Balance of Drug Residence and Diffusion in Lacrimal Fluid Determine Ocular Bioavailability in In Situ Gels Incorporating Tranilast Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Preparation of Tra-NP-Incorporated ISNG
2.4. Characteristics of the Tra-NP-Incorporated ISNGs
2.5. Corneal Toxicity of Tra-NP-Incorporated ISNGs
2.6. Release of Tra from Tra-NP-Incorporated ISNGs
2.7. Tra Contents in Lacrimal Fluid, Blood, Cornea, and Conjunctiva
2.8. Vessel Leakage in Inflammation Using EB
2.9. Measurement of NO and TNF-α Levels
2.10. Statistical Analysis
3. Results
3.1. Evaluation of Physical Properties in the Tra-NP-Incorporated ISNGs
3.2. Evaluation of Dispersibility in the Tra-NP-Incorporated ISNGs
3.3. Effect of the Tra-NP-Incorporated ISNGs on Corneal Toxicity in HCE-T Cell and Rat Corneas
3.4. Release of Tra from Tra-NP-Incorporated ISNGs
3.5. Drug Behavior in Rat Eyes Instilled with Tra-NP-Incorporated ISNGs
3.6. Preventive Effect of the Instillation of Tra-NP-Incorporated ISNGs on Inflammation in the Conjunctiva
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Boddu, S.H.; Gupta, H.; Patel, S. Drug delivery to the back of the eye following topical administration: An update on research and patenting activity. Recent Pat. Drug Deliv. Formul. 2014, 8, 27–36. [Google Scholar] [CrossRef]
- Li, C.C.; Chauhan, A. Modeling ophthalmic drug delivery by soaked contact lenses. Ind. Eng. Chem. Res. 2006, 45, 3718–3734. [Google Scholar] [CrossRef]
- Kim, J.; Chauhan, A. Dexamethasone transport and ocular delivery from poly(hydroxyethyl methacrylate) gels. Int. J. Pharm. 2008, 353, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, Y.; Chauhan, A. Ophthalmic delivery of Cyclosporine A from Brij-97 microemulsion and surfactant-laden p-HEMA hydrogels. Int. J. Pharm. 2008, 361, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Morrison, P.W.J.; Khutoryanskiy, V.V. Advances in ophthalmic drug delivery. Ther. Deliv. 2014, 5, 1297–1315. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.M.; Olejnik, O.; Chang-Lin, J.-E.; Wilson, C.G. Topical and systemic drug delivery to the posterior segments. Adv. Drug Deliv. Rev. 2005, 57, 2010–2032. [Google Scholar] [CrossRef]
- Järvinen, K.; Järvinen, T.; Urtti, A. Ocular absorption following topical delivery. Adv. Drug Deliv. Rev. 1995, 16, 3–19. [Google Scholar] [CrossRef]
- Urtti, A.; Pipkin, J.D.; Rork, G.; Repta, A.J. Controlled drug delivery devices for experimental ocular studies with timolol 1. In vitro release studies. Int. J. Pharm. 1990, 61, 235–240. [Google Scholar] [CrossRef]
- Zimmer, A.; Kreuter, J. Microspheres and nanoparticles used in ocular delivery systems. Adv. Drug Deliv. Rev. 1995, 16, 61–73. [Google Scholar] [CrossRef]
- Gan, L.; Wang, J.; Jiang, M.; Bartlett, H.; Ouyang, D.; Eperjesi, F.; Liu, J.; Gan, Y. Recent advances in topical ophthalmic drug delivery with lipid-based nanocarriers. Drug Discov. Today 2013, 18, 290–297. [Google Scholar] [CrossRef]
- Souto, E.B.; Doktorovova, S.; Gonzalez-Mira, E.; Egea, M.A.; Garcia, M.L. Feasibility of lipid nanoparticles for ocular delivery of anti-inflammatory drugs. Curr. Eye Res. 2010, 35, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Diebold, Y.; Calonge, M. Applications of nanoparticles in ophthalmology. Prog. Retin. Eye Res. 2010, 29, 596–609. [Google Scholar] [CrossRef]
- Li, N.; Zhang, C.; Wang, M.; Sun, X.; Nie, S.; Pan, W. Liposome coated with low molecular weight chitosan and its potential use in ocular drug delivery. Int. J. Pharm. 2009, 379, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Meisner, D.; Mezei, M. Liposome ocular delivery systems. Adv. Drug Deliv. Rev. 1995, 16, 75–93. [Google Scholar] [CrossRef]
- Bauer, M.; Karch, R.; Tournier, N.; Cisternino, S.; Wadsak, W.; Hacker, M.; Marhofer, P.; Zeitlinger, M.; Langer, O. Assessment of P-Glycoprotein Transport Activity at the Human Blood–Retina Barrier with (R)-11C-Verapamil PET. J. Nucl. Med. 2017, 58, 678–681. [Google Scholar] [CrossRef]
- Chapy, H.; Saubaméa, B.; Tournier, N.; Bourasset, F.; Behar-Cohen, X.; Declèves, X.; Scherrmann, J.-M.; Cisternino, S. Blood-brain and retinal barriers show dissimilar ABC transporter impacts and concealed effect of P-glycoprotein on a novel verapamil influx carrier. Br. J. Pharmacol. 2016, 173, 497–510. [Google Scholar] [CrossRef]
- Nagai, N.; Minami, M.; Deguchi, S.; Otake, H.; Sasaki, H.; Yamamoto, N. An in Situ Gelling System based on Methylcellulose and Tranilast Solid Nanoparticles Enhances Ocular Residence Time and Drug Absorption into the Cornea and Conjunctiva. Front. Bioeng. Biotechnol. 2020, 8, 764. [Google Scholar] [CrossRef]
- Minami, M.; Seiriki, R.; Otake, H.; Nakazawa, Y.; Kanai, K.; Tanino, T.; Nagai, N. Development of Sustained-Release Ophthalmic Formulation Based on Tranilast Solid Nanoparticles. Materials 2020, 13, 1675. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ono, H.; Hashino, M.; Ito, Y.; Okamoto, N.; Shimomura, Y. Improved corneal toxicity and permeability of tranilast by the preparation of ophthalmic formulations containing its nanoparticles. J. Oleo Sci. 2014, 63, 177–186. [Google Scholar] [CrossRef]
- Nagai, N.; Isaka, T.; Deguchi, S.; Minami, M.; Yamaguchi, M.; Otake, H.; Okamoto, N.; Nakazawa, Y. In Situ Gelling Systems Using Pluronic F127 Enhance Corneal Permeability of Indomethacin Nanocrystals. Int. J. Mol. Sci. 2020, 21, 7083. [Google Scholar] [CrossRef]
- Thrimawithana, T.R.; Rupenthal, I.D.; Young, S.A.; Alany, R.G. Environment-sensitive polymers for ophthalmic drug delivery. J. Drug Deliv. Sci. Technol. 2012, 22, 117–124. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Das, M.; Jain, S. In situ gel systems as ‘smart’ carriers for sustained ocular drug delivery. Expert Opin. Drug Deliv. 2012, 9, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Edsman, K.; Carlfors, J.; Petersson, R. Rheological evaluation of poloxamer as an in situ gel for ophthalmic use. Eur. J. Pharm. Sci. 1998, 6, 105–112. [Google Scholar] [CrossRef]
- Gilbert, J.C.; Richardson, J.C.; Davies, M.C.; Palin, K.J.; Hadgraft, J. The effect of solutes and polymers on the gelation properties of pluronic F-127 solutions for controlled drug delivery. J. Control. Release 1987, 5, 113–118. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Sousa Lobo, J.M. Influence of drug incorporation, temperature and storage time on the ph, textural and rheological properties of different poloxamer hydrogels. Curr. Drug Deliv. 2013, 10, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chávez, J.J.; López-Cervantes, M.; Naïk, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of thermoreversible Pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar]
- Baeyens, V.; Percicot, C.; Zignani, M.; Deshpande, A.A.; Kaltsatos, V.; Gurny, R. Ocular drug delivery in veterinary medicine. Adv. Drug Deliv. Rev. 1997, 28, 335–361. [Google Scholar] [CrossRef]
- Khateb, K.A.; Ozhmukharnetova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef]
- El-Kamel, A.H. In vitro and in vivo evaluation of Pluronic F127-based ocular delivery system for timolol maleate. Int. J. Pharm. 2002, 241, 47–55. [Google Scholar] [CrossRef]
- Zhang, M.; Djabourov, M.; Bourgaux, C.; Bouchemal, K. Nanostructured fluids from pluronic mixtures. Int. J. Pharm. 2013, 454, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.R.; Sung, K.C. Carbopol/pluronic phase change solutions for ophthalmic drug delivery. J. Control. Release 2000, 69, 379–388. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Sousa Lobo, J.M. In situ gelling systems: A strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov. Today 2014, 19, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Sousa Lobo, J.M. Applications of poloxamers in ophthalmic pharmaceutical formulations: An overview. Expert. Opin. Drug Deliv. 2013, 10, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent advances in ocular drug delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [Google Scholar] [CrossRef]
- Ito, S.; Sakamoto, T.; Tahara, Y.; Goto, Y.; Akazawa, K.; Ishibashi, T.; Inomata, H. The effect of tranilast on experimental proliferative vitreoretinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 1999, 237, 691–696. [Google Scholar] [CrossRef]
- Isaji, M.; Aruga, N.; Naito, J.; Miyata, H. Inhibition by tranilast of collagen accumulation in hypersensitive granulomatous inflammation in vivo and of morphological changes and functions of fibroblasts in vitro. Life Sci. 1994, 55, PL287–PL292. [Google Scholar] [CrossRef]
- García, M.M. New approach to the mechanism of antiasthmatic action of tranilast. Allergol. Immunopathol. 1990, 18, 53–56. [Google Scholar]
- Ogawa, Y.; Dogru, M.; Uchino, M.; Tatematsu, Y.; Kamoi, M.; Yamamoto, Y.; Ogawa, J.; Ishida, R.; Kaido, M.; Hara, S.; et al. Topical tranilast for treatment of the early stage of mild dry eye associated with chronic GVHD. Bone Marrow Transplant. 2010, 45, 565–569. [Google Scholar] [CrossRef]
- Mukai, S.; Ogawa, Y.; Saya, H.; Kawakami, Y.; Tsubota, K. Therapeutic potential of tranilast for the treatment of chronic graft-versus-host disease in mice. PLoS ONE 2018, 13, e0203742. [Google Scholar] [CrossRef]
- Fernandez-Robredo, P.; Recalde, S.; Moreno-Orduña, M.; García-García, L.; Zarranz-Ventura, J.; García-Layana, A. Azithromycin reduces inflammation in a rat model of acute conjunctivitis. Mol. Vis. 2013, 19, 153–165. [Google Scholar]
- Chen, Y.; Hong, X. Effects of carvedilol reduce conjunctivitis through changes in inflammation, NGF and VEGF levels in a rat model. Exp. Ther. Med. 2016, 11, 1987–1992. [Google Scholar] [CrossRef][Green Version]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Energy-dependent endocytosis is responsible for drug transcorneal penetration following the instillation of ophthalmic formulations containing indomethacin nanoparticles. Int. J. Nanomed. 2019, 14, 1213–1227. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology 2014, 319, 53–62. [Google Scholar] [CrossRef]
- Araki-Sasaki, K.; Ohashi, Y.; Sasabe, T.; Hayashi, K.; Watanabe, H.; Tano, Y.; Handa, H. An SV40-immortalized human corneal epithelial cell line and its charac-terization. Investig. Ophthalmol. Vis. Sci. 1995, 36, 614–621. [Google Scholar]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) triblock copolymers in aqueous solutions: Thermodynamics of copolymer association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Mengi, S.A.; Deshpande, S.G. Comparative evaluation of Butea frondosa and flurbiprofen for ocular anti-inflammatory activity in rabbits. J. Pharm. Pharmacol. 1995, 47, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.D.; Blanchard, J. In vitro evaluation of Pluronic F127-based controlled-release ocular delivery systems for pilocarpine. J. Pharm. Sci. 1998, 87, 226–230. [Google Scholar] [CrossRef]
- Aka-Any-Grah, A.; Bouchemal, K.; Koffi, A.; Agnely, F.; Zhang, M.; Djabourov, M.; Ponchel, G. Formulation of mucoadhesive vaginal hydrogels insensitive to dilution with vaginal fluids. Eur. J. Pharm. Biopharm. 2010, 76, 296–303. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, N.; Hua, H.; Liu, T.; Tang, Y.; Fu, L.; Yang, Y.; Ma, X.; Zhao, Y. Preparation, pharmacokinetics and pharmacodynamics of ophthalmic thermosensitive in situ hydrogel of betaxolol hydrochloride. Biomed. Pharmacother. 2016, 83, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Kawase, K.; Lin, W.; Aoyama, Y.; Yamamoto, T.; Shimazawa, M.; Hara, H. Effects of timolol-related ophthalmic solutions on cultured human conjunctival cells. Jpn. J. Ophthalmol. 2010, 54, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chiu, K.T.; Sun, Y.T.; Chen, W.C. Role of the cyclic AMP-protein kinase A pathway in lipopolysaccharide-induced nitric oxide synthase expression in RAW 264.7 macrophages. Involvement of cyclooxygenase-2. J. Biol. Chem. 1999, 274, 31559–31564. [Google Scholar] [CrossRef] [PubMed]
- Shapira, L.; Soskolne, W.A.; Houri, Y.; Barak, V.; Halabi, A.; Stabholz, A. Protection against endotoxic shock and lipopolysaccharide-induced local inflammation by tetracycline: Correlation with inhibition of cytokine secretion. Infect. Immun. 1996, 64, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Hodyl, N.A.; Krivanek, K.M.; Lawrence, E.; Clifton, V.L.; Hodgson, D.M. Prenatal exposure to a pro-inflammatory stimulus causes delays in the development of the innate immune response to LPS in the offspring. J. Neuroimmunol. 2007, 190, 61–71. [Google Scholar] [CrossRef]
- Allon, N.; Chapman, S.; Shalem, Y.; Brandeis, R.; Weissman, B.A.; Amir, A. Lipopolysaccharide induced protection against sulfur mustard cytotoxicity in RAW264.7 cells through generation of TNF-alpha. J. Toxicol. Sci. 2010, 35, 345–355. [Google Scholar] [CrossRef]
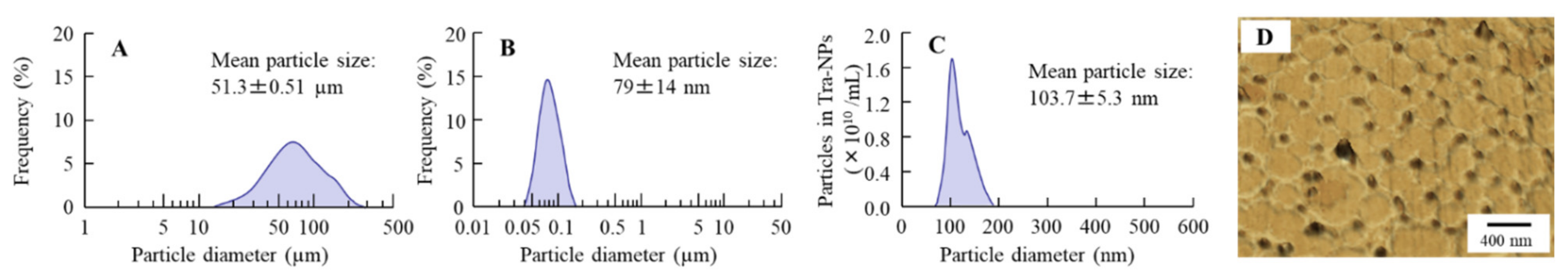



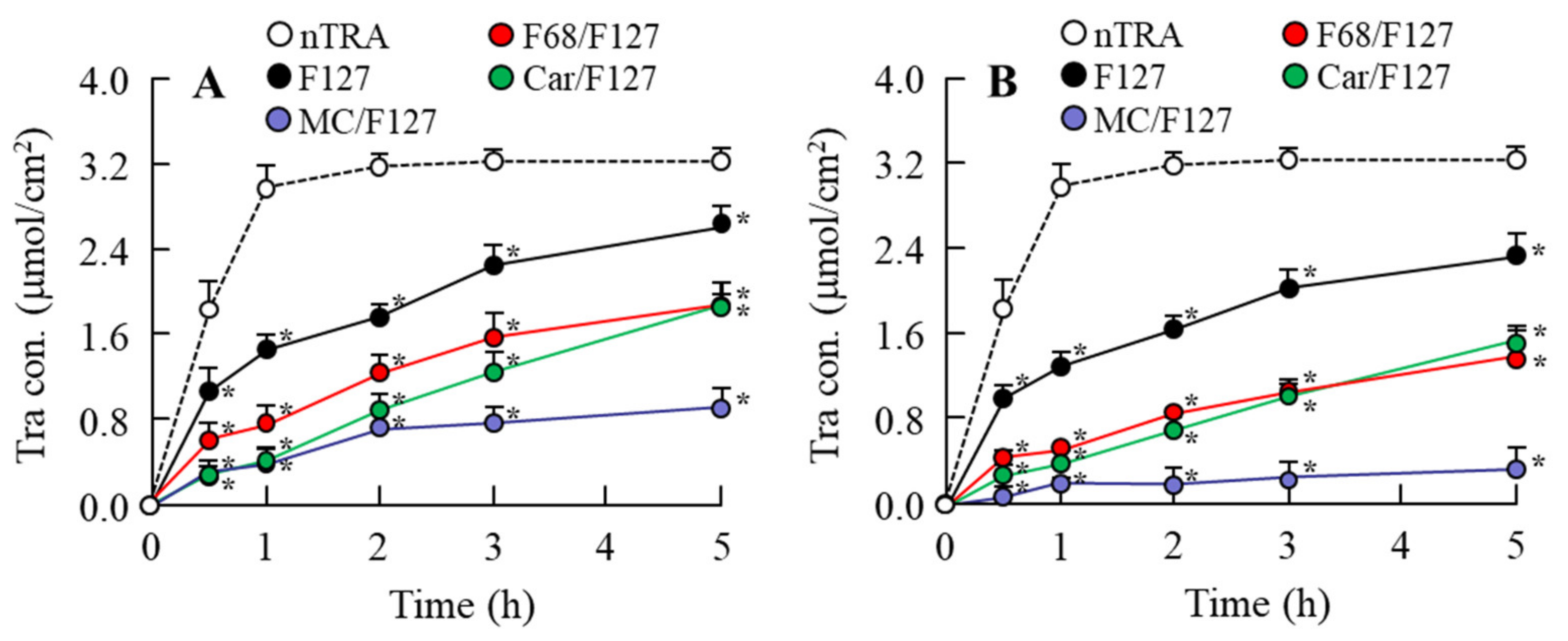
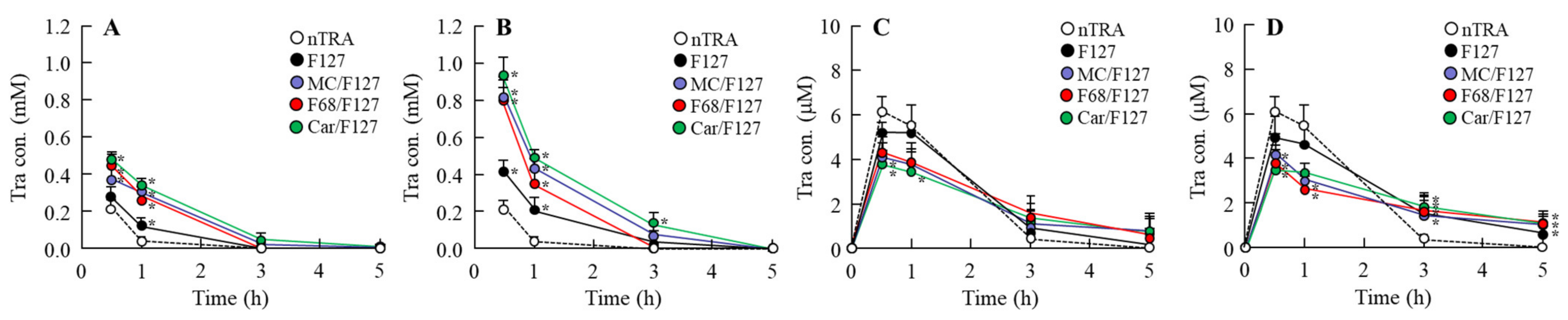



| Formulation | Treatment (w/v %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tra | BAC | Man | HPβCD | F127 | MC | F68 | Car | |||
| Non-ISG | mTRA | 0.5 | 0.001 | 0.1 | 5 | - | - | - | - | - |
| nTRA | 0.5 | 0.001 | 0.1 | 5 | - | - | - | - | Bead mill | |
| Low ISG base | nTRA-F127-L | 0.5 | 0.001 | 0.1 | 5 | 10 | - | - | Bead mill | |
| nTRA-MC/F127-L | 0.5 | 0.001 | 0.1 | 5 | 10 | 3 | - | - | Bead mill | |
| nTRA-F68/F127-L | 0.5 | 0.001 | 0.1 | 5 | 10 | - | 3 | - | Bead mill | |
| nTRA-Car/F127-L | 0.5 | 0.001 | 0.1 | 5 | 10 | - | - | 0.2 | Bead mill | |
| High ISG base | nTRA-F127-H | 0.5 | 0.001 | 0.1 | 5 | 15 | - | - | - | Bead mill |
| nTRA-MC/F127-H | 0.5 | 0.001 | 0.1 | 5 | 15 | 3 | - | - | Bead mill | |
| nTRA-F68/F127-H | 0.5 | 0.001 | 0.1 | 5 | 15 | - | 3 | - | Bead mill | |
| nTRA-Car/F127-H | 0.5 | 0.001 | 0.1 | 5 | 15 | - | - | 0.2 | Bead mill | |
| Formulation | Particle Size (µm) | NP Number (×1011 Particles/mL) | Zeta Potential (mV) | Solubility (mM) | Viscosity (mPa·s) | ||
|---|---|---|---|---|---|---|---|
| 4 °C | 20 °C | 37 °C, pH6.8 | |||||
| nTRA | 103 ± 5.3 | 10 ± 1.1 | –55 ± 0.9 | 0.30 ± 0.05 | 1.4 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 |
| nTRA-F127-L | 117 ± 4.8 | 10 ± 0.8 | –50 ± 1.3 * | 0.55 ± 0.05 * | 6.6 ± 0.5 * | 5.9 ± 0.6 * | 5.7 ± 0.4 * |
| nTRA-F127-H | 110 ± 6.9 | 10 ± 1.1 | –46 ± 1.4 *,# | 0.77 ± 0.06 *,# | 20 ± 1.8 *,# | 16 ± 0.8 *,# | 65 ± 6.1 *,#,$ |
| nTRA-MC/F127-L | 108 ± 4.5 | 9.3 ± 0.6 | –56 ± 1.0 | 0.56 ± 0.05 * | 8.9 ± 1.1 * | 8.0 ± 1.0 * | 12 ± 1.0 *,$ |
| nTRA-MC/F127-H | 92 ± 5.7 | 9.0 ± 0.4 | –45 ± 0.5 *,# | 0.78 ± 0.06 *,# | 87 ± 5.4 *,# | 76 ± 5.6 *,# | 119 ± 8.9 *,#,$ |
| nTRA-F68/F127-L | 102 ± 4.2 | 8.8 ± 0.6 | –52 ± 1.2 * | 0.85 ± 0.06 * | 8.5 ± 0.6 * | 7.6 ± 0.8 * | 9.3 ± 0.9 * |
| nTRA-F68/F127-H | 103 ± 7.2 | 8.2 ± 1.7 | –38 ± 1.8 *,# | 0.87 ± 0.08 * | 73 ± 5.3 *,# | 70 ± 4.9 *,# | 106 ± 8.7 *,#,$ |
| nTRA-Car/F127-L | 106 ± 6.4 | 8.4 ± 1.3 | –78 ± 1.1 * | 1.62 ± 0.18 * | 8.6 ± 0.6 * | 7.7 ± 0.6 * | 28.8 ± 2.1 *,$ |
| nTRA-Car/F127-H | 94 ± 7.8 | 11 ± 1.9 | –89 ± 1.8 *,# | 2.34 ± 0.23 *,# | 78 ± 5.1 *,# | 70 ± 5.7 *,# | 123 ± 7.8 *,#,$ |
| Formulation | nTRA | nTRA-F127 | nTRA-MC/F12 | nTRA-F68/F127 | nTRA-Car/F127 | ||||
|---|---|---|---|---|---|---|---|---|---|
| L | H | L | H | L | H | L | H | ||
| Particle size (nm) | 238 ± 3.2 | 203 ± 7.7 | 173 ± 3.5 | 204 ± 6.1 | 157 ± 3.4 | 203 ± 7.0 | 186 ± 2.3 | 173 ± 5.8 | 173 ± 3.7 |
| NP number (×109 particles/mL) | 17 ± 0.8 | 13 ± 1.1 | 11 ± 0.7 | 3.5 ± 0.2 | 2.9 ± 0.2 | 8.7 ± 0.6 | 6.3 ± 0.2 | 9.2 ± 0.9 | 7.6 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minami, M.; Otake, H.; Nakazawa, Y.; Okamoto, N.; Yamamoto, N.; Sasaki, H.; Nagai, N. Balance of Drug Residence and Diffusion in Lacrimal Fluid Determine Ocular Bioavailability in In Situ Gels Incorporating Tranilast Nanoparticles. Pharmaceutics 2021, 13, 1425. https://doi.org/10.3390/pharmaceutics13091425
Minami M, Otake H, Nakazawa Y, Okamoto N, Yamamoto N, Sasaki H, Nagai N. Balance of Drug Residence and Diffusion in Lacrimal Fluid Determine Ocular Bioavailability in In Situ Gels Incorporating Tranilast Nanoparticles. Pharmaceutics. 2021; 13(9):1425. https://doi.org/10.3390/pharmaceutics13091425
Chicago/Turabian StyleMinami, Misa, Hiroko Otake, Yosuke Nakazawa, Norio Okamoto, Naoki Yamamoto, Hiroshi Sasaki, and Noriaki Nagai. 2021. "Balance of Drug Residence and Diffusion in Lacrimal Fluid Determine Ocular Bioavailability in In Situ Gels Incorporating Tranilast Nanoparticles" Pharmaceutics 13, no. 9: 1425. https://doi.org/10.3390/pharmaceutics13091425
APA StyleMinami, M., Otake, H., Nakazawa, Y., Okamoto, N., Yamamoto, N., Sasaki, H., & Nagai, N. (2021). Balance of Drug Residence and Diffusion in Lacrimal Fluid Determine Ocular Bioavailability in In Situ Gels Incorporating Tranilast Nanoparticles. Pharmaceutics, 13(9), 1425. https://doi.org/10.3390/pharmaceutics13091425








