Combined Action of Anti-MUC1 Monoclonal Antibody and Pyrazole-Platinum(II) Complexes Reveals Higher Effectiveness towards Apoptotic Response in Comparison with Monotherapy in AGS Gastric Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Assay
2.3. Annexin V Binding Assessment
2.4. RNA Isolation and Quantitative Real-Time PCR
2.5. Western Blot Analysis
2.6. ELISA Tests
2.7. Statistical Analysis
3. Results
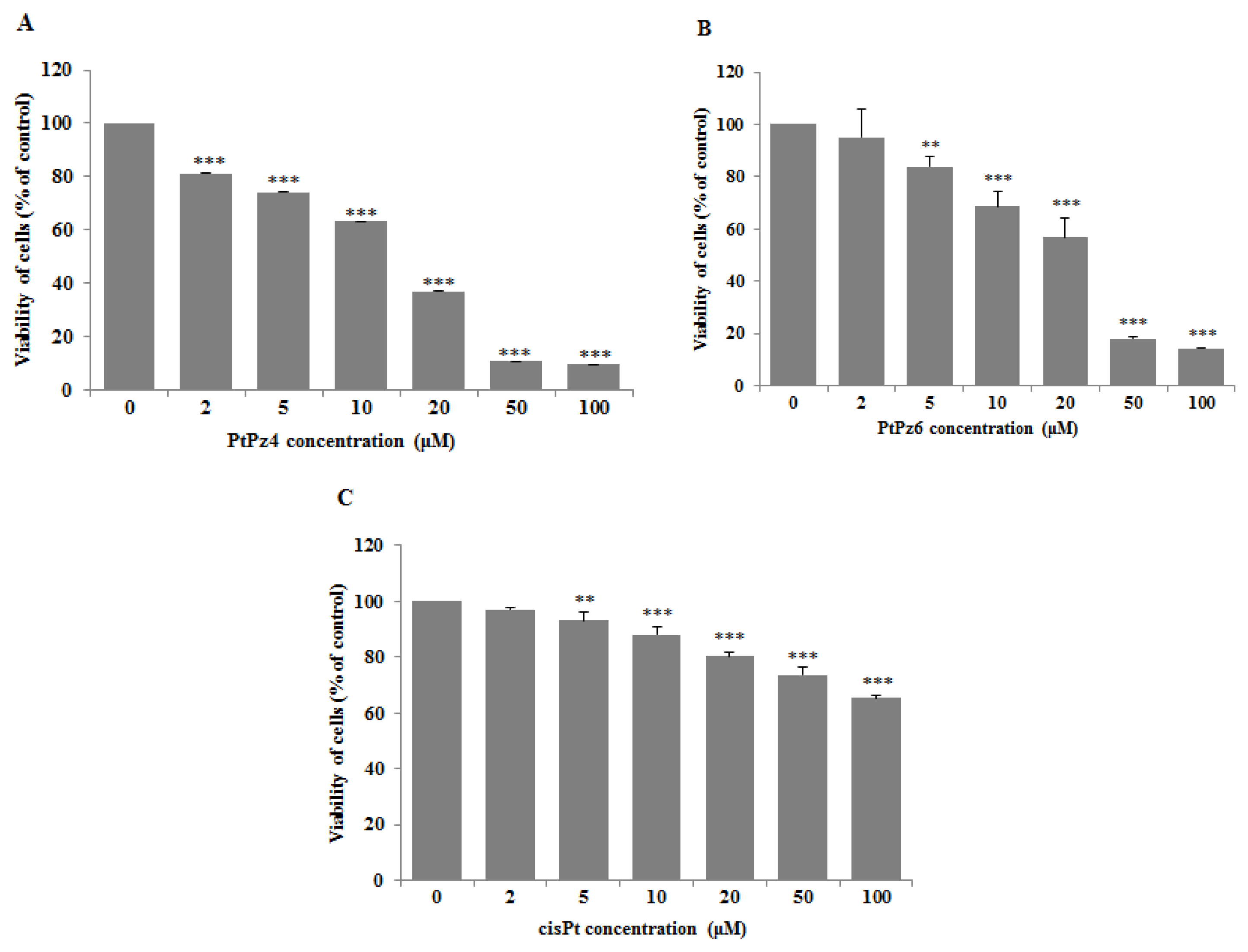
3.1. Cytotoxic Effects of PtPz4, PtPz6, cisPt, and Anti-MUC1
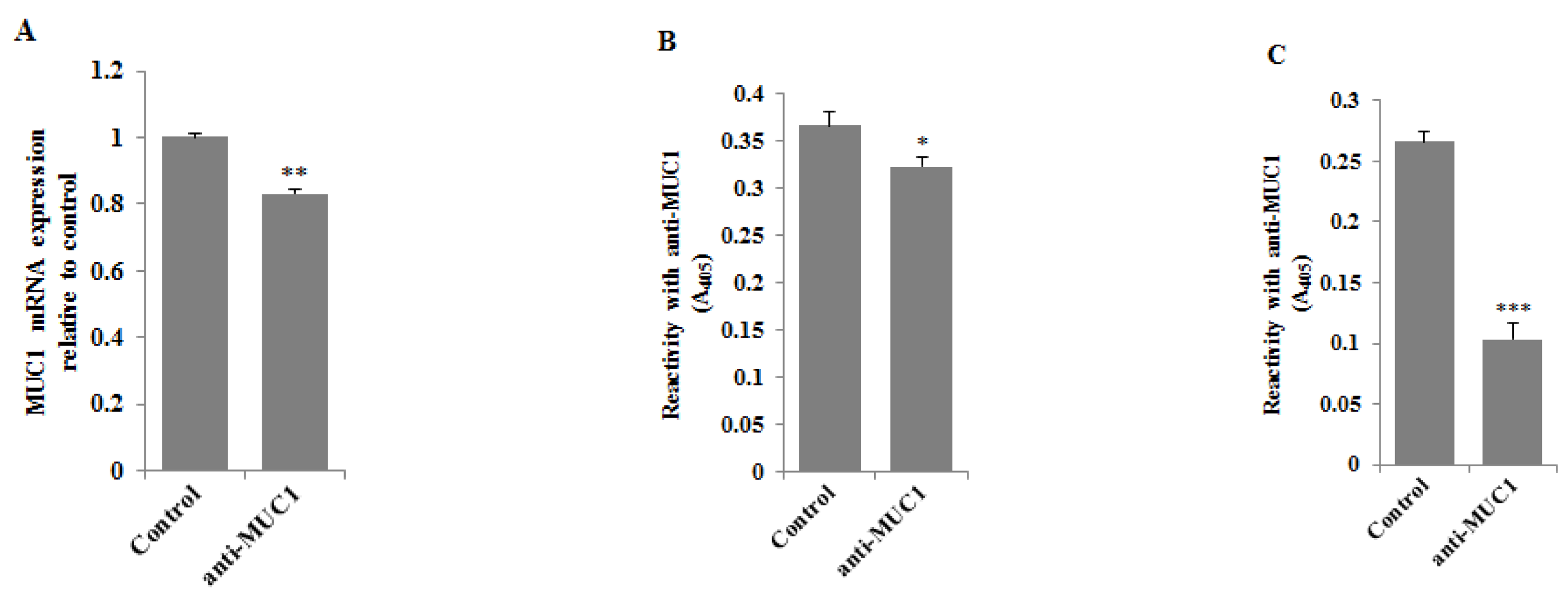
3.2. Inhibitory Effect of Anti-MUC1 Monoclonal Antibody on MUC1
3.3. The Effect of PtPz4, PtPz6, cisPt, and Anti-MUC1 on the MUC1 Cytoplasmic Domain
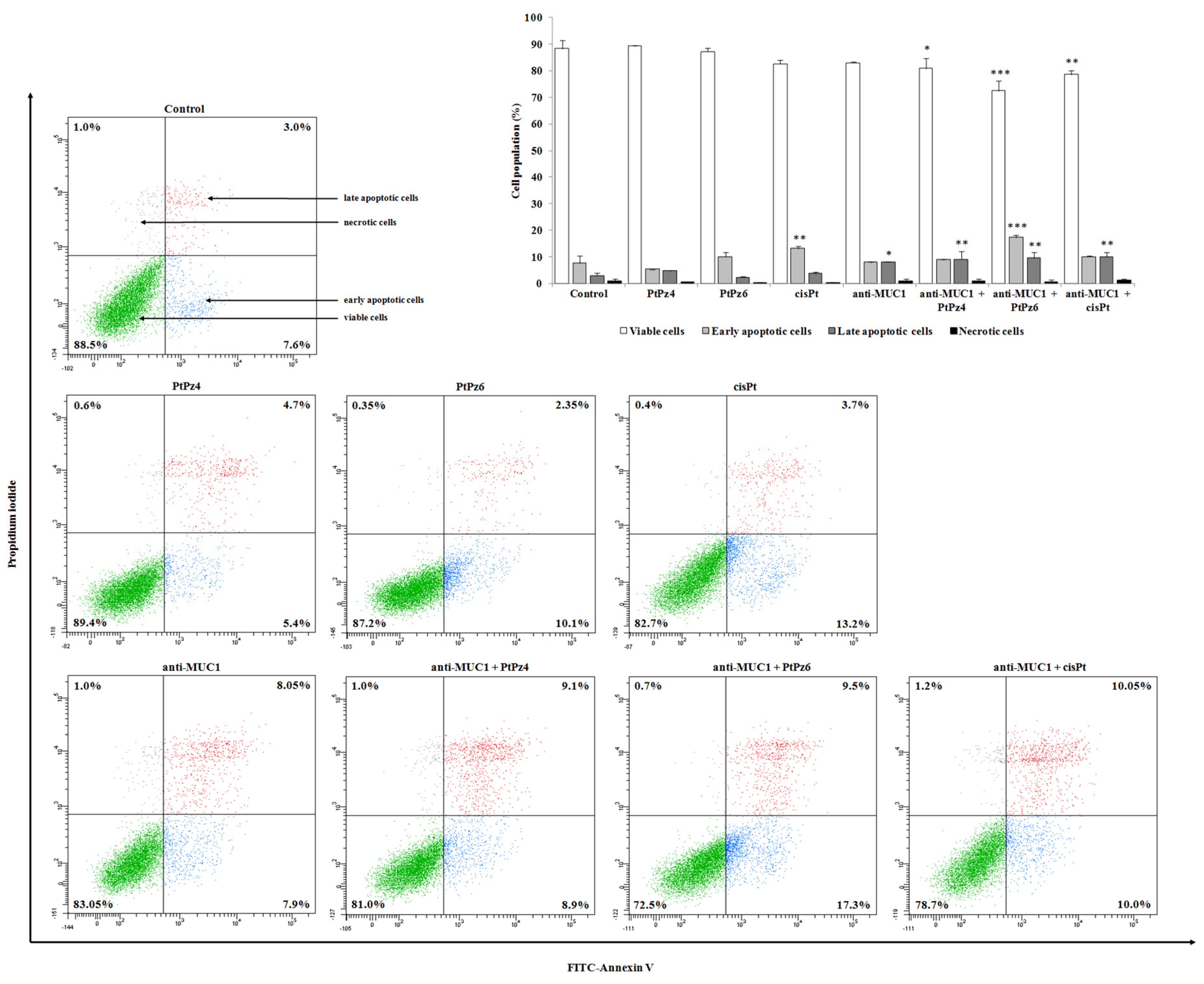
3.4. Impact of PtPz4, PtPz6, cisPt, and Anti-MUC1 on Apoptosis
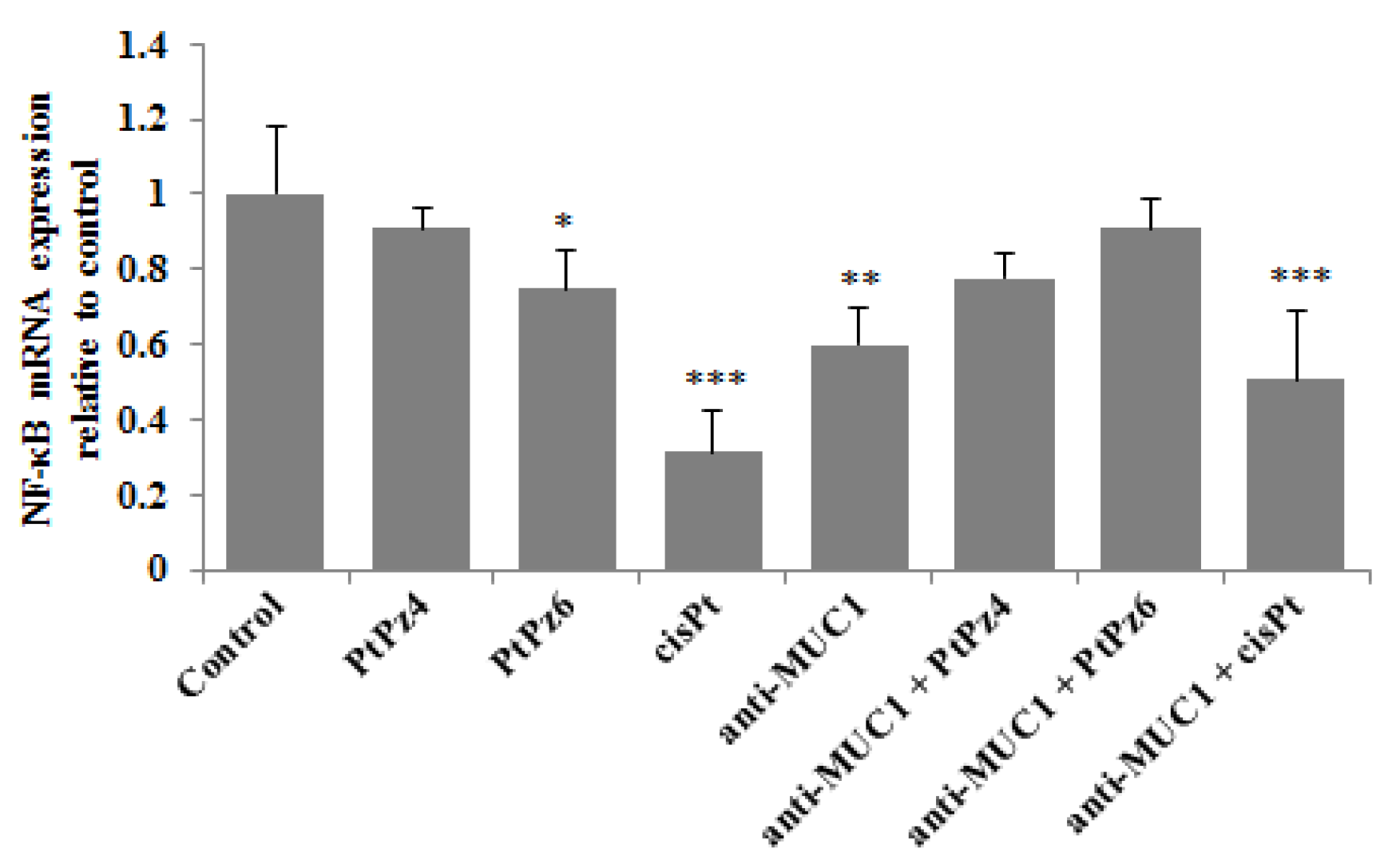
3.5. The Effect of PtPz4, PtPz6, cisPt, and Anti-MUC1 on NF-κB mRNA
3.6. The Effect of PtPz4, PtPz6, cisPt, and Anti-MUC1 on AKT mRNA
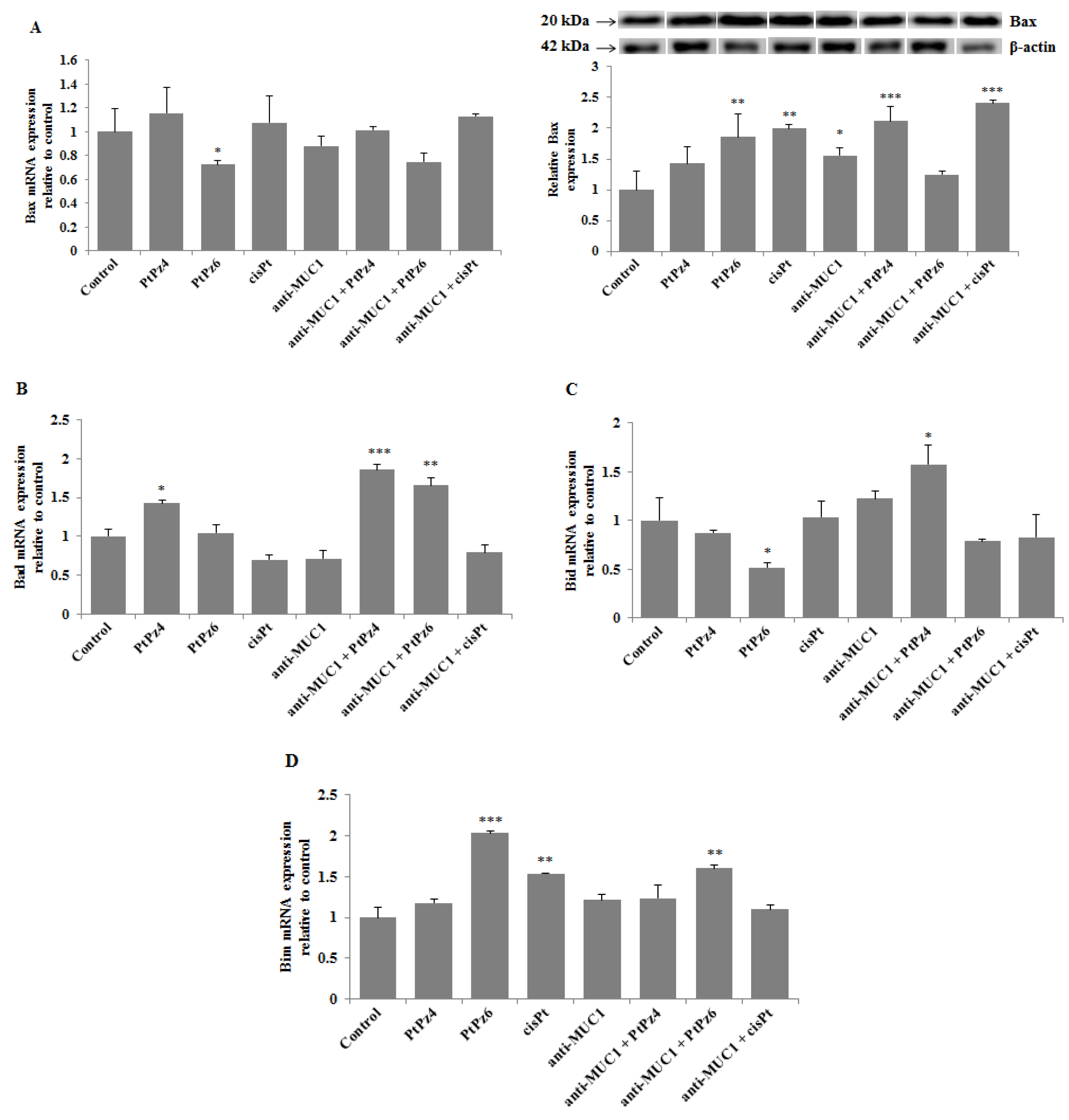
3.7. The Effect of PtPz4, PtPz6, cisPt, and Anti-MUC1 on Pro-Apoptotic Factors
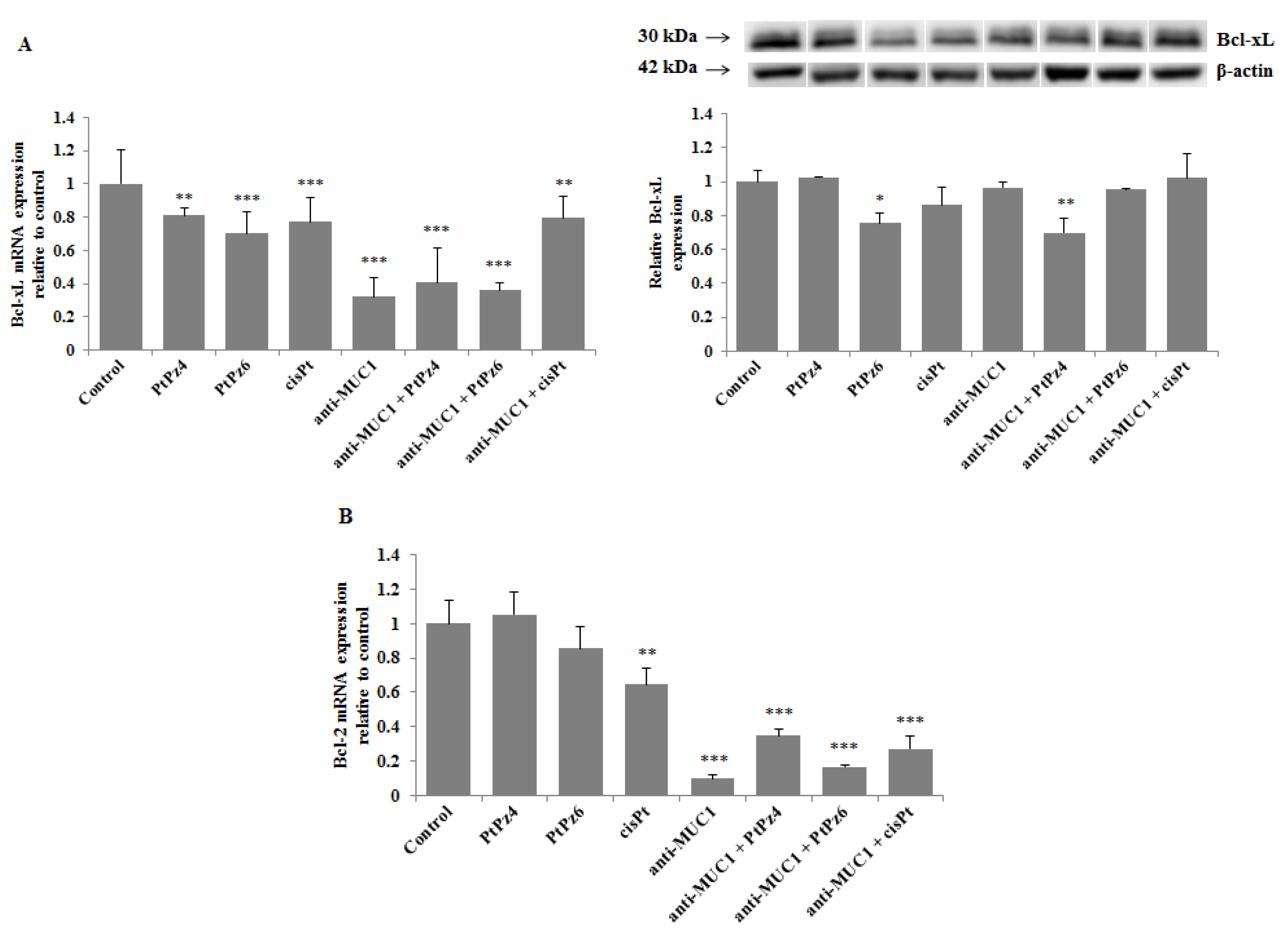
3.8. The Effect of PtPz4, PtPz6, cisPt, and Anti-MUC1 on Anti-Apoptotic Factors
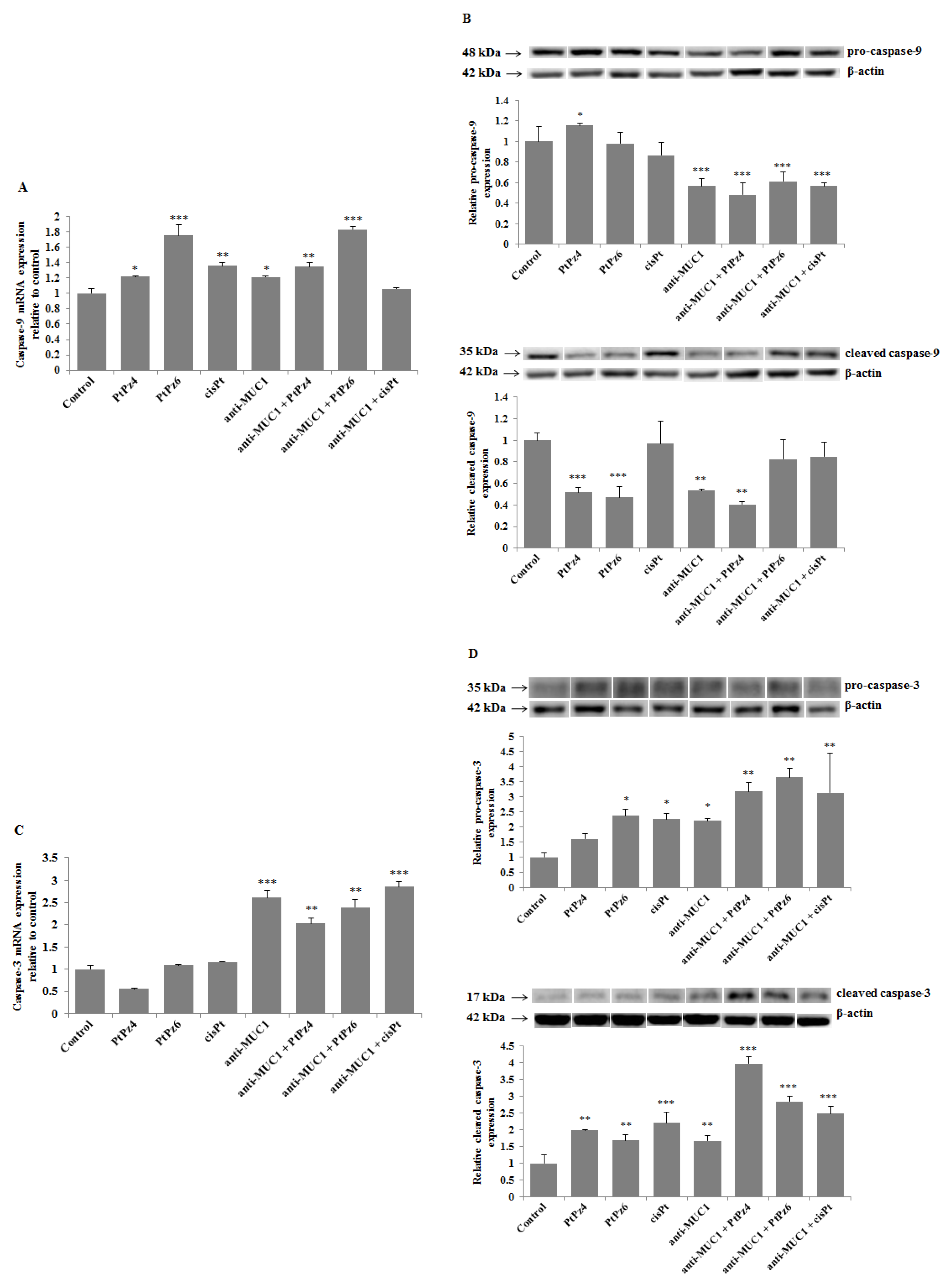
3.9. The Effect of PtPz4, PtPz6, cisPt, and Anti-MUC1 on Caspases
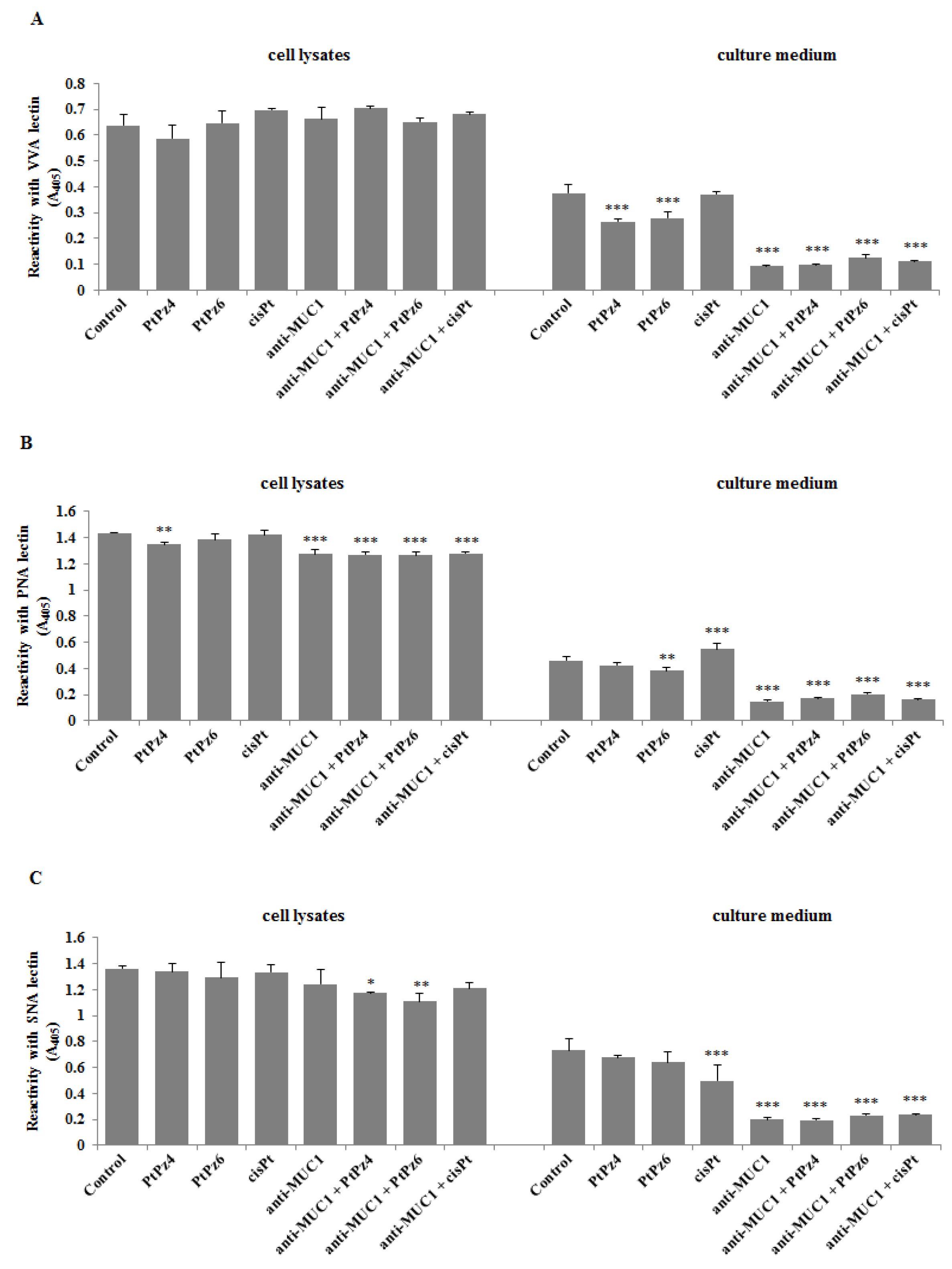
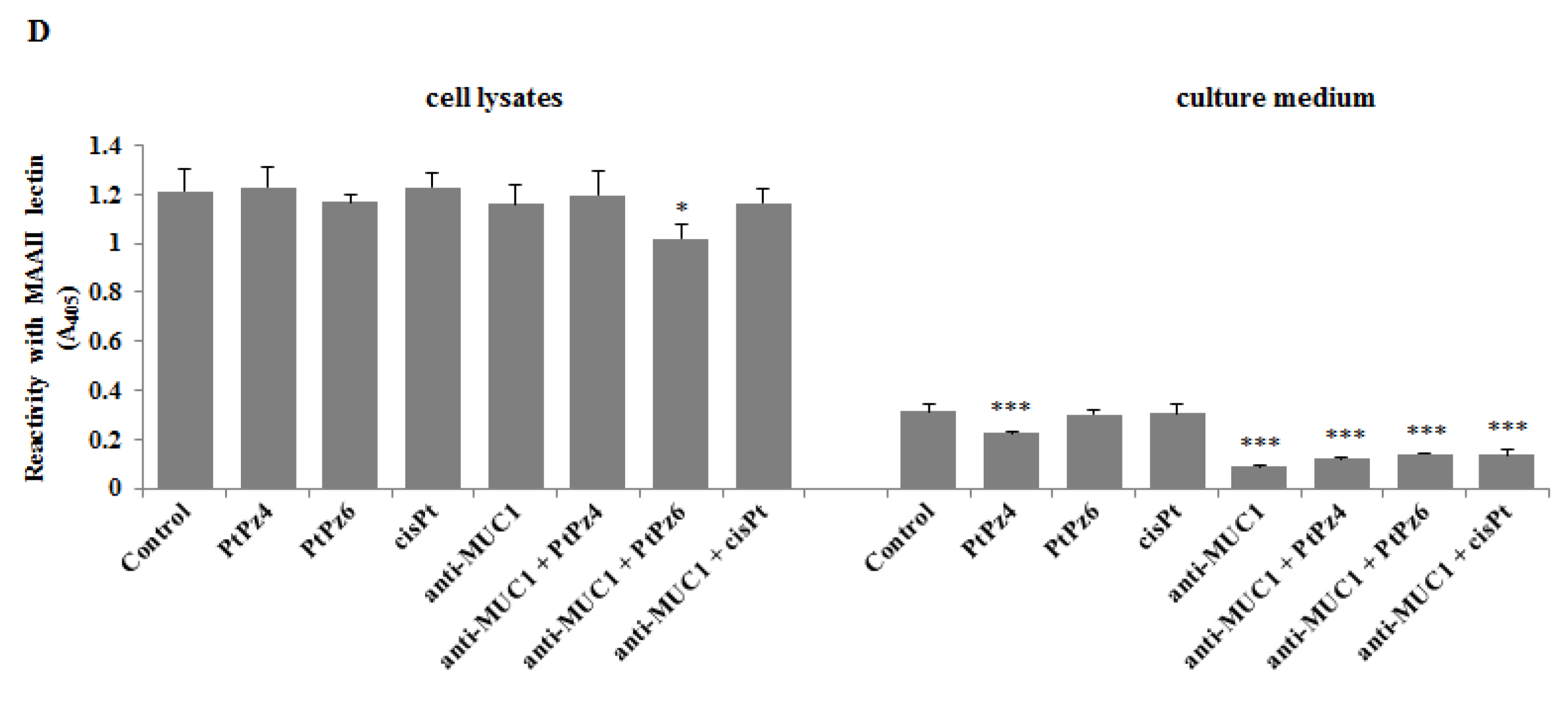
3.10. The Effect of PtPz4, PtPz6, cisPt, and Anti-MUC1 on Cancer Related Carbohydrate Antigens Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnston, F.M.; Beckman, M. Updates on management of gastric cancer. Curr. Oncol. Rep. 2019, 21, 67. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Tan, T.; Li, S.; Tang, S.; Chen, X. Sinomenine sensitizes human gastric cancer cells to cisplatin through negative regulation of PI3K/AKT/Wnt signaling pathway. Anti-Cancer Drugs 2019, 30, 983–990. [Google Scholar] [CrossRef]
- Qian, K.; Qian, H.; Cai, J.; Yue, W.; Yu, X.; Liu, B. Evaluation of cisplatin-hydrogel for improving localized antitumor efficacy in gastric cancer. Pathol. Res. Pract. 2019, 215, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kang, Y.; Chen, L.; Wang, H.; Liu, J.; Zeng, S.; Yu, L. The drug-resistance mechanisms of five platinum-based antitumor agents. Front. Pharmacol. 2020, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Dilruba, S.; Kalayda, G.V. Platinum-based drugs: Past, present and future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef]
- Czaronomysy, R.; Surażyński, A.; Muszyńska, A.; Gornowicz, A.; Bielawska, A.; Bielawski, K. A novel serious of pyrazole-platinum(II) complexes as potential anti-cancer agents that induce cell cycle arrest and apoptosis in breast cancer cells. J. Enzyme Inhib. Med. Chem. 2018, 33, 1006–1023. [Google Scholar] [CrossRef]
- Nath, S.; Mukherjee, P. MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef]
- Cascio, S.; Finn, O.J. Intra- and extra-cellular events related to altered glycosylation of MUC1 promote chronic inflammation, tumor progression, invasion, and metastasis. Biomolecules 2016, 6, 39. [Google Scholar] [CrossRef]
- Pinho, S.S.; Carvalho, S.; Marcos-Pinto, R.; Magalhaes, A.; Oliveira, C.; Gu, J.; Dinis-Ribeiro, M.; Carneiro, F.; Seruca, R.; Reis, C.A. Gastric cancer: Adding glycosylation to the equation. Trends Mol. Med. 2013, 19, 664–676. [Google Scholar] [CrossRef]
- Wei, X.; Xu, H.; Kufe, D. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell 2005, 7, 167–178. [Google Scholar] [CrossRef]
- Ahmad, R.; Raina, D.; Trivedi, V.; Ren, J.; Rajabi, H.; Kharbanda, S.; Kufe, D. MUC1 oncoprotein activates the IkappaB kinase beta complex and constitutive NF-kappaB signalling. Nat. Cell Biol. 2007, 9, 1419–1427. [Google Scholar] [CrossRef]
- Bose, M.; Mukherjee, P. Potential of anti-MUC1 antibodies as a targeted therapy for gastrointestinal cancers. Vaccines 2020, 8, 659. [Google Scholar] [CrossRef] [PubMed]
- Agata, N.; Ahmad, R.; Kawano, T.; Raina, D.; Kharbanda, S.; Kufe, D. MUC1 oncoprotein blocks death receptor-mediated apoptosis by inhibiting recruitment of caspase-8. Cancer Res. 2008, 68, 6136–6144. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Denda-Nagai, K.; Takahashi, Y.; Nagashima, I.; Shimizu, H.; Kishimoto, T.; Noji, M.; Shichino, S.; Chiba, Y.; Irimura, T. Products of chemoenzymatic synthesis representing MUC1 tandem repeat unit with T-ST- or STn-antigen revealed distinct specificities of anti-MUC1 antibodies. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Runcie, K.; Budman, D.R.; John, V.; Seetharamu, N. Bi-specific and tri-specific antibodies—The next big thing in solid tumor therapeutics. Mol. Med. 2018, 24, 50. [Google Scholar] [CrossRef]
- Gornowicz, A.; Bielawska, A.; Czarnomysy, R.; Gabryel-Porowska, H.; Muszyńska, A.; Bielawski, K. The combined treatment with novel platinum(II) complex and anti-MUC1 increases apoptotic response in MDA-MB-231 breast cancer cells. Mol. Cell Biochem. 2015, 408, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Gornowicz, A.; Bielawska, A.; Szymanowski, W.; Gabryel-Porowska, H.; Czarnomysy, R.; Bielawski, K. Mechanism of anticancer action of novel berenil complex of platinum(II) combined with anti-MUC1 in MCF-7 breast cancer cells. Oncol. Lett. 2018, 15, 2340–2348. [Google Scholar] [CrossRef]
- Carmichael, J.; Degraff, W.; Gazdar, A.; Minna, J.; Mitchell, J. Evaluation of a tetrazolium-based semi-automated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Radziejewska, I.; Supruniuk, K.; Bielawska, A. Anti-cancer effect of combined action of anti-MUC1 and rosmarinic acid in AGS gastric cancer cells. Eur. J. Pharmacol. 2021, 902, 174119. [Google Scholar] [CrossRef]
- Towbin, T.; Stachelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Hannon, M.J. Metal-based anticancer drugs: From a past anchored in platinum chemistry to a post-genomic future of diverse chemistry and biology. Pure Appl. Chem. 2007, 79, 2243–2261. [Google Scholar] [CrossRef]
- Gosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Nabavinia, M.S.; Gholoobi, A.; Charbgoo, F.; Nabavinia, M.; Ramezani, M.; Abnous, K. Anti-MUC1 aptamer: A potential opportunity for cancer treatment. Med. Res. Rev. 2017, 37, 1518–1539. [Google Scholar] [CrossRef]
- Pichinuk, E.; Chalik, M.; Benhar, I.; Ginat-Koton, R.; Ziv, R.; Smorodinsky, N.I.; Haran, G.; Garbar, C.; Bensussan, A.; Meeker, A.; et al. In vivo anti-MUC1+ tumor activity and sequences of high-affinity anti-MUC1-SEA antibodies. Cancer Immunol. Immunother. 2020, 69, 1337–1352. [Google Scholar] [CrossRef] [PubMed]
- Rivalland, G.; Loveland, B.; Mitchel, P. Update on Mucin-1 immunotherapy in cancer: A clinical perspective. Exp. Opin. Biol. Ther. 2015, 15, 1773–1787. [Google Scholar] [CrossRef] [PubMed]
- Birrer, M.J.; Moore, K.N.; Betella, I.; Bates, R.C. Antibody-drug conjugate-based therapeutics: State of the science. J. Natl. Cancer Inst. 2019, 111, 538–549. [Google Scholar] [CrossRef]
- Redman, J.M.; Hill, E.M.; AIDeghaither, D.; Weiner, L.M. Mechanisms of action of therapeutic antibodies for cancer. Mol. Immunol. 2015, 67, 28–45. [Google Scholar] [CrossRef]
- Fiedler, W.; DeDosso, S.; Cresta, S.; Weidmann, J.; Tessari, A.; Salzberg, M.; Dietrich, B.; Baumeister, H.; Goletz, S.; Gianni, L.; et al. A phase I study of PankoMab-GEX, a humanised glyco-optimised monoclonal antibody to a novel tumour-specific MUC1 glycopeptide epitope in patients with advanced carcinomas. Eur. J. Cancer 2016, 63, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Maharjan, S.; Kim, D.; Kim, J.N.; Park, B.K.; Koh, H.; Moon, K.; Lee, Y.; Kwon, H.J. A novel monoclonal antibody targets mucin1 and attenuates growth in pancreatic cancer model. Int. J. Mol. Sci. 2018, 19, 2004. [Google Scholar] [CrossRef]
- Hisatsune, A.; Kawasaki, M.; Nakayama, H.; Mikami, Y.; Miyata, T.; Isohama, Y.; Katsuki, H.; Kim, K.C. Internalization of MUC1 by anti-MUC1 antibody from cell membrane through the macropinocytic pathway. Biochem. Biophys. Res. Commun. 2009, 388, 677–682. [Google Scholar] [CrossRef]
- Bafna, S.; Kaur, S.; Batra, S.K. Membrane-bound mucins: The mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene 2010, 29, 2893–2904. [Google Scholar] [CrossRef] [PubMed]
- Cascio, S.; Zhang, L.; Finn, O.J. MUC1 protein expression in tumor cells regulates transcription of proinflammatory cytokines by forming a complex with Nuclear Factor-κB and binding to cytokine promoters. J. Biol. Chem. 2011, 286, 42248–42256. [Google Scholar] [CrossRef] [PubMed]
- Endo, F.; Nishizuka, S.S.; Kume, K.; Ishida, K.; Katagiri, H.; Ishida, K.; Sato, K.; Iwaya, T.; Koeda, K.; Wakabayashi, G. A compensatory role of NF-B to p53 in response to 5-FU-based chemotherapy for gastric cancer cell lines. PLoS ONE 2014, 9, e90155. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, O.; Naumann, M. NF-κB signaling in gastric cancer. Toxins 2017, 9, 119. [Google Scholar] [CrossRef]
- Tian, R.; Jiang, H.; Shao, L.; Yu, Y.; Guo, Q.; Cao, B.; Guo, S. miR193b Promotes Apoptosis of Gastric Cancer Cells via Directly Mediating the Akt Pathway. BioMed Res. Int. 2020, 2020, 2863236. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, S.; Amjadi-Moheb, F.; Tabaripour, R.; Ashrafi, G.H.; Akhavan-Niaki, H. PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci. 2020, 262, 118513. [Google Scholar] [CrossRef]
- Qi, X.J.; Wildey, G.M.; Howe, P.H. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J. Biol. Chem. 2006, 281, 813–823. [Google Scholar] [CrossRef]
- Wu, N.; Huang, Y.; Zou, Z.; Gimenez-Capitan, A.; Yu, L.; Hu, W.; Zhu, L.; Sun, X.; Sanchez, J.J.; Guan, W.; et al. High MIM mRNA levels are associated with longer survival in advanced gastric cancer. Oncol. Lett. 2017, 13, 1826–1834. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.; Ding, Y.; Ye, N.; Wild, C.; Chen, H.; Zhou, J. Direct activation of Bax protein for cancer therapy. Med. Res. Rev. 2016, 36, 313–341. [Google Scholar] [CrossRef]
- Khodapasand, E.; Jafarzadeh, N.; Farrokhi, F.; Kamalideghhan, B.; Houshmand, M. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran. Biomed. J. 2015, 19, 69–75. [Google Scholar]
- Raisova, M.; Hossini, A.M.; Eberle, J.; Riebeling, C.; Wieder, T.; Sturm, I.; Daniel, P.T.; Orfanos, C.E.; Geilen, C.C. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J. Investig. Dermatol. 2001, 117, 333–340. [Google Scholar] [CrossRef]
- Gryko, M.; Pryczynicz, A.; Zareba, K.; Kędra, B.; Kemona, A.; Guzińska-Ustymowicz, K. The expression of Bcl-2 and BID in gastric cancer cells. J. Immunol. Res. 2014, 2014, 953203. [Google Scholar] [CrossRef] [PubMed]
- Boice, A.; Bouchier-Hayes, L. Targeting apoptotic caspases in cancer. BBA Mol. Cell Res. 2020, 1867, 118688. [Google Scholar] [CrossRef]
- Jager, R.; Zwacka, R.M. The enigmatic roles of caspases in tumor development. Cancers 2010, 2, 1952–1979. [Google Scholar] [CrossRef] [PubMed]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Opdenbosch, N.V.; Lamkanfi, M. Caspases in cell death, inflammation and disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, F.; Liu, X.; Li, W.; Shi, W.; Liu, F.F.; O’Sullivan, B.; He, Z.; Peng, Y.; Tan, A.C.; et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 2011, 17, 860–866. [Google Scholar] [CrossRef]
- Rashidijahanabad, Z.; Huang, X. Recent advances in tumor associated carbohydrate antigen based chimeric receptor T cells and bispecific antibodies for anti-cancer immunotherapy. Semin. Immunol. 2020, 47, 101390. [Google Scholar] [CrossRef]
- Beckwith, D.M.; Cudic, M. Tumor-associated O-glycans of MUC1: Carriers of the glycol-code and targets for cancer vaccine design. Semin. Immunol. 2020, 47, 101389. [Google Scholar] [CrossRef]
- Chia, J.; Goh, G.; Bard, F. Short O-GalNAc glycans: Regulation and role in tumor development and clinical perspectives. Biochim. Biophys. Acta 2016, 1860, 1623–1639. [Google Scholar] [CrossRef] [PubMed]
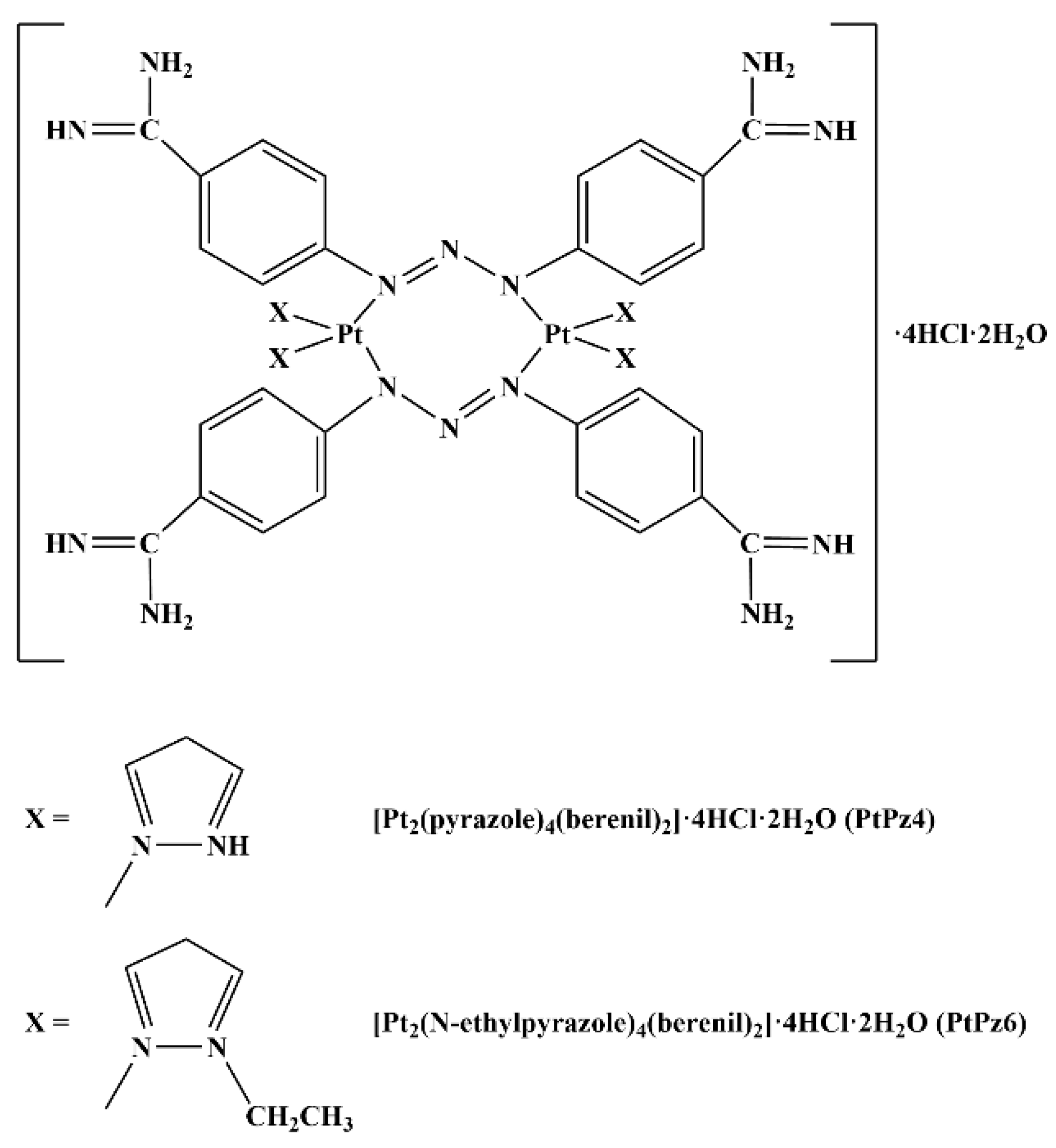


| Gene | Forward Primer (5′→ 3′) | Reverse Primer (5′→ 3′) |
|---|---|---|
| MUC1 | TGCCTTGGCTGTCTGTCAGT | GTAGGTATCCCGGGCTGGAA |
| Akt | TCTATGGCGCTGAGATTGTG | CTTAATGTGCCCGTCCTTGT |
| NF-κB | CTGAACCAGGGCATACCTGT | GAGAAGTCCATGTCCGCAAT |
| Caspase-3 | CAGTGGAGGCCGACTTCTTG | TGGCACAAAGCGACTGGAT |
| Caspase-9 | CCCATATGATCGAGGACATCCA | ACAACTTTGCTGCTTGCCTGTTAG |
| Bcl-2 | GCTGAAGATTGATGGGATCG | TACAGCATGATCCTCTGTCAAG |
| Bcl-xL | TGACGTGGACATCCGC | CTGGAAGGTGGACAGCGAGC |
| Bid | CCTACCCTAGAGACATGGAGAAG | TTTCTGGCTAAGCTCCTCACG |
| Bad | CCCAGAGTTTGAGCCGAGTG | CCCATCCCTTCGTCGTCCT |
| Bim | TAGGTGAGCGGGAGGCTAGGGATCA | GTGCAGGCTCGGACAGGTAAAGGC |
| Bax | TTGCTTCAGGGTTTCATCCA | CAGCCTTGAGCACCAGTTTG |
| GAPDH | GTGAACCATGAGAAGTATGACAA | CATGAGTCCTTCCACGATAC |
| Antibody | Clone | Source |
|---|---|---|
| Anti-MUC1; extracellular domain (mouse IgG) | BC2 | Abcam |
| Anti-MUC1; cytoplasmic tail (Armenian hamster IgG) | CT2 | Abcam |
| Anti-Caspase-3 (mouse IgG) | B-4 | Santa Cruz Biotech |
| Anti-Cleaved caspase-3 (rabbit IgG) | 5A1E | Cell Sign Tech |
| Anti-Caspase-9 (mouse IgG) | C9 | Cell Sign Tech |
| Anti-Cleaved caspase-9 (rabbit IgG) | E5Z7N | Cell Sign Tech |
| Anti-Bax (rabbit IgG) | D2E11 | Cell Sign Tech |
| Anti-Bcl-xL (mouse IgG) | 7B2.5 | Santa Cruz Biotech |
| Anti-β-actin (rabbit IgG) | Sigma | |
| Anti-mouse IgG peroxidase conjugated | Sigma | |
| Anti-rabbit IgG peroxidase conjugated | Sigma | |
| Anti-Armenian hamster IgG peroxidase conjugated | Abcam |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supruniuk, K.; Czarnomysy, R.; Muszyńska, A.; Radziejewska, I. Combined Action of Anti-MUC1 Monoclonal Antibody and Pyrazole-Platinum(II) Complexes Reveals Higher Effectiveness towards Apoptotic Response in Comparison with Monotherapy in AGS Gastric Cancer Cells. Pharmaceutics 2021, 13, 968. https://doi.org/10.3390/pharmaceutics13070968
Supruniuk K, Czarnomysy R, Muszyńska A, Radziejewska I. Combined Action of Anti-MUC1 Monoclonal Antibody and Pyrazole-Platinum(II) Complexes Reveals Higher Effectiveness towards Apoptotic Response in Comparison with Monotherapy in AGS Gastric Cancer Cells. Pharmaceutics. 2021; 13(7):968. https://doi.org/10.3390/pharmaceutics13070968
Chicago/Turabian StyleSupruniuk, Katarzyna, Robert Czarnomysy, Anna Muszyńska, and Iwona Radziejewska. 2021. "Combined Action of Anti-MUC1 Monoclonal Antibody and Pyrazole-Platinum(II) Complexes Reveals Higher Effectiveness towards Apoptotic Response in Comparison with Monotherapy in AGS Gastric Cancer Cells" Pharmaceutics 13, no. 7: 968. https://doi.org/10.3390/pharmaceutics13070968
APA StyleSupruniuk, K., Czarnomysy, R., Muszyńska, A., & Radziejewska, I. (2021). Combined Action of Anti-MUC1 Monoclonal Antibody and Pyrazole-Platinum(II) Complexes Reveals Higher Effectiveness towards Apoptotic Response in Comparison with Monotherapy in AGS Gastric Cancer Cells. Pharmaceutics, 13(7), 968. https://doi.org/10.3390/pharmaceutics13070968






