Direct Powder Extrusion 3D Printing of Praziquantel to Overcome Neglected Disease Formulation Challenges in Paediatric Populations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Powdered Materials
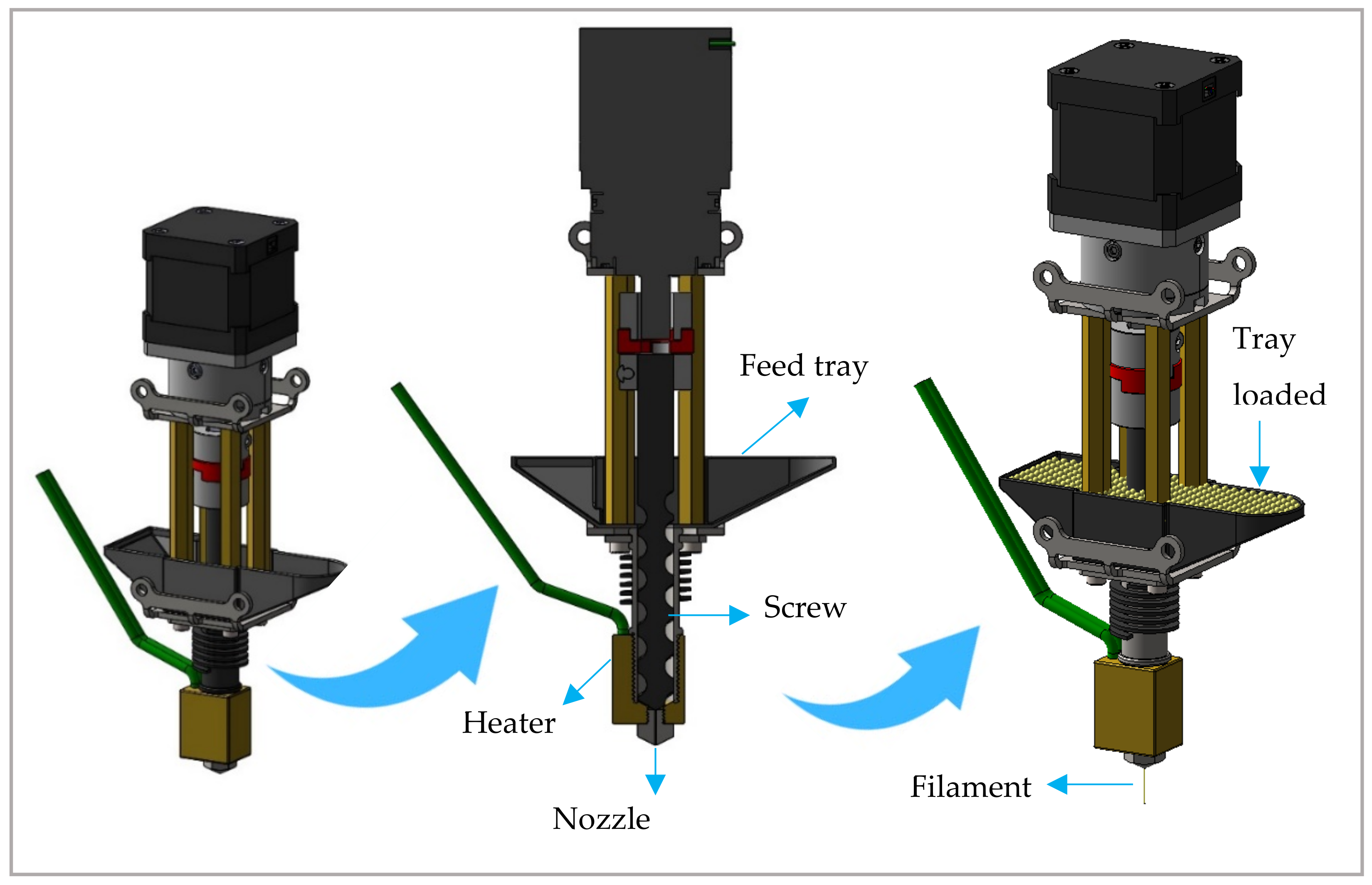
2.2.2. DPE 3D Printing
2.2.3. Printlet Characterisation
Dimensions
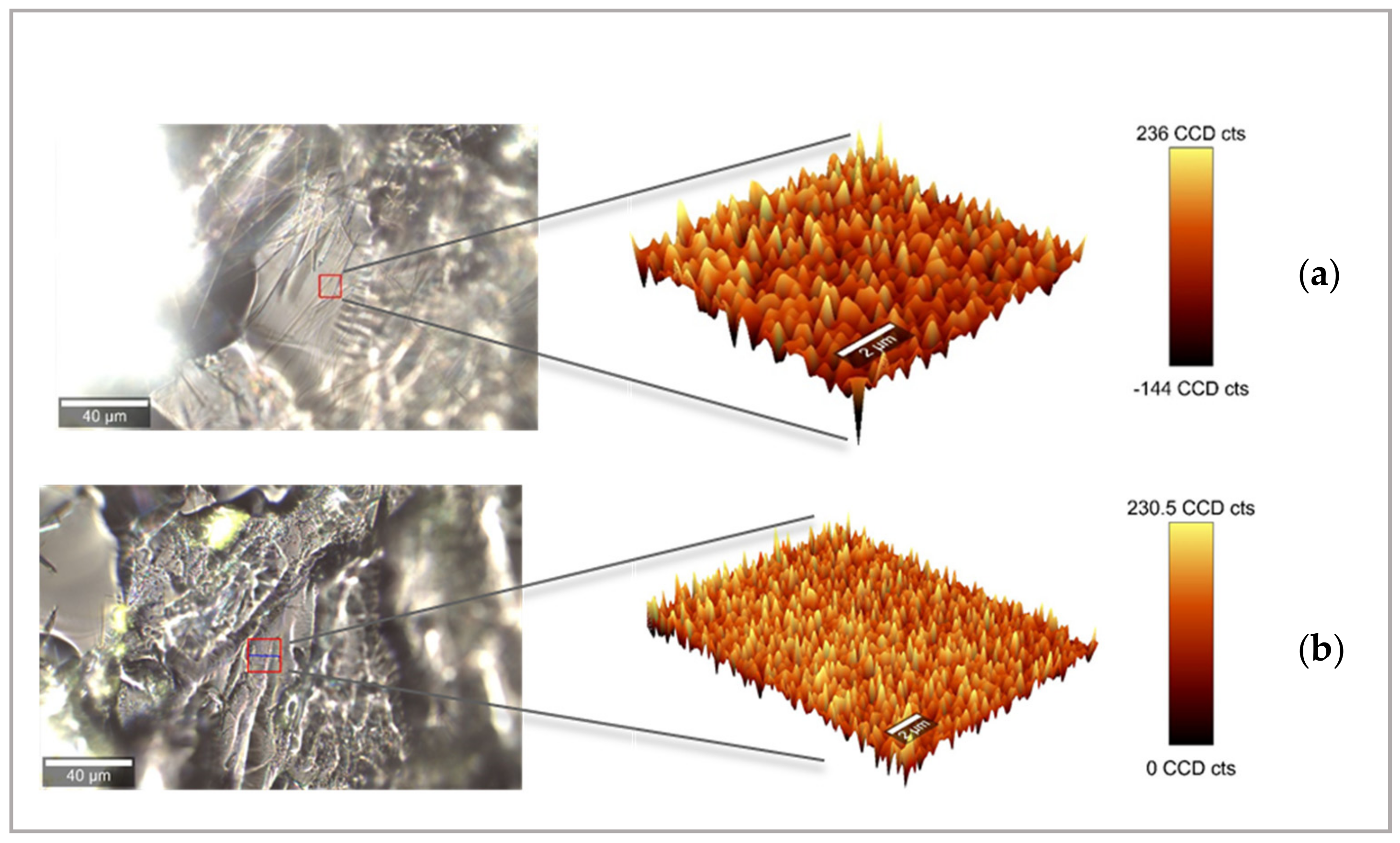
Optical Microscopy
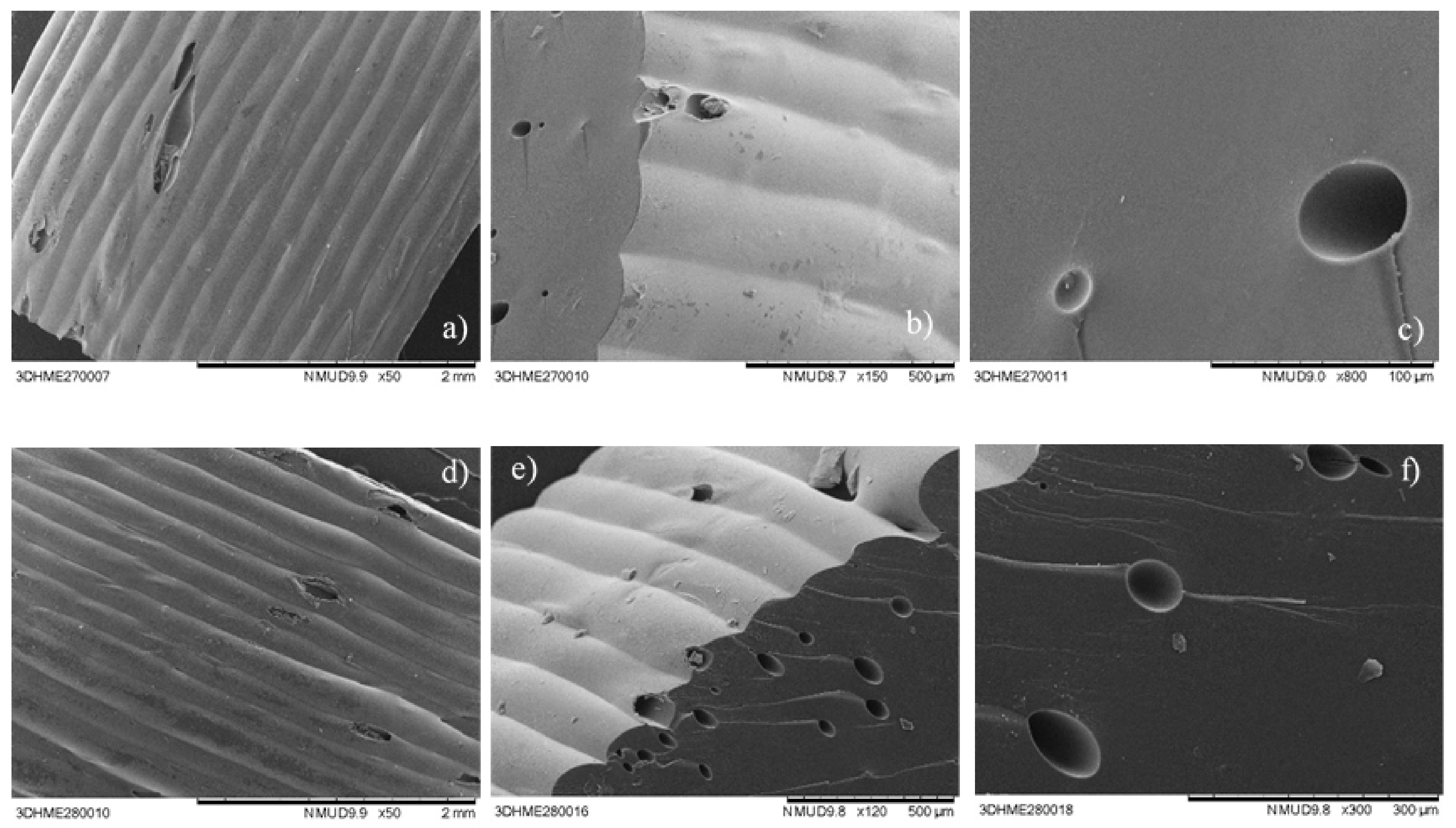
Scanning Electronic Microscopy (SEM)
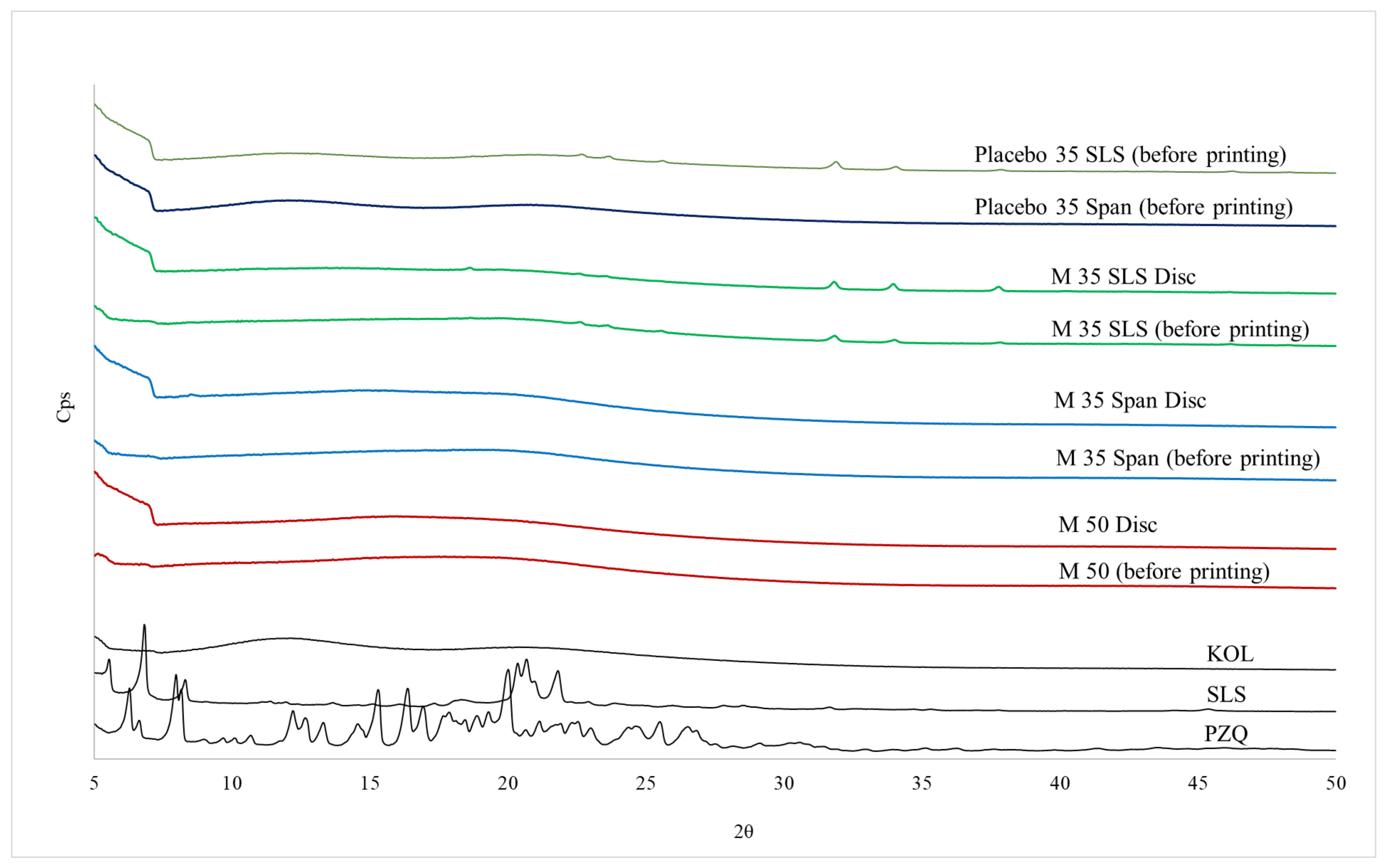
X-ray Powder Diffraction (XRPD) Analysis
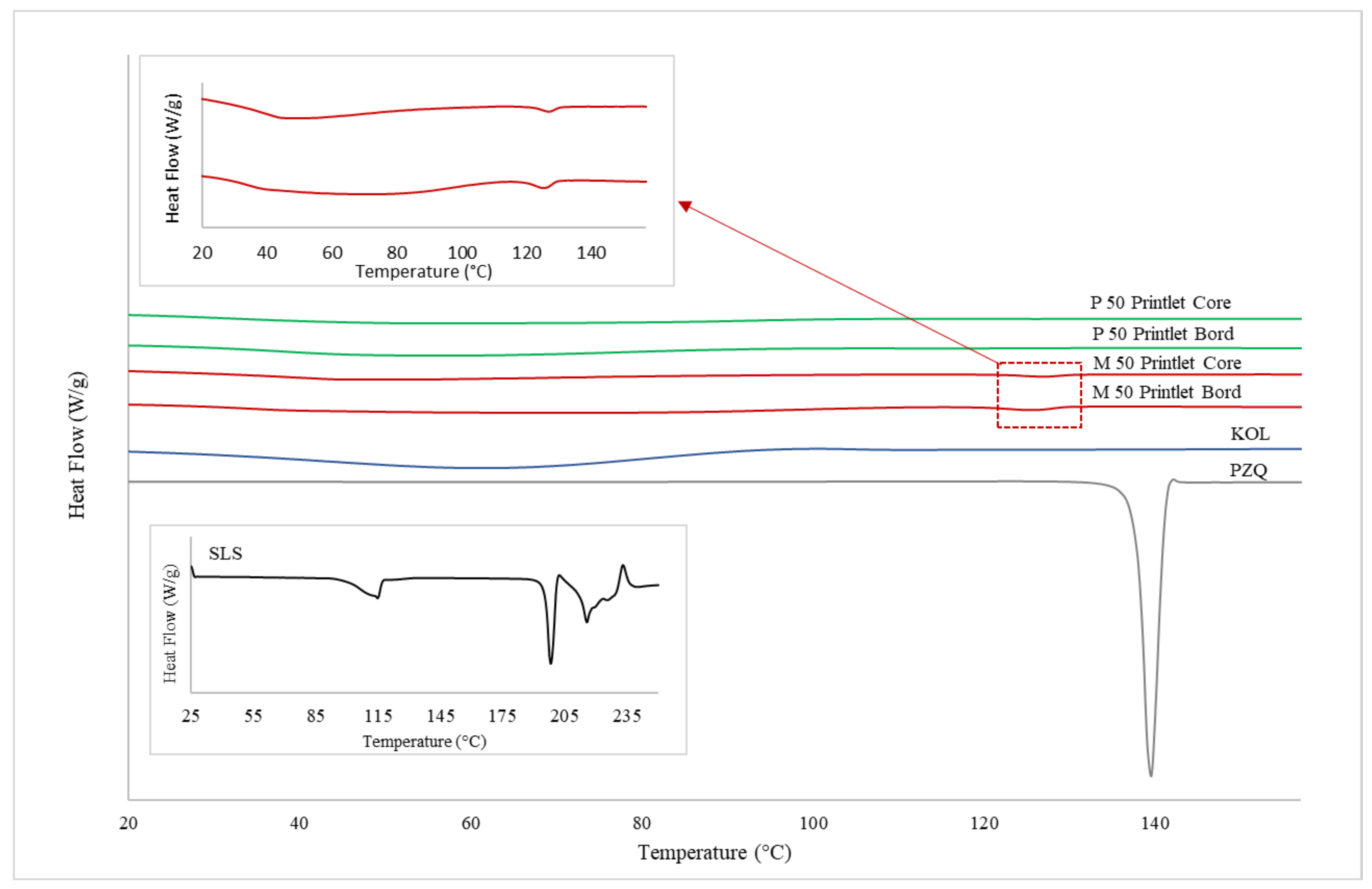
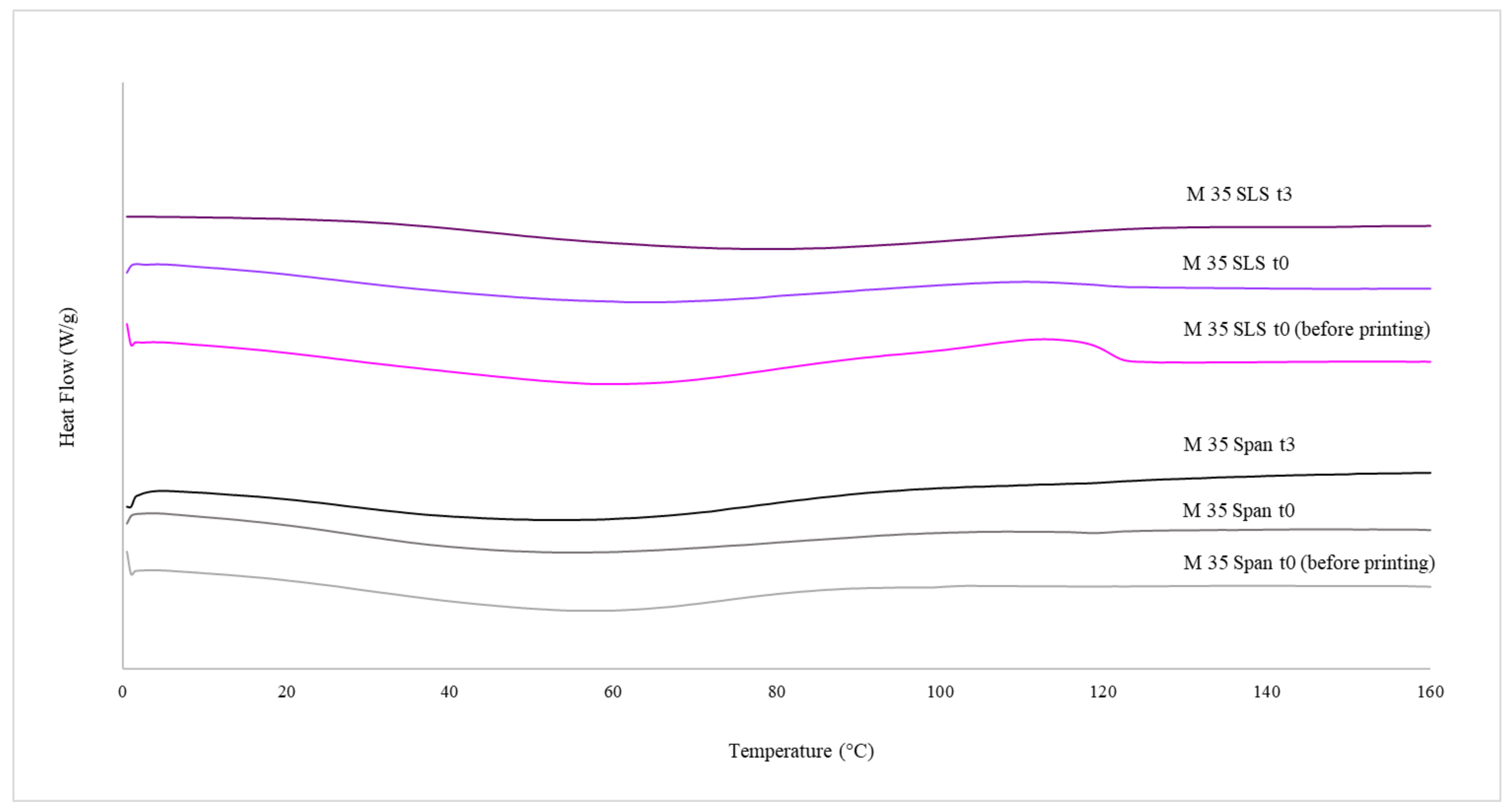
Differential Scanning Calorimetry (DSC)
Raman Microscopy
Determination of Drug Loading
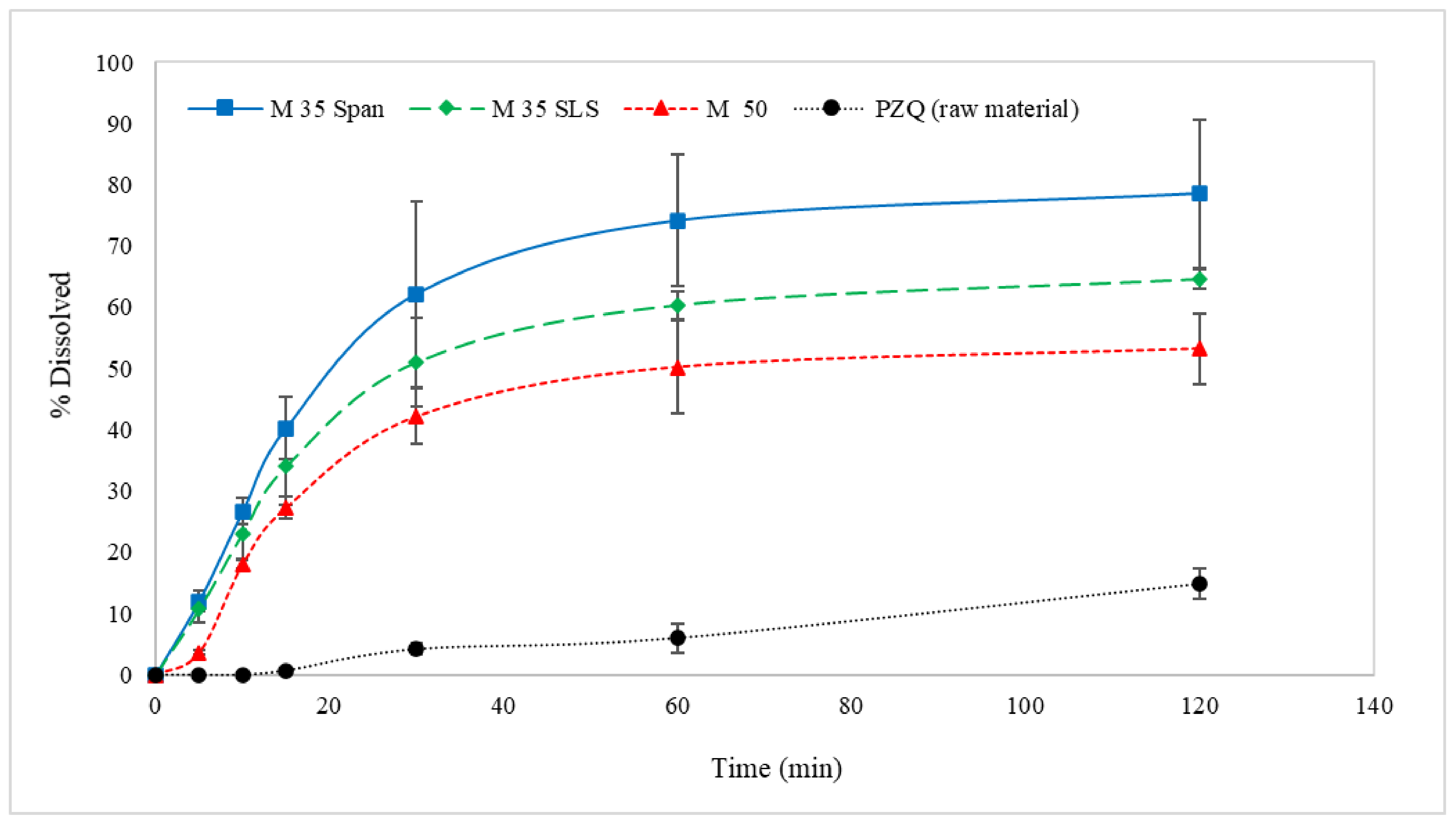
In Vitro Drug Release Studies
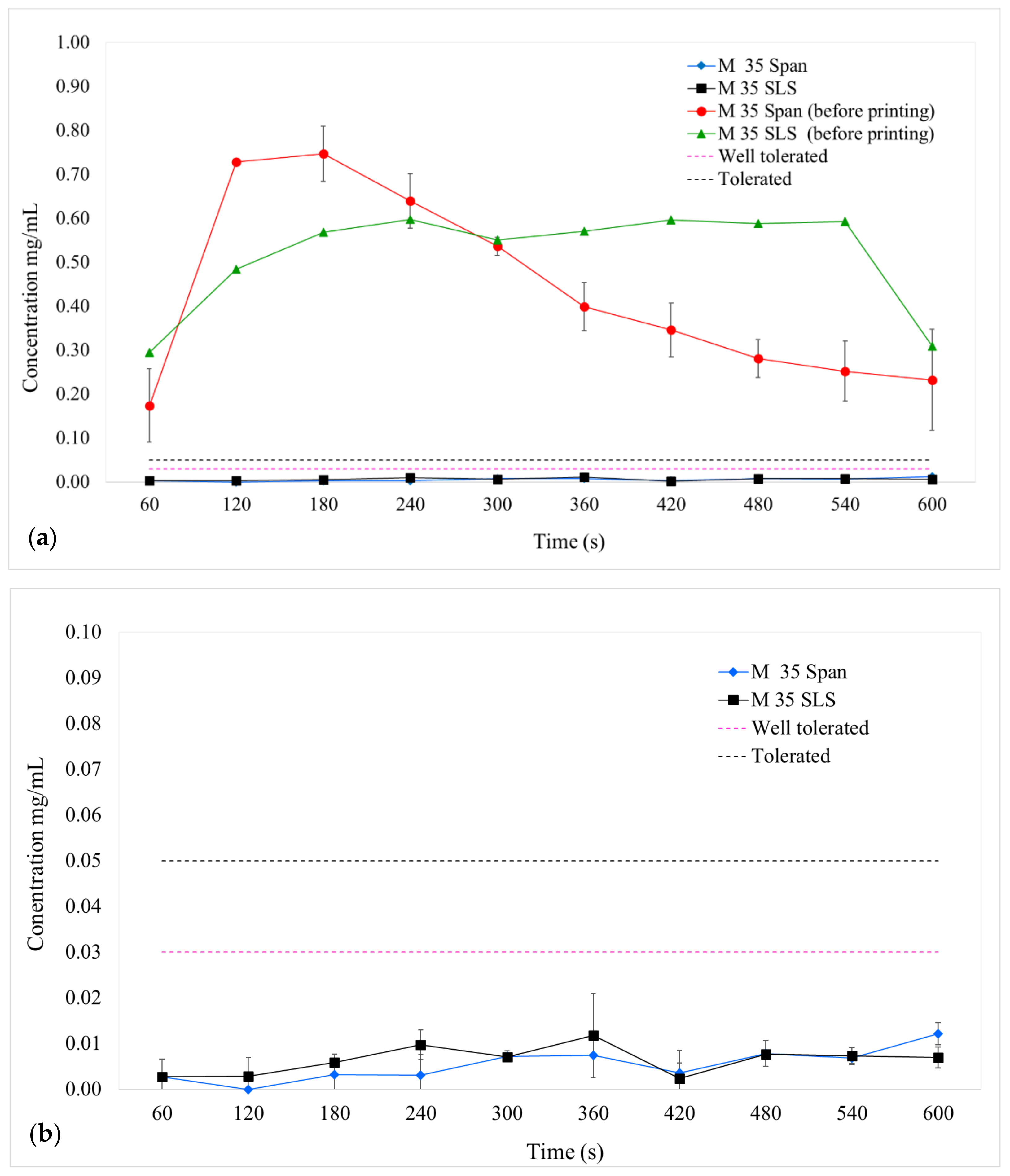
Assessment of Taste Masking Efficiency Using a Novel Biorelevant Buccal Dissolution Test
Stability Study
3. Results
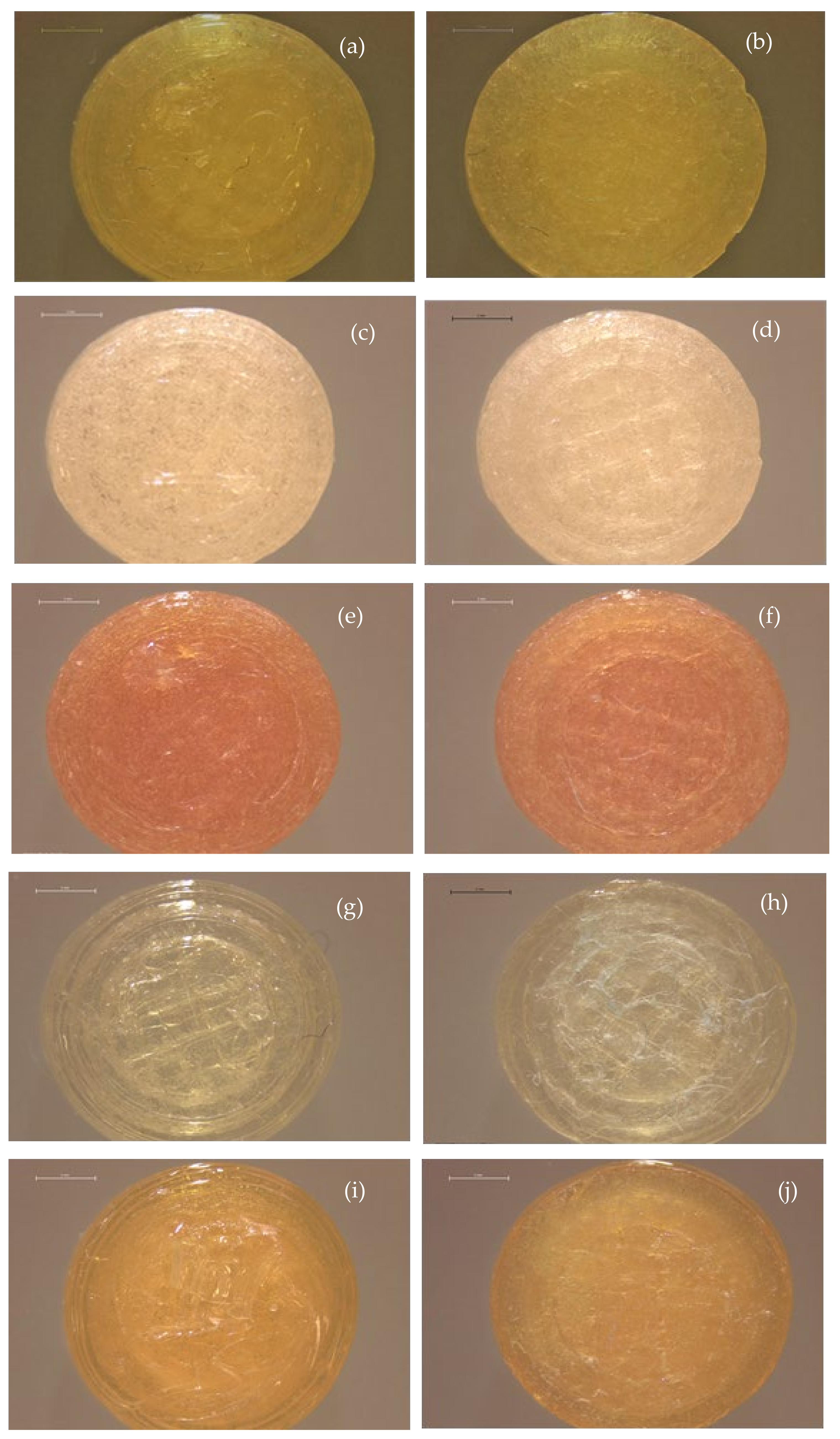
3.1. Physical Printlet Characteristics
3.2. Physicochemical Characterisations
3.3. Dissolution Profiles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Karunamoorthi, K.; Almalki, M.J.; Ghailan, K.Y. Schistosomiasis: A neglected tropical disease of poverty: A call for intersectoral mitigation strategies for better health. J. Health Res. Rev. 2018, 5, 1. [Google Scholar] [CrossRef]
- Mondiale, O.; La Santé, D. Schistosomiasis: Number of people treated worldwide in 2014 Schistosomiase: Nombre de personnes traitées dans le monde en 2014. Wkly. Epidemiol. Rec. 2015, 30, 25–31. [Google Scholar]
- Coulibaly, J.T.; Panic, G.; Silué, K.D.; Kovač, J.; Hattendorf, J.; Keiser, J. Efficacy and safety of praziquantel in preschool-aged and school-aged children infected with Schistosoma mansoni: A randomised controlled, parallel-group, dose-ranging, phase 2 trial. Lancet Glob. Health 2017, 5, e688–e698. [Google Scholar] [CrossRef] [Green Version]
- Hoekstra, P.T.; Partal, M.C.; Van Lieshout, L.; Corstjens, P.L.A.M.; Tsonaka, R.; Assaré, R.K.; Silué, K.D.; Meité, A.; N’Goran, E.K.; N’Gbesso, Y.K.; et al. Efficacy of single versus four repeated doses of praziquantel against Schistosoma mansoni infection in school-aged children from Côte d’Ivoire based on Kato-Katz and POC-CCA: An open-label, randomised controlled trial (RePST). PLoS Negl. Trop. Dis. 2020, 14, e0008189. [Google Scholar] [CrossRef] [PubMed]
- Osakunor, D.N.M.; Woolhouse, M.; Mutapi, F. Paediatric schistosomiasis: What we know and what we need to know. PLoS Negl. Trop. Dis. 2018, 12, e0006144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pediatric Praziquantel Consortium. Schistosomiasis in Children: A Major Public Health Problem. Available online: https://www.pediatricpraziquantelconsortium.org/ (accessed on 19 May 2021).
- Eyayu, T.; Zeleke, A.J.; Worku, L. Current status and future prospects of protein vaccine candidates against Schistosoma mansoni infection. Parasite Epidemiol. Control 2020, 11, e00176. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.I.N.; Salazar, G.D.O.; La Corte, R. Retrocesso do Programa de Controle da Esquistossomose no estado de maior prevalência da doença no Brasil. Rev. Pan Amaz. Saúde 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Meyer, T.; Sekljic, H.; Fuchs, S.; Bothe, H.; Schollmeyer, D.; Miculka, C. Taste, A New Incentive to Switch to (R)-Praziquantel in Schistosomiasis Treatment. PLoS Negl. Trop. Dis. 2009, 3, e357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, J.C.S.; Bernardes, M.V.A.D.S.; de Melo, F.L.; Sá, M.P.; Carvalho, B.M. Praziquantel versus praziquantel associated with immunomodulators in mice infected with schistosoma mansoni: A systematic review and meta-analysis. Acta Trop. 2020, 204, 105359. [Google Scholar] [CrossRef]
- Navaratnam, A.; Sousa-Figueiredo, J.; Stothard, R.; Kabatereine, N.; Fenwick, A.; Mutumba-Nakalembe, M. Efficacy of praziquantel syrup versus crushed praziquantel tablets in the treatment of intestinal schistosomiasis in Ugandan preschool children, with observation on compliance and safety. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 400–407. [Google Scholar] [CrossRef]
- Winzenburg, G.; Desset-Brèthes, S. Industry perspective on palatability testing in children—Two case studies. Int. J. Pharm. 2012, 435, 139–142. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Guideline on Pharmaceutical Development of Medicines for Paediatric Use; UK, 2013; pp. 1–24, [Online]. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-pharmaceutical-development-medicines-paediatric-use_en.pdf (accessed on 10 March 2021).
- Borrego-Sánchez, A.; Carazo, E.; Albertini, B.; Passerini, N.; Perissutti, B.; Cerezo, P.; Viseras, C.; Hernández-Laguna, A.; Aguzzi, C.; Sainz-Díaz, C.I. Conformational polymorphic changes in the crystal structure of the chiral antiparasitic drug praziquantel and interactions with calcium carbonate. Eur. J. Pharm. Biopharm. 2018, 132, 180–191. [Google Scholar] [CrossRef]
- Malaquias, L.F.; Schulte, H.L.; Chaker, J.A.; Karan, K.; Durig, T.; Marreto, R.; Gratieri, T.; Gelfuso, G.; Cunha-Filho, M. Hot Melt Extrudates Formulated Using Design Space: One Simple Process for Both Palatability and Dissolution Rate Improvement. J. Pharm. Sci. 2018, 107, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Jacobs, E.; Jones, D.S.; McCoy, C.P.; Wu, H.; Andrews, G.P. The design and development of high drug loading amorphous solid dispersion for hot-melt extrusion platform. Int. J. Pharm. 2020, 586, 119545. [Google Scholar] [CrossRef]
- Zhao, M.; You, D.; Yin, J.; Sun, W.; Yin, T.; Gou, J.; Zhang, Y.; Wang, Y.; He, H.; Tang, X. Quaternary enteric solid dispersion prepared by hot-melt extrusion to mask the bitter taste and enhance drug stability. Int. J. Pharm. 2021, 597, 120279. [Google Scholar] [CrossRef]
- Pandi, P.; Bulusu, R.; Kommineni, N.; Khan, W.; Singh, M. Amorphous solid dispersions: An update for preparation, characterization, mechanism on bioavailability, stability, regulatory considerations and marketed products. Int. J. Pharm. 2020, 586, 119560. [Google Scholar] [CrossRef] [PubMed]
- Kanaujia, P.; Poovizhi, P.; Ng, W.; Tan, R. Amorphous formulations for dissolution and bioavailability enhancement of poorly soluble APIs. Powder Technol. 2015, 285, 2–15. [Google Scholar] [CrossRef]
- Al-Kasmi, B.; Alsirawan, M.H.D.B.; Paradkar, A.; Nattouf, A.-H.; El-Zein, H. Aqueous and pH dependent coacervation method for taste masking of paracetamol via amorphous solid dispersion formation. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Boateng, J.; Chowdhry, B.Z.; Snowden, M.; Douroumis, D. A review on the taste masking of bitter APIs: Hot-melt extrusion (HME) evaluation. Drug Dev. Ind. Pharm. 2013, 40, 145–156. [Google Scholar] [CrossRef]
- Januskaite, P.; Xu, X.; Ranmal, S.R.; Gaisford, S.; Basit, A.W.; Tuleu, C.; Goyanes, A. I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets. Pharmaceutics 2020, 12, 1100. [Google Scholar] [CrossRef]
- Nordenmalm, S.; Kimland, E.; Ligas, F.; Lehmann, B.; Claverol, J.; Nafria, B.; Tötterman, A.M.; Pelle, B. Children’s views on taking medicines and participating in clinical trials. Arch. Dis. Child. 2019, 104, 900–905. [Google Scholar] [CrossRef]
- Lyons, J.G.; Hallinan, M.; Kennedy, J.E.; Devine, D.M.; Geever, L.M.; Blackie, P.; Higginbotham, C.L. Preparation of monolithic matrices for oral drug delivery using a supercritical fluid assisted hot melt extrusion process. Int. J. Pharm. 2007, 329, 62–71. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharm. 2017, 519, 186–197. [Google Scholar] [CrossRef]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int. J. Pharm. 2019, 567, 118471. [Google Scholar] [CrossRef]
- Capel, A.J.; Rimington, R.P.; Lewis, M.P.; Christie, S.D.R. 3D printing for chemical, pharmaceutical and biological applications. Nat. Rev. Chem. 2018, 2, 422–436. [Google Scholar] [CrossRef]
- Goyanes, A.; Fernández-Ferreiro, A.; Majeed, A.; Gomez-Lado, N.; Awad, A.; Luaces-Rodríguez, A.; Gaisford, S.; Aguiar, P.; Basit, A.W. PET/CT imaging of 3D printed devices in the gastrointestinal tract of rodents. Int. J. Pharm. 2018, 536, 158–164. [Google Scholar] [CrossRef]
- Elbadawi, M.; McCoubrey, L.E.; Gavins, F.K.; Ong, J.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Harnessing artificial intelligence for the next generation of 3D printed medicines. Adv. Drug Deliv. Rev. 2021, 175, 113805. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advances in powder bed fusion 3D printing in drug delivery and healthcare. Adv. Drug Deliv. Rev. 2021, 174, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Viaño, I.; Trenfield, S.J.; Basit, A.W.; Goyanes, A. Translating 3D printed pharmaceuticals: From hype to real-world clinical applications. Adv. Drug Deliv. Rev. 2021, 174, 553–575. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Yao, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Tablets (Printlets) with Braille and Moon Patterns for Visually Impaired Patients. Pharmaceutics 2020, 12, 172. [Google Scholar] [CrossRef] [Green Version]
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.W.; Gaisford, S.; Boyd, B.J. A Proof of Concept for 3D Printing of Solid Lipid-Based Formulations of Poorly Water-Soluble Drugs to Control Formulation Dispersion Kinetics. Pharm. Res. 2019, 36, 102. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Gómez-Lado, N.; Lázare-Iglesias, H.; García-Otero, X.; Antúnez-López, J.R.; Ruibal, Á.; Varela-Correa, J.J.; Aguiar, P.; Basit, A.W.; Otero-Espinar, F.J.; et al. 3D Printed Tacrolimus Rectal Formulations Ameliorate Colitis in an Experimental Animal Model of Inflammatory Bowel Disease. Biomedicines 2020, 8, 563. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Rowland, M.; Gaisford, S.; Basit, A.W. 3D Printing of Tunable Zero-Order Release Printlets. Polymers 2020, 12, 1769. [Google Scholar] [CrossRef]
- Elbadawi, M.; McCoubrey, L.E.; Gavins, F.K.; Ong, J.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Disrupting 3D printing of medicines with machine learning. Trends Pharmacol. Sci. 2021. [Google Scholar] [CrossRef]
- Mathew, E.; Pitzanti, G.; Larrañeta, E.; Lamprou, D.A. 3D Printing of Pharmaceuticals and Drug Delivery Devices. Pharmaceutics 2020, 12, 266. [Google Scholar] [CrossRef] [Green Version]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Tabriz, A.G.; Nandi, U.; Hurt, A.P.; Hui, H.-W.; Karki, S.; Gong, Y.; Kumar, S.; Douroumis, D. 3D printed bilayer tablet with dual controlled drug release for tuberculosis treatment. Int. J. Pharm. 2021, 593, 120147. [Google Scholar] [CrossRef] [PubMed]
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Gioumouxouzis, C.I.; Katsamenis, O.L.; Bouropoulos, N.; Fatouros, D.G. 3D printed oral solid dosage forms containing hydrochlorothiazide for controlled drug delivery. J. Drug Deliv. Sci. Technol. 2017, 40, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Palekar, S.; Nukala, P.K.; Mishra, S.M.; Kipping, T.; Patel, K. Application of 3D printing technology and quality by design approach for development of age-appropriate pediatric formulation of baclofen. Int. J. Pharm. 2019, 556, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Briatico-Vangosa, F.; Uboldi, M.; Parietti, F.; Turchi, M.; von Zeppelin, D.; Maroni, A.; Zema, L.; Gazzaniga, A.; Zidan, A. Quality considerations on the pharmaceutical applications of fused deposition modeling 3D printing. Int. J. Pharm. 2021, 592, 119901. [Google Scholar] [CrossRef]
- Elbadawi, M.; Castro, B.M.; Gavins, F.K.; Ong, J.J.; Gaisford, S.; Pérez, G.; Basit, A.W.; Cabalar, P.; Goyanes, A. M3DISEEN: A novel machine learning approach for predicting the 3D printability of medicines. Int. J. Pharm. 2020, 590, 119837. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.J.; Awad, A.; Martorana, A.; Gaisford, S.; Stoyanov, E.; Basit, A.W.; Goyanes, A. 3D printed opioid medicines with alcohol-resistant and abuse-deterrent properties. Int. J. Pharm. 2020, 579, 119169. [Google Scholar] [CrossRef] [PubMed]
- Keeley, A.; Teo, M.; Ali, Z.; Frost, J.; Ghimire, M.; Rajabi-Siahboomi, A.; Orlu, M.; Tuleu, C. In Vitro Dissolution Model Can Predict the in Vivo Taste Masking Performance of Coated Multiparticulates. Mol. Pharm. 2019, 16, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolut. Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, A.H.; Soto, J.; Ernest, T.; Tuleu, C. Non-human tools for the evaluation of bitter taste in the design and development of medicines: A systematic review. Drug Discov. Today 2016, 21, 1170–1180. [Google Scholar] [CrossRef]
- Spath, S.; Seitz, H. Influence of grain size and grain-size distribution on workability of granules with 3D printing. Int. J. Adv. Manuf. Technol. 2014, 70, 135–144. [Google Scholar] [CrossRef]
- Bharate, S.S.; Bharate, S.B.; Bajaj, A.N. Interactions and incompatibilities of pharmaceutical excipients with active pharmaceutical ingredients: A comprehensive review. J. Excipients Food Chem. 2010, 1, 3–26. [Google Scholar]
- Rowe, R.C.; Sheskey, P.J.; Owen, S.C. Handbook of Pharmaceutical Excipients, 5th ed.; Pharmaceutical Press: London, UK, 2006. [Google Scholar]
- Schlindwein, W.; Bezerra, M.; Almeida, J.; Berghaus, A.; Owen, M.; Muirhead, G. In-Line UV-Vis Spectroscopy as a Fast-Working Process Analytical Technology (PAT) during Early Phase Product Development Using Hot Melt Extrusion (HME). Pharmaceutics 2018, 10, 166. [Google Scholar] [CrossRef] [Green Version]
- Goyanes, A.; Madla, C.M.; Umerji, A.; Piñeiro, G.D.; Montero, J.M.G.; Diaz, M.J.L.; Barcia, M.G.; Taherali, F.; Sánchez-Pintos, P.; Couce, M.-L.; et al. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: First single-centre, prospective, crossover study in patients. Int. J. Pharm. 2019, 567, 118497. [Google Scholar] [CrossRef]
- Ivanovska, V.; Rademaker, C.M.; Van Dijk, L.; Mantel-Teeuwisse, A.K. Pediatric Drug Formulations: A Review of Challenges and Progress. Pediatrics 2014, 134, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Gerrard, S.E.; Walsh, J.; Bowers, N.; Salunke, S.; Hershenson, S. Innovations in Pediatric Drug Formulations and Administration Technologies for Low Resource Settings. Pharmaceutics 2019, 11, 518. [Google Scholar] [CrossRef] [Green Version]
- Pawar, J.N.; Shete, R.T.; Gangurde, A.B.; Moravkar, K.; Javeer, S.D.; Jaiswar, D.R.; Amin, P. Development of amorphous dispersions of artemether with hydrophilic polymers via spray drying: Physicochemical and in silico studies. Asian J. Pharm. Sci. 2016, 11, 385–395. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.S.; Meena, A.; Parikh, T.; Serajuddin, A.T.M. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion—I: Polyvinylpyrrolidone and related polymers. J. Excip. Food Chem. 2014, 5, 32–45. [Google Scholar]
- Zanolla, D.; Perissutti, B.; Vioglio, P.C.; Chierotti, M.R.; Gigli, L.; Demitri, N.; Passerini, N.; Albertini, B.; Franceschinis, E.; Keiser, J.; et al. Exploring mechanochemical parameters using a DoE approach: Crystal structure solution from synchrotron XRPD and characterization of a new praziquantel polymorph. Eur. J. Pharm. Sci. 2019, 140, 105084. [Google Scholar] [CrossRef] [PubMed]
- Zanolla, D.; Hasa, D.; Arhangelskis, M.; Schneider-Rauber, G.; Chierotti, M.R.; Keiser, J.; Voinovich, D.; Jones, W.; Perissutti, B. Mechanochemical Formation of Racemic Praziquantel Hemihydrate with Improved Biopharmaceutical Properties. Pharmaceutics 2020, 12, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanolla, D.; Perissutti, B.; Passerini, N.; Chierotti, M.R.; Hasa, D.; Voinovich, D.; Gigli, L.; Demitri, N.; Geremia, S.; Keiser, J.; et al. A new soluble and bioactive polymorph of praziquantel. Eur. J. Pharm. Biopharm. 2018, 127, 19–28. [Google Scholar] [CrossRef] [PubMed]
- De Lima, Á.A.N. Avaliação dos Aspectos Físico-Químicos do Antichagásico Benznidazol, Diferentes Sistemas Carreadores e Formas Farmacêuticas, Através de Técnicas Analíticas e de Imagens Avaliação dos Aspectos Físico-Químicos do Antichagásico Benznidazol, Diferentes sis. Ph.D. Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2012. [Google Scholar]
- Mendonsa, N.; Almutairy, B.; Kallakunta, V.R.; Sarabu, S.; Thipsay, P.; Bandari, S.; Repka, M.A. Manufacturing strategies to develop amorphous solid dispersions: An overview. J. Drug Deliv. Sci. Technol. 2020, 55, 101459. [Google Scholar] [CrossRef] [PubMed]
- Münster, M.; Ahmed, A.M.; Immohr, L.I.; Schoch, C.; Schmidt, C.; Tuleu, C.; Breitkreutz, J. Comparative in vitro and in vivo taste assessment of liquid praziquantel formulations. Int. J. Pharm. 2017, 529, 310–318. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. “Report of A Meeting to Review the Results of Studies on the Treatment of Schistosomiasis in Preschool-Age Children”. 2010. Available online: https://apps.who.int/iris/bitstream/handle/10665/44639/9789241501880_eng.pdf?sequence=1 (accessed on 10 May 2021).
- Olliaro, P.L.; Coulibaly, J.T.; Garba, A.; Halleux, C.; Keiser, J.; King, C.; Mutapi, F.; N’Goran, E.K.; Raso, G.; Scherrer, A.U.; et al. Efficacy and safety of single-dose 40 mg/kg oral praziquantel in the treatment of schistosomiasis in preschool-age versus school-age children: An individual participant data meta-analysis. PLoS Negl. Trop. Dis. 2020, 14, e0008277. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, J.T.; Panić, G.; Yapi, R.B.; Kovac, J.; Barda, B.; N’Gbesso, Y.K.; Hattendorf, J.; Keiser, J. Efficacy and safety of ascending doses of praziquantel against Schistosoma haematobium infection in preschool-aged and school-aged children: A single-blind randomised controlled trial. BMC Med. 2018, 16, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boniatti, J. Taste evaluation of three different commercial tablets for paediatric patients for neglected tropical diseases. In Proceedings of the 11th European Paediatric Formulation Initiative (EUPFI), Malmo, Sweden, 10–12 September 2019. [Google Scholar]

| Formulation Code | Composition (wt%) | Printing Parameters | |||||
|---|---|---|---|---|---|---|---|
| PZQ | KOL | SLS | Span | Printing Temperature (°C) | Flow Rate (%) | Feed Rate (%) | |
| Physical mixtures (PM) | |||||||
| PM 50 | 50 | 50 | - | - | 145–170 | 100–140 | 100 |
| PM 35 | 35 | 65 | - | - | 145–170 | 100–140 | 100 |
| PM 35 SLS | 35 | 60 | 5 | - | 140–200 | 100–140 | 100 |
| Pellets (P) and milled powder (M) obtained from HME extrudates produced in a twin-screw extruder | |||||||
| P 50 | 50 | 50 | - | - | 140 | 90 | 100 |
| M 50 | 50 | 50 | - | - | 135 | 75 | 100 |
| M 35 Span | 35 | 60 | - | 5 | 130 | 75 | 100 |
| M 35 SLS | 35 | 60 | 5 | - | 130 | 75 | 100 |
| Measured Characteristics of Printlets | |||
|---|---|---|---|
| Printlet Formulation | Weight (g) | Diameter (mm) | Height (mm) |
| P 50 * | 0.270 ± 0.02 | 9.720 ± 0.22 | 3.579 ± 0.05 |
| M 50 * | 0.298 ± 0.01 | 9.810 ± 0.16 | 3.554 ± 0.14 |
| M 35 Span ** | 0.297 ± 0.02 | 9.982 ± 0.25 | 3.507 ± 0.09 |
| M 35 SLS ** | 0.290 ± 0.04 | 9.841 ± 0.22 | 3.591 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boniatti, J.; Januskaite, P.; Fonseca, L.B.d.; Viçosa, A.L.; Amendoeira, F.C.; Tuleu, C.; Basit, A.W.; Goyanes, A.; Ré, M.-I. Direct Powder Extrusion 3D Printing of Praziquantel to Overcome Neglected Disease Formulation Challenges in Paediatric Populations. Pharmaceutics 2021, 13, 1114. https://doi.org/10.3390/pharmaceutics13081114
Boniatti J, Januskaite P, Fonseca LBd, Viçosa AL, Amendoeira FC, Tuleu C, Basit AW, Goyanes A, Ré M-I. Direct Powder Extrusion 3D Printing of Praziquantel to Overcome Neglected Disease Formulation Challenges in Paediatric Populations. Pharmaceutics. 2021; 13(8):1114. https://doi.org/10.3390/pharmaceutics13081114
Chicago/Turabian StyleBoniatti, Janine, Patricija Januskaite, Laís B. da Fonseca, Alessandra L. Viçosa, Fábio C. Amendoeira, Catherine Tuleu, Abdul W. Basit, Alvaro Goyanes, and Maria-Inês Ré. 2021. "Direct Powder Extrusion 3D Printing of Praziquantel to Overcome Neglected Disease Formulation Challenges in Paediatric Populations" Pharmaceutics 13, no. 8: 1114. https://doi.org/10.3390/pharmaceutics13081114
APA StyleBoniatti, J., Januskaite, P., Fonseca, L. B. d., Viçosa, A. L., Amendoeira, F. C., Tuleu, C., Basit, A. W., Goyanes, A., & Ré, M.-I. (2021). Direct Powder Extrusion 3D Printing of Praziquantel to Overcome Neglected Disease Formulation Challenges in Paediatric Populations. Pharmaceutics, 13(8), 1114. https://doi.org/10.3390/pharmaceutics13081114









