Long-Acting Risperidone Dual Control System: Preparation, Characterization and Evaluation In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Microspheres
2.3. Implant Preparation
2.4. Mathematical Model
2.5. Surface Morphology Analysis
2.6. Particle Size and Distribution
2.7. Drug Loading and Entrapment Efficiency
2.8. Thermal Analyses
2.9. X-ray Powder Diffraction
2.10. Residual Solvent
2.11. In-Vitro Experiments
2.12. In-Vivo Experiments
2.12.1. Grouping and Administration
2.12.2. Biological Sample Analysis
2.12.3. Statistical Analysis
2.13. Polymer Degradation Test
2.14. Irritation Evaluation
2.15. Stability of Products
3. Results and Discussion
3.1. Microsphere Preparation
3.2. Thermal Analysis
3.3. Residual Solvent
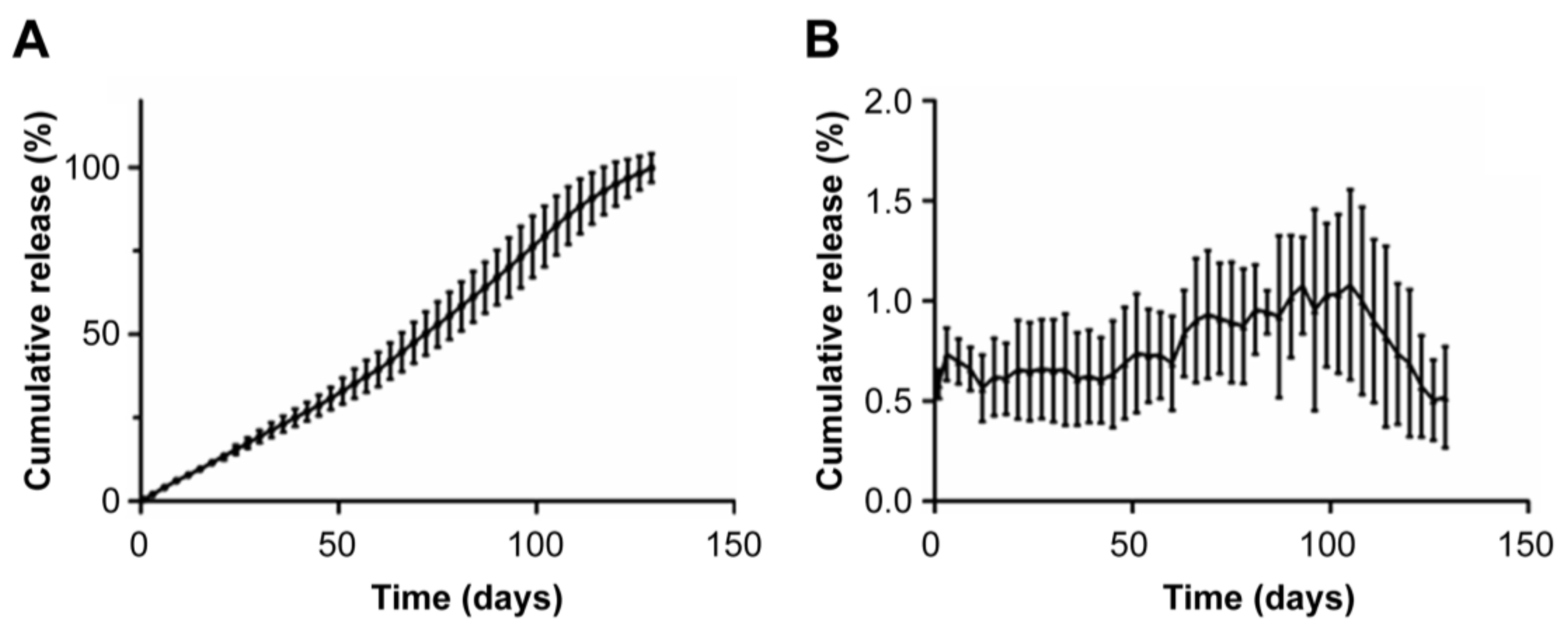
3.4. In-Vitro Dissolution
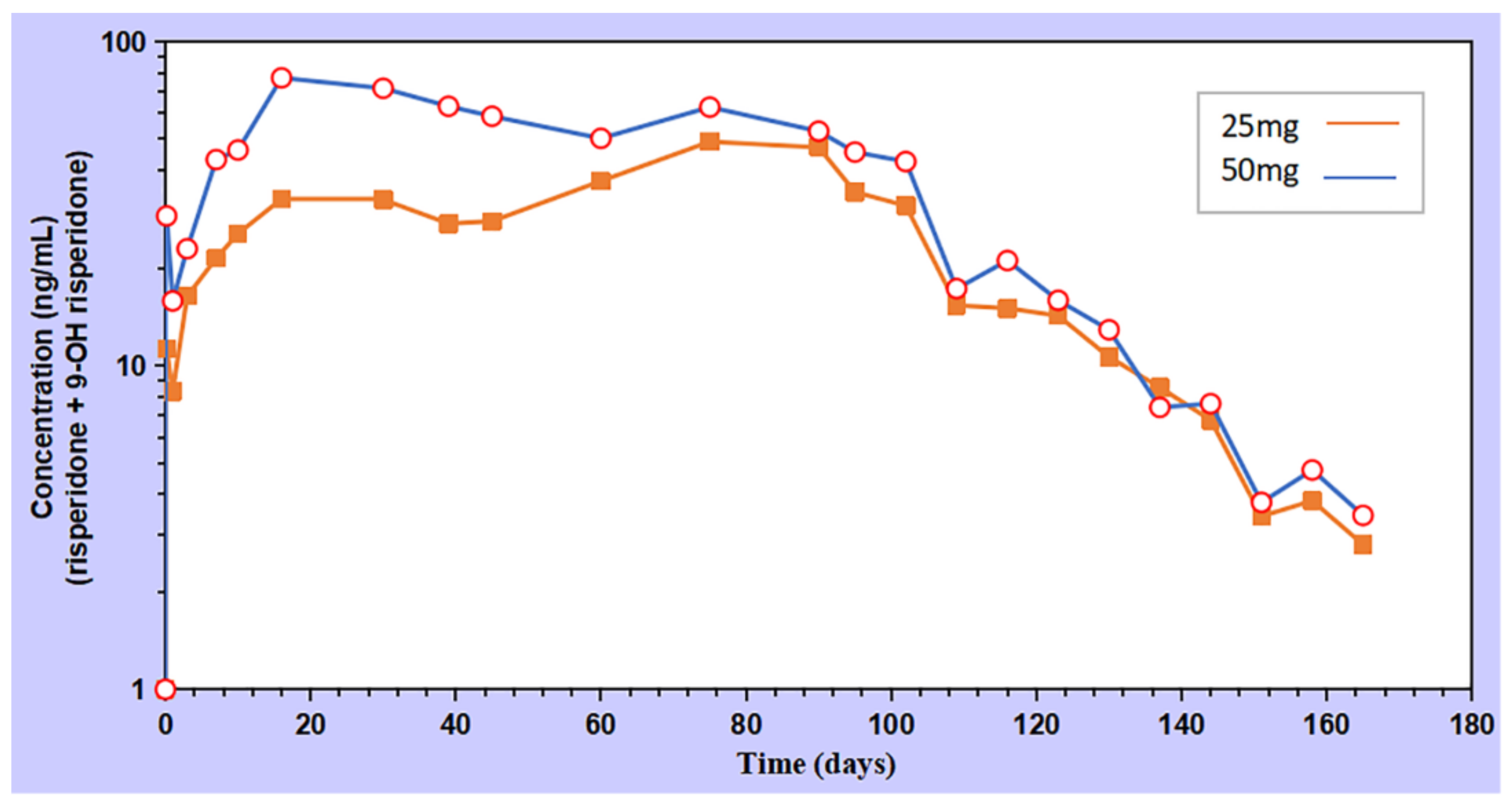
3.5. In-Vivo Pharmacokinetics
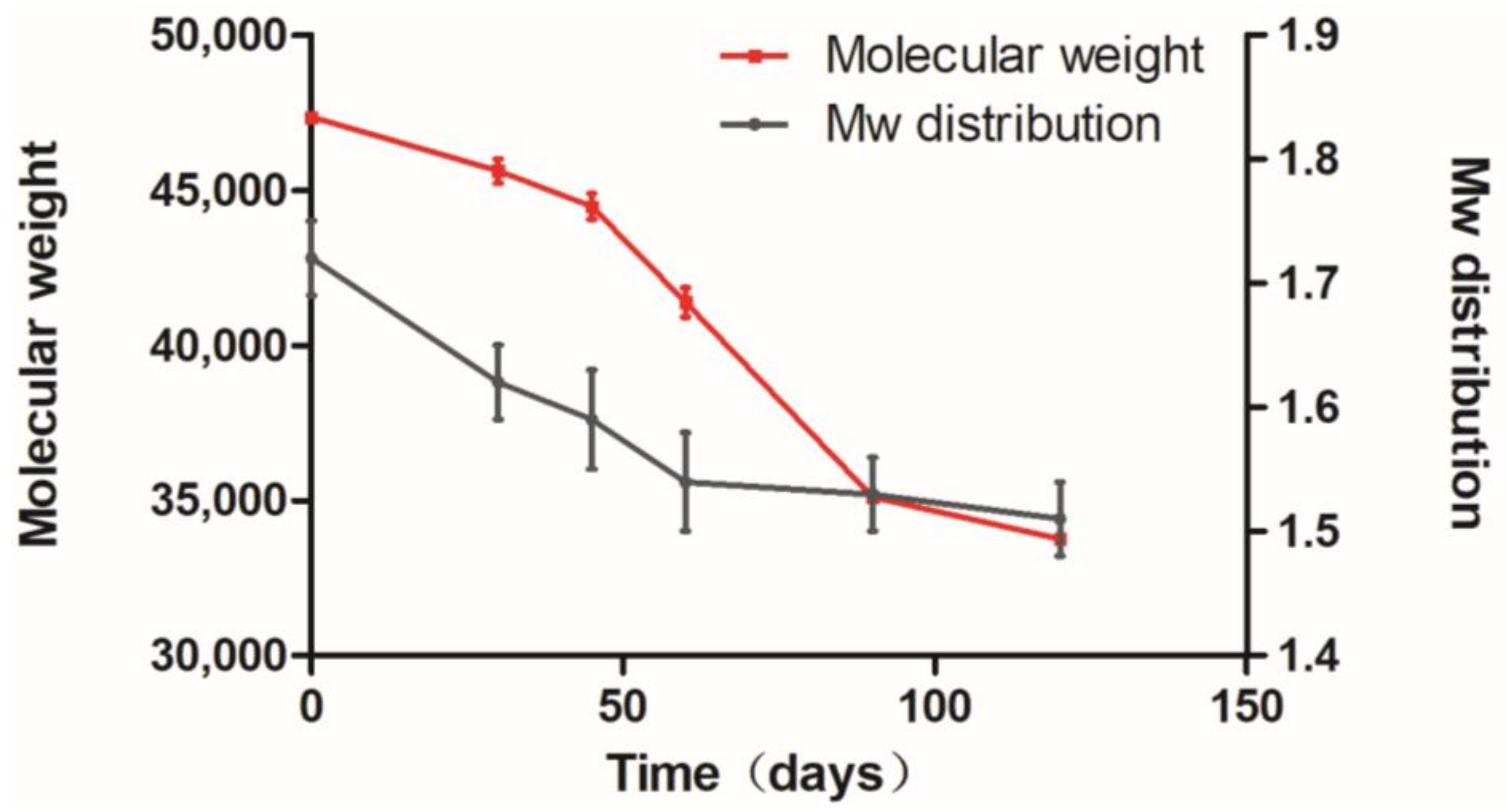
3.6. Polymer Degradation Analysis
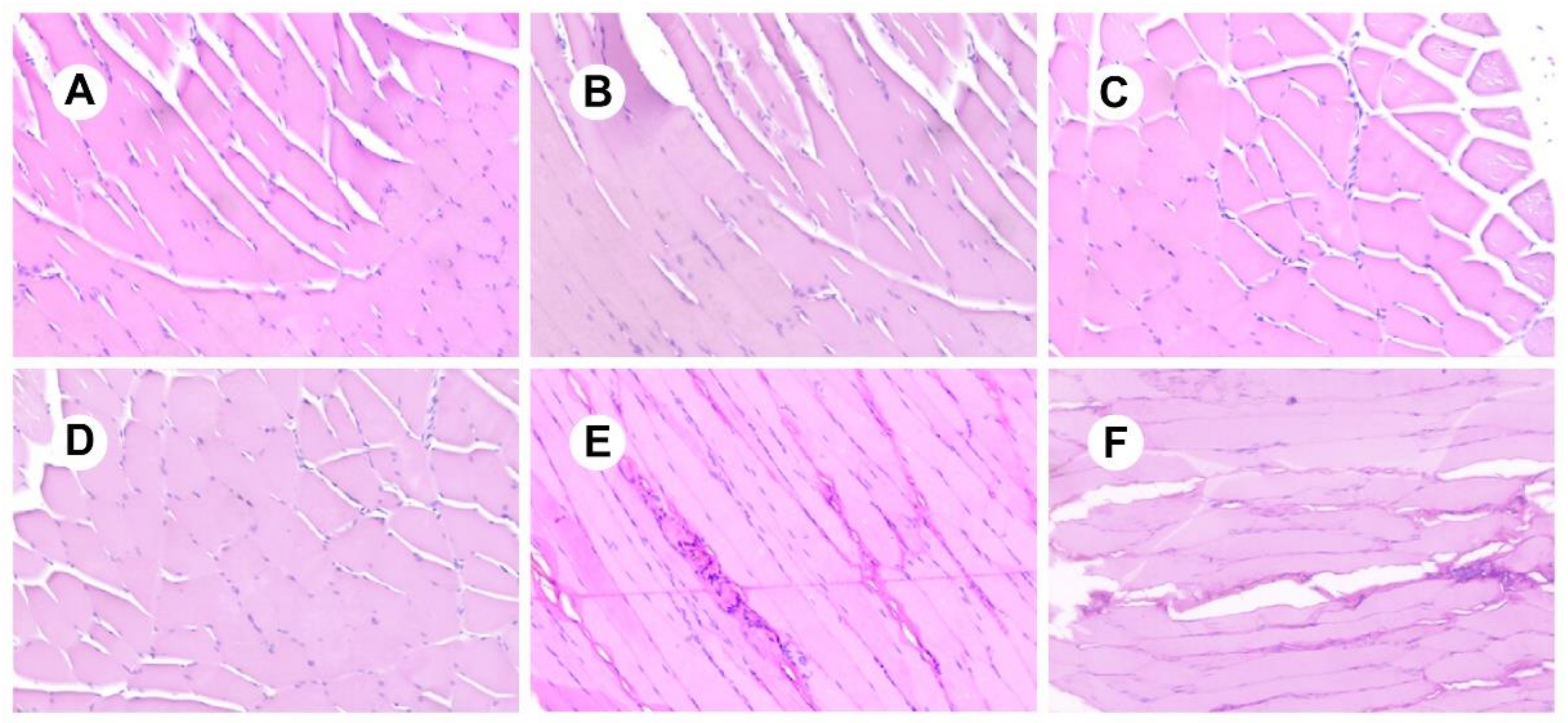
3.7. Analysis of Irritation Experiments
3.8. Studying the Stability of RIS-IMs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD; Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Schultz, S.; North, S.; Shields, C. Schizophrenia: A review. Am. Fam. Physician 2007, 75, 1821–1829. [Google Scholar]
- Laursen, T.M.; Nordentoft, M.; Mortensen, P.B. Excess early mortality in schizophrenia. Annu. Rev. Clin. Psychol. 2014, 10, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Conley, R.R.; Ascher-Svanum, H.; Zhu, B.; Faries, D.E.; Kinon, B.J. The burden of depressive symptoms in the long-term treatment of patients with schizophrenia. Schizophr. Res. 2007, 90, 186–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, Y.; Li, S.; Liu, J.; Li, X.; Luo, X.J. Functional genomics reveal gene regulatory mechanisms underlying schizophrenia risk. Nat. Commun. 2019, 10, 670. [Google Scholar] [CrossRef]
- Vilain, J.; Galliot, A.M.; Durand-Roger, J.; Leboyer, M.; Llorca, P.M.; Schürhoff, F.; Szöke, A. Environmental risk factors for schizophrenia: A review. Encephale 2013, 39, 19–28. [Google Scholar] [CrossRef]
- Moran, P.; Stokes, J.; Marr, J.; Bock, G.; Desbonnet, L.; Waddington, J.; O’Tuathaigh, C. Gene × environment interactions in schizophrenia: Evidence from genetic mouse models. Neural. Plast. 2016, 2016, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Ayhan, Y.; McFarland, R.; Pletnikov, M.V. Animal models of gene-environment interaction in schizophrenia: A dimensional perspective. Prog. Neurobiol. 2016, 136, 1–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solmi, M.; Murru, A.; Pacchiarotti, I.; Undurraga, J.; Veronese, N.; Fornaro, M.; Stubbs, B.; Monaco, F.; Vieta, E.; Seeman, M.V.; et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: A state-of-the-art clinical review. Ther. Clin. Risk Manag. 2017, 13, 757–777. [Google Scholar] [CrossRef] [Green Version]
- Leucht, S.; Corves, C.; Arbter, D.; Engel, R.R.; Li, C.; Davis, J.M. Second-generation versus first-generation antipsychotic drugs for schizophrenia: A meta-analysis. Lancet 2009, 373, 31–41. [Google Scholar] [CrossRef]
- Aravagiri, M.; Yuwiler, A.; Marder, S.R. Distribution after repeated oral administration of different dose levels of risperidone and 9-hydroxy-risperidone in the brain and other tissues of rat. Psychopharmacology 1998, 139, 356–363. [Google Scholar] [CrossRef]
- Schoretsanitis, G.; de Leon, J.; Haen, E.; Stegmann, B.; Hiemke, C.; Gründer, G.; Paulzen, M. Pharmacokinetics of risperidone in different application forms—Comparing long-acting injectable and oral formulations. Eur. Neuropsychopharmacol. 2018, 28, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Ramachandran, C.; Bermejo, M.; Yamashita, S.; Yu, L.X.; Amidon, G.L. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol. Pharm. 2006, 3, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Sussman, N. Choosing an atypical antipsychotic. Int. Clin. Psychopharmacol. 2002, 17, S2933. [Google Scholar]
- FDA. 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021346s063lbl.pdf (accessed on 13 March 2021).
- FDA. 2003. Pharmacology Review(s). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21346_Risperdal%20Consta%20Long-Acting%20Injection_pharmr.pdf (accessed on 13 March 2021).
- Yerragunta, B.; Jogala, S.; Chinnala, K.M.; Aukunuru, J. Development of a novel 3-month drug releasing risperidone microspheres. J. Pharm. Bioallied. Sci. 2015, 7, 37–44. [Google Scholar] [PubMed]
- Chaurasia, S.; Mounika, K.; Bakshi, V.; Prasad, V. 3-month parenteral PLGA microsphere formulations of risperidone: Fabrication, characterization and neuropharmacological assessments. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 75, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable materials for bone repair and tissue engineering applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Larsen, M.W.; Pietrzak, W.S.; DeLee, J.C. Fixation of osteochondritis dissecans lesions using poly(L-lactic acid)/poly(glycolic acid) copolymer bioabsorbable screws. Am. J. Sports Med. 2005, 33, 68–76. [Google Scholar] [CrossRef]
- Afsharzadeh, M.; Hashemi, M.; Babaei, M.; Abnous, K.; Ramezani, M. PEG-PLA nanoparticles decorated with small-molecule PSMA ligand for targeted delivery of galbanic acid and docetaxel to prostate cancer cells. J. Cell. Physiol. 2020, 235, 4618–4630. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, L.; Zhou, J.; Fan, X.; Xie, H.; Zhang, H.; Zhang, G.; Shi, X. Influence of polylactide (PLA) stereocomplexation on the microstructure of PLA/PBS blends and the cell morphology of their microcellular foams. Polymers 2020, 12, 2362. [Google Scholar] [CrossRef] [PubMed]
- Repp, L.; Rasoulianboroujeni, M.; Lee, H.J.; Kwon, G.S. Acyl and oligo(lactic acid) prodrugs for PEG-b-PLA and PEG-b-PCL nano-assemblies for injection. J. Control. Release 2021, 330, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- FDA. 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020517s042,019732s044lbl.pdf (accessed on 13 March 2021).
- Rong, X.; Yuan, W.; Lu, Y.; Mo, X. Safety evaluation of poly(lactic-co-glycolic acid)/poly(lactic-acid) microspheres through intravitreal injection in rabbits. Int. J. Nanomed. 2014, 9, 3057–3068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, C.M.; Quiroga, R.M. Assessment of the safety and efficacy of poly-L-lactic acid for the treatment of HIV-associated facial lipoatrophy. J. Am. Acad. Dermatol. 2005, 52, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Kim, S.H.; Ha, H.J.; Ko, Y.K.; So, J.W.; Kim, M.S.; Yang, Y.I.; Khang, G.; Rhee, J.M.; Lee, H.B. Reduction of inflammatory reaction of poly(D,L-lactic-co-glycolic acid) using demineralized bone particles. Tissue Eng. Part A 2008, 14, 539–547. [Google Scholar] [CrossRef]
- Dailey, L.A.; Jekel, N.; Fink, L.; Gessler, T.; Schmehl, T.; Wittmar, M.; Kissel, T.; Seeger, W. Investigation of the proinflammatory potential of biodegradable nanoparticle drug delivery systems in the lung. Toxicol. Appl. Pharmacol. 2006, 215, 100–108. [Google Scholar] [CrossRef]
- Colucci, D.; Fissore, D.; Barresi, A.A.; Braatz, R.D. A new mathematical model for monitoring the temporal evolution of the ice crystal size distribution during freezing in pharmaceutical solutions. Eur. J. Pharm. Biopharm. 2020, 148, 148–159. [Google Scholar] [CrossRef]
- Fridgeirsdottir, G.A.; Harris, R.J.; Dryden, I.L.; Fischer, P.M.; Roberts, C.J. Multiple linear regression modeling to predict the stability of polymer-drug solid dispersions: Comparison of the effects of polymers and manufacturing methods on solid dispersion stability. Mol. Pharm. 2018, 15, 1826–1841. [Google Scholar] [CrossRef]
- Lopez-Palau, N.E.; Olais-Govea, J.M. Mathematical model of blood glucose dynamics by emulating the pathophysiology of glucose metabolism in type 2 diabetes mellitus. Sci. Rep. 2020, 10, 12697. [Google Scholar] [CrossRef]
- Gorry, C.; McCullagh, L.; Barry, M. Economic evaluation of systemic treatments for advanced melanoma: A systematic review. Value Health 2020, 23, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Janich, C.; Friedmann, A.; Martins de Souza, E.S.J.; Silva, J.; Santos de Oliveira, C.; Souza, L.E.; Rujescu, D.; Hildebrandt, C.; Beck-Broichsitter, M.; Schmelzer, C.E.H.; et al. Risperidone-loaded PLGA-lipid particles with improved release kinetics: Manufacturing and detailed characterization by electron microscopy and nano-CT. Pharmaceutics 2019, 11, 665. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Prog. Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Li, K.; Yu, L.; Liu, X.; Chen, C.; Chen, Q.; Ding, J. A long-acting formulation of a polypeptide drug exenatide in treatment of diabetes using an injectable block copolymer hydrogel. Biomaterials 2013, 34, 2834–2842. [Google Scholar] [CrossRef]
- Huang, S.; Huang, Z.; Fu, Z.; Shi, Y.; Dai, Q.; Tang, S.; Gu, Y.; Xu, Y.; Chen, J.; Wu, X.; et al. A novel drug delivery carrier comprised of nimodipine drug solution and a nanoemulsion: Preparation, characterization, In Vitro, and In Vivo studies. Int. J. Nanomed. 2020, 15, 1161–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ICH. Q1A(R2): Stability Testing of New Drug Substances and Products. 2003. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-1-r2-stability-testing-new-drug-substances-products-step-5_en.pdf (accessed on 13 March 2021).
- Kabacoff, R. R in Action: Data Analysis and Graphics with R; Manning Publications: New York, NY, USA, 2011. [Google Scholar]
- Al-Akayleh, F.; Adwan, S.; Khanfar, M.; Idkaidek, N.; Al-Remawi, M. A novel eutectic-based transdermal delivery system for risperidone. AAPS Pharm. Sci. Tech. 2020, 22, 4. [Google Scholar] [CrossRef]
- Tran, P.; Pyo, Y.C.; Kim, D.H.; Lee, S.E.; Kim, J.K.; Park, J.S. Overview of the manufacturing methods of solid dispersion technology for improving the solubility of poorly water-soluble drugs and application to anticancer drugs. Pharmaceutics 2019, 11, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, Z.; Zidan, A.S.; Khan, M.A. Risperidone solid dispersion for orally disintegrating tablet: Its formulation design and non-destructive methods of evaluation. Int. J. Pharm. 2010, 400, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Widjaja, E.; Boey, F.; Loo, S.C.J. Spectroscopy techniques for analyzing the hydrolysis of PLGA and PLLA. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 433–440. [Google Scholar] [CrossRef] [PubMed]
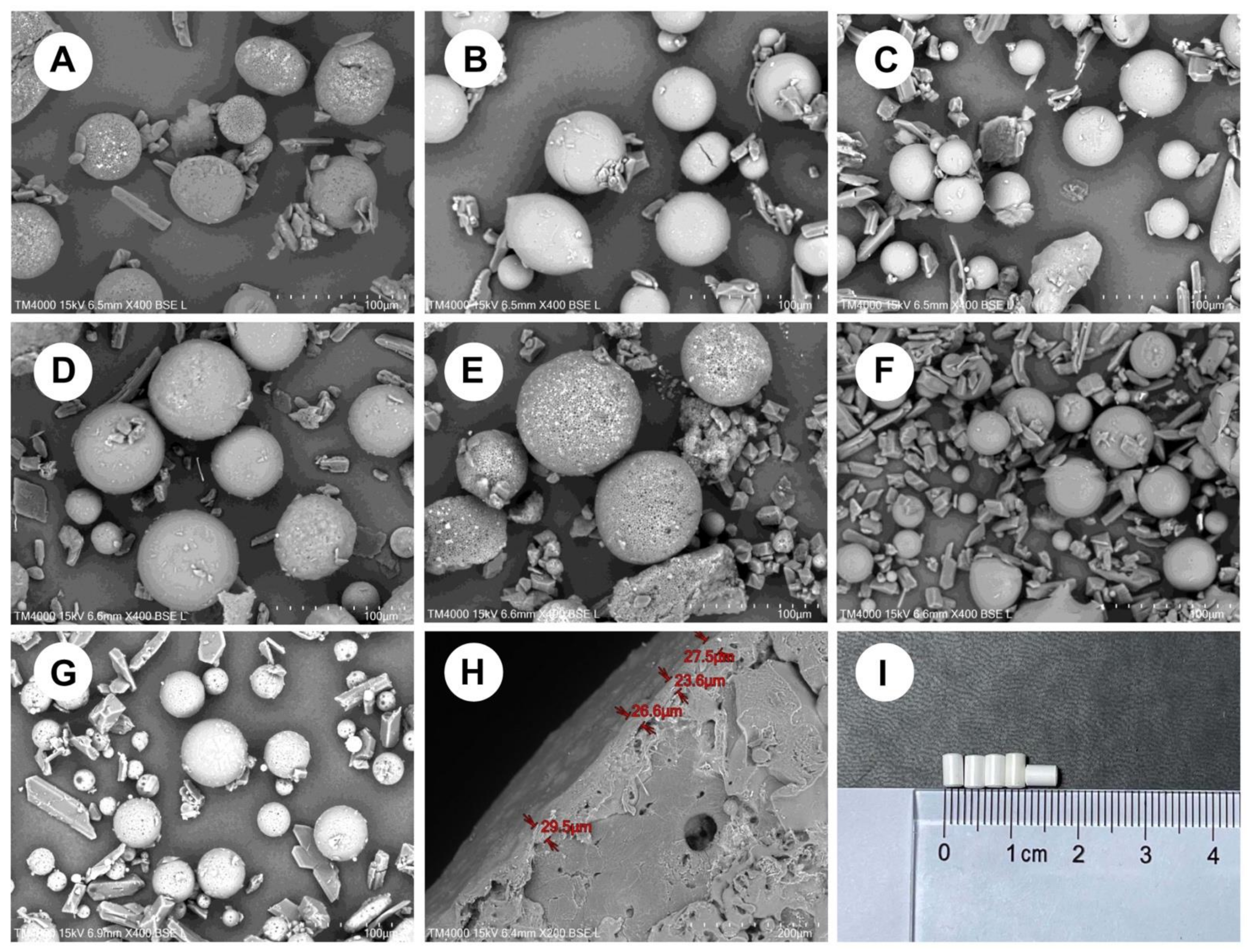
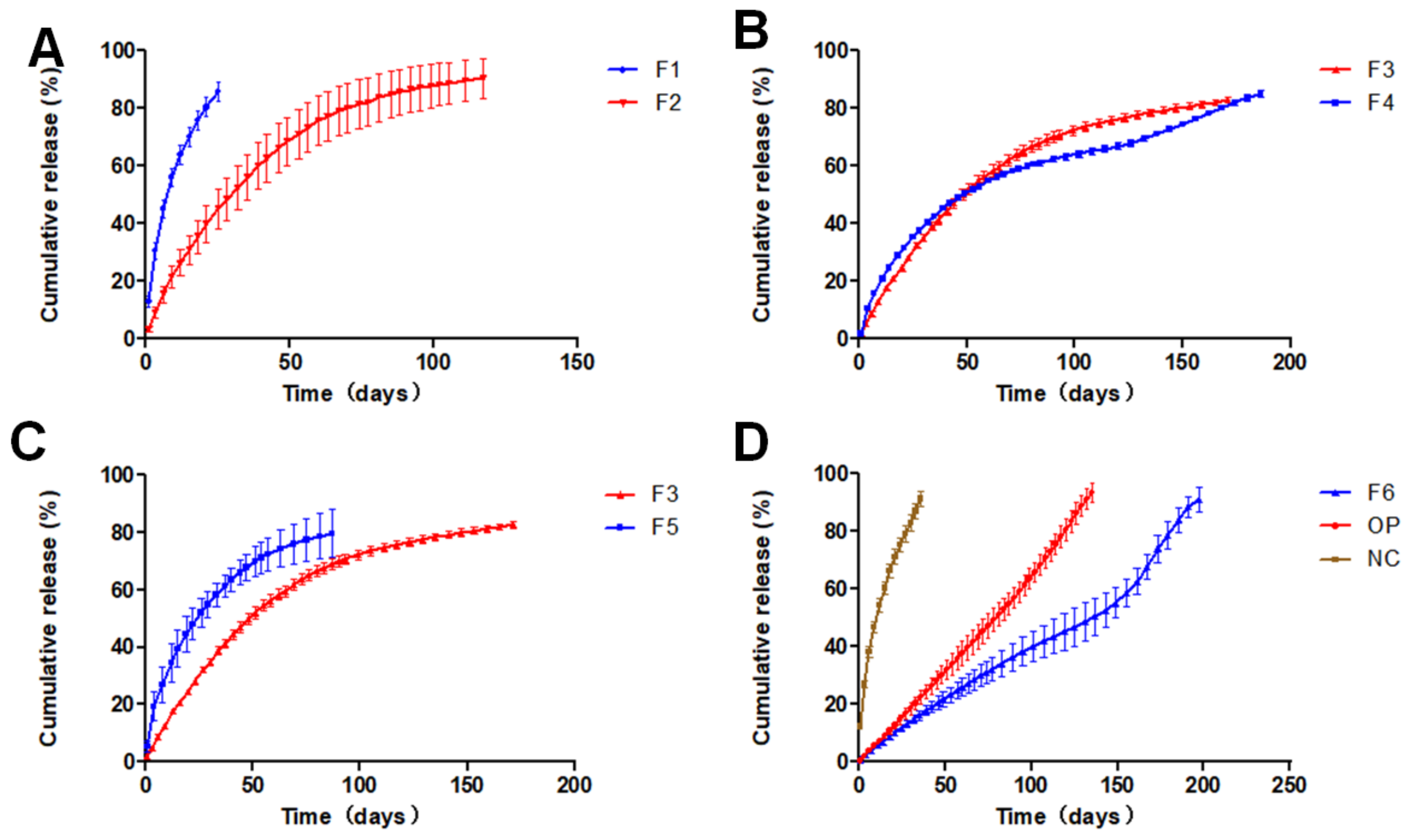
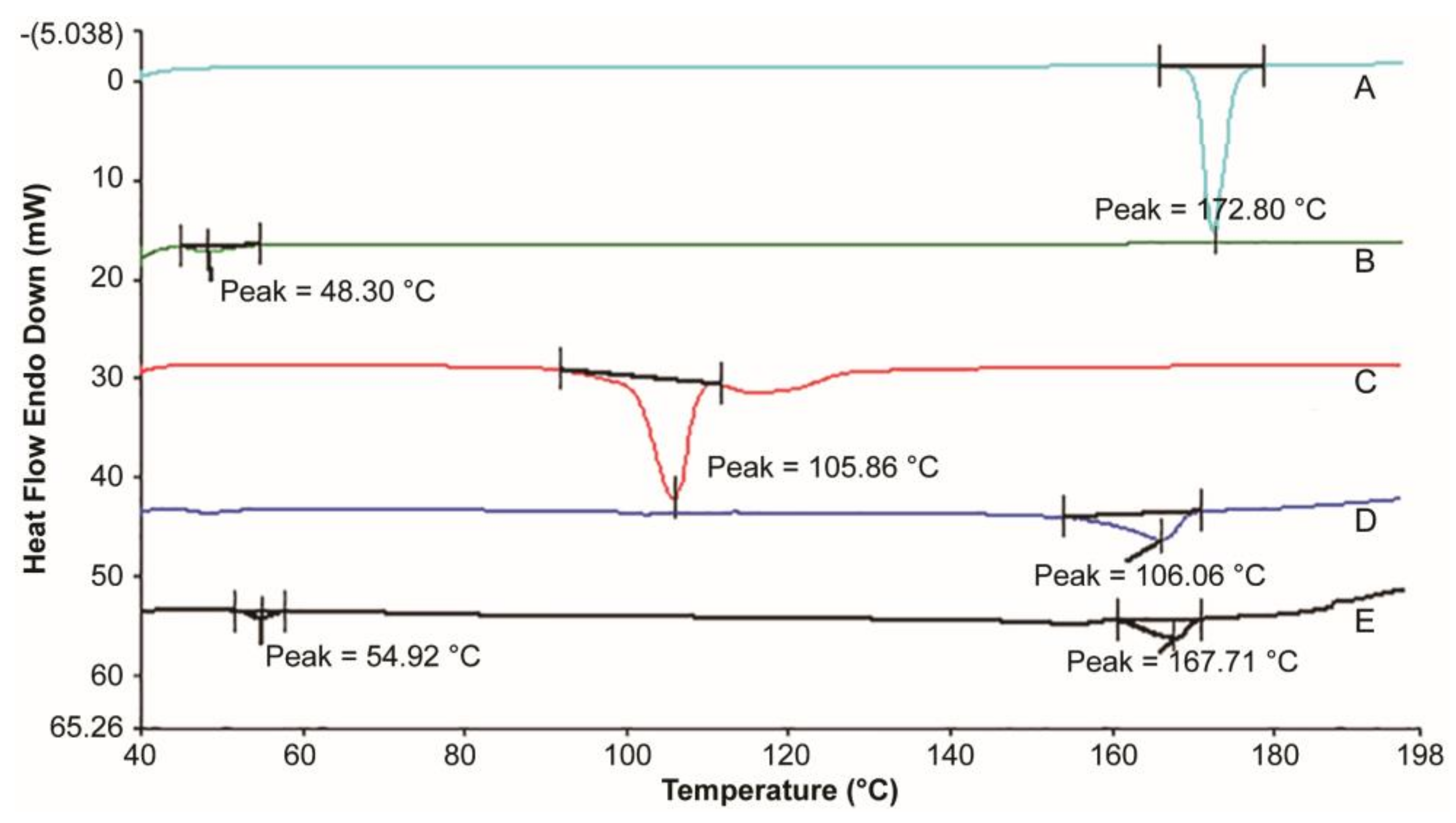
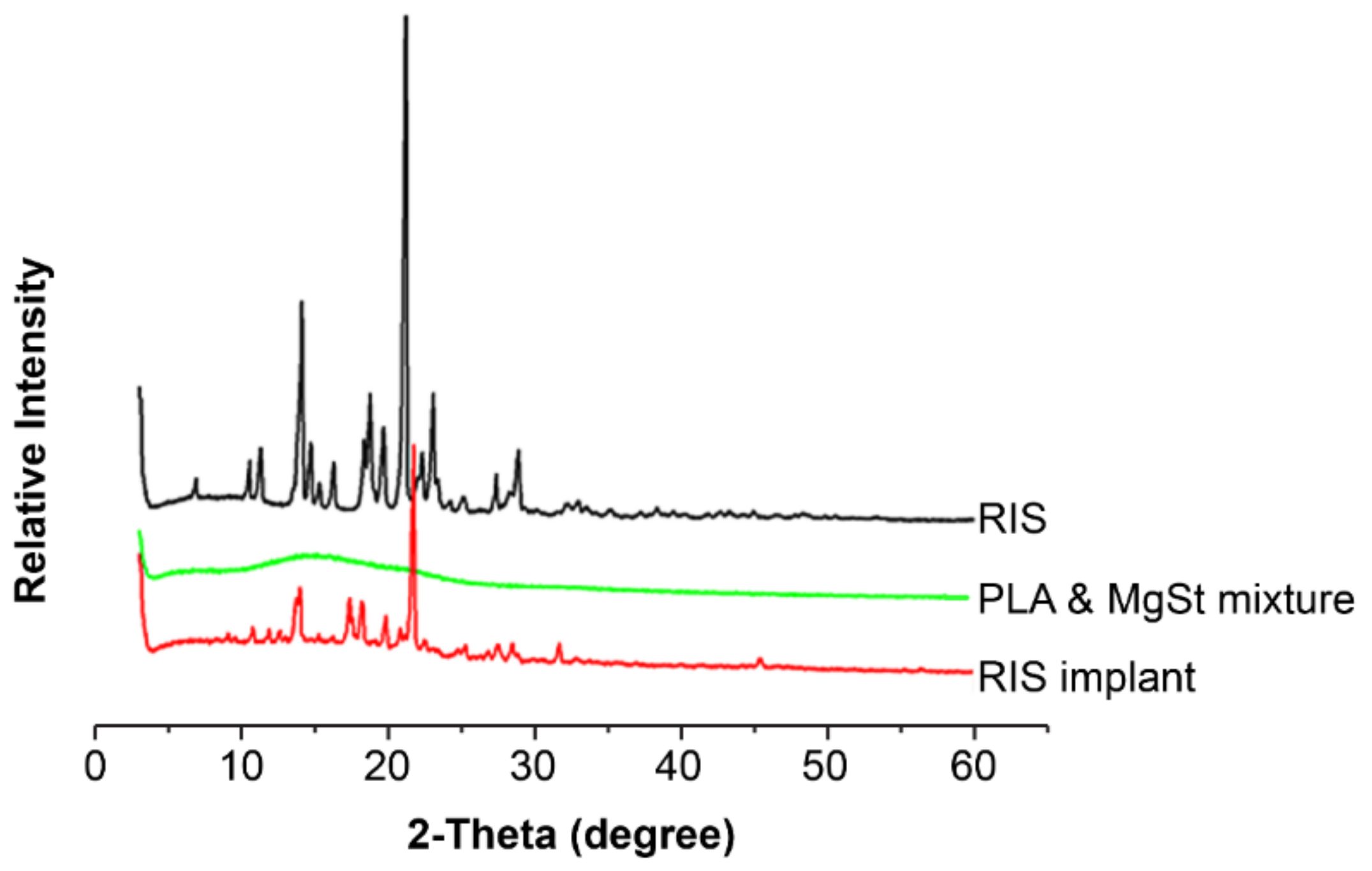
| Formulation | Oil Phase | Inner Water Phase | Internal Water Phase/Oil Phase (w/o) ** | |||
|---|---|---|---|---|---|---|
| RIS (g) | PLA (g) | DCM (mL) | Types of Salt | Concentration (%) | ||
| F1 | 5 | 5 | 30 | Sucrose | 10 | 1:10 |
| F2 | 5 | 5 | 30 | Sodium chloride | 10 | 1:10 |
| F3 | 7.5 | 7.5 | 25 | Sodium chloride | 5 | 1:10 |
| F4 | 6.0 | 9.0 | 25 | Sodium chloride | 5 | 1:10 |
| F5 | 7.5 | 7.5 | 25 | Sodium chloride | 20 | 1:10 |
| F6 * | 7.5 | 7.5 | 25 | - | 0 | 0 |
| Risperidone/PLA | DCM | PLA Concentration | Sodium Chloride Concentration | w/o | R2 | |
|---|---|---|---|---|---|---|
| Risperidone/PLA | 1.000 | 0.333 | −1.000 | 0.192 | −0.293 | 0.205 |
| DCM | 0.333 | 1.000 | −0.333 | −0.192 | 0.410 | −0.381 |
| PLA concentration | −1.000 | −0.333 | 1.000 | −0.192 | 0.293 | −0.205 |
| Sodium chloride concentration | 0.192 | −0.192 | −0.192 | 1.000 | 0.542 | −0.636 |
| w/o | −0.293 | 0.410 | 0.293 | 0.542 | 1.000 | −0.992 |
| R2 | 0.205 | −0.381 | −0.205 | −0.636 | −0.992 | 1.000 |
| Value | p-Value | Decision | |
|---|---|---|---|
| Global Stat | 1.366 | 0.850 | Assumptions acceptable. |
| Skewness | 0.202 | 0.653 | Assumptions acceptable. |
| Kurtosis | 0.159 | 0.690 | Assumptions acceptable. |
| Link Function | 0.208 | 0.648 | Assumptions acceptable. |
| Heteroscedasticity | 0.797 | 0.372 | Assumptions acceptable. |
| Formulation | Particle Size (µm) | Specific Surface Area (m2/kg) | Drug Loading of Microsphere (%) | Drug Loading of the Implant (%) | Entrapment Efficiency of Implant (%) | Correlation Coefficient (R2) |
|---|---|---|---|---|---|---|
| F1 | 133.7 ± 5.2 | 65.44 | 25.5 ± 3.2 | 32.9 ± 2.0 | 65.8 ± 4.0 | 0.918 |
| F2 | 138.9 ± 6.2 | 38.24 | 34.6 ± 3.1 | 41.0 ± 3.1 | 82.0 ± 6.2 | 0.860 |
| F3 | 125.5 ± 5.2 | 29.39 | 38.1 ± 3.5 | 42.2 ± 3.1 | 84.4 ± 6.2 | 0.875 |
| F4 | 139.0 ± 6.8 | 28.88 | 31.1 ± 3.5 | 36.9 ± 1.6 | 92.3 ± 4.0 | 0.977 |
| F5 | 166.7 ± 8.7 | 21.68 | 29.3 ± 5.8 | 37.3 ± 3.1 | 74.6 ± 1.6 | 0.858 |
| F6 | 135.4 ± 4.5 | 29.77 | 40.0 ± 4.3 | 45.3 ± 2.7 | 90.6 ± 5.4 | 0.918 |
| OP | 134.4 ± 3.5 | 30.77 | 42.1 ± 4.2 | 46.7 ± 2.3 | 93.4 ± 4.6 | 0.997 |
| Parameter | Unit | Dose | |
|---|---|---|---|
| 25 mg | 50 mg | ||
| t1/2 | d | 19.00 | 18.40 |
| Tmax | d | 75.00 | 16.00 |
| Cmax | ng/mL | 49.07 | 77.22 |
| AUC0-t | ng/mL·d | 4197 | 6594 |
| AUC 0-inf_obs | ng/mL·d | 4274 | 6685 |
| AUC 0-t/0-inf_obs | 0.9820 | 0.9864 | |
| MRT 0-inf_obs | d | 69.57 | 60.68 |
| Vz/F_obs | (mg)/(ng/mL) | 0.1603 | 0.1981 |
| Cl/F_obs | (mg)/(ng/mL)/d | 5.800 × 10−3 | 7.5 × 10−3 |
| Packaging Material | Storage Conditions | Time | Experimental Project | Results | |
|---|---|---|---|---|---|
| Accelerated test | Brown 10-mL vial rubber stopper | 40 °C 60% RH | 6 months | Assay, Impurities, Dissolution | The Mw of PLA decreased from 48,000 to 43,500, and the drug release and impurities of the implants did not change significantly. |
| Long-term test | Brown 10-mL vial rubber stopper | 5 °C | 6 months | Assay, Impurities, Dissolution | There was no significant change in the Mw of PLA, drug release, and impurities. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Wang, S.; Sun, K. Long-Acting Risperidone Dual Control System: Preparation, Characterization and Evaluation In Vitro and In Vivo. Pharmaceutics 2021, 13, 1210. https://doi.org/10.3390/pharmaceutics13081210
Yan X, Wang S, Sun K. Long-Acting Risperidone Dual Control System: Preparation, Characterization and Evaluation In Vitro and In Vivo. Pharmaceutics. 2021; 13(8):1210. https://doi.org/10.3390/pharmaceutics13081210
Chicago/Turabian StyleYan, Xieguo, Shiqiang Wang, and Kaoxiang Sun. 2021. "Long-Acting Risperidone Dual Control System: Preparation, Characterization and Evaluation In Vitro and In Vivo" Pharmaceutics 13, no. 8: 1210. https://doi.org/10.3390/pharmaceutics13081210
APA StyleYan, X., Wang, S., & Sun, K. (2021). Long-Acting Risperidone Dual Control System: Preparation, Characterization and Evaluation In Vitro and In Vivo. Pharmaceutics, 13(8), 1210. https://doi.org/10.3390/pharmaceutics13081210





