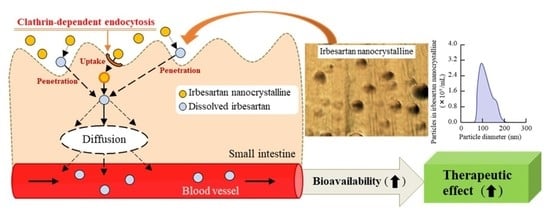
Nanocrystalline Suspensions of Irbesartan Enhance Oral Bioavailability by Improving Drug Solubility and Leading Endocytosis Uptake into the Intestine
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Production of IRB-NC Suspensions
2.4. Measurement of Irbesartan by the HPLC Method
2.5. Evaluation of the Characteristics in the IRB-NC Suspensions
2.6. In Vitro Intestinal Penetration of Irbesartan in Rats
2.7. Measurement of the Irbesartan Concentration in Rat Blood
2.8. Measurement of Blood Pressure (BP) in SHR-SP Rats Orally Administered Irbesartan
2.9. Statistical Analysis
3. Results
3.1. Changes in the Characteristics of Irbesartan Particles with or without Bead Mill Treatment
3.2. Relationships between Energy-Dependent Endocytosis and the Transintestinal Penetration of the IRB-NC Suspensions in Rat Intestines
3.3. Changes in the Therapeutic Effect of the IRB-NC Suspensions in the Intestines of SHR-SP Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dahlof, B.; Hansson, L.; Lindholm, L.H.; Schersten, B.; Ekbom, T.; Wester, P.O. Swedish Trial in Old Patients with Hypertension (STOP-Hypertension) analyses performed up to 1992. Clin. Exp. Hypertens. 1993, 15, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991, 265, 3255–3264. [CrossRef]
- Jansook, P.; Muankaew, C.; Stefánsson, E.; Loftsson, T. Development of eye drops containing antihypertensive drugs: Formulation of aqueous irbesartan/γCD eye drops. Pharm. Dev. Technol. 2015, 20, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Necciari, J.; Denolle, T.; Le Coz, F. Pharmacokinetics of SR 47436 (BMS 186295), a new angiotensin II receptor antagonist in man. J. Hypertens. 1994, 12, 88. [Google Scholar]
- Ribstein, J.; Sissmann, J.; Picard, A.; Bouroudian, M.; Mimran, A. Effects of the angiotensin II antagonist SR 47436 (BMS 186295) on the pressor response to exogenous angiotensin II and the renin-angiotensin system in sodium replete normal subjects. J. Hypertens. 1994, 12, 131. [Google Scholar]
- Cazaubon, C.; Gougat, J.; Bousquet, F.; Guiraudou, P.; Lacour, C.; Roccon, A.; Galindo, G.; Barthelemy, G.; Gautret, B. Pharmacological characterization of SR 47436, a new nonpeptide AT1 subtype angiotensin II receptor antagonist. J. Pharmacol. Exp. Ther. 1993, 265, 826–834. [Google Scholar]
- Ramos, J.J.M.; Diogo, H.P. Thermal behavior and molecular mobility in the glassy state of three anti-hypertensive pharmaceutical ingredients. RSC Adv. 2017, 7, 10831–10840. [Google Scholar] [CrossRef]
- Pan, D.; Crull, G.; Yin, S.; Grosso, J. Low level drug product API form analysis–Avalide tablet NIR quantitative method development and robustness challenges. J. Pharm. Biomed. Anal. 2014, 89, 268–275. [Google Scholar] [CrossRef]
- Brunner, H.R. The new angiotensin II receptor antagonist, irbesartan: Pharmacokinetic and pharmacodynamic considerations. Am. J. Hypertens. 1997, 10, 311S–317S. [Google Scholar] [CrossRef]
- Hedaya, M.A.; Helmy, S.A. Modeling of the pharmacokinetic/pharmacodynamics interaction between irbesartan and hydrochlorothiazide in normotensive subjects. Biopharm. Drug Dispos. 2015, 36, 216–231. [Google Scholar] [CrossRef]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Impact of particle elasticity on particle-based drug delivery systems. Adv. Drug Deliv. Rev. 2017, 108, 51–67. [Google Scholar] [CrossRef]
- Liversidge, G.G.; Cundy, K.C. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int. J. Pharm. 1995, 125, 91–97. [Google Scholar] [CrossRef]
- Kesisoglou, F.; Mitra, A. Crystalline nanosuspensions as potential toxicology and clinical oral formulations for BCS II/IV compounds. AAPS J. 2012, 14, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Jinno, J.-I.; Kamada, N.; Miyake, M.; Yamada, K.; Mukai, T.; Odomi, M.; Toguchi, H.; Liversidge, G.G.; Higaki, K.; Kimura, T. Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogs. J. Control. Release 2006, 111, 56–64. [Google Scholar] [CrossRef]
- Kipp, J.E. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int. J. Pharm. 2004, 284, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jog, R.; Shen, J.; Zolnik, B.; Sadrieh, N.; Burgess, D.J. In Vitro and in Vivo Performance of Different Sized Spray-Dried Crystalline Itraconazole. J. Pharm. Sci. 2015, 104, 3018–3028. [Google Scholar] [CrossRef]
- Jog, R.; Burgess, D.J. Comprehensive quality by design approach for stable nanocrystalline drug products. Int. J. Pharm. 2019, 564, 426–460. [Google Scholar] [CrossRef]
- Verma, S.; Gokhale, R.; Burgess, D.J. A comparative study of top-down and bottom-up approaches for the preparation of micro/nanosuspensions. Int. J. Pharm. 2009, 380, 216–222. [Google Scholar] [CrossRef]
- Ravichandran, R. Nanoparticles in drug delivery: Potential green nanobiomedicine applications. Int. J. Green Nanotechnol. Biomed. 2009, 1, B108–B130. [Google Scholar] [CrossRef]
- Ishii, M.; Fukuoka, Y.; Deguchi, S.; Otake, H.; Tanino, T.; Nagai, N. Energy-Dependent Endocytosis is Involved in the Absorption of Indomethacin Nanoparticles in the Small Intestine. Int. J. Mol. Sci. 2019, 20, 476. [Google Scholar] [CrossRef]
- Nagai, N.; Minami, M.; Deguchi, S.; Otake, H.; Sasaki, H.; Yamamoto, N. An In Situ Gelling System based on Methylcellulose and Tranilast Solid Nanoparticles Enhances Ocular Residence Time and Drug Absorption into the Cornea and Conjunctiva. Front. Bioeng. Biotechnol. 2020, 8, 764. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Energy-dependent endocytosis is responsible for drug transcorneal penetration following the instillation of ophthalmic formulations containing indomethacin nanoparticles. Int. J. Nanomed. 2019, 14, 1213–1227. [Google Scholar] [CrossRef]
- Nagai, R.; Nagata, S.; Fukuya, F.; Higaki, J.; Rakugi, H.; Ogihara, T. Changes in autonomic activity and baroreflex sensitivity with the hypertension process and age in rats. Clin. Exp. Pharmacol. Physiol. 2003, 30, 419–425. [Google Scholar] [CrossRef]
- Griffin, K.A.; Churchill, P.C.; Picken, M.; Webb, R.C.; Kurtz, T.W.; Bidani, A.K. Differential salt-sensitivity in the pathogenesis of renal damage in SHR and stroke prone SHR. Am. J. Hypertens. 2001, 14, 311–320. [Google Scholar] [CrossRef]
- Nagai, N.; Seiriki, R.; Deguchi, S.; Otake, H.; Hiramatsu, N.; Sasaki, H.; Yamamoto, N. Hydrogel Formulations Incorporating Drug Nanocrystals Enhance the Therapeutic Effect of Rebamipide in a Hamster Model for Oral Mucositis. Pharmaceutics 2020, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, K.; Manaloto, E.; Casey, A.; Cribaro, G.P.; Byrne, H.J.; Tian, F.; Baracia, C.; Conway, G.E.; Cullen, P.J.; et al. Cold Atmospheric Plasma Induces ATP-Dependent Endocytosis of Nanoparticles and Synergistic U373MG Cancer Cell Death. Sci. Rep. 2018, 8, 5298. [Google Scholar] [CrossRef]
- Mäger, I.; Langel, K.; Lehto, T.; Eiríksdóttir, E.; Langel, U. The role of endocytosis on the uptake kinetics of luciferin-conjugated cell-penetrating peptides. Biochim. Biophys. Acta 2012, 1818, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Malomouzh, A.I.; Mukhitov, A.R.; Proskurina, S.E.; Vyskocil, F.; Nikolsky, E.E. The effect of dynasore, a blocker of dynamin-dependent endocytosis, on spontaneous quantal and non-quantal release of acetylcholine in murine neuromuscular junctions. Dokl. Biol. Sci. 2014, 459, 330–333. [Google Scholar] [CrossRef]
- Hufnagel, H.; Hakim, P.; Lima, A.; Hollfelder, F. Fluid phase endocytosis contributes to transfection of DNA by PEI-25. Mol. Ther. 2009, 17, 1411–1417. [Google Scholar] [CrossRef]
- Kumar, S.; Burgess, D.J. Wet milling induced physical and chemical instabilities of naproxen nano-crystalline suspensions. Int. J. Pharm. 2014, 466, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology 2014, 319, 53–62. [Google Scholar] [CrossRef]
- Araya-Sibaja, A.M.; de Campos, C.E.M.; Fandaruff, C.; Vega-Baudrit, J.R.; Guillén-Girón, T.; Navarro-Hoyos, M.; Cuffini, S.L. Irbesartan desmotropes: Solid-state characterization, thermodynamic study and dissolution properties. J. Pharm. Anal. 2019, 9, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Lykotrafitis, G.; Bao, G.; Suresh, S. Size-dependent endocytosis of nanoparticles. Adv. Mater. 2009, 21, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Pouleur, H.G. Clinical overview of irbesartan: A new angiotensin II receptor antagonist. Am. J. Hypertens. 1997, 10, 318S–324S. [Google Scholar] [CrossRef][Green Version]
- Lipinski, C.A. Poor aqueous solubility—An industry wide problem in drug discovery. Am. Pharm. Rev. 2002, 5, 82–85. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deguchi, S.; Ogata, F.; Watanabe, M.; Otake, H.; Yamamoto, N.; Kawasaki, N.; Nagai, N. Nanocrystalline Suspensions of Irbesartan Enhance Oral Bioavailability by Improving Drug Solubility and Leading Endocytosis Uptake into the Intestine. Pharmaceutics 2021, 13, 1404. https://doi.org/10.3390/pharmaceutics13091404
Deguchi S, Ogata F, Watanabe M, Otake H, Yamamoto N, Kawasaki N, Nagai N. Nanocrystalline Suspensions of Irbesartan Enhance Oral Bioavailability by Improving Drug Solubility and Leading Endocytosis Uptake into the Intestine. Pharmaceutics. 2021; 13(9):1404. https://doi.org/10.3390/pharmaceutics13091404
Chicago/Turabian StyleDeguchi, Saori, Fumihiko Ogata, Masaki Watanabe, Hiroko Otake, Naoki Yamamoto, Naohito Kawasaki, and Noriaki Nagai. 2021. "Nanocrystalline Suspensions of Irbesartan Enhance Oral Bioavailability by Improving Drug Solubility and Leading Endocytosis Uptake into the Intestine" Pharmaceutics 13, no. 9: 1404. https://doi.org/10.3390/pharmaceutics13091404
APA StyleDeguchi, S., Ogata, F., Watanabe, M., Otake, H., Yamamoto, N., Kawasaki, N., & Nagai, N. (2021). Nanocrystalline Suspensions of Irbesartan Enhance Oral Bioavailability by Improving Drug Solubility and Leading Endocytosis Uptake into the Intestine. Pharmaceutics, 13(9), 1404. https://doi.org/10.3390/pharmaceutics13091404