.; Vervaet, C.; Remon, J.P.; De Beer, T. Visualization and process understanding of material behavior in the extrusion barrel during a hot-melt extrusion process using raman spectroscopy. Anal. Chem. 2013, 85, 5420–5429. [Google Scholar] [CrossRef] [PubMed]
- Saerens, L.; Dierickx, L.; Quinten, T.; Adriaensens, P.; Carleer, R.; Vervaet, C.; Remon, J.P.; De Beer, T. In-line NIR spectroscopy for the understanding of polymer-drug interaction during pharmaceutical hot-melt extrusion. Eur. J. Pharm. Biopharm. 2012, 81, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Aho, J.; Syrjälä, S. Shear viscosity measurements of polymer melts using injection molding machine with adjustable slit die. Polym. Test. 2011, 30, 595–601. [Google Scholar] [CrossRef]
- Dealy, J.M.; Broadhead, T.O. Process rheometers for molten plastics: A survey of existing technology. Polym. Eng. Sci. 1993, 33, 1513–1523. [Google Scholar] [CrossRef]
- Ponrajan, A.; Tonner, T.; Okos, M.; Campanella, O.; Narsimhan, G. Comparing inline extrusion viscosity for different operating conditions to offline capillary viscosity measurements. J. Food Process Eng. 2019, e13199. [Google Scholar] [CrossRef]
- Chen, Z.L.; Chao, P.Y.; Chiu, S.H. Proposal of an empirical viscosity model for quality control in the polymer extrusion process. Polym. Test. 2003, 22, 601–607. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Repka, M.A.; Gerding, T.G.; Repka, S.L.; McGinity, J.W. Influence of plasticizers and drugs on the physical-mechanical properties of hydroxypropylcellulose films prepared by hot melt extrusion. Drug Dev. Ind. Pharm. 1999, 25, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Low, A.Q.J.; Parmentier, J.; Khong, Y.M.; Chai, C.C.E.; Tun, T.Y.; Berania, J.E.; Liu, X.; Gokhale, R.; Chan, S.Y. Effect of type and ratio of solubilising polymer on characteristics of hot-melt extruded orodispersible films. Int. J. Pharm. 2013, 455, 138–147. [Google Scholar] [CrossRef]
- Vo, A.Q.; Feng, X.; Morott, J.T.; Pimparade, M.B.; Tiwari, R.V.; Zhang, F.; Repka, M.A. A novel floating controlled release drug delivery system prepared by hot-melt extrusion. Eur. J. Pharm. Biopharm. 2016, 98, 108–121. [Google Scholar] [CrossRef]
- Patil, H.; Feng, X.; Ye, X.; Majumdar, S.; Repka, M.A. Continuous Production of Fenofibrate Solid Lipid Nanoparticles by Hot-Melt Extrusion Technology: A Systematic Study Based on a Quality by Design Approach. AAPS J. 2015, 17, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Guidance for Industry, PAT-A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance. In FDA/RPSGB Guidance Workshop; No. September; 2004. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070305.pdf (accessed on 14 October 2020).
- Chirkot, T.; Halsey, S.; Swanborough, A. Monitoring the Output of Pharmaceutical Hot Melt Extruders with near Infrared Spectroscopy. NIR News 2014, 25, 15–18. [Google Scholar] [CrossRef]
- Whitaker, D.A.; Buchanan, F.; Billham, M.; McAfee, M. A UV-Vis spectroscopic method for monitoring of additive particle properties during polymer compounding. Polym. Test. 2018, 67, 392–398. [Google Scholar] [CrossRef]
- Kelly, A.L.; Gough, T.; Isreb, M.; Dhumal, R.; Jones, J.W.; Nicholson, S.; Dennis, A.B.; Paradkar, A. In-process rheometry as a PAT tool for hot melt extrusion. Drug Dev. Ind. Pharm. 2018, 44, 670–676. [Google Scholar] [CrossRef]
- Mulrennan, K.; Donovan, J.; Creedon, L.; Rogers, I.; Lyons, J.G.; McAfee, M. A soft sensor for prediction of mechanical properties of extruded PLA sheet using an instrumented slit die and machine learning algorithms. Polym. Test. 2018, 69, 462–469. [Google Scholar] [CrossRef]
- Vo, A.Q.; He, H.; Zhang, J.; Martin, S.; Chen, R.; Repka, M.A. Application of FT-NIR Analysis for In-line and Real-Time Monitoring of Pharmaceutical Hot Melt Extrusion: A Technical Note. AAPS PharmSciTech 2018, 19, 3425–3429. [Google Scholar] [CrossRef]
- Almeida, J.; Bezerra, M.; Markl, D.; Berghaus, A.; Borman, P.; Schlindwein, W. Development and validation of an in-line API quantification method using AQbD principles based on UV-vis spectroscopy to monitor and optimise continuous hot melt extrusion process. Pharmaceutics 2020, 12, 150. [Google Scholar] [CrossRef]
- Guo, X.; Lin, Z.; Wang, Y.; He, Z.; Wang, M.; Jin, G. In-line monitoring the degradation of polypropylene under multiple extrusions based on Raman spectroscopy. Polymers 2019, 11, 1698. [Google Scholar] [CrossRef]
- Montano-herrera, L.; Pratt, S.; Arcos-hernandez, M.V.; Halley, P.J.; Lant, P.A.; Werker, A.; Laycock, B. In-line monitoring of thermal degradation of PHA during melt-processing by Near-Infrared spectroscopy. New Biotechnol. 2013, 31, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Abeykoon, C.; Martin, P.J.; Li, K.; Kelly, A.L. Dynamic modelling of die melt temperature profile in polymer extrusion: Effects of process settings, screw geometry and material. Appl. Math. Model. 2014, 38, 1224–1236. [Google Scholar] [CrossRef]
- Abeykoon, C.; Li, K.; Martin, P.J.; Kelly, A.L. Monitoring and modelling of the effects of process settings and screw geometry on melt pressure generation in polymer extrusion. Int. J. Syst. Control Inf. Process. 2012, 1, 71. [Google Scholar] [CrossRef]
- Liu, X.; Li, K.; McAfee, M.; Deng, J. “Soft-sensor” for real-time monitoring of melt viscosity in polymer extrusion process. In Proceedings of the 49th IEEE conference on decision and control (CDC), Atlanta, GA, USA, 15–17 December 2010; pp. 3469–3474. [Google Scholar]
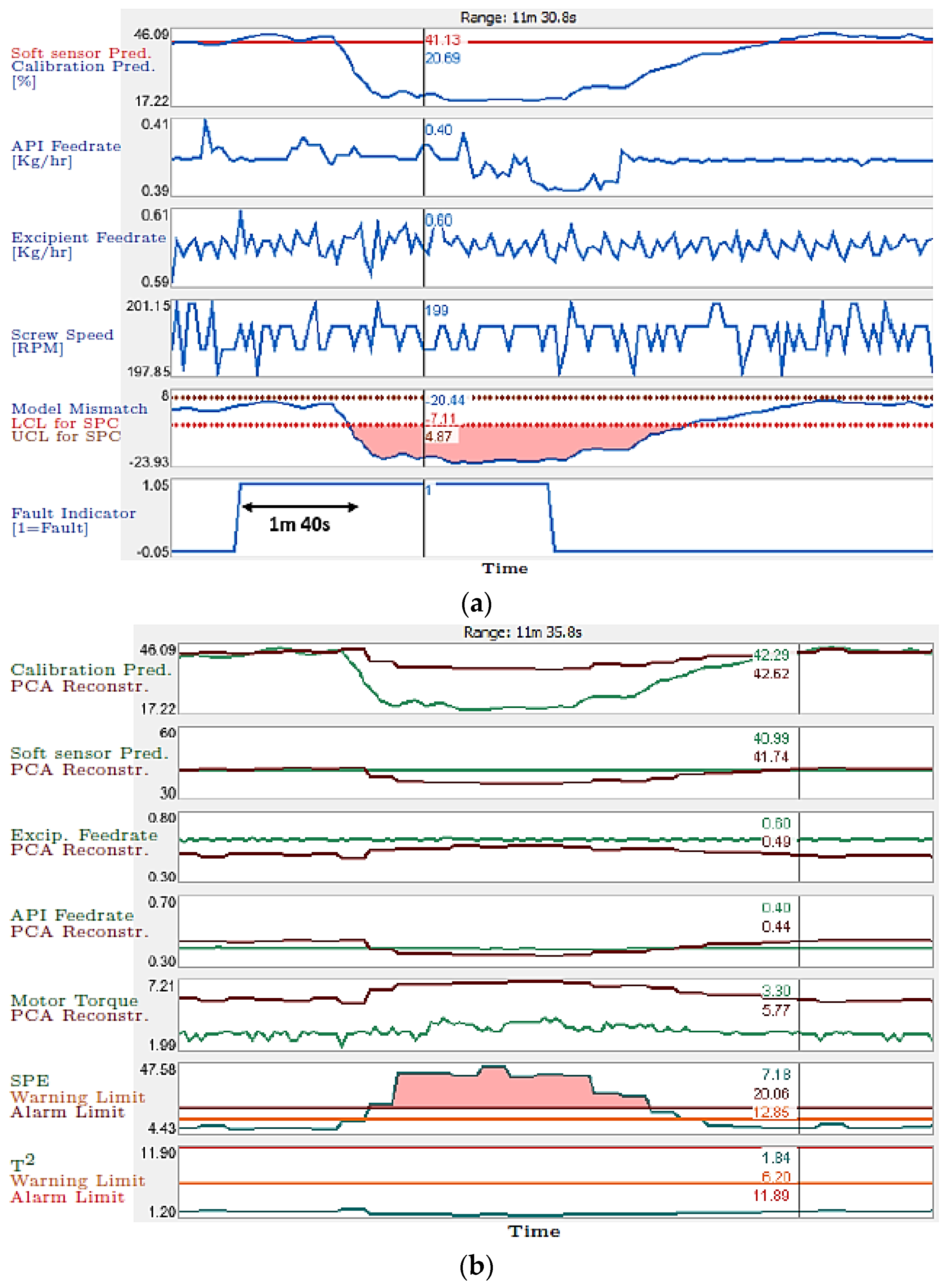
- Tahir, F.; Islam, M.T.; Mack, J.; Robertson, J.; Lovett, D. Process monitoring and fault detection on a hot-melt extrusion process using in-line Raman spectroscopy and a hybrid soft sensor. Comput. Chem. Eng. 2019, 125, 400–414. [Google Scholar] [CrossRef]
- Vynckier, A.K.; Dierickx, L.; Voorspoels, J.; Gonnissen, Y.; Remon, J.P.; Vervaet, C. Hot-melt co-extrusion: Requirements, challenges and opportunities for pharmaceutical applications. J. Pharm. Pharmacol. 2014, 66, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Stanković, M.; Frijlink, H.W.; Hinrichs, W.L.J. Polymeric formulations for drug release prepared by hot melt extrusion: Application and characterization. Drug Discov. Today 2015, 20, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Maddineni, S.; Lu, J.; Repka, M.A. Melt extrusion with poorly soluble drugs. Int. J. Pharm. 2013, 453, 233–252. [Google Scholar] [CrossRef]
- Saerens, L.; Vervaet, C.; Remon, J.P.; De Beer, T. Process monitoring and visualization solutions for hot-melt extrusion: A review. J. Pharm. Pharmacol. 2014, 66, 180–203. [Google Scholar] [CrossRef]
- Repka, M.A.; Bandari, S.; Kallakunta, V.R.; Vo, A.Q.; McFall, H.; Pimparade, M.B.; Bhagurkar, A.M. Melt extrusion with poorly soluble drugs—An integrated review. Int. J. Pharm. 2018, 535, 68–85. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Boateng, J.S.; Snowden, M.J.; Douroumis, D. A Review of Hot-Melt Extrusion: Process Technology to Pharmaceutical Products. ISRN Pharm. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Netchacovitch, L.; Thiry, J.; De Bleye, C.; Chavez, P.F.; Krier, F.; Sacré, P.Y.; Evrard, B.; Hubert, P.; Ziemons, E. Vibrational spectroscopy and microspectroscopy analyzing qualitatively and quantitatively pharmaceutical hot melt extrudates. J. Pharm. Biomed. Anal. 2015, 113, 21–33. [Google Scholar] [CrossRef]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-Melt Extrusion: From Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef]
- Lang, B.; McGinity, J.W.; Williams, R.O. Hot-melt extrusion-basic principles and pharmaceutical applications. Drug Dev. Ind. Pharm. 2014, 40, 1133–1155. [Google Scholar] [CrossRef]
- Maniruzzaman, M. Pharmaceutical applications of hot-melt extrusion: Continuous manufacturing, twin-screw granulations, and 3D printing. Pharmaceutics 2019, 11, 218. [Google Scholar] [CrossRef]
- LaFountaine, J.S.; McGinity, J.W.; Williams, R.O. Challenges and Strategies in Thermal Processing of Amorphous Solid Dispersions: A Review. AAPS PharmSciTech 2016, 17, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, R.; Zia, H. Hot-Melt Extrusion Technique: A review. Iran. J. Pharm. Res. 2011, 5, 1–21. [Google Scholar]
- Jani, R.; Patel, D. Hot melt extrusion: An industrially feasible approach for casting orodispersible film. Asian J. Pharm. Sci. 2014, 10, 292–305. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef]
- Bhairav, B.A.; Kokane, P.A.; Saudagar, R.B. Hot Melt Extrusion Technique—A Review. Res. J. Sci. Technol. 2016, 8, 155. [Google Scholar] [CrossRef]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Battu, S.K.; McGinity, J.W.; Martin, C. Pharmaceutical applications of hot-melt extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Breukelaar, A.H.B. Hot Melt Extrusion Technique. WebmedCentral Pharm. Sci. 2011, 2, 135–139. [Google Scholar] [CrossRef]
- Schmidt, J.; Marques, M.R.G.; Botti, S.; Marques, M.A.L. Recent advances and applications of machine learning in solid-state materials science. NPJ Comput. Mater. 2019, 5, 83. [Google Scholar] [CrossRef]
- Dey, A. Machine Learning Algorithms: A Review. Int. J. Comput. Sci. Inf. Technol. 2016, 7, 1174–1179. [Google Scholar]
- Wosiak, A.; Zamecznik, A.; Niewiadomska-Jarosik, K. Supervised and unsupervised machine learning for improved identification of intrauterine growth restriction types. In Proceedings of the 2016 Federated Conference on Computer Science and Information Systems (FedCSIS), Gdansk, Poland, 11–14 September 2016; Volume 8, pp. 323–329. [Google Scholar] [CrossRef]
- Uddin, S.; Khan, A.; Hossain, M.E.; Moni, M.A. Comparing different supervised machine learning algorithms for disease prediction. BMC Med. Inform. Decis. Mak. 2019, 19, 281. [Google Scholar] [CrossRef]
- Kotsiantis, S.B.; Zaharakis, I.D.; Pintelas, P.E. Machine learning: A review of classification and combining techniques. Artif. Intell. Rev. 2006, 26, 159–190. [Google Scholar] [CrossRef]
- Partheniadis, I.; Toskas, M.; Stavras, F.M.; Menexes, G.; Nikolakakis, I. Impact of hot-melt-extrusion on solid-state properties of pharmaceutical polymers and classification using hierarchical cluster analysis. Processes 2020, 8, 1208. [Google Scholar] [CrossRef]
- López del Val, J.A.; Alonso Pérez de Agreda, J.P. Principal components analysis. Aten. Primaria 1993, 12, 333–338. [Google Scholar] [CrossRef]
- Hammoudeh, A. A Concise Introduction to Reinforcement Learning; Princess Suamaya University for Technology: Amman, Jordan, 2018. [Google Scholar] [CrossRef]
- Morales, E.F.; Zaragoza, J.H. An introduction to reinforcement learning. Decis. Theory Model. Appl. Artif. Intell. Concepts Solut. 2011, 63–80. [Google Scholar] [CrossRef]
- Rinnan, Å.; van den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, Y.P.; Tang, L.J.; Wu, H.L.; Jiang, J.H.; Shen, G.L.; Yu, R.Q. Ensemble preprocessing of near-infrared (NIR) spectra for multivariate calibration. Anal. Chim. Acta 2008, 616, 138–143. [Google Scholar] [CrossRef]
- Almeida, A.; Saerens, L.; De Beer, T.; Remon, J.P.; Vervaet, C. Upscaling and in-line process monitoring via spectroscopic techniques of ethylene vinyl acetate hot-melt extruded formulations. Int. J. Pharm. 2012, 439, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Zeaiter, M.; Roger, J.M.; Bellon-Maurel, V. Robustness of models developed by multivariate calibration. Part II: The influence of pre-processing methods. TrAC Trends Anal. Chem. 2005, 24, 437–445. [Google Scholar] [CrossRef]
- Bi, Y.; Yuan, K.; Xiao, W.; Wu, J.; Shi, C.; Xia, J.; Chu, G.; Zhang, G.; Zhou, G. A local pre-processing method for near-infrared spectra, combined with spectral segmentation and standard normal variate transformation. Anal. Chim. Acta 2016, 909, 30–40. [Google Scholar] [CrossRef]
- De Maesschalck, R.; Estienne, F.; Verdú-Andrés, J.; Candolfi, A.; Centner, V.; Despagne, F.; Jouan-Rimbaud, D.; Walczak, B.; Massart, D.; De Jong, S. The development of calibration models for spectroscopic data using principal component regression. Internet J. Chem. 1999, 2, 1. [Google Scholar]
- Blazhko, U.; Shapaval, V.; Kovalev, V.; Kohler, A. Comparison of augmentation and pre-processing for deep learning and chemometric classification of infrared spectra. Chemom. Intell. Lab. Syst. 2021, 215, 104367. [Google Scholar] [CrossRef]
- Fearn, T.; Riccioli, C.; Garrido-Varo, A.; Guerrero-Ginel, J.E. On the geometry of SNV and MSC. Chemom. Intell. Lab. Syst. 2009, 96, 22–26. [Google Scholar] [CrossRef]
- Radhakrishna Rao, C. The use and interpretation of principal component analysis in applied research. Sankhya Ser. A 1964, 26, 329–358. [Google Scholar]
- Maćkiewicz, A.; Ratajczak, W. Principal Components Analysis (PCA). Comput. Geosci. 1993, 19, 303–342. [Google Scholar] [CrossRef]
- Biancolillo, A.; Marini, F. Chemometric methods for spectroscopy-based pharmaceutical analysis. Front. Chem. 2018, 6, 576. [Google Scholar] [CrossRef] [PubMed]
- Pawar, H.A. Chemometrics and its Application in Pharmaceutical Field. J. Phys. Chem. Biophys. 2014, 4, 4–6. [Google Scholar] [CrossRef]
- Singh, I.; Juneja, P.; Kaur, B.; Kumar, P. Pharmaceutical Applications of Chemometric Techniques. ISRN Anal. Chem. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Sartorius Stedim Data Analytics Simca® 15 User Guide. Sartorius Stedim Data Analytics AB. Available online: https://www.sartorius.com/download/544940/simca-15-user-guide-en-b-00076-sartorius-data.pdf2017 (accessed on 15 April 2021).
- Markl, D.; Wahl, P.R.; Menezes, J.C.; Koller, D.M.; Kavsek, B.; Francois, K.; Roblegg, E.; Khinast, J.G. Supervisory control system for monitoring a pharmaceutical hot melt extrusion process. AAPS PharmSciTech 2013, 14, 1034–1044. [Google Scholar] [CrossRef]
- El-Gindy, A.; Hadad, G.M. Chemometrics in pharmaceutical analysis: An introduction, review, and future perspectives. J. AOAC Int. 2012, 95, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Dadou, S.M.; Tian, Y.; Li, S.; Jones, D.S.; Andrews, G.P. The optimization of process analytical technology for the inline quantification of multiple drugs in fixed dose combinations during continuous processing. Int. J. Pharm. 2021, 592, 120024. [Google Scholar] [CrossRef]
- Tumuluri, S.V.S. Off-line and On-line Measurements of Drug-loaded Hot-Melt Extruded Films Using Raman Spectroscopy. Int. J. Pharm. 2008, 357, 77–84. [Google Scholar] [CrossRef]
- Kelly, A.L.; Halsey, S.A.; Bottom, R.A.; Korde, S.; Gough, T.; Paradkar, A. A novel transflectance near infrared spectroscopy technique for monitoring hot melt extrusion. Int. J. Pharm. 2015, 496, 117–123. [Google Scholar] [CrossRef]
- Kelly, A.L.; Gough, T.; Dhumal, R.S.; Halsey, S.A.; Paradkar, A. Monitoring ibuprofen-nicotinamide cocrystal formation during solvent free continuous cocrystallization (SFCC) using near infrared spectroscopy as a PAT tool. Int. J. Pharm. 2012, 426, 15–20. [Google Scholar] [CrossRef]
- Wood, C.; Alwati, A.; Halsey, S.; Gough, T.; Brown, E.; Kelly, A.; Paradkar, A. Near infra red spectroscopy as a multivariate process analytical tool for predicting pharmaceutical co-crystal concentration. J. Pharm. Biomed. Anal. 2016, 129, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Jafari, M.; Soto, R.; Albadarin, A.B.; Croker, D.; Walker, G. In-line Raman spectroscopy and chemometrics for monitoring cocrystallisation using hot melt extrusion. Int. J. Pharm. 2021, 601, 120555. [Google Scholar] [CrossRef] [PubMed]
- Rohe, T.; Becker, W.; Kölle, S.; Eisenreich, N.; Eyerer, P. Near infrared (NIR) spectroscopy for in-line monitoring of polymer extrusion processes. Talanta 1999, 50, 283–290. [Google Scholar] [CrossRef]
- Barnes, S.E.; Sibley, M.G.; Edwards, H.G.M.; Coates, P.D. Process monitoring of polymer melts using in-line spectroscopy. Trans. Inst. Meas. Control 2007, 29, 453–465. [Google Scholar] [CrossRef]
- Witschnigg, A.; Laske, S.; Kracalik, M.; Feuchter, M.; Pinter, G.; Maier, G.; Marzinger, W.; Haberkorn, M.; Langecker, G.R.; Holzer, C. In-line characterization of polypropylene nanocomposites using FT-NIR. J. Appl. Polym. Sci. 2010, 117, 3047–3053. [Google Scholar] [CrossRef]
- Benneyan, J.C.; Lloyd, R.C.; Plsek, P.E. Statistical process control as a tool for research and healthcare improvement. Qual. Saf. Heal. Care 2003, 12, 458–464. [Google Scholar] [CrossRef]
- Brereton, R.G. Hotelling’s T squared distribution, its relationship to the F distribution and its use in multivariate space. J. Chemom. 2016, 30, 18–21. [Google Scholar] [CrossRef]
- Ghorbani, H. Mahalanobis Distance and Its Application for. Facta Univ. Ser. Math. Inform. 2019, 34, 583–595. [Google Scholar]
- Liu, X.; Li, K.; McAfee, M.; Deng, J. Application of nonlinear PCA for fault detection in polymer extrusion processes. Neural Comput. Appl. 2012, 21, 1141–1148. [Google Scholar] [CrossRef]
- Kazmer, D.O.; Johnston, S.; Hazen, D.; Ambrozic, C. Multivariate Modelling, Fault Detection and Validation for the Exrusion Proces; University of Massachusetts Lowell: Pawtucket St, Lowell, MA, USA, 2012. [Google Scholar]
- Biswas, R.K. Shewhart control chart for individual measurement: An application in a weaving mill. Australas. J. Business, Soc. Sci. Inf. Technol. 2016, 2, 89–93. [Google Scholar]
- Wood, A.K.; Rasid, R. Effect of process variables on melt velocity profiles in extrusion process using single screw plastics extruder. Plast. Rubber Compos. 2003, 32, 193–198. [Google Scholar] [CrossRef]
- Abeykoon, C.; Martin, P.J.; Kelly, A.L.; Brown, E.C. A review and evaluation of melt temperature sensors for polymer extrusion. Sensors Actuators A Phys. 2012, 182, 16–27. [Google Scholar] [CrossRef]
- Abeykoon, C.; Li, K.; McAfee, M.; Martin, P.J.; Deng, J.; Kelly, A.L. Modelling the effects of operating conditions on die melt temperature homogeneity in single screw extrusion. IET Semin. Dig. 2010, 2010, 42–47. [Google Scholar] [CrossRef]
- McAfee, M.; Thompson, S. A novel approach to dynamic modelling of polymer extrusion for improved process control. Proc. Inst. Mech. Eng. Part I J. Syst. Control Eng. 2007, 221, 617–628. [Google Scholar] [CrossRef]
- McAfee, M.; Thompson, S. A Soft Sensor for viscosity control of polymer extrusion. In Proceedings of the 2007 European Control Conference (ECC), Kos, Greece, 2–5 July 2007; pp. 5671–5678. [Google Scholar] [CrossRef]
- Kugler, C.; Dietl, K.; Hochrein, T.; Heidemeyer, P.; Bastian, M. Robust soft sensor based on an artificial neural network for real-time determination of the melt viscosity of polymers. AIP Conf. Proc. 2014, 1593, 213–216. [Google Scholar] [CrossRef]
- McKinley, D.A.; Patel, S.K.; Regev, G.; Rohan, L.C.; Akil, A. Delineating the effects of hot-melt extrusion on the performance of a polymeric film using artificial neural networks and an evolutionary algorithm. Int. J. Pharm. 2019, 571, 118715. [Google Scholar] [CrossRef] [PubMed]
- García, V.; Sánchez, J.S.; Rodríguez-Picón, L.A.; Méndez-González, L.C.; Ochoa-Domínguez, H. de J. Using regression models for predicting the product quality in a tubing extrusion process. J. Intell. Manuf. 2019, 30, 2535–2544. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Z.; Shi, S.; Wang, M.; Jin, G. Fusion of near-infrared and raman spectroscopy for in-line measurement of component content of molten polymer blends. Sensors 2019, 19, 3463. [Google Scholar] [CrossRef] [PubMed]
- Saerens, L.; Segher, N.; Vervaet, C.; Remon, J.P.; De Beer, T. Validation of an in-line Raman spectroscopic method for continuous active pharmaceutical ingredient quantification during pharmaceutical hot-melt extrusion. Anal. Chim. Acta 2014, 806, 180–187. [Google Scholar] [CrossRef]
- Netchacovitch, L.; Thiry, J.; De Bleye, C.; Dumont, E.; Cailletaud, J.; Sacré, P.Y.; Evrard, B.; Hubert, P.; Ziemons, E. Global approach for the validation of an in-line Raman spectroscopic method to determine the API content in real-time during a hot-melt extrusion process. Talanta 2017, 171, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Abeykoon, C. Design and Applications of Soft Sensors in Polymer Processing: A Review. IEEE Sens. J. 2019, 19, 2801–2813. [Google Scholar] [CrossRef]
- Shah, D.; Wang, J.; He, Q.P. A feature-based soft sensor for spectroscopic data analysis. J. Process Control 2019, 78, 98–107. [Google Scholar] [CrossRef]
- Leardl, R.; Norgaard, L. Sequential application of backward interval partial least squares and genetic algorithms for the selection of relevant spectral regions. J. Chemom. 2004, 18, 486–497. [Google Scholar] [CrossRef]
- Marini, F.; Bucci, R.; Ginevro, I.; Magrì, A.L. Coupling of IR measurements and multivariate calibration techniques for the determination of enantiomeric excess in pharmaceutical preparations. Chemom. Intell. Lab. Syst. 2009, 97, 52–63. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, Z.; Zhang, C.; Yuan, S.; Zhu, Y.; Wang, J. PVAm–PIP/PS composite membrane with high performance for CO2/N2 separation. AIChE J. 2012, 59, 215–228. [Google Scholar] [CrossRef]
- Peter He, Q.; Wang, J. Statistics Pattern Analysis: A Statistical Process Monitoring Tool for Smart Manufacturing; Elsevier: Amsterdam, The Netherlands, 2018; Volume 44, ISBN 9780444642417. [Google Scholar]
- Tschudi, J.; O’Farrell, M.; Hestnes Bakke, K.A. Inline Spectroscopy: From Concept to Function. Appl. Spectrosc. 2018, 72, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, M.; Van Hauwermeiren, D.; Hellings, M.; Hermans, E.; Geens, J.; Vervaet, C.; Nopens, I.; De Beer, T. Model-based NIR spectroscopy implementation for in-line assay monitoring during a pharmaceutical suspension manufacturing process. Int. J. Pharm. 2018, 546, 247–254. [Google Scholar] [CrossRef]
- Kazemi, P.; Khalid, M.H.; Gago, A.P.; Kleinebudde, P.; Jachowicz, R.; Szlęk, J.; Mendyk, A. Effect of roll compaction on granule size distribution of microcrystalline cellulose–mannitol mixtures: Computational intelligence modeling and parametric analysis. Drug Des. Dev. Ther. 2017, 11, 241–251. [Google Scholar] [CrossRef]
- Shirazian, S.; Kuhs, M.; Darwish, S.; Croker, D.; Walker, G.M. Artificial neural network modelling of continuous wet granulation using a twin-screw extruder. Int. J. Pharm. 2017, 521, 102–109. [Google Scholar] [CrossRef]
- Khalid, M.H.; Tuszyński, P.K.; Kazemi, P.; Szlek, J.; Jachowicz, R.; Mendyk, A. Transparent computational intelligence models for pharmaceutical tableting process. Complex Adapt. Syst. Model. 2016, 4, 7. [Google Scholar] [CrossRef][Green Version]
- Dengler, S.; Lahriri, S.; Trunzer, E.; Vogel-Heuser, B. Applied machine learning for a zero defect tolerance system in the automated assembly of pharmaceutical devices. Decis. Support Syst. 2021, 146, 113540. [Google Scholar] [CrossRef]
- He, Y.; Ye, Z.; Liu, X.; Wei, Z.; Qiu, F.; Li, H.F.; Zheng, Y.; Ouyang, D. Can machine learning predict drug nanocrystals? J. Control. Release 2020, 322, 274–285. [Google Scholar] [CrossRef]
- Harms, Z.D.; Shi, Z.; Kulkarni, R.A.; Myers, D.P. Characterization of Near-Infrared and Raman Spectroscopy for In-Line Monitoring of a Low-Drug Load Formulation in a Continuous Manufacturing Process. Anal. Chem. 2019, 91, 8045–8053. [Google Scholar] [CrossRef]
- Muteki, K.; Blackwood, D.O.; Maranzano, B.; Zhou, Y.; Liu, Y.A.; Leeman, K.R.; Reid, G.L. Mixture component prediction using iterative optimization technology (Calibration-Free/Minimum Approach). Ind. Eng. Chem. Res. 2013, 52, 12258–12268. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Z.; Su, Y.; Zhao, Q.; Li, X.; Ouyang, D. Deep learning for in vitro prediction of pharmaceutical formulations. Acta Pharm. Sin. B 2019, 9, 177–185. [Google Scholar] [CrossRef]
- Kneale, C.; Brown, S.D. Small moving window calibration models for soft sensing processes with limited history. Chemom. Intell. Lab. Syst. 2018, 183, 36–46. [Google Scholar] [CrossRef]
- Agency, E.M. European Medicines Agency: An unacceptable choice. Prescrire Int. 2011, 20, 278. [Google Scholar]
- Hubert, P.; Nguyen-Huu, J.J.; Boulanger, B.; Chapuzet, E.; Chiap, P.; Cohen, N.; Compagnon, P.A.; Dewé, W.; Feinberg, M.; Lallier, M.; et al. Harmonization of strategies for the validation of quantitative analytical procedures: A SFSTP proposal—Part I. J. Pharm. Biomed. Anal. 2004, 36, 579–586. [Google Scholar] [CrossRef]
- De Bleye, C.; Chavez, P.F.; Mantanus, J.; Marini, R.; Hubert, P.; Rozet, E.; Ziemons, E. Critical review of near-infrared spectroscopic methods validations in pharmaceutical applications. J. Pharm. Biomed. Anal. 2012, 69, 125–132. [Google Scholar] [CrossRef]
- Hubert, P.; Nguyen-Huu, J.J.; Boulanger, B.; Chapuzet, E.; Cohen, N.; Compagnon, P.A.; Dewé, W.; Feinberg, M.; Laurentie, M.; Mercier, N.; et al. Harmonization of strategies for the validation of quantitative analytical procedures. A SFSTP proposal—Part III. J. Pharm. Biomed. Anal. 2007, 45, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Hubert, P.; Nguyen-Huu, J.J.; Boulanger, B.; Chapuzet, E.; Chiap, P.; Cohen, N.; Compagnon, P.A.; Dewé, W.; Feinberg, M.; Lallier, M.; et al. Harmonization of strategies for the validation of quantitative analytical procedures. A SFSTP proposal—Part II. J. Pharm. Biomed. Anal. 2007, 45, 70–81. [Google Scholar] [CrossRef]

| Young’s Modulus | Interlayer Distance | Drawing Force | ||||
|---|---|---|---|---|---|---|
| R2 | RMSECV | R2 | RMSECV | R2 | RMSECV | |
| Geometry 1 | 97.70% | 30 MPa | 93.44% | 0.0.13 nm | 94.59% | 2.64 mN |
| Geometry 2 | 90.55% | 94 MPa | 93.51% | 0.019 nm | 97.50% | 1.98 mN |
| Validation Set | ELM | ANN | PLS | |
|---|---|---|---|---|
| Data | RMSEP | RMSEP | RMSEP | |
| Raman spectra | Validation set 1 | 0.978 | 1.691 | 10.536 |
| Validation set 2 | 2.357 | 3.13 | 25.755 | |
| Validation set 3 | 1.313 | 2.232 | 13.916 | |
| NIR spectra | Validation set 1 | 1.024 | 1.221 | 2.372 |
| Validation set 2 | 2.186 | 2.381 | 3.079 | |
| Validation set 3 | 3.124 | 3.507 | 2.5311 | |
| Low-level data fusion (sample = 500, spectra = 657, NIR spectra = 125, Raman = 532) | Validation set 1 | 0.658 | 1.087 | 1.601 |
| Validation set 2 | 0.95 | 1.291 | 2.02 | |
| Validation set 3 | 1.74 | 1.838 | 5.119 | |
| Mid-level data fusion (sample = 500, spectra = 10, 5 features each from NIR and Raman) | Validation set 1 | 0.992 | 0.941 | 1.915 |
| Validation set 2 | 1.411 | 1.375 | 2.459 | |
| Validation set 3 | 1.68 | 1.617 | 5.45 |
| Algorithm Used | In/On-Line Monitoring | Purpose | Pre-Processing | RMSE on Unseen Data | Polymer | Drug | Software Used | Reference |
|---|---|---|---|---|---|---|---|---|
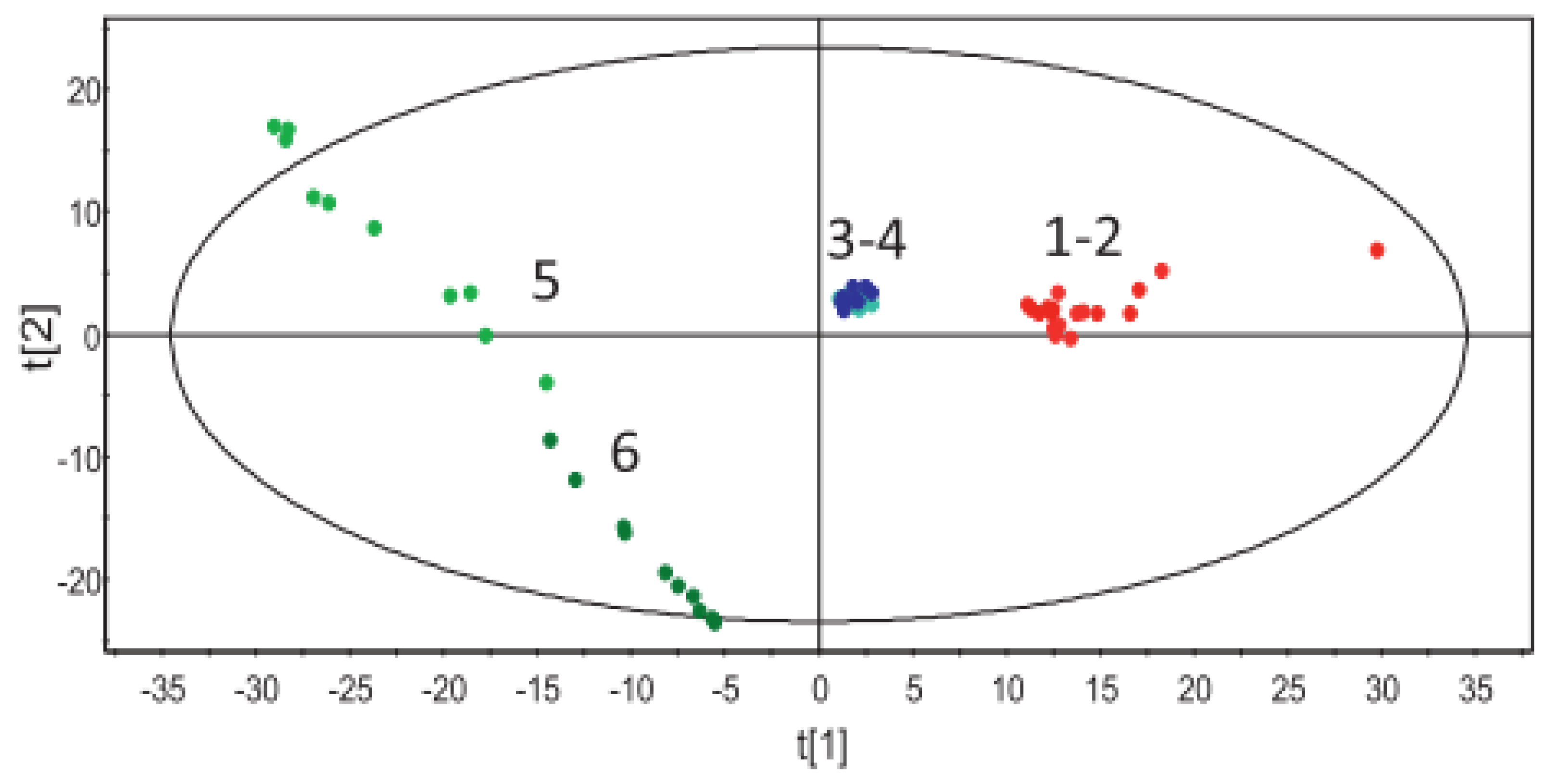
| PCA | Raman and NIR | Solid state | SNV | - | EVA | MPT | SIMCA P+ | [70] |
| Raman | Solid state | SNV | - | Eudragit | MPT | SIMCA P+ | [10] | |
| Raman | Solid state | SNV and mean centring | - | Eudragit | CEL | SIMCA P+ | [2] | |
| Raman | Solid state | SNV | - | Eudragit | MPT | SIMCA P+ | [3] | |
| Raman | Fault detection | - | - | Affinsole | Paracetamol | PharmaMV (Perceptive APC) | [41] | |
| - | API concentration | - | - | Calcium stearate | Paracetamol | SIMCA-Q | [82] | |
| PLS | Raman | API concentration | Second derivative | ketoprofen = 0.94%, clotrimazole = 0.97% | PEO | Ketoprofen, Clotrimazole | Grams™ | [85] |
| Raman | API concentration | SNV, SG | 0.59% | Eudragit | MPT | SIMCA P+ | [9] | |
| NIR | API concentration | MSC, second derivative | 1.54% | Kollidon | MPT | SIMCA P+ | [11] | |
| NIR | Co-crystal concentration | Second derivative | R2 = 0.99 | Nicotinamide | Ibuprofen | TQ Analyst™ | [87] | |
| NIR | Co-crystal concentration | SNV, Second derivative, NS and SGS | 0.95% (Ibuprofen), 3.53% (Carbamazepine) | Nicotinamide | Ibuprofen and Carbamazepine | TQ Analyst™ | [88] | |
| Raman | Co-crystal concentration | SNV | 0.83% | Nicotinamide | Ibuprofen | MATLAB | [89] | |
| Raman | API concentration | MCR | 1.09% | Eudragit | MPT | SIMCA P+ | [108] | |
| UV-Vis | API concentration | Normalisation | Kollidon | Piroxicam | MATLAB | [35] | ||
| PLS | FT-NIR | API concentration | Norris second derivative, SNV, | 0.62% | Eudragit | Ketoprofen | TQ Analyst™ | [34] |
| Raman | API concentration | - | - | Soluplus | Itraconazole | MATLAB | [109] | |
| NIR | API/plasticiser concentration | Second derivative | PEG = 0.67%, CBZ = 1.06% | Kollidon | Carbamazepine | TQ Analyst™ | [86] | |
| NIR Raman | API concentration API concentration | Second derivative SNV, ID, MSC, SG | 0.40% RMP = 1.007% HCTZ = 1.237% | Kollidon Eudragit | Ibuprofen RMP HCTZ | TQ Analyst™ SIMCA | [30] [84] | |
| ANN | - | Dissolution profile, puncture strength and drug content | - | 8.71, 15.46 and 5.32 | PEG, HPC, and vitamin E | Dapivirine | Python | [105] |
| Algorithm Used | In/On-Line Monitoring | Purpose | Pre-Processing | RMSE on Unseen Data | Polymer | Reference |
|---|---|---|---|---|---|---|
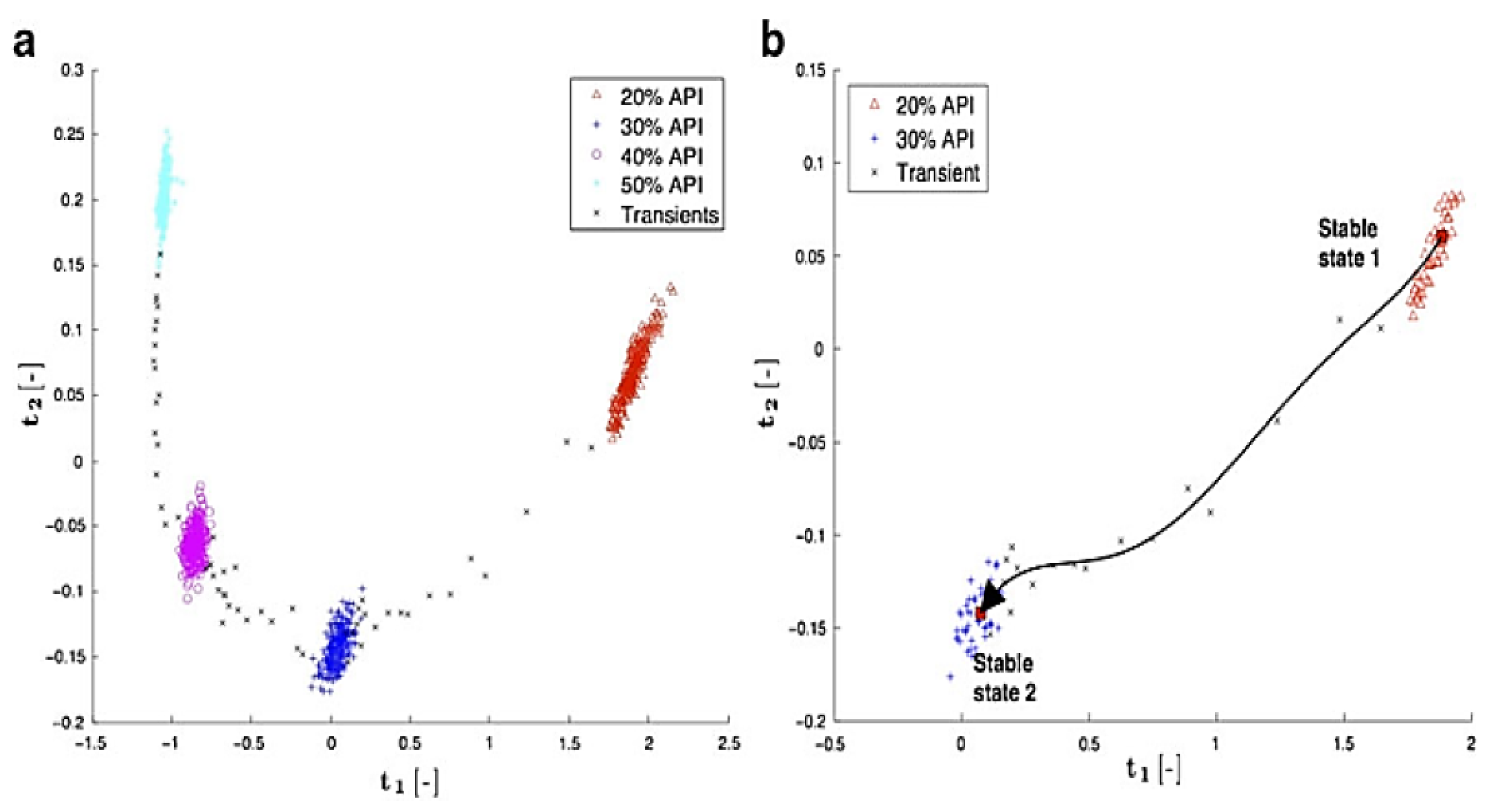
| PCA | Slit die | Fault detection | - | - | LDPE | [96] |
| - | Fault detection | - | - | LDPE, LLDPE | [97] | |
| PLS | NIR | Additive concentration | Spectral averaging and smoothing | 0.38% | PP/PE | [90] |
| PLS | FT-NIR | Filler concentration | - | 0.27% | LDPE | [91] |
| PLS | FT-NIR, Raman | VA monomer contents | Baseline correction (for FT-NIR spectra), MSC (for Raman spectra) | 0.38% (FT-NIR), 0.187% (for Raman) | EVA | [91] |
| PLS | Raman | Degradation | Baseline correction | 1.72% | PP | [36] |
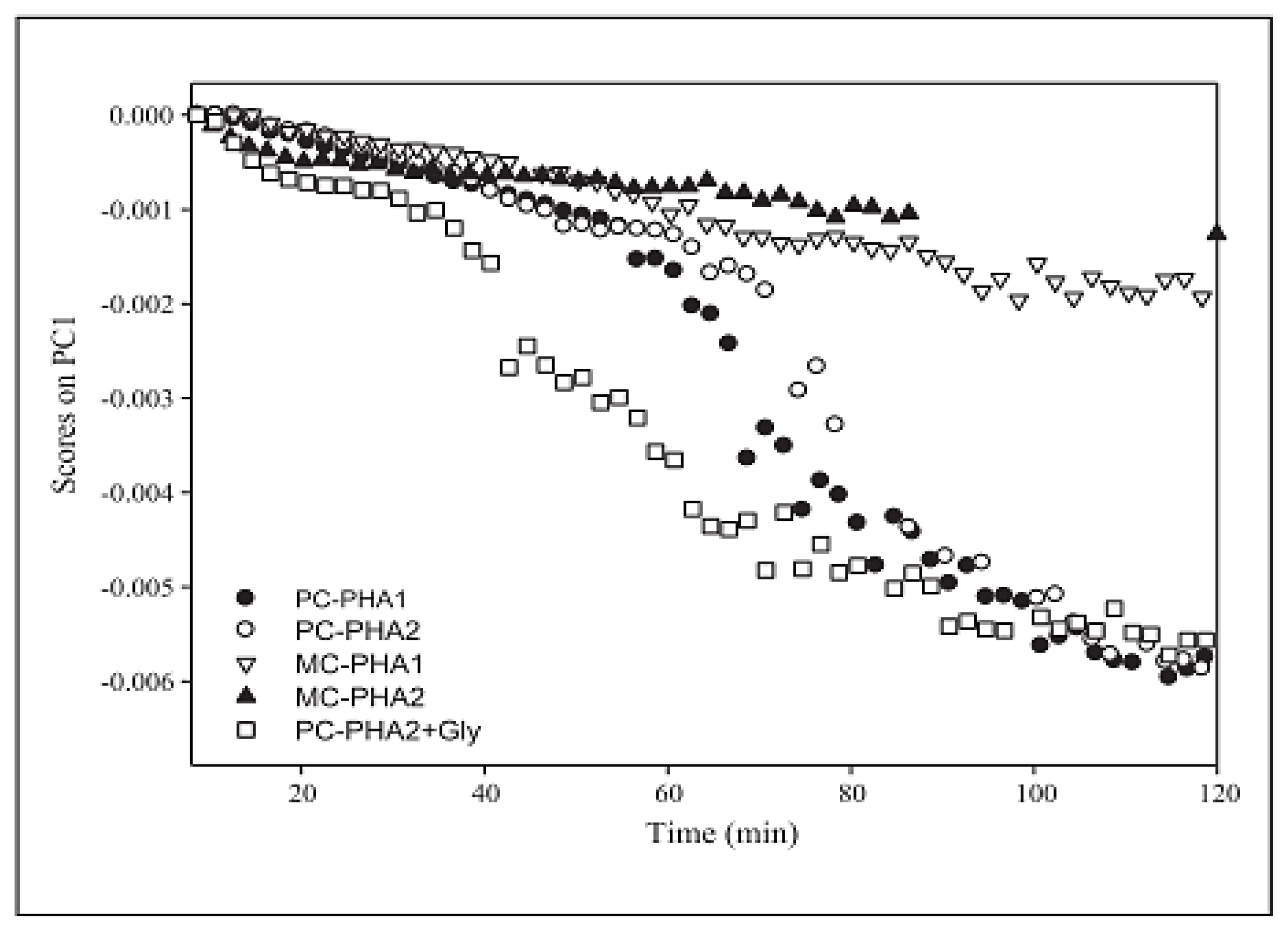
| PLS | NIR | Degradation | MSC, second derivative, mean centring | 0.0126 | PHA | [37] |
| LDA and PLS | UV-Vis | Particle size | - | - | PLA | [37] |
| PLS | NIR | Mechanical properties | Mean centring, SNV, normalisation | - | PP | [92] |
| PCA-Random forest | Slit die | Yield stress | - | 0.25% | PLA | [33] |
| k-NN, SVR, LR | - | Inner and outer diameter of tube | Normalisation | 0.00965 (outer diameter), 0.00107 (inner diameter) | - | [106] |
| ANN, PLS, and ELM | NIR and Raman | Polymer blend concentration | Baseline correction, and normalisation | 0.6583 (best result) | PP/PS | [107] |
| Drug | Original Features | Features Selected by Bi-PLS | Features Selected by GA | RMSEP of Final PLS Model | RMSEP of PLS Model (with All Features) |
|---|---|---|---|---|---|
| Ketoprofen | 1661 | 196 | 9 | 2.12 | 2.32 |
| Mandelic acid | 1391 | 121 | 31 | 4.57 | 6.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munir, N.; Nugent, M.; Whitaker, D.; McAfee, M. Machine Learning for Process Monitoring and Control of Hot-Melt Extrusion: Current State of the Art and Future Directions. Pharmaceutics 2021, 13, 1432. https://doi.org/10.3390/pharmaceutics13091432
Munir N, Nugent M, Whitaker D, McAfee M. Machine Learning for Process Monitoring and Control of Hot-Melt Extrusion: Current State of the Art and Future Directions. Pharmaceutics. 2021; 13(9):1432. https://doi.org/10.3390/pharmaceutics13091432
Chicago/Turabian StyleMunir, Nimra, Michael Nugent, Darren Whitaker, and Marion McAfee. 2021. "Machine Learning for Process Monitoring and Control of Hot-Melt Extrusion: Current State of the Art and Future Directions" Pharmaceutics 13, no. 9: 1432. https://doi.org/10.3390/pharmaceutics13091432
APA StyleMunir, N., Nugent, M., Whitaker, D., & McAfee, M. (2021). Machine Learning for Process Monitoring and Control of Hot-Melt Extrusion: Current State of the Art and Future Directions. Pharmaceutics, 13(9), 1432. https://doi.org/10.3390/pharmaceutics13091432







