Inclusion of Medium-Chain Triglyceride in Lipid-Based Formulation of Cannabidiol Facilitates Micellar Solubilization In Vitro, but In Vivo Performance Remains Superior with Pure Sesame Oil Vehicle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lipid-Based Formulations
2.3. In Vitro Lipolysis
2.4. Animal Studies
2.5. Bioanalytical Conditions
2.5.1. Sample Preparation
2.5.2. Chromatography Conditions
2.6. Triglycerides Level Determinations
2.7. Data Analysis
3. Results
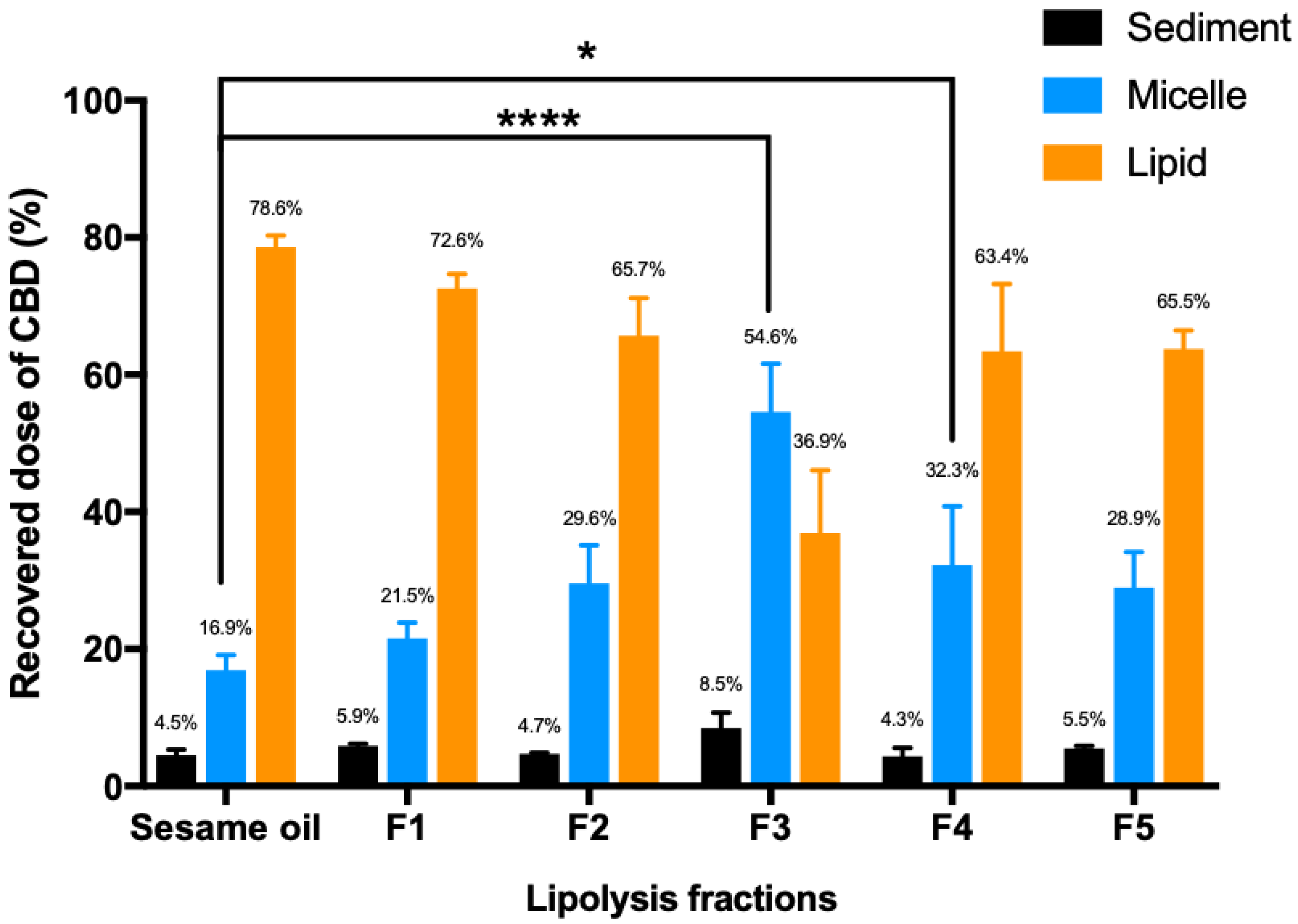
3.1. In Vitro Lipolysis of Lipid-Based Formulations
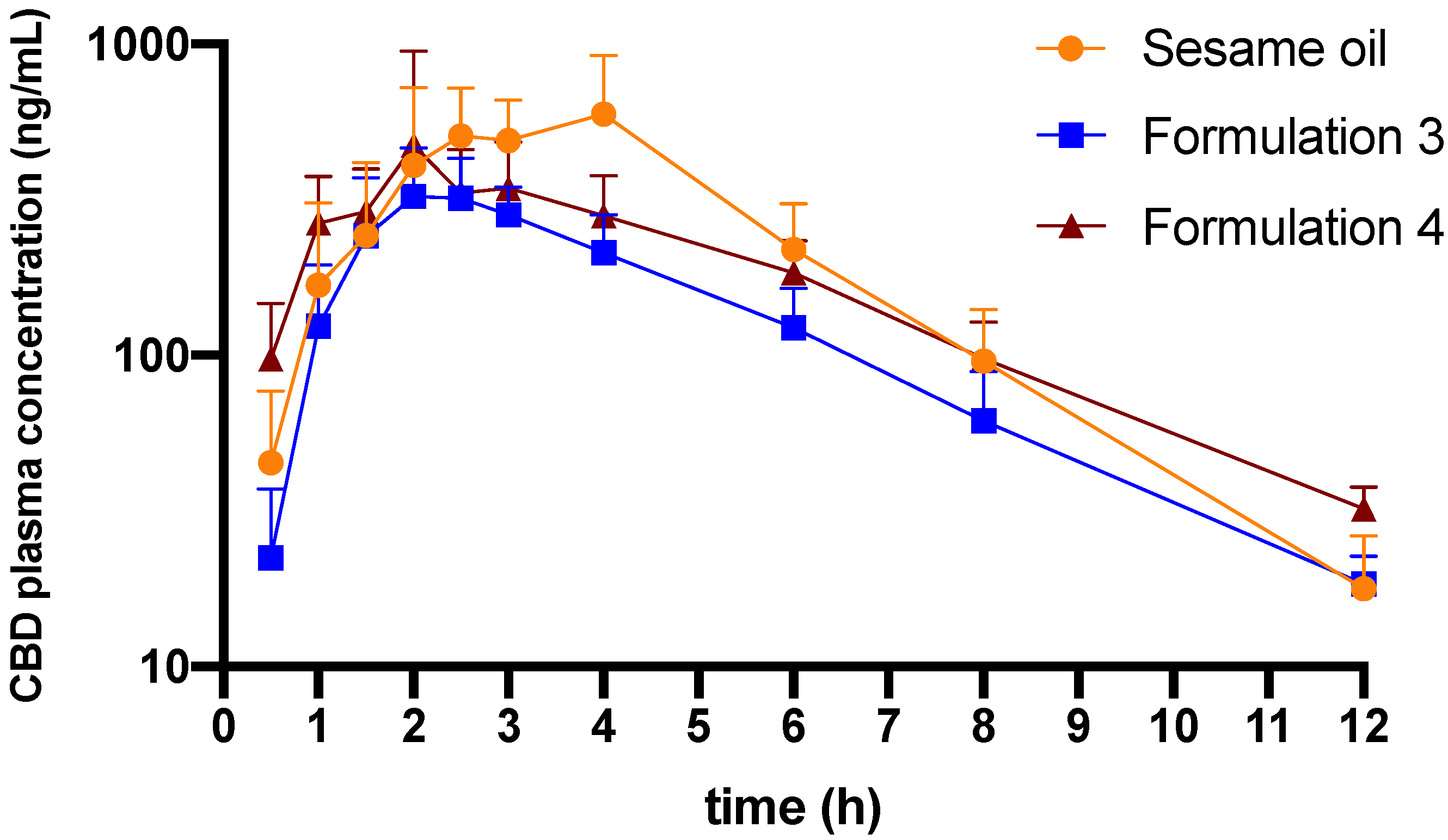
3.2. In Vivo Pharmacokinetics
3.3. Biodistribution
4. Discussion
4.1. The Design of Lipid-Based Formulations
4.2. In Vitro Lipolysis
4.3. In Vivo Pharmacokinetics and Biodistribution
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hauss, D.J. Oral lipid-based formulations. Adv. Drug Deliv. Rev. 2007, 59, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Hauss, D.J. Oral Lipi-Based Formulations Enhancing the Bioavailability of Poorly Water-Soluble Drugs; CRC Press: Boca Raton, FL, USA, 2007; Available online: http://library1.nida.ac.th/termpaper6/sd/2554/19755.pdf (accessed on 12 December 2020).
- Khoo, S.; Shackleford, D.M.; Porter, C.; Edwards, G.A.; Charman, W.N. Intestinal Lymphatic Transport of Halofantrine Occurs After Oral Administration of a Unit-Dose Lipid-Based Formulation to Fasted Dogs. Pharm. Res. 2003, 20, 1460–1465. [Google Scholar] [CrossRef]
- Patel, V.; Lalani, R.; Bardoliwala, D.; Ghosh, S.; Misra, A. Lipid-Based Oral Formulation Strategies for Lipophilic Drugs. AAPS PharmSciTech 2018, 19, 3609–3630. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Qin, C.; Chu, Y.; Berton, M.; Lee, J.B.; Zgair, A.; Bettonte, S.; Stocks, M.J.; Constantinescu, C.S.; Barrett, D.A.; et al. Natural sesame oil is superior to pre-digested lipid formulations and purified triglycerides in promoting the intestinal lymphatic transport and systemic bioavailability of cannabidiol. Eur. J. Pharm. Biopharm. 2021, 162, 43–49. [Google Scholar] [CrossRef]
- Zgair, A.; Lee, J.B.; Wong, J.C.M.; Taha, D.; Aram, J.; Di Virgilio, D.; McArthur, J.W.; Cheng, Y.-K.; Hennig, I.M.; Barrett, D.A.; et al. Oral administration of cannabis with lipids leads to high levels of cannabinoids in the intestinal lymphatic system and prominent immunomodulation. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zgair, A.; Wong, J.C.; Lee, J.B.; Mistry, J.; Sivak, O.; Wasan, K.M.; Hennig, I.M.; Barrett, D.; Constantinescu, C.; Fischer, P.M.; et al. Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicines. Am. J. Transl. Res. 2016, 8, 3448–3459. [Google Scholar]
- Hussain, M.M. A proposed model for the assembly of chylomicrons. Atherosclerosis 2000, 148, 1–15. [Google Scholar] [CrossRef]
- Randolph, G.J.; Miller, N.E.; Randolph, G.J.; Miller, N.E. Lymphatic transport of high-density lipoproteins and chylomicrons Find the latest version: Review series Lymphatic transport of high-density lipoproteins and chylomicrons. J. Clin. Investig. 2014, 124, 929–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mechoulam, R.; Parker, L.A.; Gallily, R. Cannabidiol: An Overview of Some Pharmacological Aspects. J. Clin. Pharmacol. 2002, 42, 11S–19S. [Google Scholar] [CrossRef]
- Wong, J.; Sivak, O.; Wasan, K.M.; Fischer, P.M.; Gershkovich, P. Intestinal absorption of lipophilic cannabinoids: The role of lymphatic transport. In Proceedings of the 5th World Conference on Drug Absorption, Transport and Delivery, Uppsala, Sweden, 24–26 June 2013. [Google Scholar]
- Gershkovich, P.; Fanous, J.; Qadri, B.; Yacovan, A.; Amselem, S.; Hoffman, A. The role of molecular physicochemical properties and apolipoproteins in association of drugs with triglyceride-rich lipoproteins: In-silico prediction of uptake by chylomicrons. J. Pharm. Pharmacol. 2009, 61, 31–39. [Google Scholar] [CrossRef]
- Holm, R. Examination of oral absorption and lymphatic transport of halofantrine in a triple-cannulated canine model after administration in self-microemulsifying drug delivery systems (SMEDDS) containing structured triglycerides. Eur. J. Pharm. Sci. 2003, 20, 91–97. [Google Scholar] [CrossRef]
- Dahan, A.; Hoffman, A. Evaluation of a chylomicron flow blocking approach to investigate the intestinal lymphatic transport of lipophilic drugs. Eur. J. Pharm. Sci. 2005, 24, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; De Filippis, D.; Cirillo, C.; Iuvone, T.; Capoccia, E.; Scuderi, C.; Steardo, A.; Cuomo, R.; Steardo, L. Cannabidiol in Inflammatory Bowel Diseases: A Brief Overview. Phytother. Res. 2012, 27, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Colleoni, M.; Conti, S.; Parolaro, D.; Franke, C.; Trovato, A.E.; Giagnoni, G. Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn-Schmiedeberg Arch. Pharmacol. 2004, 369, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M.; Brennan, F.M.; Foxwell, B.M.; Taylor, P.C.; Williams, R.O.; Maini, R. Anti-TNF therapy: Where have we got to in 2005? J. Autoimmun. 2005, 25, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Corey-Bloom, J.; Wolfson, T.; Gamst, A.; Jin, S.; Marcotte, T.D.; Bentley, H.; Gouaux, B. Smoked cannabis for spasticity in multiple sclerosis: A randomized, placebo-controlled trial. Can. Med. Assoc. J. 2012, 184, 1143–1150. [Google Scholar] [CrossRef] [Green Version]
- Nichols, J.M.; Kaplan, B.L. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, B.; Springs, A.E.; Kaminski, N.E. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT). Biochem. Pharmacol. 2008, 76, 726–737. [Google Scholar] [CrossRef] [Green Version]
- Watzl, B.; Scuderi, P.; Watson, R.R. Marijuana components stimulate human peripheral blood mononuclear cell secretion of interferon-gamma and suppress interleukin-1 alpha in vitro. Int. J. Immunopharmacol. 1991, 13, 1091–1097. [Google Scholar] [CrossRef]
- Izgelov, D.; Davidson, E.; Barasch, D.; Regev, A.; Domb, A.J.; Hoffman, A. Pharmacokinetic investigation of synthetic cannabidiol oral formulations in healthy volunteers. Eur. J. Pharm. Biopharm. 2020, 154, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Benito-Gallo, P.; Franceschetto, A.; Wong, J.C.; Marlow, M.; Zann, V.; Scholes, P.; Gershkovich, P. Chain length affects pancreatic lipase activity and the extent and pH–time profile of triglyceride lipolysis. Eur. J. Pharm. Biopharm. 2015, 93, 353–362. [Google Scholar] [CrossRef]
- Dahan, A.; Hoffman, A. The effect of different lipid based formulations on the oral absorption of lipophilic drugs: The ability of in vitro lipolysis and consecutive ex vivo intestinal permeability data to predict in vivo bioavailability in rats. Eur. J. Pharm. Biopharm. 2007, 67, 96–105. [Google Scholar] [CrossRef]
- Griffin, W.C. Classification of Surface-Active Agents by “HLB”. J. Cosmet. Sci. 1949, 1, 311–326. [Google Scholar]
- Chun, A.H.C.; Martin, A.N. Measurement of Hydrophile-Lipophile Balance of Surface-Active Agents. J. Pharm. Sci. 1961, 50, 732–736. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, J.; Tan, S.; Otieno, B.O.; Zhang, Z. The applications of Vitamin E TPGS in drug delivery. Eur. J. Pharm. Sci. 2013, 49, 175–186. [Google Scholar] [CrossRef]
- Zuccari, G.; Alfei, S.; Zorzoli, A.; Marimpietri, D.; Turrini, F.; Baldassari, S.; Marchitto, L.; Caviglioli, G. Increased Water-Solubility and Maintained Antioxidant Power of Resveratrol by Its Encapsulation in Vitamin E TPGS Micelles: A Potential Nutritional Supplement for Chronic Liver Disease. Pharmaceutics 2021, 13, 1128. [Google Scholar] [CrossRef]
- Zuccari, G.; Baldassari, S.; Alfei, S.; Marengo, B.; Valenti, G.; Domenicotti, C.; Ailuno, G.; Villa, C.; Marchitto, L.; Caviglioli, G. D-α-Tocopherol-Based Micelles for Successful Encapsulation of Retinoic Acid. Pharmaceutics 2021, 14, 212. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, X.; Liu, J.; Yong, H.; Gao, L.; Liu, J. Development of antioxidant and antimicrobial packaging films based on chitosan, D-α-tocopheryl polyethylene glycol 1000 succinate and silicon dioxide nanoparticles. Food Packag. Shelf Life 2020, 24, 100503. [Google Scholar] [CrossRef]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Bussche, A.V.D.; Kane, A.B.; Hurt, R.H. Tocopheryl polyethylene glycol succinate as a safe, antioxidant surfactant for processing carbon nanotubes and fullerenes. Carbon 2007, 45, 2463–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.; Wu, J.; Fang, X.; Sha, X. A new function of Vitamin E-TPGS in the intestinal lymphatic transport of lipophilic drugs: Enhancing the secretion of chylomicrons. Int. J. Pharm. 2013, 445, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.T.; Sassene, P.; Müllertz, A. In vitro lipolysis models as a tool for the characterization of oral lipid and surfactant based drug delivery systems. Int. J. Pharm. 2011, 417, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Kaukonen, A.M.; Taillardat-Bertschinger, A.; Boyd, B.; O’Connor, J.M.; Edwards, G.A.; Charman, W. Use of in vitro lipid digestion data to explain the in vivo performance of triglyceride-based oral lipid formulations of poorly water-soluble drugs: Studies with halofantrine. J. Pharm. Sci. 2004, 93, 1110–1121. [Google Scholar] [CrossRef]
- Fatouros, D.G.; Bergenstahl, B.; Mullertz, A. Morphological observations on a lipid-based drug delivery system during in vitro digestion. Eur. J. Pharm. Sci. 2007, 31, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.Ø.; Schultz, K.; Mollgaard, B.; Kristensen, H.G.; Müllertz, A. Solubilisation of poorly water-soluble drugs during in vitro lipolysis of medium- and long-chain triacylglycerols. Eur. J. Pharm. Sci. 2004, 23, 287–296. [Google Scholar] [CrossRef]
- Rezhdo, O.; Di Maio, S.; Le, P.; Littrell, K.C.; Carrier, R.L.; Chen, S.-H. Characterization of colloidal structures during intestinal lipolysis using small-angle neutron scattering. J. Colloid Interface Sci. 2017, 499, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Zgair, A.; Wong, J.C.; Sabri, A.; Fischer, P.M.; Barrett, D.A.; Constantinescu, C.S.; Gershkovich, P. Development of a simple and sensitive HPLC–UV method for the simultaneous determination of cannabidiol and Δ9-tetrahydrocannabinol in rat plasma. J. Pharm. Biomed. Anal. 2015, 114, 145–151. [Google Scholar] [CrossRef]
- Grove, M.; Pedersen, G.P.; Nielsen, J.L.; Müllertz, A. Bioavailability of seocalcitol I: Relating solubility in biorelevant media with oral bioavailability in rats—Effect of medium and long chain triglycerides. J. Pharm. Sci. 2005, 94, 1830–1838. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Katare, O.; Singh, B. Optimized self nano-emulsifying systems of ezetimibe with enhanced bioavailability potential using long chain and medium chain triglycerides. Colloids Surf. B Biointerfaces 2012, 100, 50–61. [Google Scholar] [CrossRef]
- Han, S.-F.; Yao, T.-T.; Zhang, X.-X.; Gan, L.; Zhu, C.; Yu, H.-Z.; Gan, Y. Lipid-based formulations to enhance oral bioavailability of the poorly water-soluble drug anethol trithione: Effects of lipid composition and formulation. Int. J. Pharm. 2009, 379, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Izgelov, D.; Shmoeli, E.; Domb, A.J.; Hoffman, A. The effect of medium chain and long chain triglycerides incorporated in self-nano emulsifying drug delivery systems on oral absorption of cannabinoids in rats. Int. J. Pharm. 2020, 580, 119201. [Google Scholar] [CrossRef]
- Mohsin, K. Design of Lipid-Based Formulations for Oral Administration of Poorly Water-Soluble Drug Fenofibrate: Effects of Digestion. AAPS PharmSciTech 2012, 13, 637–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahan, A.; Hoffman, A. Rationalizing the selection of oral lipid based drug delivery systems by an in vitro dynamic lipolysis model for improved oral bioavailability of poorly water soluble drugs. J. Control Release 2008, 129, 1–10. [Google Scholar] [CrossRef]
- Boggs, D.L.; Surti, T.; Gupta, A.; Gupta, S.; Niciu, M.; Pittman, B.; Martin, A.M.S.; Thurnauer, H.; Davies, A.; D’Souza, D.C.; et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology 2018, 235, 1923–1932. [Google Scholar] [CrossRef]
- Hardy, J.; Haywood, A.; Gogna, G.; Martin, J.; Yates, P.; Greer, R.; Good, P. Oral medicinal cannabinoids to relieve symptom burden in the palliative care of patients with advanced cancer: A double-blind, placebo-controlled, randomised clinical trial of efficacy and safety of 1:1 delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Trials 2020, 21, 611. [Google Scholar] [CrossRef]
- White, C.M. A Review of Human Studies Assessing Cannabidiol’s (CBD) Therapeutic Actions and Potential. J. Clin. Pharmacol. 2019, 59, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Sholler, D.J.; Schoene, L.; Spindle, T.R. Therapeutic Efficacy of Cannabidiol (CBD): A Review of the Evidence from Clinical Trials and Human Laboratory Studies. Curr. Addict. Rep. 2020, 7, 405–412. [Google Scholar] [CrossRef]
- Linares, I.M.; Zuardi, A.W.; Pereira, L.C.; Queiroz, R.H.; Mechoulam, R.; Guimarães, F.S.; Crippa, J.A. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Rev. Bras. Psiquiatr. 2019, 41, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Zuardi, A.W.; Rodrigues, N.P.; Silva, A.L.; Bernardo, S.A.; Hallak, J.E.C.; Guimarães, F.S.; Crippa, J.A.S. Inverted U-Shaped Dose-Response Curve of the Anxiolytic Effect of Cannabidiol during Public Speaking in Real Life. Front. Pharmacol. 2017, 8, 259. [Google Scholar] [CrossRef] [Green Version]
- Zgair, A. Intestinal Lymphatic Transport of Cannabinoids: Implications for People with Autoimmune Diseases and Immunocompromised Individuals; University of Nottingham: Nottingham, UK, 2017. [Google Scholar]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granger, C.; Barey, P.; Combe, N.; Veschambre, P.; Cansell, M. Influence of the fat characteristics on the physicochemical behavior of oil-in-water emulsions based on milk proteins-glycerol esters mixtures. Colloids Surf. B Biointerfaces 2003, 32, 353–363. [Google Scholar] [CrossRef]
- Speranza, A.; Corradini, M.; Hartman, T.G.; Ribnicky, D.; Oren, A.; Rogers, M.A. Influence of Emulsifier Structure on Lipid Bioaccessibility in Oil–Water Nanoemulsions. J. Agric. Food Chem. 2013, 61, 6505–6515. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.M.; Raab, T.W.; Chuat, J.Y.; Leser, M.E.; Miller, R.; Watzke, H.J.; Holmberg, K. Influence of Surfactants on Lipase Fat Digestion in a Model Gastro-intestinal System. Food Biophys. 2008, 3, 370–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuiné, J.F.; Charman, W.; Pouton, C.; Edwards, G.A.; Porter, C. Increasing the Proportional Content of Surfactant (Cremophor EL) Relative to Lipid in Self-emulsifying Lipid-based Formulations of Danazol Reduces Oral Bioavailability in Beagle Dogs. Pharm. Res. 2007, 24, 748–757. [Google Scholar] [CrossRef]
- Christiansen, A.; Backensfeld, T.; Weitschies, W. Effects of non-ionic surfactants on in vitro triglyceride digestion and their susceptibility to digestion by pancreatic enzymes. Eur. J. Pharm. Sci. 2010, 41, 376–382. [Google Scholar] [CrossRef]
- MacGregor, K.J.; Embleton, J.K.; Lacy, J.E.; Perry, E.A.; Solomon, L.J.; Seager, H.; Pouton, C.W. Influence of lipolysis on drug absorption from the gastro-intestinal tract. Adv. Drug Deliv. Rev. 1997, 27, 33–46. [Google Scholar] [CrossRef]
- Dahan, A.; Hoffman, A. Use of a Dynamic in Vitro Lipolysis Model to Rationalize Oral Formulation Development for Poor Water Soluble Drugs: Correlation with in Vivo Data and the Relationship to Intra-Enterocyte Processes in Rats. Pharm. Res. 2006, 23, 2165–2174. [Google Scholar] [CrossRef]
- Lee, J.B.; Kim, T.H.; Feng, W.; Choi, H.G.; Zgair, A.; Shin, S.; Yoo, S.D.; Gershkovich, P.; Shin, B.S. Quantitative Prediction of Oral Bioavailability of a Lipophilic Antineoplastic Drug Bexarotene Administered in Lipidic Formulation Using a Combined In Vitro Lipolysis/Microsomal Metabolism Approach. J. Pharm. Sci. 2019, 108, 1047–1052. [Google Scholar] [CrossRef]
- Benito-Gallo, P.; Marlow, M.; Zann, V.; Scholes, P.; Gershkovich, P. Linking in Vitro Lipolysis and Microsomal Metabolism for the Quantitative Prediction of Oral Bioavailability of BCS II Drugs Administered in Lipidic Formulations. Mol. Pharm. 2016, 13, 3526–3540. [Google Scholar] [CrossRef] [Green Version]
- Gershkovich, P.; Hoffman, A. Uptake of lipophilic drugs by plasma derived isolated chylomicrons: Linear correlation with intestinal lymphatic bioavailability. Eur. J. Pharm. Sci. 2005, 26, 394–404. [Google Scholar] [CrossRef]
- Griffin, B.T.; O’Driscoll, C. A comparison of intestinal lymphatic transport and systemic bioavailability of saquinavir from three lipid-based formulations in the anaesthetised rat model. J. Pharm. Pharmacol. 2010, 58, 917–925. [Google Scholar] [CrossRef]




| Formulation No. | Control | F1 | F2 | F3 | F4 | F5 | HLB | |
|---|---|---|---|---|---|---|---|---|
| Lipid | Sesame oil (v/v, %) | 100 | 50 | 50 | 50 | 40 | 50 | 7 |
| Tri-C8 (v/v, %) | - | 30 | 30 | 50 | 40 | 30 | 7 | |
| Surfactant | Tween® 85 (v/v, %) | - | - | 20 | - | 20 | 10 | 11 |
| Tween® 80 (v/v, %) | - | 20 | - | - | - | - | 15 | |
| Span® 20 (v/v, %) | - | - | - | - | - | 10 | 8.6 | |
| TPGS (mg/mL) | - | 10 | 10 | - | 10 | 10 | 13.2 | |
| Administration/ Formulation | t1/2 a (h) | tmax b (h) | Cmax c (ng/mL) | AUC0-∞ d (h×ng/mL) | n |
|---|---|---|---|---|---|
| Sesame oil | 1.6 ± 0.1 | 2.5–4 | 724 ± 318 | 2702 ± 909 | 5 |
| Formulation 3 | 2.3 ± 0.3 | 2–2.5 | 371 ± 85 | 1512 ± 224 ** | 6 |
| Formulation 4 | 2.5 ± 0.5 | 2–3 | 562 ± 390 | 2131 ± 373 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, W.; Qin, C.; Cipolla, E.; Lee, J.B.; Zgair, A.; Chu, Y.; Ortori, C.A.; Stocks, M.J.; Constantinescu, C.S.; Barrett, D.A.; et al. Inclusion of Medium-Chain Triglyceride in Lipid-Based Formulation of Cannabidiol Facilitates Micellar Solubilization In Vitro, but In Vivo Performance Remains Superior with Pure Sesame Oil Vehicle. Pharmaceutics 2021, 13, 1349. https://doi.org/10.3390/pharmaceutics13091349
Feng W, Qin C, Cipolla E, Lee JB, Zgair A, Chu Y, Ortori CA, Stocks MJ, Constantinescu CS, Barrett DA, et al. Inclusion of Medium-Chain Triglyceride in Lipid-Based Formulation of Cannabidiol Facilitates Micellar Solubilization In Vitro, but In Vivo Performance Remains Superior with Pure Sesame Oil Vehicle. Pharmaceutics. 2021; 13(9):1349. https://doi.org/10.3390/pharmaceutics13091349
Chicago/Turabian StyleFeng, Wanshan, Chaolong Qin, Elena Cipolla, Jong Bong Lee, Atheer Zgair, Yenju Chu, Catherine A. Ortori, Michael J. Stocks, Cris S. Constantinescu, David A. Barrett, and et al. 2021. "Inclusion of Medium-Chain Triglyceride in Lipid-Based Formulation of Cannabidiol Facilitates Micellar Solubilization In Vitro, but In Vivo Performance Remains Superior with Pure Sesame Oil Vehicle" Pharmaceutics 13, no. 9: 1349. https://doi.org/10.3390/pharmaceutics13091349
APA StyleFeng, W., Qin, C., Cipolla, E., Lee, J. B., Zgair, A., Chu, Y., Ortori, C. A., Stocks, M. J., Constantinescu, C. S., Barrett, D. A., Fischer, P. M., & Gershkovich, P. (2021). Inclusion of Medium-Chain Triglyceride in Lipid-Based Formulation of Cannabidiol Facilitates Micellar Solubilization In Vitro, but In Vivo Performance Remains Superior with Pure Sesame Oil Vehicle. Pharmaceutics, 13(9), 1349. https://doi.org/10.3390/pharmaceutics13091349









