Evaluation of the Influence of a Hydrogel Containing AMPD on the Stability of Tetracycline Hydrochloride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Hydrogels and Solutions
2.3. HPLC Analysis of Hydrogels and Solutions
2.3.1. Samples Preparation
2.3.2. Chromatographic System
2.4. Macroscopic Observations of Changes in the Solutions and Hydrogel Samples
2.5. SEM Study of Freeze-Dried Hydrogel Samples
2.6. Powder X-ray Diffraction Analysis (XRPD)
3. Results
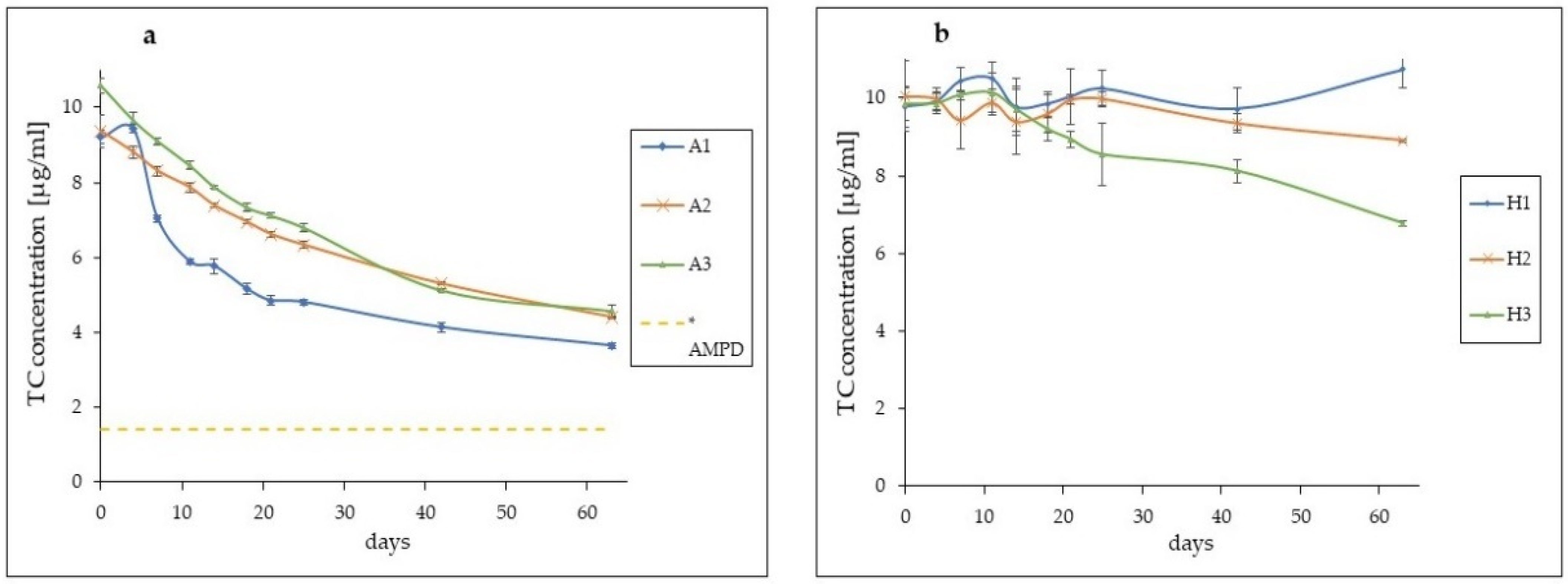
3.1. Evaluation of TC Stability Based on HPLC Analysis
3.2. Macroscopic Observations of Changes in the Hydrogel Samples and Solutions
3.3. Evaluation of SEM Study
3.4. Powder X-ray Diffraction Analysis (XRPD)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [Green Version]
- Sapadin, A.N.; Fleischmajer, R. Tetracyclines: Nonantibiotic properties and their clinical implications. J. Am. Acad. Dermatol. 2006, 54, 258–265. [Google Scholar] [CrossRef]
- Gu, Y.; Walker, C.; Ryan, M.E.; Payne, J.B.; Golub, L.M. Non-antibacterial tetracycline formulations: Clinical applications in dentistry and medicine. J. Oral Microbiol. 2012, 4, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonart, T.; Dramaix, M.; De Maertelaer, V. Efficacy of tetracyclines in the treatment of acne vulgaris: A review. Br. J. Dermatol. 2008, 158, 208–216. [Google Scholar] [CrossRef]
- Dréno, B.; Pécastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platsidaki, E.; Dessinioti, C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne [version 1; referees: 2 approved]. F1000Research 2018, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pena, A.; Carmona, A.; Barbosa, A.; Lino, C.M.; Silveira, M.I.; Castillo, B. Determination of tetracycline and its major degradation products by liquid chromatography with fluorescence detection. J. Pharm. Biomed. Anal. 1998, 18, 839–845. [Google Scholar] [CrossRef] [Green Version]
- Hasan, T.; Allen, M.; Cooperman, B.S. Anhydrotetracycline is a Major Product of Tetracycline Photolysis. J. Org. Chem. 1985, 50, 1755–1757. [Google Scholar] [CrossRef]
- Wu, Y.; Fassihi, R. Stability of metronidazole, tetracycline HCl and famotidine alone and in combination. Int. J. Pharm. 2005, 290, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schlecht, K.D.; Frank, C.W. Dehydration of Tetracycline. J. Pharm. Sci. 1975, 64, 352–354. [Google Scholar] [CrossRef]
- Walton, V.C.; Howlett, M.R.; Selzer, G.B. Anhydrotetracycline and 4-epianhydrotetracycline in market tetracyclines and aged tetracycline products. J. Pharm. Sci. 1970, 59, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Morrison, H.; Olack, G.; Xiao, C. Photochemical and Photophysical Studies of Tetracycline. J. Am. Chem. Soc. 1991, 113, 8110–8118. [Google Scholar] [CrossRef]
- Pena, A.; Palilis, L.P.; Lino, C.M.; Silveira, M.I.; Calokerinos, A.C. Determination of tetracycline and its major degradation products by chemiluminescence. Anal. Chim. Acta 2000, 405, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.G. Determination of Anhydrotetracycline and 4-Epianhydrotetracycline in a Tetracycline Mixture. J. Pharm. Sci. 1964, 53, 1551–1552. [Google Scholar] [CrossRef] [PubMed]
- Benitz, K.F.; Diermeier, H.F. Renal Toxicity of Tetracycline Degradation Products. Soc. Exp. Biol. Med. 1964, 115, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Aszalos, A. Fast determination of tetracycline antibiotics in different media by high-performance liquid chromatography. Chromatographia 1985, 20, 313–322. [Google Scholar] [CrossRef]
- Kostrzębska, A.; Musiał, W. The influence of increasing concentrations of AMPD on the efficacy of its penetration into a model skin sebum layer. Pharmaceutics 2020, 12, 1228. [Google Scholar] [CrossRef]
- Musial, W.; Kubis, A. Preliminary assessment of alginic acid as a factor buffering triethanolamine interacting with artificial skin sebum. Eur. J. Pharm. Biopharm. 2003, 55, 237–240. [Google Scholar] [CrossRef]
- Musial, W.; Kubis, A. Preliminary evaluation of interactions between selected alcoholamines and model skin sebum components. Chem. Pharm. Bull. 2006, 54, 1076–1081. [Google Scholar] [CrossRef] [Green Version]
- Stefaniak, A.B.; Harvey, C.J. Dissolution of materials in artificial skin surface film liquids. Toxicol. Vitr. 2006, 20, 1265–1283. [Google Scholar] [CrossRef]
- Musial, W.; Kubis, A. Carbopols as factors buffering triethanolamine interacting with artificial skin sebum. Polim. Med. 2004, 34, 17–30. [Google Scholar] [PubMed]
- Meidan, V.M.; Bonner, M.C.; Michniak, B.B. Transfollicular drug delivery—Is it a reality? Int. J. Pharm. 2005, 306, 1–14. [Google Scholar] [CrossRef]
- Lindauer, R.F.; Cohen, D.M.; Munnelly, K.P. Determination of Anhydrotetracyclines in Tetracycline by High-Pressure Liquid Chromatography. Anal. Chem. 1976, 48, 1731–1734. [Google Scholar] [CrossRef]
- Liang, Y.; Denton, M.B.; Bates, R.B. Stability studies of tetracycline in methanol solution. J. Chromatogr. A 1998, 827, 45–55. [Google Scholar] [CrossRef]
- Younis, U.S.; Fazel, M.; Myrdal, P.B. Characterization of Tetracycline Hydrochloride Compounded in a Miracle Mouthwash Formulation. AAPS PharmSciTech 2019, 20, 1–8. [Google Scholar] [CrossRef]
- Drexel, R.E.; Olack, G.; Jones, C.; Santim, R.; Morrison, H.; Chmurny, G. Lumitetracycline: A Novel New Tetracycline Photoproduct. J. Org. Chem. 1990, 55, 2471–2478. [Google Scholar] [CrossRef]
- Davies, A.K.; McKellar, J.F.; Phillips, G.O.; Reid, A.G. Photochemical Oxidation of Tetracycline in Aqueous Solution. J. Chem. Soc. Perkin Trans. 2 1979, 369–375. [Google Scholar] [CrossRef]
- Oka, H.; Ikai, Y.; Kawamura, N.; Yamada, M.; Harada, K.; Ito, S.; Suzuki, M. Photodecomposition Products of Tetracycline in Aqueous Solution. J. Agric. Food Chem. 1989, 37, 226–231. [Google Scholar] [CrossRef]
- Miskoski, S.; Sanchez, E.; Garavano, M.; López, M.; Soltermann, A.T.; Garcia, N.A. Singlet molecular oxygen-mediated photo-oxidation of tetracyclines: Kinetics, mechanism and microbiological implications. J. Photochem. Photobiol. B Biol. 1998, 43, 164–171. [Google Scholar] [CrossRef]
- Varanda, F.; Pratas De Melo, M.J.; Caço, A.I.; Dohrn, R.; Makrydaki, F.A.; Voutsas, E.; Tassios, D.; Marrucho, I.M. Solubility of antibiotics in different solvents. 1. Hydrochloride forms of tetracycline, moxifloxacin, and ciprofloxacin. Ind. Eng. Chem. Res. 2006, 45, 6368–6374. [Google Scholar] [CrossRef]
- Caira, M.R.; Nassimbeni, L.R.; Russell, J.C. The Crystal and Molecular Structure of Tetracycline Hexahydrate. Acta Crystallogr. Sect. B 1977, B33, 1171–1176. [Google Scholar] [CrossRef] [Green Version]
- Hoener, B.-A.; Sokoloski, T.D.; Mitscher, L.A.; Malspeis, L. Kinetics of dehydration of epitetracycline in solution. J. Pharm. Sci. 1974, 63, 1901–1904. [Google Scholar] [CrossRef] [PubMed]
- Grobben-Verpoorten, A.; Dihuidi, K.; Roets, E.; Hoogmartens, J.; Vanderhaeghe, H. Determination of the stability of tetracycline suspensions by high performance liquid chromatography. Pharm. Weekbl. Sci. Ed. 1985, 7, 104–108. [Google Scholar] [CrossRef]
- Khan, N.H.; Wera, P.; Roets, E.; Hoogmartens, J. Quantitative analysis of tetracycline by high performance liquid chromatography on polystyrene-divinylbenzene packing materials. J. Liq. Chromatogr. 1990, 13, 1351–1374. [Google Scholar] [CrossRef]
- Ouyang, H.; Ding, S.; Quak, N.; Su, M.; Chin, Y.; Ooi, S.M.; Choo, W.; Li, B.K.; Wan, P.; Heng, S.; et al. Reformulation of extemporaneous tetracycline mouthwash to improve its stability for pemphigus patients. J. Asian Assoc. Sch. Pharm. 2021, 10, 9–16. [Google Scholar]
- Hussien, E.M. HPLC method validation for modernization of the tetracycline hydrochloride capsule USP monograph. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Furusawa, N. Validation/suitability of HPLC system with an isocratic 100% aqueous mobile phase for detecting tetracycline and its 4-epimer, 4-epi-tetracycline. Chem. Res. J. 2019, 4, 129–134. [Google Scholar]
- Chu, J.; Lee, H.; Kim, H.; Lee, Y.W. Recrystallization of tetracycline hydrochloride using supercritical anti-solvent process. Korean J. Chem. Eng. 2009, 26, 1119–1124. [Google Scholar] [CrossRef]
- Yoon, T.J.; Son, W.S.; Park, H.J.; Seo, B.; Kim, T.; Lee, Y.W. Tetracycline nanoparticles precipitation using supercritical and liquid CO2 as antisolvents. J. Supercrit. Fluids 2016, 107, 51–60. [Google Scholar] [CrossRef]
- Burton, J. A Placebo-Controlled Study to Evaluate the Efficacy of Topical Tetracycline and Oral Tetracycline in the Treatment of Mild to Moderate Acne. J. Int. Med. Res. 1990, 18, 94–103. [Google Scholar] [CrossRef]
- Inoue, Y.; Furuya, K.; Maeda, R.; Murata, I.; Kanamoto, I. Assessment of the physical properties and stability of mixtures of tetracycline hydrochloride ointment and acyclovir cream. Int. J. Pharm. 2013, 447, 158–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Formulation | Composition | Storage Conditions | ||||
|---|---|---|---|---|---|---|
| TC [g] | Water [g] | Carbopol 980 NF [g] | AMPD [g] | Temperature [°C] | Photoprotection | |
| A1 | 0.20 | 99.80 | - | - | 5 | Yes |
| A2 | 0.20 | 99.80 | - | - | 23 | Yes |
| A3 | 0.20 | 99.80 | - | - | 23 | No |
| H1 | 0.20 | 97.80 | 1.00 | 1.00 | 5 | Yes |
| H2 | 0.20 | 97.80 | 1.00 | 1.00 | 23 | Yes |
| H3 | 0.20 | 97.80 | 1.00 | 1.00 | 23 | No |
| AMPD sol | 0.20 | 98.80 | - | 1.00 | 5 | Yes |
| Sample | Peak 1 2Θ (°) | I 1 (a.u) | Peak 2 2Θ (°) | I 2 (a.u) | Peak 3 2Θ (°) | I 3 (a.u) |
|---|---|---|---|---|---|---|
| H1 | 9.15 | 1193 | 11.66 | 1077 | 17.79 | 2459 |
| H2 | 8.91 | 770 | 11.60 | 680 | 17.73 | 1346 |
| H3 | 8.89 | 803 | 11.60 | 707 | 17.67 | 1501 |
| TC | 8.65 | 1875 | 11.74 | 1886 | 18.20 | 2191 |
| Day | A1% * | A2% * | A3% * | H1% ** | H2% ** | H3% ** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 100.00 | SD = 0.19 | 100.00 | SD = 0.43 | 100.00 | SD = 0.19 | 100.00 | SD = 0.52 | 100.00 | SD = 0.90 | 100.00 | SD = 0.43 |
| 4 | 102.42 | SD = 0.10 | 93.97 | SD = 0.16 | 91.25 | SD = 0.21 | 101.51 | SD = 0.25 | 99.21 | SD = 0.28 | 100.17 | SD = 0.27 |
| 7 | 76.32 | SD = 0.08 | 88.64 | SD = 0.12 | 86.00 | SD = 0.09 | 106.88 | SD = 0.35 | 93.80 | SD = 0.72 | 102.32 | SD = 0.13 |
| 11 | 63.94 | SD = 0.08 | 83.88 | SD = 0.14 | 79.78 | SD = 0.11 | 107.67 | SD = 0.40 | 98.31 | SD = 0.33 | 102.96 | SD = 0.51 |
| 14 | 62.64 | SD = 0.20 | 78.81 | SD = 0.06 | 74.38 | SD = 0.04 | 99.98 | SD = 0.74 | 93.39 | SD = 0.84 | 98.74 | SD = 0.57 |
| 18 | 56.02 | SD = 0.15 | 74.14 | SD = 0.06 | 69.27 | SD = 0.12 | 100.82 | SD = 0.33 | 95.38 | SD = 0.49 | 93.48 | SD = 0.29 |
| 21 | 52.60 | SD = 0.13 | 70.60 | SD = 0.08 | 67.15 | SD = 0.08 | 102.72 | SD = 0.73 | 99.12 | SD = 0.13 | 90.75 | SD = 0.20 |
| 25 | 52.16 | SD = 0.08 | 67.57 | SD = 0.09 | 64.17 | SD = 0.10 | 104.89 | SD = 0.48 | 99.35 | SD = 0.18 | 86.80 | SD = 0.80 |
| 42 | 44.85 | SD = 0.12 | 56.53 | SD = 0.05 | 48.33 | SD = 0.04 | 99.47 | SD = 0.55 | 93.00 | SD = 0.25 | 82.49 | SD = 0.30 |
| 63 | 39.51 | SD = 0.07 | 47.01 | SD = 0.03 | 43.08 | SD = 0.16 | 110.02 | SD = 0.47 | 88.60 | SD = 0.03 | 68.79 | SD = 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostrzębska, A.; Złocińska, A.; Musiał, W. Evaluation of the Influence of a Hydrogel Containing AMPD on the Stability of Tetracycline Hydrochloride. Pharmaceutics 2021, 13, 1381. https://doi.org/10.3390/pharmaceutics13091381
Kostrzębska A, Złocińska A, Musiał W. Evaluation of the Influence of a Hydrogel Containing AMPD on the Stability of Tetracycline Hydrochloride. Pharmaceutics. 2021; 13(9):1381. https://doi.org/10.3390/pharmaceutics13091381
Chicago/Turabian StyleKostrzębska, Agnieszka, Adrianna Złocińska, and Witold Musiał. 2021. "Evaluation of the Influence of a Hydrogel Containing AMPD on the Stability of Tetracycline Hydrochloride" Pharmaceutics 13, no. 9: 1381. https://doi.org/10.3390/pharmaceutics13091381
APA StyleKostrzębska, A., Złocińska, A., & Musiał, W. (2021). Evaluation of the Influence of a Hydrogel Containing AMPD on the Stability of Tetracycline Hydrochloride. Pharmaceutics, 13(9), 1381. https://doi.org/10.3390/pharmaceutics13091381






