Schisandrol A Exhibits Estrogenic Activity via Estrogen Receptor α-Dependent Signaling Pathway in Estrogen Receptor-Positive Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
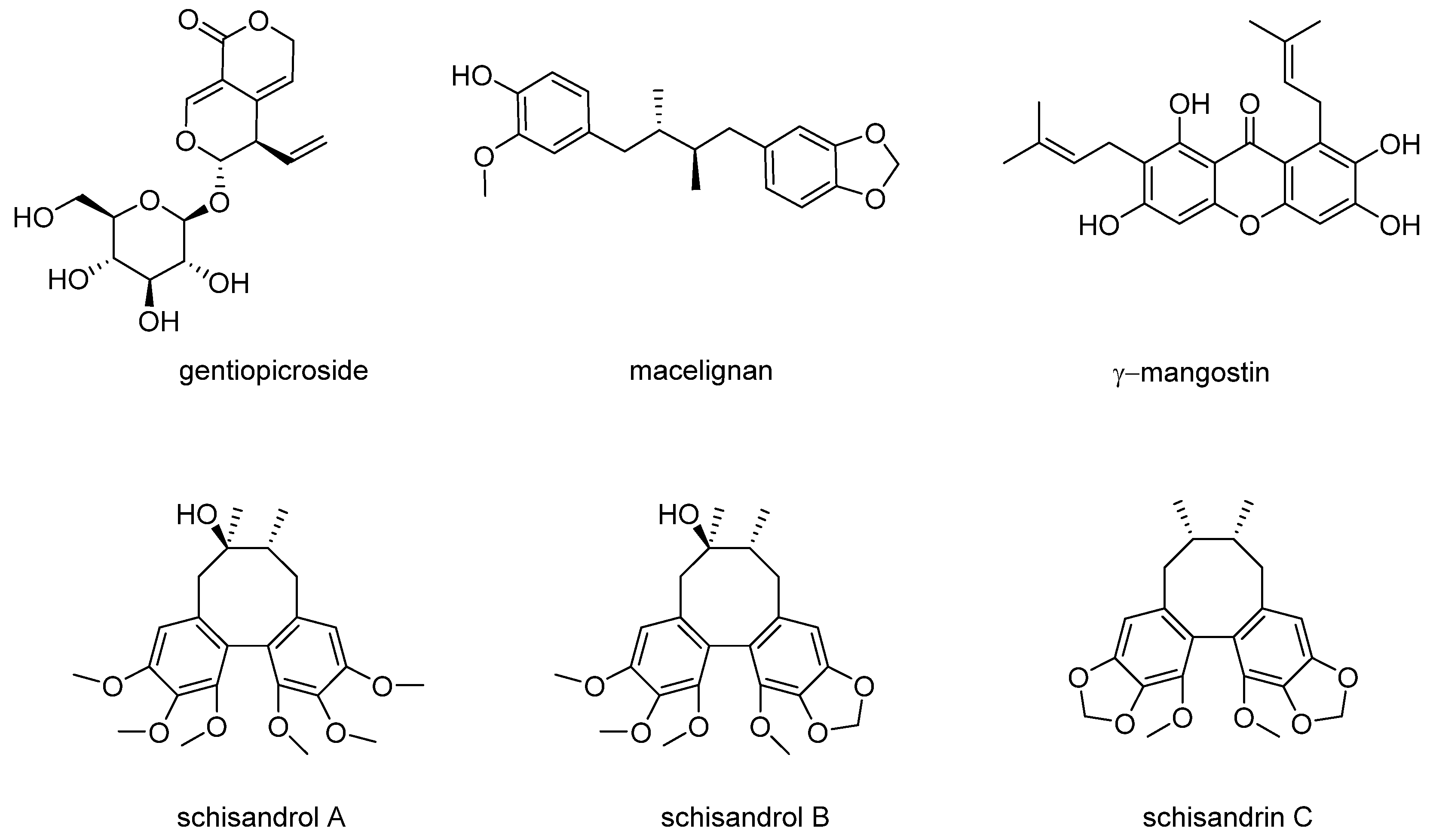
2.2. Extraction and Isolation of Compounds
2.3. Purity Analysis of Compounds
2.4. Cell Culture
2.5. E-Screen Assay
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
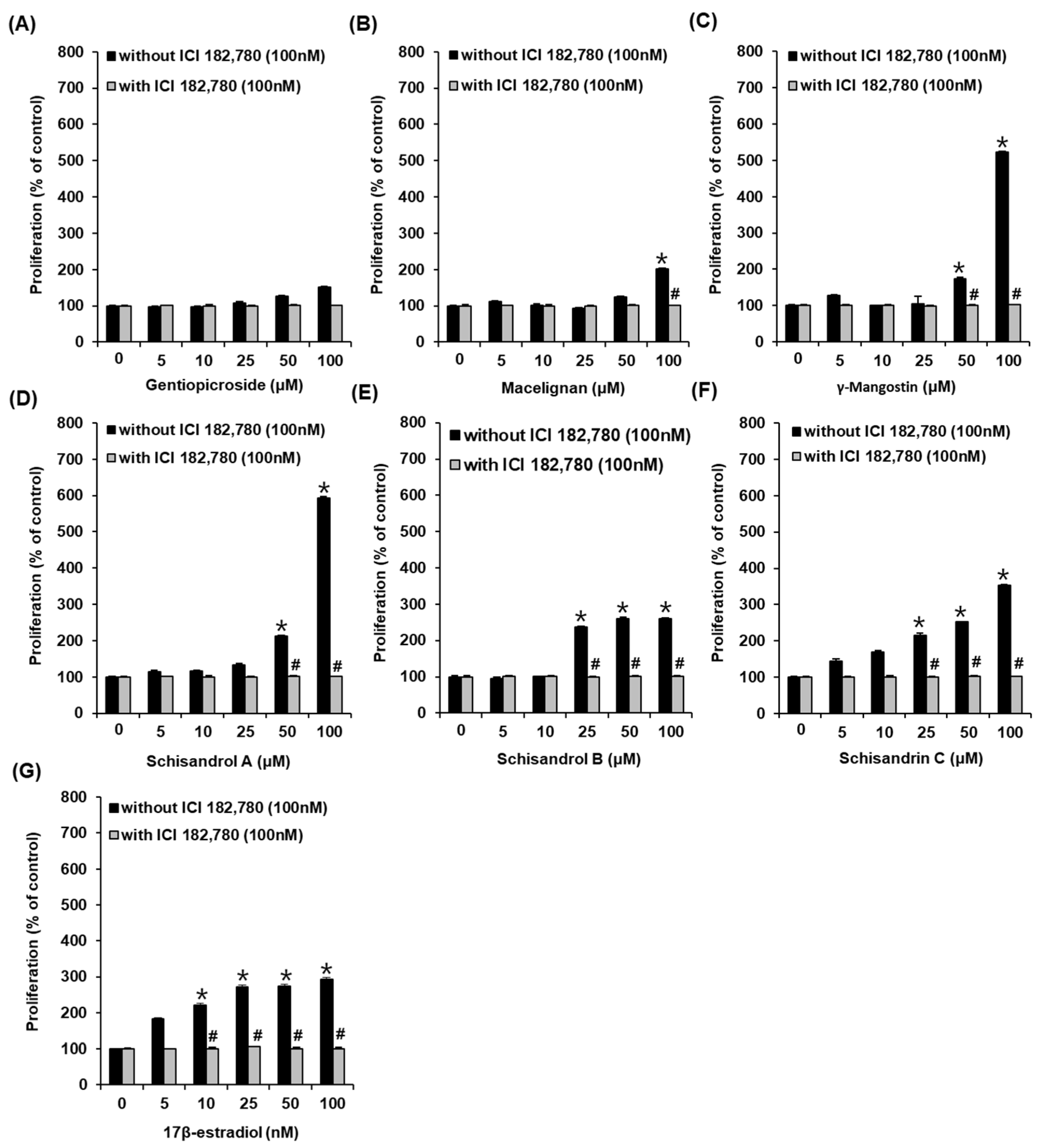
3.1. Effects of Compounds on the Proliferation of MCF-7 Cells
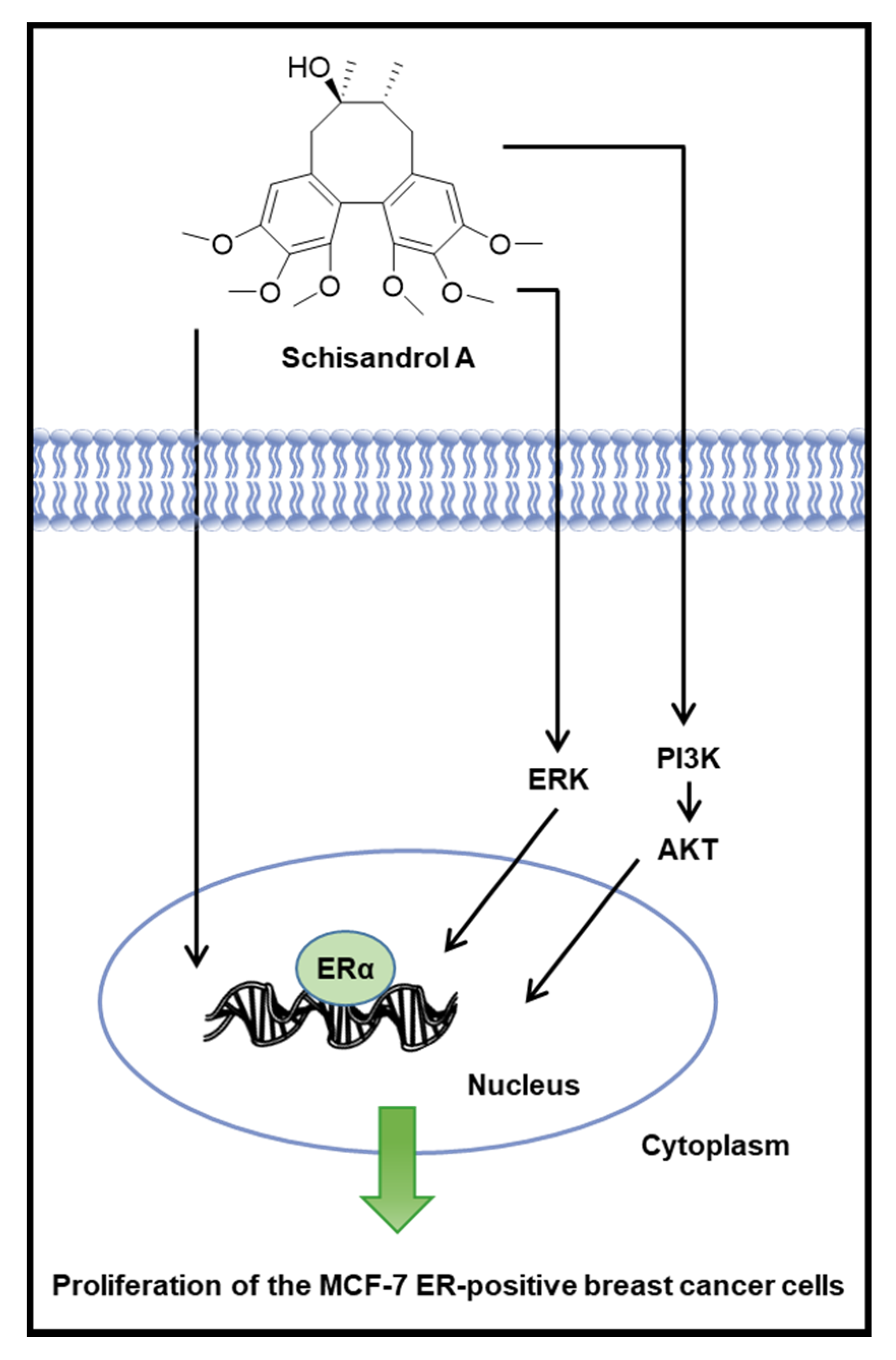
3.2. Effect of Schisandrol A on the Protein Expression of p-PI3K, PI3K, p-Akt, Akt, p-ERα, and ERα
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, H.-R.; Kim, T.-H.; Choi, K.-C. Functions and physiological roles of two types of estrogen receptors, ERα and ERβ, identified by estrogen receptor knockout mouse. Lab. Anim. Res. 2012, 28, 71. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Galluzzo, P.; Ascenzi, P. Estrogen signaling multiple pathways to impact gene transcription. Curr. Genet. 2006, 7, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Kumar, K.; Purohit, D.; Verma, R.; Pandey, P.; Bhatia, S.; Malik, V.; Mittal, V.; Rahman, M.H.; Albadrani, G.M. Exploration of therapeutic applicability and different signaling mechanism of various phytopharmacological agents for treatment of breast cancer. Biomed. Pharmacother. 2021, 139, 111584. [Google Scholar] [CrossRef]
- Sunita, P.; Pattanayak, S. Phytoestrogens in postmenopausal indications: A theoretical perspective. Pharmacogn. Rev. 2011, 5, 41. [Google Scholar] [CrossRef]
- Fait, T. Menopause hormone therapy: Latest developments and clinical practice. Drugs Context 2019, 8, 212551. [Google Scholar] [CrossRef]
- Barrett-Connor, E.L. The risks and benefits of long-term estrogen replacement therapy. Public Health Rep. 1989, 104, 62. [Google Scholar]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef]
- Lagari, V.S.; Levis, S. Phytoestrogens for menopausal bone loss and climacteric symptoms. J. Steroid Biochem. Mol. Biol. 2014, 139, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Glazier, M.G.; Bowman, M.A. A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Arch. Intern. Med. 2001, 161, 1161–1172. [Google Scholar] [CrossRef]
- Keinan-Boker, L.; van Der Schouw, Y.T.; Grobbee, D.E.; Peeters, P.H. Dietary phytoestrogens and breast cancer risk. Am. J. Clin. Nutr. 2004, 79, 282–288. [Google Scholar] [CrossRef]
- Kiyama, R. Biological effects induced by estrogenic activity of lignans. Trends Food Sci. Technol. 2016, 54, 186–196. [Google Scholar] [CrossRef]
- Saleem, M.; Kim, H.J.; Ali, M.S.; Lee, Y.S. An update on bioactive plant lignans. Nat. Prod. Rep. 2005, 22, 696–716. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef]
- McCann, S.E.; Muti, P.; Vito, D.; Edge, S.B.; Trevisan, M.; Freudenheim, J.L. Dietary lignan intakes and risk of pre-and postmenopausal breast cancer. Int. J. Cancer Res. 2004, 111, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Silva, I.; Mangtani, P.; McCormack, V.; Bhakta, D.; McMichael, A.J.; Sevak, L. Phyto-oestrogen intake and breast cancer risk in South Asian women in England: Findings from a population-based case-control study. Cancer Causes Control 2004, 15, 805–818. [Google Scholar] [CrossRef]
- McCann, S.E.; Kulkarni, S.; Trevisan, M.; Vito, D.; Nie, J.; Edge, S.B.; Muti, P.; Freudenheim, J.L. Dietary lignan intakes and risk of breast cancer by tumor estrogen receptor status. Breast Cancer Res. Treat. 2006, 99, 309–311. [Google Scholar] [CrossRef]
- Aehle, E.; Müller, U.; Eklund, P.C.; Willför, S.M.; Sippl, W.; Dräger, B. Lignans as food constituents with estrogen and antiestrogen activity. Phytochemistry 2011, 72, 2396–2405. [Google Scholar] [CrossRef]
- Leusch, F.D.; De Jager, C.; Levi, Y.; Lim, R.; Puijker, L.; Sacher, F.; Tremblay, L.A.; Wilson, V.S.; Chapman, H.F. Comparison of five in vitro bioassays to measure estrogenic activity in environmental waters. Environ. Sci. Technol. 2010, 44, 3853–3860. [Google Scholar] [CrossRef]
- Ito-Harashima, S.; Matano, M.; Onishi, K.; Nomura, T.; Nakajima, S.; Ebata, S.; Shiizaki, K.; Kawanishi, M.; Yagi, T. Construction of reporter gene assays using CWP and PDR mutant yeasts for enhanced detection of various sex steroids. Genes Environ. 2020, 42, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.M.; Sonnenschein, C.; Chung, K.L.; Fernandez, M.F.; Olea, N.; Serrano, F.O. The E-SCREEN assay as a tool to identify estrogens: An update on estrogenic environmental pollutants. Environ. Health Perspect. 1995, 103, 113–122. [Google Scholar] [PubMed]
- Bitsch, N.; Dudas, C.; Körner, W.; Failing, K.; Biselli, S.; Rimkus, G.; Brunn, H. Estrogenic activity of musk fragrances detected by the E-screen assay using human mcf-7 cells. Arch. Environ. Contam. Toxicol. 2002, 43, 0257–0264. [Google Scholar] [CrossRef]
- Rasmussen, T.H.; Nielsen, J.B. Critical parameters in the MCF-7 cell proliferation bioassay (E-Screen). Biomarkers 2002, 7, 322–336. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.-Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Guo, M.; An, F.; Wei, X.; Hong, M.; Lu, Y. Comparative effects of schisandrin A, B, and C on acne-related inflammation. Inflammation 2017, 40, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-Y.; Kim, Y.H.; Bae, D.S.; Um, B.H.; Pan, C.-H.; Kim, C.Y.; Lee, H.J.; Lee, J.K. Anti-inflammatory effects of gomisin N, gomisin J, and schisandrin C isolated from the fruit of Schisandra chinensis. Biosci. Biotechnol. Biochem. 2010, 74, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-K. Effects of macelignan isolated from Myristica fragrans Houtt. on UVB-induced matrix metalloproteinase-9 and cyclooxygenase-2 in HaCaT cells. J. Dermatol. Sci. 2010, 57, 114–122. [Google Scholar]
- Zhao, L.; Ye, J.; Wu, G.-T.; Peng, X.-J.; Xia, P.-F.; Ren, Y. Gentiopicroside prevents interleukin-1 beta induced inflammation response in rat articular chondrocyte. J. Ethnopharmacol. 2015, 172, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Aisha, A.F.; Abu-Salah, K.M.; Ismail, Z.; Majid, A.M.S.A. In vitro and in vivo anti-colon cancer effects of Garcinia mangostana xanthones extract. BMC Complement. Altern. Med. 2012, 12, 1–10. [Google Scholar] [CrossRef]
- Itoh, T.; Ohguchi, K.; Iinuma, M.; Nozawa, Y.; Akao, Y. Inhibitory effect of xanthones isolated from the pericarp of Garcinia mangostana L. on rat basophilic leukemia RBL-2H3 cell degranulation. Bioorg. Med. Chem. 2008, 16, 4500–4508. [Google Scholar] [CrossRef]
- Han, Y.-H.; Mun, J.-G.; Jeon, H.D.; Park, J.; Kee, J.-Y.; Hong, S.-H. Gomisin A ameliorates metastatic melanoma by inhibiting AMPK and ERK/JNK-mediated cell survival and metastatic phenotypes. Phytomedicine 2020, 68, 153147. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Q.; Zheng, H.; Ye, Q.; Li, R.-Y.; Chen, Y. Chloroquinone inhibits cell proliferation and induces apoptosis in nasopharyngeal carcinoma cell lines. Trop. J. Pharm. Res. 2015, 14, 2187–2192. [Google Scholar] [CrossRef]
- Lee, G.T.; Cha, H.J.; Lee, K.S.; Lee, K.K.; Hong, J.T.; Ahn, K.J.; An, I.-S.; An, S.; Bae, S. Arctiin induces an UVB protective effect in human dermal fibroblast cells through microRNA expression changes. Int. J. Mol. Med. 2014, 33, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.J.; Johnson, M.D.; Lee, A.V.; Oesterreich, S. Endocrine response phenotypes are altered by charcoal-stripped serum variability. Endocrinology 2016, 157, 3760–3766. [Google Scholar] [CrossRef] [PubMed]
- Dinda, S.; Sanchez, A.; Moudgil, V. Estrogen-like effects of thyroid hormone on the regulation of tumor suppressor proteins, p53 and retinoblastoma, in breast cancer cells. Oncogene 2002, 21, 761–768. [Google Scholar] [CrossRef]
- Howell, A.; Osborne, C.K.; Morris, C.; Wakeling, A.E. ICI 182,780 (Faslodex™) development of a novel,“pure” antiestrogen. Cancer 2000, 89, 817–825. [Google Scholar] [CrossRef]
- Zhao, L.; O’Neill, K.; Brinton, R.D. Estrogenic agonist activity of ICI 182,780 (Faslodex) in hippocampal neurons: Implications for basic science understanding of estrogen signaling and development of estrogen modulators with a dual therapeutic profile. J. Pharmacol. Exp. Ther. 2006, 319, 1124–1132. [Google Scholar] [CrossRef]
- Ahn, S.-Y.; Jo, M.S.; Lee, D.; Baek, S.-E.; Baek, J.; Yu, J.S.; Jo, J.; Yun, H.; Kang, K.S.; Yoo, J.-E. Dual effects of isoflavonoids from Pueraria lobata roots on estrogenic activity and anti-proliferation of MCF-7 human breast carcinoma cells. Bioorg. Chem. 2019, 83, 135–144. [Google Scholar] [CrossRef]
- Trinh, T.A.; Park, E.-J.; Lee, D.; Song, J.H.; Lee, H.L.; Kim, K.H.; Kim, Y.; Jung, K.; Kang, K.S.; Yoo, J.-E. Estrogenic activity of sanguiin H-6 through activation of estrogen receptor α coactivator-binding site. Nat. Prod. Sci. 2019, 25, 28–33. [Google Scholar] [CrossRef][Green Version]
- Lee, D.; Park, S.; Choi, S.; Kim, S.H.; Kang, K.S. In Vitro Estrogenic and Breast Cancer Inhibitory Activities of Chemical Constituents Isolated from Rheum undulatum L. Molecules 2018, 23, 1215. [Google Scholar] [CrossRef]
- Lai, Q.; Yuan, G.-Y.; Wang, H.; Liu, Z.-L.; Kou, J.-P.; Yu, B.-Y.; Li, F. Exploring the protective effects of schizandrol a in acute myocardial ischemia mice by comprehensive metabolomics profiling integrated with molecular mechanism studies. Acta Pharmacol. Sin. 2020, 41, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Huo, D.-S.; Cai, Z.-P.; Shao, G.; Wang, H.; Zhao, Z.-Y.; Yang, Z.-J. The effect of schizandrol A-induced DNA methylation on SH-SY5YAB 1-40 altered neuronal cell line: A potential use in alzheimer’s disease. J. Toxicol. Environ. Health 2015, 78, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Wang, W.; Bian, Z.; Ren, Z. Effects of schizandrol on the central nervous system. Acta Pharm. Sin. 1983, 18, 416–421. [Google Scholar]
- Zhang, L.; Niu, X. Effects of schizandrol A on monoamine neurotransmitters in the central nervous system. Acta Acad. Med. Sin. 1991, 13, 13–16. [Google Scholar]
- Song, L.; Yao, L.; Zhang, L.; Piao, Z.; Lu, Y. Schizandrol A protects against Aβ 1–42-induced autophagy via activation of PI3K/AKT/mTOR pathway in SH-SY5Y cells and primary hippocampal neurons. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1739–1752. [Google Scholar] [CrossRef] [PubMed]
- Wang, O.; Cheng, Q.; Liu, J.; Wang, Y.; Zhao, L.; Zhou, F.; Ji, B. Hepatoprotective effect of Schisandra chinensis (Turcz.) Baill. lignans and its formula with Rubus idaeus on chronic alcohol-induced liver injury in mice. Food Funct. 2014, 5, 3018–3025. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, X.; Shi, Y.; Wang, D.; Gu, Y.; Li, S.; Liang, X.; Wang, Z.; Wang, C. Protection of seven dibenzocyclooctadiene lignans from Schisandra chinensis against serum and glucose deprivation injury in SH-SY5Y cells. Cell Biol. Int. 2015, 39, 1418–1424. [Google Scholar]
- Levenson, A.S.; Jordan, V.C. MCF-7: The first hormone-responsive breast cancer cell line. Cancer Res. 1997, 57, 3071–3078. [Google Scholar] [PubMed]
- Luo, J.; Liu, D. Does GPER Really Function as a G Protein-Coupled Estrogen Receptor in vivo? Front. Endocrinol. 2020, 11, 148. [Google Scholar] [CrossRef]
- Barton, M.; Filardo, E.J.; Lolait, S.J.; Thomas, P.; Maggiolini, M.; Prossnitz, E.R. Twenty years of the G protein-coupled estrogen receptor GPER: Historical and personal perspectives. J. Steroid Biochem. Mol. Biol. 2018, 176, 4–15. [Google Scholar] [CrossRef]
- Lei, B.; Sun, S.; Zhang, X.; Feng, C.; Xu, J.; Wen, Y.; Huang, Y.; Wu, M.; Yu, Y. Bisphenol AF exerts estrogenic activity in MCF-7 cells through activation of Erk and PI3K/Akt signals via GPER signaling pathway. Chemosphere 2019, 220, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Prossnitz, E.R.; Arterburn, J.B.; Smith, H.O.; Oprea, T.I.; Sklar, L.A.; Hathaway, H.J. Estrogen signaling through the transmembrane G protein–coupled receptor GPR30. Annu. Rev. Physiol. 2008, 70, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Rieber, M.; Strasberg-Rieber, M. p53 inactivation decreases dependence on estrogen/ERK signalling for proliferation but promotes EMT and susceptility to 3-bromopyruvate in ERα+ breast cancer MCF-7 cells. Biochem. Pharmacol. 2014, 88, 169–177. [Google Scholar] [CrossRef]
- Watson, C.S.; Jeng, Y.-J.; Hu, G.; Wozniak, A.; Bulayeva, N.; Guptarak, J. Estrogen-and xenoestrogen-induced ERK signaling in pituitary tumor cells involves estrogen receptor-α interactions with G protein-αi and caveolin I. Steroids 2012, 77, 424–432. [Google Scholar] [CrossRef]
- Welsh, T.; Johnson, M.; Yi, L.; Tan, H.; Rahman, R.; Merlino, A.; Zakar, T.; Mesiano, S. Estrogen receptor (ER) expression and function in the pregnant human myometrium: Estradiol via ERa activates ERK1/2 signaling in term myometrium. J. Endocrinol. 2012, 212, 227–238. [Google Scholar] [CrossRef]
- Jeon, N.R.; Kang, K.; Jho, E.H.; Lee, H.J.; Kim, C.Y.; Nho, C.W. Phytoestrogenic activity of ethanol extract from Korean wild vegetable Disporum uniflorum. Food Sci. Biotechnol. 2010, 19, 1543–1550. [Google Scholar] [CrossRef]
- Wang, G.-S.; Huang, Y.-G.; Li, H.; Bi, S.-J.; Zhao, J.-L. ERK/CANP rapid signaling mediates 17β-estradiol-induced proliferation of human breast cancer cell line MCF-7 cells. Int. J. Clin. Exp. Med. 2014, 7, 156. [Google Scholar]
- Kang, K.; Lee, S.B.; Jung, S.H.; Cha, K.H.; Park, W.D.; Sohn, Y.C.; Nho, C.W. Tectoridin, a poor ligand of estrogen receptor α, exerts its estrogenic effects via an ERK-dependent pathway. Mol. Cells 2009, 27, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, K.; Kim, K.; Bender, J. Estrogen receptor-mediated rapid signaling. Endocrinology 2006, 147, 5557–5563. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Zhang, K.; Hu, X.; Sun, Y.; Sheng, J.; Fu, X. Black tea and D. candidum extracts play estrogenic activity via estrogen receptor α-dependent signaling pathway. Am. J. Transl. Res. 2018, 10, 114. [Google Scholar]
- Chen, F.-P.; Chien, M.-H.; Chern, I.Y.-Y. Impact of low concentrations of phthalates on the effects of 17β-estradiol in MCF-7 breast cancer cells. Taiwan. J. Obstet. Gynecol. 2016, 55, 826–834. [Google Scholar] [CrossRef]
- Chen, F.; Chien, M. Phytoestrogens induce differential effects on both normal and malignant human breast cells in vitro. Climacteric 2014, 17, 682–691. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, H.; Kong, Q.; Jiang, Y. Mechanisms for estrogen receptor expression in human cancer. Exp Hematol Oncol. 2018, 7, 24. [Google Scholar] [CrossRef]
- Girgert, R.; Emons, G.; Gründker, C. Estrogen signaling in ERα-negative breast cancer: ERβ and GPER. Front. Endocrinol. 2019, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- JavanMoghadam, S.; Weihua, Z.; Hunt, K.K.; Keyomarsi, K. Estrogen receptor alpha is cell cycle-regulated and regulates the cell cycle in a ligand-dependent fashion. Cell Cycle 2016, 15, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Katchy, A.; Pinto, C.; Jonsson, P.; Nguyen-Vu, T.; Pandelova, M.; Riu, A.; Schramm, K.-W.; Samarov, D.; Gustafsson, J.-Å.; Bondesson, M. Coexposure to phytoestrogens and bisphenol a mimics estrogenic effects in an additive manner. Toxicol. Sci. 2014, 138, 21–35. [Google Scholar] [CrossRef]
- Movérare-Skrtic, S.; Börjesson, A.E.; Farman, H.H.; Sjögren, K.; Windahl, S.H.; Lagerquist, M.K.; Andersson, A.; Stubelius, A.; Carlsten, H.; Gustafsson, J.-Å. The estrogen receptor antagonist ICI 182,780 can act both as an agonist and an inverse agonist when estrogen receptor α AF-2 is modified. Proc. Natl. Acad. Sci. USA 2014, 111, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.; Skouv, J.; Lundholt, B.; Lykkesfeldt, A. Differential regulation of specific genes in MCF-7 and the ICI 182780-resistant cell line MCF-7/182 R-6. Br. J. Cancer 1999, 79, 386–392. [Google Scholar] [CrossRef] [PubMed][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Kim, Y.-M.; Chin, Y.-W.; Kang, K.S. Schisandrol A Exhibits Estrogenic Activity via Estrogen Receptor α-Dependent Signaling Pathway in Estrogen Receptor-Positive Breast Cancer Cells. Pharmaceutics 2021, 13, 1082. https://doi.org/10.3390/pharmaceutics13071082
Lee D, Kim Y-M, Chin Y-W, Kang KS. Schisandrol A Exhibits Estrogenic Activity via Estrogen Receptor α-Dependent Signaling Pathway in Estrogen Receptor-Positive Breast Cancer Cells. Pharmaceutics. 2021; 13(7):1082. https://doi.org/10.3390/pharmaceutics13071082
Chicago/Turabian StyleLee, Dahae, Young-Mi Kim, Young-Won Chin, and Ki Sung Kang. 2021. "Schisandrol A Exhibits Estrogenic Activity via Estrogen Receptor α-Dependent Signaling Pathway in Estrogen Receptor-Positive Breast Cancer Cells" Pharmaceutics 13, no. 7: 1082. https://doi.org/10.3390/pharmaceutics13071082
APA StyleLee, D., Kim, Y.-M., Chin, Y.-W., & Kang, K. S. (2021). Schisandrol A Exhibits Estrogenic Activity via Estrogen Receptor α-Dependent Signaling Pathway in Estrogen Receptor-Positive Breast Cancer Cells. Pharmaceutics, 13(7), 1082. https://doi.org/10.3390/pharmaceutics13071082








