Assessing the Functional Redundancy between P-gp and BCRP in Controlling the Brain Distribution and Biliary Excretion of Dual Substrates with PET Imaging in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Radiotracer Synthesis and Formulation
2.2. PET Imaging
2.3. Analysis of PET Data
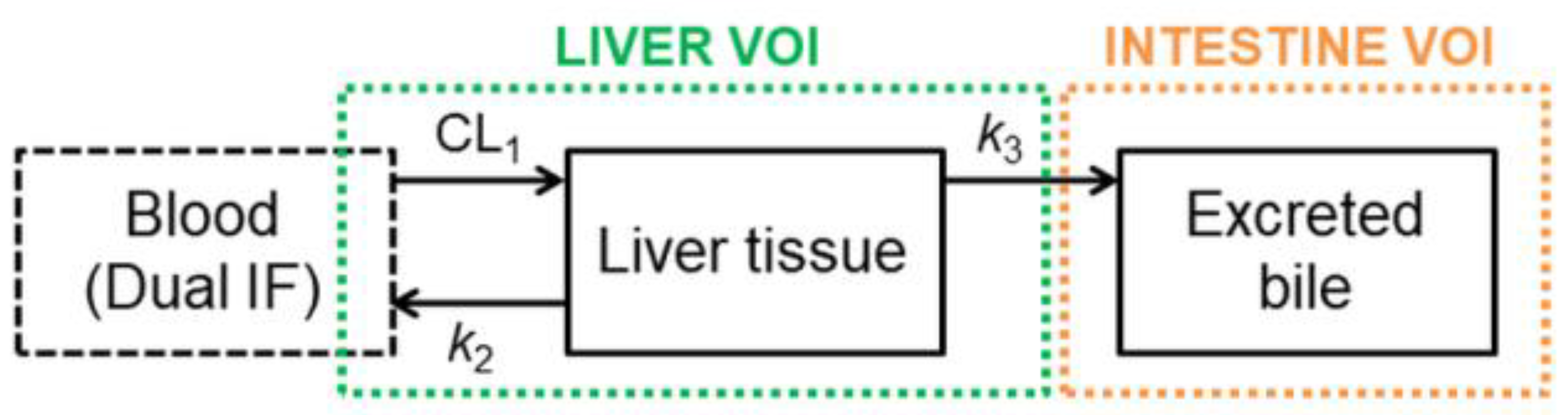
2.4. Pharmacokinetic Modeling
2.5. Statistical Analysis
3. Results
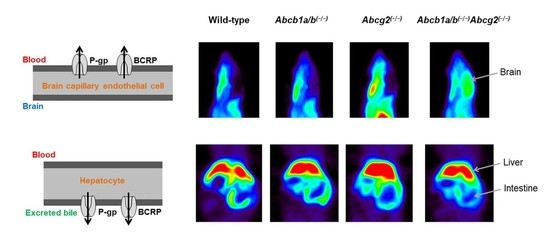
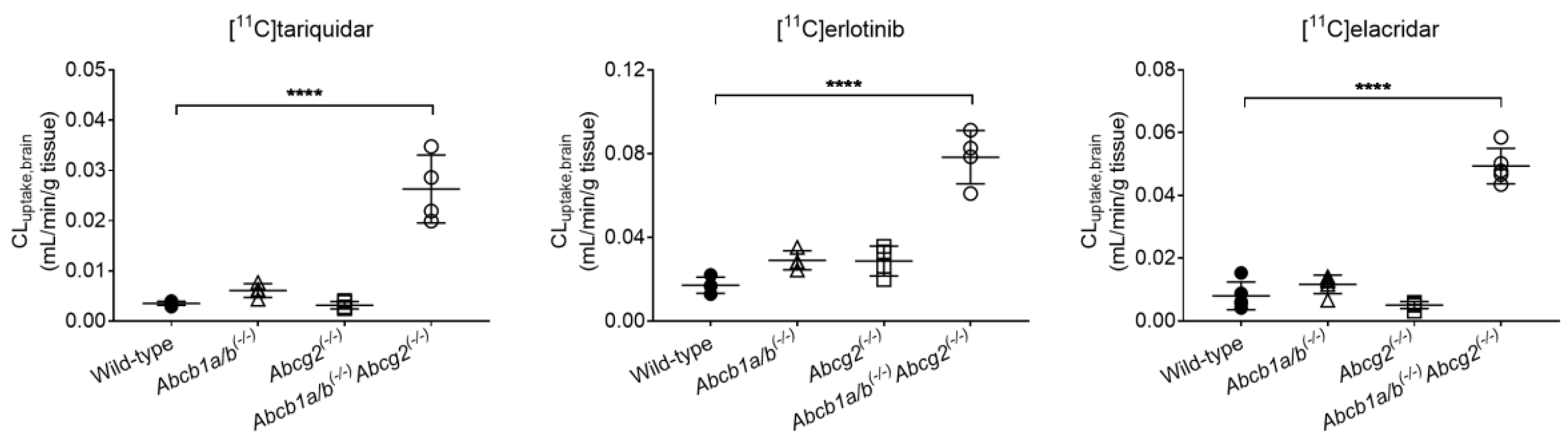
3.1. Functional Redundancy between P-gp and BCRP in Controlling the Brain Distribution of [11C]tariquidar, [11C]erlotinib, and [11C]elacridar
3.2. Functional Redundancy between P-gp and BCRP in Mediating the Biliary Excretion of [11C]tariquidar, [11C]erlotinib, and [11C]elacridar
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Giacomini, K.; Huang, S.; Tweedie, D. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar]
- Agarwal, S.; Hartz, A.M.S.; Elmquist, E.F.; Bauer, B. Breast cancer resistance protein and p-glycoprotein in brain cancer: Two gatekeepers team up. Curr. Pharm. Des. 2011, 17, 2793–2802. [Google Scholar] [CrossRef] [PubMed]
- Durmus, S.; Hendrikx, J.J.; Schinkel, A.H. Apical ABC transporters and cancer chemotherapeutic drug disposition. Adv. Cancer. Res. 2015, 125, 1–41. [Google Scholar] [PubMed]
- Kalvass, J.C.; Polli, J.W.; Bourdet, D.L.; Feng, B.; Huang, S.M.; Liu, X.; Smith, Q.R.; Zhang, L.K.; Zamek-Gliszczynski, M.J.; International Transporter, C. Why clinical modulation of efflux transport at the human blood-brain barrier is unlikely: The ITC evidence-based position. Clin. Pharmacol. Ther. 2013, 94, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Polli, J.W.; Olson, K.L.; Chism, J.P.; John-Williams, L.S.; Yeager, R.L.; Woodard, S.M.; Otto, V.; Castellino, S.; Demby, V.E. An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino }methyl)-2-furyl]-4-quinazolinamine; GW572016). Drug Metab. Dispos. 2009, 37, 439–442. [Google Scholar] [PubMed]
- Kodaira, H.; Kusuhara, H.; Ushiki, J.; Fuse, E.; Sugiyama, Y. Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J. Pharmacol. Exp. Ther. 2010, 333, 788–796. [Google Scholar] [CrossRef]
- Bauer, M.; Romermann, K.; Karch, R.; Wulkersdorfer, B.; Stanek, J.; Philippe, C.; Maier-Salamon, A.; Haslacher, H.; Jungbauer, C.; Wadsak, W.; et al. Pilot PET study to assess the functional interplay between ABCB1 and ABCG2 at the human blood-brain barrier. Clin. Pharmacol. Ther. 2016, 100, 131–141. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Tournier, N.; Stieger, B.; Langer, O. Imaging techniques to study drug transporter function in vivo. Pharmacol. Ther. 2018, 189, 104–122. [Google Scholar] [CrossRef]
- Hernández Lozano, I.; Langer, O. Use of imaging to assess the activity of hepatic transporters. Expert Opin. Drug Metab. Toxicol. 2020, 16, 149–164. [Google Scholar] [CrossRef]
- Bankstahl, J.P.; Bankstahl, M.; Römermann, K.; Wanek, T.; Stanek, J.; Windhorst, A.D.; Fedrowitz, M.; Erker, T.; Müller, M.; Löscher, W.; et al. Tariquidar and elacridar are dose-dependently transported by P-glycoprotein and Bcrp at the blood-brain barrier: A small-animal positron emission tomography and in vitro study. Drug Metab. Dispos. 2013, 41, 754–762. [Google Scholar] [CrossRef]
- Wanek, T.; Kuntner, C.; Bankstahl, J.P.; Mairinger, S.; Bankstahl, M.; Stanek, J.; Sauberer, M.; Filip, T.; Erker, T.; Muller, M.; et al. A novel PET protocol for visualization of breast cancer resistance protein function at the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 2002–2011. [Google Scholar] [CrossRef]
- Bauer, M.; Karch, R.; Zeitlinger, M.; Stanek, J.; Philippe, C.; Wadsak, W.; Mitterhauser, M.; Jager, W.; Haslacher, H.; Muller, M.; et al. Interaction of [11C]tariquidar and [11C]elacridar with P-glycoprotein and breast cancer resistance protein at the human blood-brain barrier. J. Nucl. Med. 2013, 54, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Traxl, A.; Mairinger, S.; Filip, T.; Sauberer, M.; Stanek, J.; Poschner, S.; Jäger, W.; Zoufal, V.; Novarino, G.; Tournier, N.; et al. Inhibition of ABCB1 and ABCG2 at the mouse blood-brain barrier with marketed drugs to improve brain delivery of the model ABCB1/ABCG2 substrate [11C]erlotinib. Mol. Pharm. 2019, 16, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Karch, R.; Wulkersdorfer, B.; Philippe, C.; Nics, L.; Klebermass, E.M.; Weber, M.; Poschner, S.; Haslacher, H.; Jager, W.; et al. A proof-of-concept study to inhibit ABCG2- and ABCB1-mediated efflux transport at the human blood-brain barrier. J. Nucl. Med. 2019, 60, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Traxl, A.; Wanek, T.; Mairinger, S.; Stanek, J.; Filip, T.; Sauberer, M.; Müller, M.; Kuntner, C.; Langer, O. Breast cancer resistance protein and P-Glycoprotein influence in vivo disposition of [11C]erlotinib. J. Nucl. Med. 2015, 56, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Tournier, N.; Goutal, S.; Mairinger, S.; Hernandez-Lozano, I.; Filip, T.; Sauberer, M.; Caille, F.; Breuil, L.; Stanek, J.; Freeman, A.F.; et al. Complete inhibition of ABCB1 and ABCG2 at the blood-brain barrier by co-infusion of erlotinib and tariquidar to improve brain delivery of the model ABCB1/ABCG2 substrate [11C]erlotinib. J. Cereb. Blood Flow Metab. 2021, 41, 1634–1646. [Google Scholar] [CrossRef]
- Ling, J.; Johnson, K.A.; Miao, Z.; Rakhit, A.; Pantze, M.P.; Hamilton, M.; Lum, B.L.; Prakash, C. Metabolism and excretion of erlotinib, a small molecule inhibitor of epidermal growth factor receptor tyrosine kinase, in healthy male volunteers. Drug Metab. Dispos. 2006, 34, 420–426. [Google Scholar] [CrossRef]
- Bauer, M.; Blaickner, M.; Philippe, C.; Wadsak, W.; Hacker, M.; Zeitlinger, M.; Langer, O. Whole-body distribution and radiation dosimetry of [11C]elacridar and [11C]tariquidar in humans. J. Nucl. Med. 2016, 57, 1265–1268. [Google Scholar] [CrossRef]
- Bauer, F.; Kuntner, C.; Bankstahl, J.P.; Wanek, T.; Bankstahl, M.; Stanek, J.; Mairinger, S.; Dörner, B.; Löscher, W.; Müller, M.; et al. Synthesis and in vivo evaluation of [11C]tariquidar, a positron emission tomography radiotracer based on a third-generation P-glycoprotein inhibitor. Bioorg. Med. Chem. 2010, 18, 5489–5497. [Google Scholar] [CrossRef]
- Philippe, C.; Mairinger, S.; Pichler, V.; Stanek, J.; Nics, L.; Mitterhauser, M.; Hacker, M.; Wanek, T.; Langer, O.; Wadsak, W. Comparison of fully-automated radiosyntheses of [11C]erlotinib for preclinical and clinical use starting from in target produced [11C]CO2 or [11C]CH4. EJNMMI Radiopharm. Chem. 2018, 3, 8. [Google Scholar] [CrossRef]
- Dörner, B.; Kuntner, C.; Bankstahl, J.P.; Bankstahl, M.; Stanek, J.; Wanek, T.; Stundner, G.; Mairinger, S.; Loscher, W.; Muller, M.; et al. Synthesis and small-animal positron emission tomography evaluation of [11C]-elacridar as a radiotracer to assess the distribution of P-glycoprotein at the blood-brain barrier. J. Med. Chem. 2009, 52, 6073–6082. [Google Scholar] [CrossRef]
- Loening, A.M.; Gambhir, S.S. AMIDE: A free software tool for multimodality medical image analysis. Mol. Imaging 2003, 2, 131–137. [Google Scholar] [CrossRef]
- Takashima, T.; Yokoyama, C.; Mizuma, H.; Yamanaka, H.; Wada, Y.; Onoe, K.; Nagata, H.; Tazawa, S.; Doi, H.; Takahashi, K.; et al. Developmental changes in P-glycoprotein function in the blood-brain barrier of nonhuman primates: PET study with R-11C-verapamil and 11C-oseltamivir. J. Nucl. Med. 2011, 52, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, H.; Suzuki, H.; Terasaki, T.; Kakee, A.; Lemaire, M.; Sugiyama, Y. P-glycoprotein mediates the efflux of quinidine across the blood-brain barrier. J. Pharmacol. Exp. Ther. 1997, 283, 574–580. [Google Scholar]
- Logan, J.; Fowler, J.S.; Volkow, N.D.; Wolf, A.P.; Dewey, S.L.; Schlyer, D.J.; MacGregor, R.R.; Hitzemann, R.; Bendriem, B.; Gatley, S.J.; et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J. Cereb. Blood Flow Metab. 1990, 10, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Hernández Lozano, I.; Bauer, M.; Wulkersdorfer, B.; Traxl, A.; Philippe, C.; Weber, M.; Häusler, S.; Stieger, B.; Jäger, W.; Mairinger, S.; et al. Measurement of hepatic ABCB1 and ABCG2 transport activity with [11C]tariquidar and PET in humans and mice. Mol. Pharm. 2020, 17, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Hernández Lozano, I.; Karch, R.; Bauer, M.; Blaickner, M.; Matsuda, A.; Wulkersdorfer, B.; Hacker, M.; Zeitlinger, M.; Langer, O. Towards improved pharmacokinetic models for the analysis of transporter-mediated hepatic disposition of drug molecules with positron emission tomography. AAPS. J. 2019, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Kamiie, J.; Ohtsuki, S.; Iwase, R.; Ohmine, K.; Katsukura, Y.; Yanai, K.; Sekine, Y.; Uchida, Y.; Ito, S.; Terasaki, T. Quantitative atlas of membrane transporter proteins: Development and application of a highly sensitive simultaneous LC/MS/MS method combined with novel in-silico peptide selection criteria. Pharm. Res. 2008, 25, 1469–1483. [Google Scholar] [CrossRef]
- Zamek-Gliszczynski, M.J.; Kalvass, J.C.; Pollack, G.M.; Brouwer, K.L. Relationship between drug/metabolite exposure and impairment of excretory transport function. Drug Metab. Dispos. 2009, 37, 386–390. [Google Scholar] [CrossRef]
- Munk, O.L.; Keiding, S.; Bass, L. Impulse-response function of splanchnic circulation with model-independent constraints: Theory and experimental validation. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, 671–680. [Google Scholar] [CrossRef][Green Version]
- Kawamura, K.; Yamasaki, T.; Konno, F.; Yui, J.; Hatori, A.; Yanamoto, K.; Wakizaka, H.; Takei, M.; Kimura, Y.; Fukumura, T.; et al. Evaluation of limiting brain penetration related to P-glycoprotein and breast cancer resistance protein using [11C]GF120918 by PET in mice. Mol. Imaging Biol. 2011, 13, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Uchida, Y.; Mittapalli, R.K.; Sane, R.; Terasaki, T.; Elmquist, W.F. Quantitative proteomics of transporter expression in brain capillary endothelial cells isolated from P-glycoprotein (P-gp), breast cancer resistance protein (Bcrp), and P-gp/Bcrp knockout mice. Drug Metab. Dispos. 2012, 40, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, H.L.; Liu, L.; Sheps, J.A.; Phillips, M.J.; Ling, V. Compensatory role of P-glycoproteins in knockout mice lacking the bile salt export pump. Hepatology 2009, 50, 948–956. [Google Scholar] [CrossRef]
- Mennone, A.; Soroka, C.J.; Harry, K.M.; Boyer, J.L. Role of breast cancer resistance protein in the adaptive response to cholestasis. Drug Metab. Dispos. 2010, 38, 1673–1678. [Google Scholar] [CrossRef]
- Fallon, J.K.; Smith, P.C.; Xia, C.Q.; Kim, M.S. Quantification of four efflux drug transporters in liver and kidney across species using targeted quantitative proteomics by isotope dilution nanoLC-MS/MS. Pharm. Res. 2016, 33, 2280–2288. [Google Scholar] [CrossRef] [PubMed]
- Badawi, R.D.; Shi, H.; Hu, P.; Chen, S.; Xu, T.; Price, P.M.; Ding, Y.; Spencer, B.A.; Nardo, L.; Liu, W.; et al. First human imaging studies with the EXPLORER total-body PET scanner. J. Nucl. Med. 2019, 60, 299–303. [Google Scholar] [CrossRef]

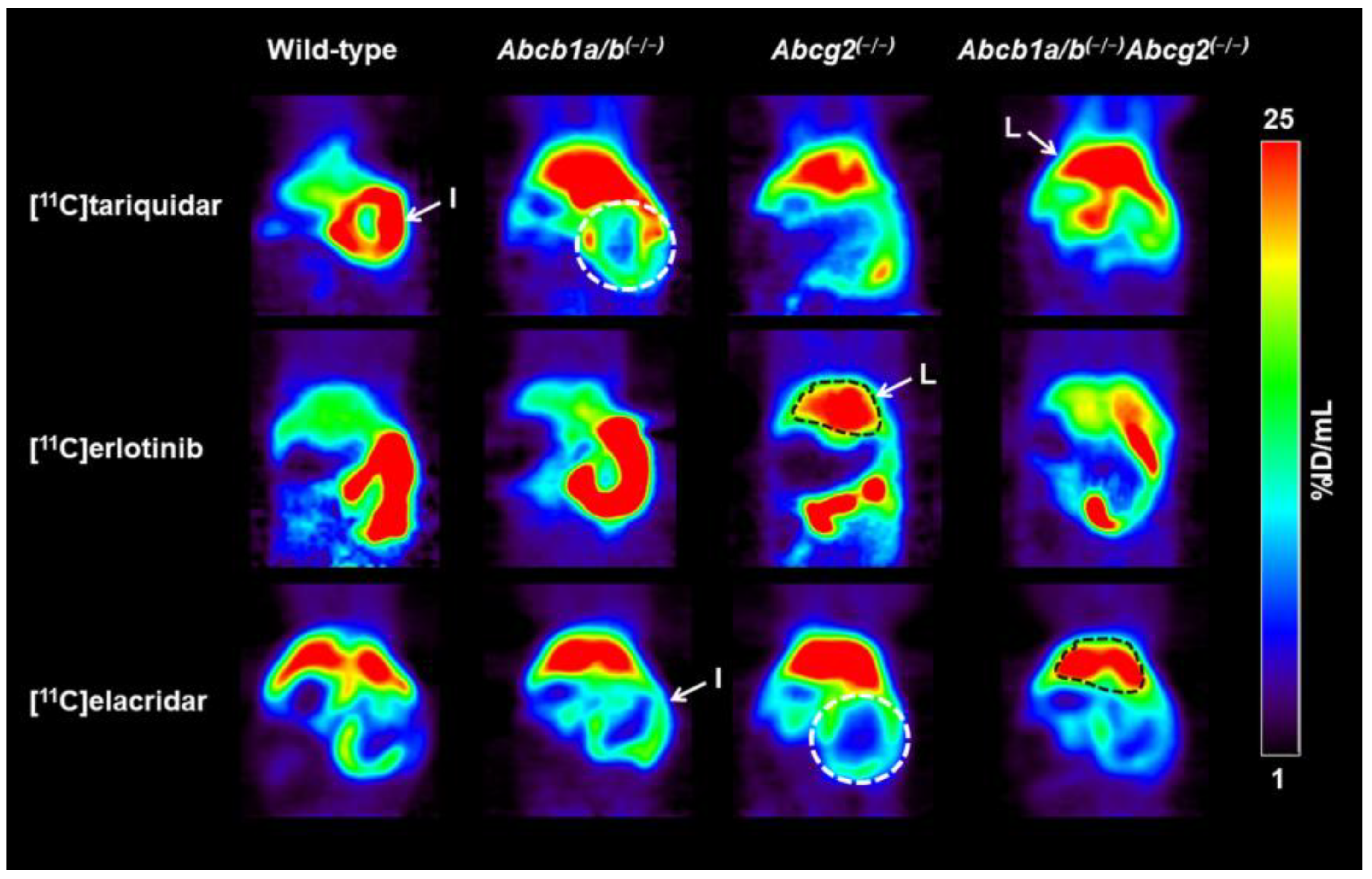

| Radiotracer | Parameter | Wild-Type | Abcb1a/b(−/−) | Abcg2(−/−) | Abcb1a/b(−/−)Abcg2(−/−) |
|---|---|---|---|---|---|
| [11C]tariquidar | CLuptake,brain (mL/min/g tissue) | 0.0035 ± 0.0004 | 0.0061 ± 0.0014 | 0.0031 ± 0.0007 | 0.0263 ± 0.0068 * |
| VT,brain | 0.1269 ± 0.0133 | 0.2169 ± 0.0227 | 0.1045 ± 0.0065 | 0.7460 ± 0.1504 * | |
| [11C]erlotinib | CLuptake,brain (mL/min/g tissue) | 0.0171 ± 0.0038 | 0.0290 ± 0.0045 | 0.0287 ± 0.0071 | 0.0784 ± 0.0128 * |
| VT,brain | 0.2016 ± 0.0141 | 0.2622 ± 0.0079 | 0.2335 ± 0.0174 | 0.5015 ± 0.0497 * | |
| [11C]elacridar | CLuptake,brain (mL/min/g tissue) | 0.0080 ± 0.0044 | 0.0116 ± 0.0030 | 0.0051 ± 0.0011 | 0.0493 ± 0.0564 * |
| VT,brain | 0.1547 ± 0.0489 | 0.2460 ± 0.0673 | 0.1097 ± 0.0315 | 0.6048 ± 0.2069 * |
| Radiotracer | Parameter | Wild-Type | Abcb1a/b(−/−) | Abcg2(−/−) | Abcb1a/b(−/−)Abcg2(−/−) |
|---|---|---|---|---|---|
| [11C]tariquidar | CL1 (mL/min) | 2.2798 ± 0.5058 (12.9–32.3) | 1.7207 ± 0.1377 (10.7–23.1) | 1.5836 ± 0.1993 (9.6–22.1) | 1.9316 ± 0.6933 (9.6–34.2) |
| k2 (min−1) | 0.2600 ± 0.0475 (11.0–26.4) | 0.2421 ± 0.0227 (10.5–19.0) | 0.3244 ± 0.0204 (9.0–20.5) | 0.3441 ± 0.1549 (9.0–29.7) | |
| k3 (min−1) | 0.0036 ± 0.0004 (4.1–7.6) | 0.0039 ± 0.0002 (4.2–7.6) | 0.0037 ± 0.0007 (2.8–9.1) | 0.0019 ± 0.0004 * (5.3–8.4) | |
| [11C]erlotinib | CL1 (mL/min) | 5.9437 ± 2.4249 (5.3–51.5) | 5.2978 ± 1.8375 (12.1–45.4) | 6.3856 ± 1.7690 (10.6–33.4) | 7.3708 ± 0.2309 (20.7–36.7) |
| k2 (min−1) | 0.6431 ± 0.2567 (5.1–47.3) | 0.5815 ± 0.1818 (10.8–41.4) | 0.6051 ± 0.0664 (10.7–30.8) | 0.5484 ± 0.1021 (17.6–32.2) | |
| k3 (min−1) | 0.0193 ± 0.0025 (1.2–4.2) | 0.0191 ± 0.0067 (1.7–4.7) | 0.0091 ± 0.0011 * (1.9–3.3) | 0.0069 ± 0.0018 * (2.4–4.1) | |
| [11C]elacridar | CL1 (mL/min) | 0.5338 ± 0.1759 (6.0–14.3) | 0.6887 ± 0.1939 (11.3–22.4) | 0.4304 ± 0.1392 (4.0–21.0) | 0.5777 ± 0.2241 (4.9–24.6) |
| k2 (min−1) | 0.1121 ± 0.0641 (7.7–13.5) | 0.1872 ± 0.1165 (10.5–17.5) | 0.1674 ± 0.1366 (4.8–20.6) | 0.1426 ± 0.0728 (7.1–18.8) | |
| k3 (min−1) | 0.0040 ± 0.0008 (4.0–6.8) | 0.0042 ± 0.0008 (5.2–8.9) | 0.0043 ± 0.0010 (3.2–18.6) | 0.0015 ± 0.0006 * (7.0–23.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Lozano, I.; Mairinger, S.; Traxl, A.; Sauberer, M.; Filip, T.; Stanek, J.; Kuntner, C.; Wanek, T.; Langer, O. Assessing the Functional Redundancy between P-gp and BCRP in Controlling the Brain Distribution and Biliary Excretion of Dual Substrates with PET Imaging in Mice. Pharmaceutics 2021, 13, 1286. https://doi.org/10.3390/pharmaceutics13081286
Hernández-Lozano I, Mairinger S, Traxl A, Sauberer M, Filip T, Stanek J, Kuntner C, Wanek T, Langer O. Assessing the Functional Redundancy between P-gp and BCRP in Controlling the Brain Distribution and Biliary Excretion of Dual Substrates with PET Imaging in Mice. Pharmaceutics. 2021; 13(8):1286. https://doi.org/10.3390/pharmaceutics13081286
Chicago/Turabian StyleHernández-Lozano, Irene, Severin Mairinger, Alexander Traxl, Michael Sauberer, Thomas Filip, Johann Stanek, Claudia Kuntner, Thomas Wanek, and Oliver Langer. 2021. "Assessing the Functional Redundancy between P-gp and BCRP in Controlling the Brain Distribution and Biliary Excretion of Dual Substrates with PET Imaging in Mice" Pharmaceutics 13, no. 8: 1286. https://doi.org/10.3390/pharmaceutics13081286
APA StyleHernández-Lozano, I., Mairinger, S., Traxl, A., Sauberer, M., Filip, T., Stanek, J., Kuntner, C., Wanek, T., & Langer, O. (2021). Assessing the Functional Redundancy between P-gp and BCRP in Controlling the Brain Distribution and Biliary Excretion of Dual Substrates with PET Imaging in Mice. Pharmaceutics, 13(8), 1286. https://doi.org/10.3390/pharmaceutics13081286