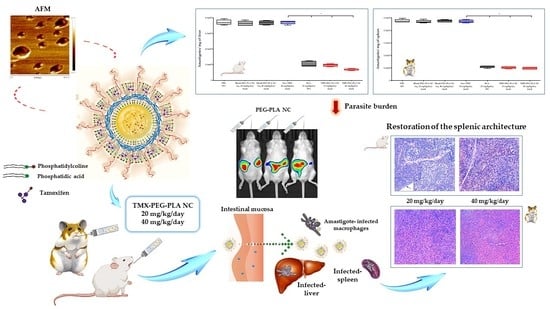
Repositioning of Tamoxifen in Surface-Modified Nanocapsules as a Promising Oral Treatment for Visceral Leishmaniasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Tamoxifen Nanocapsules
2.3. Physicochemical Characterization
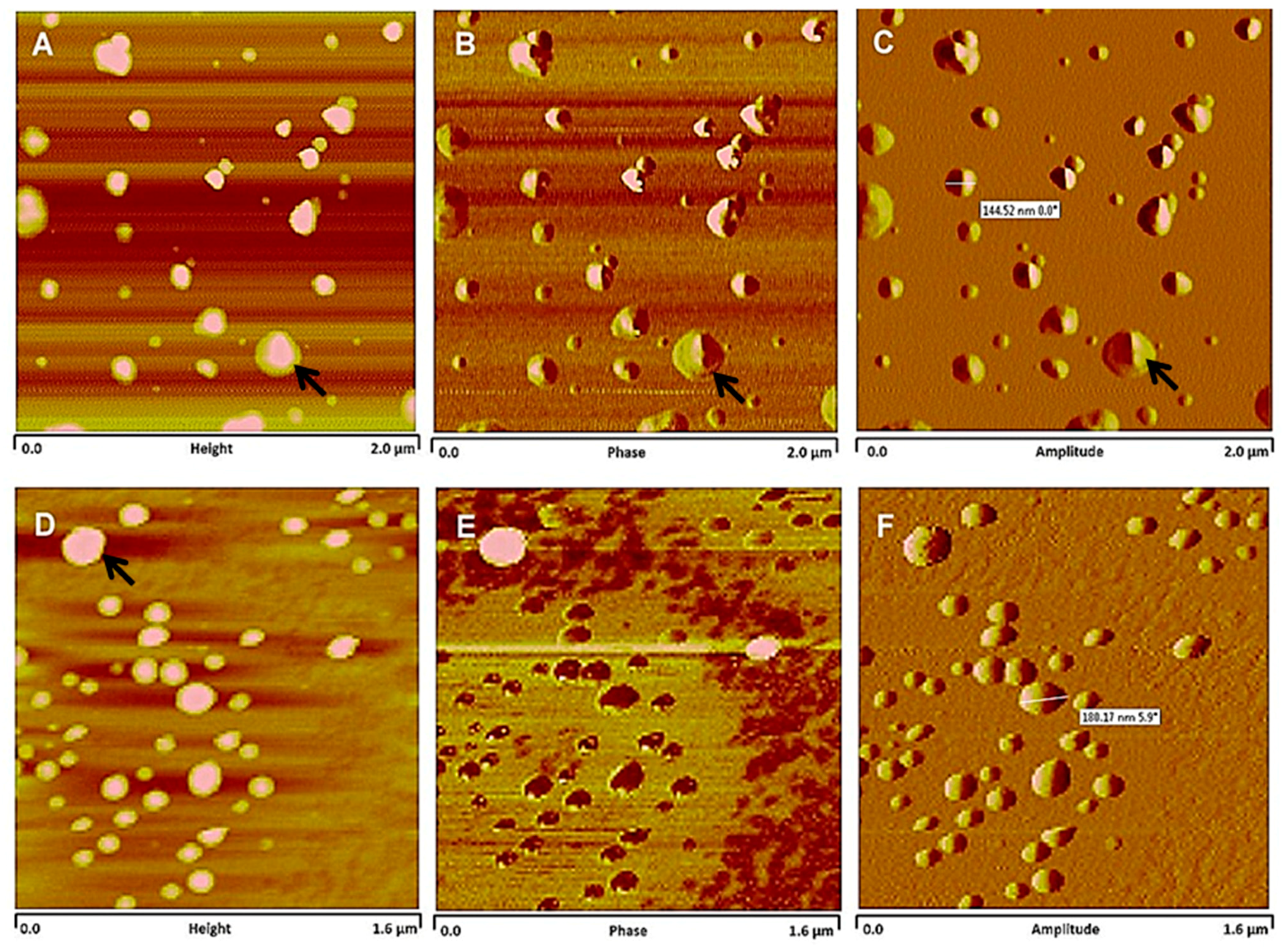
2.4. Atomic Force Microscopy Imaging and Analyses
2.5. Tamoxifen Encapsulation
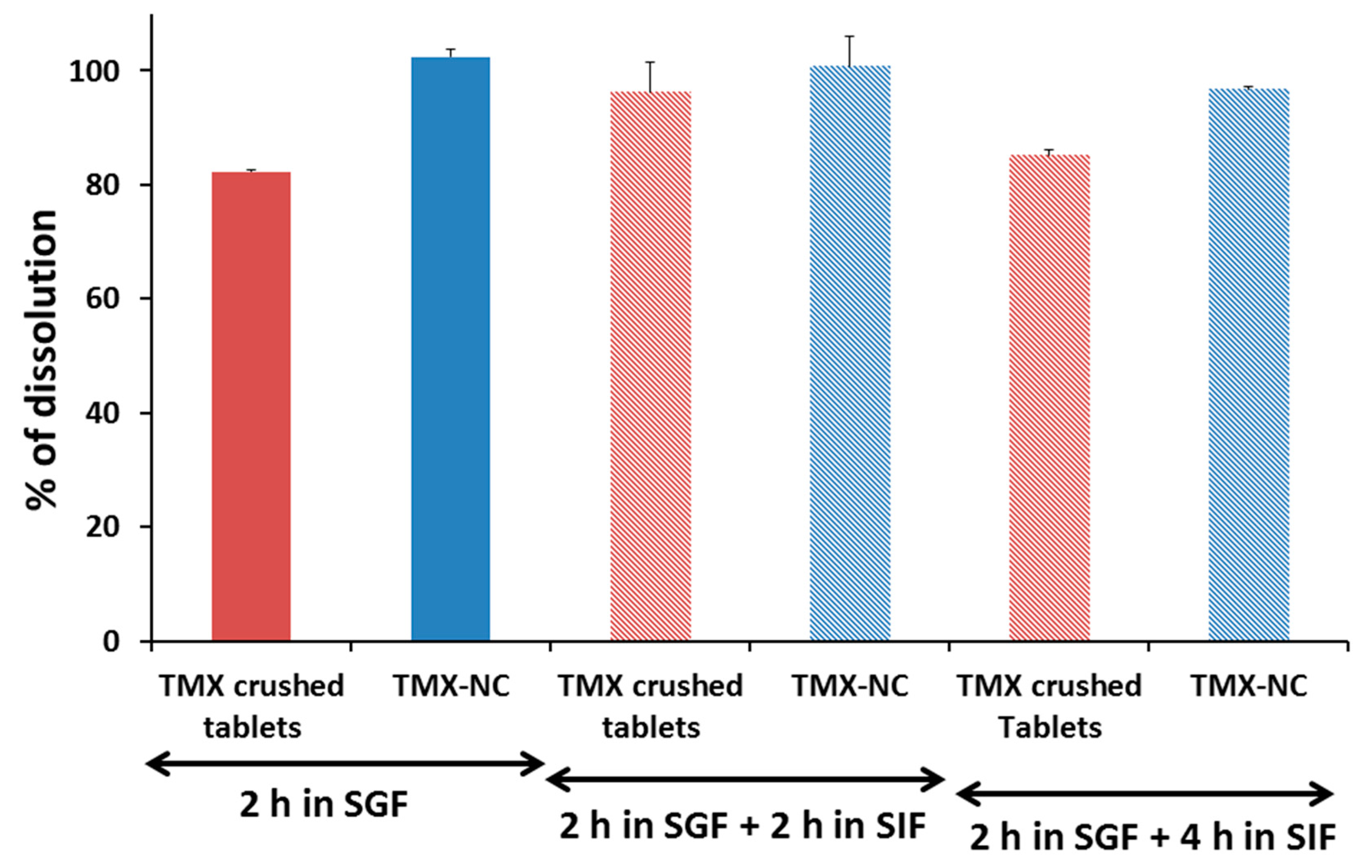
2.6. In Vitro Tamoxifen Dissolution
2.7. Parasites and Cell Culture
2.8. Experimental Animals and Ethical Statements
2.9. Infection and Treatment Regimens
2.10. Parasite Quantification in Tissues
2.11. In Vivo Imaging of Biodistribution of Fluorescently Labeled Nanocapsules after Oral Administration
2.12. Histophatological Analysis
2.13. Statistical Analysis
3. Results
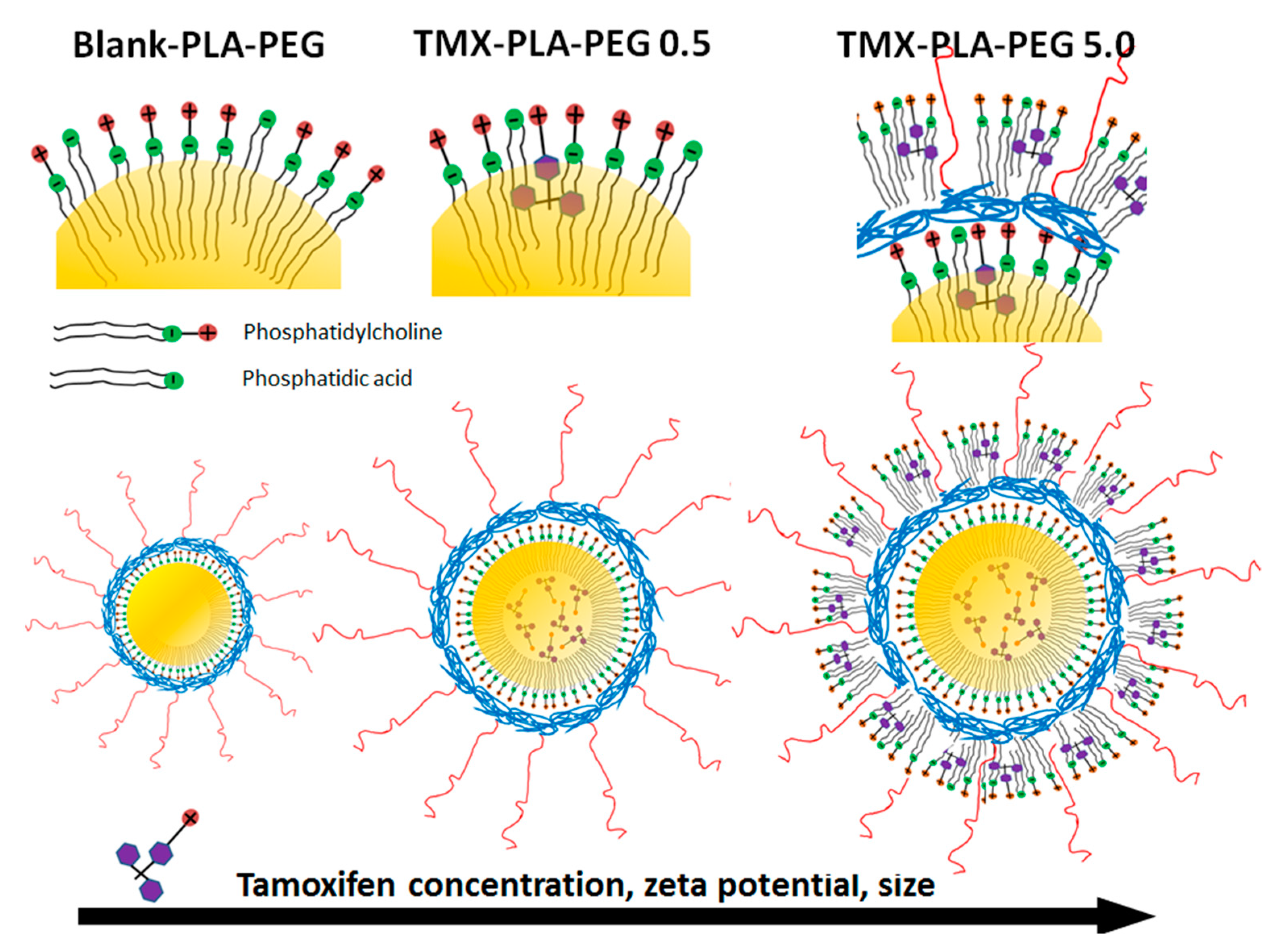
3.1. Tamoxifen PEG-PLA Nanocapsules Development
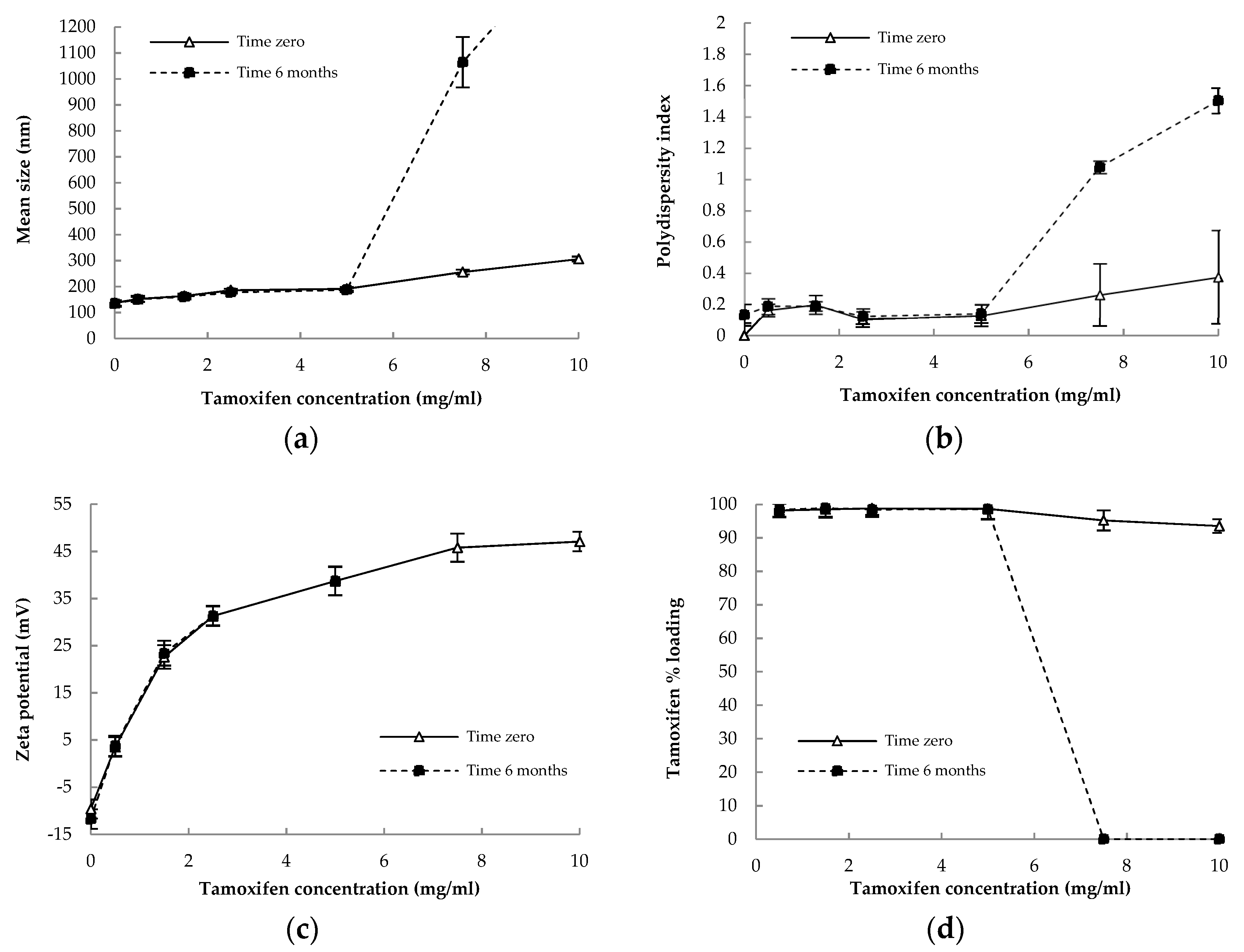
3.2. Physicochemical Characterization and Stability of Nanocapsules
3.3. In Vitro Tamoxifen Solubility and Release
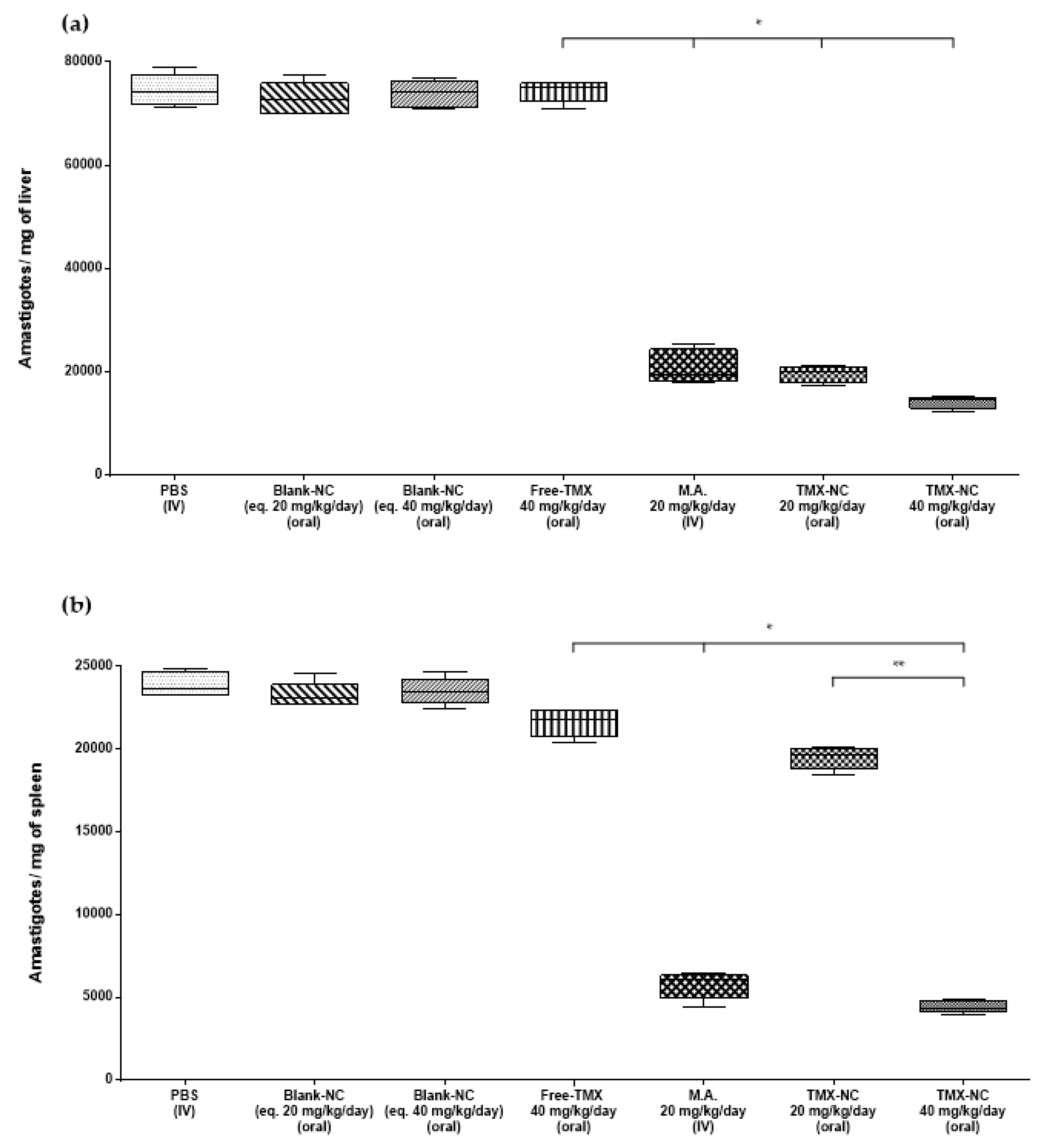
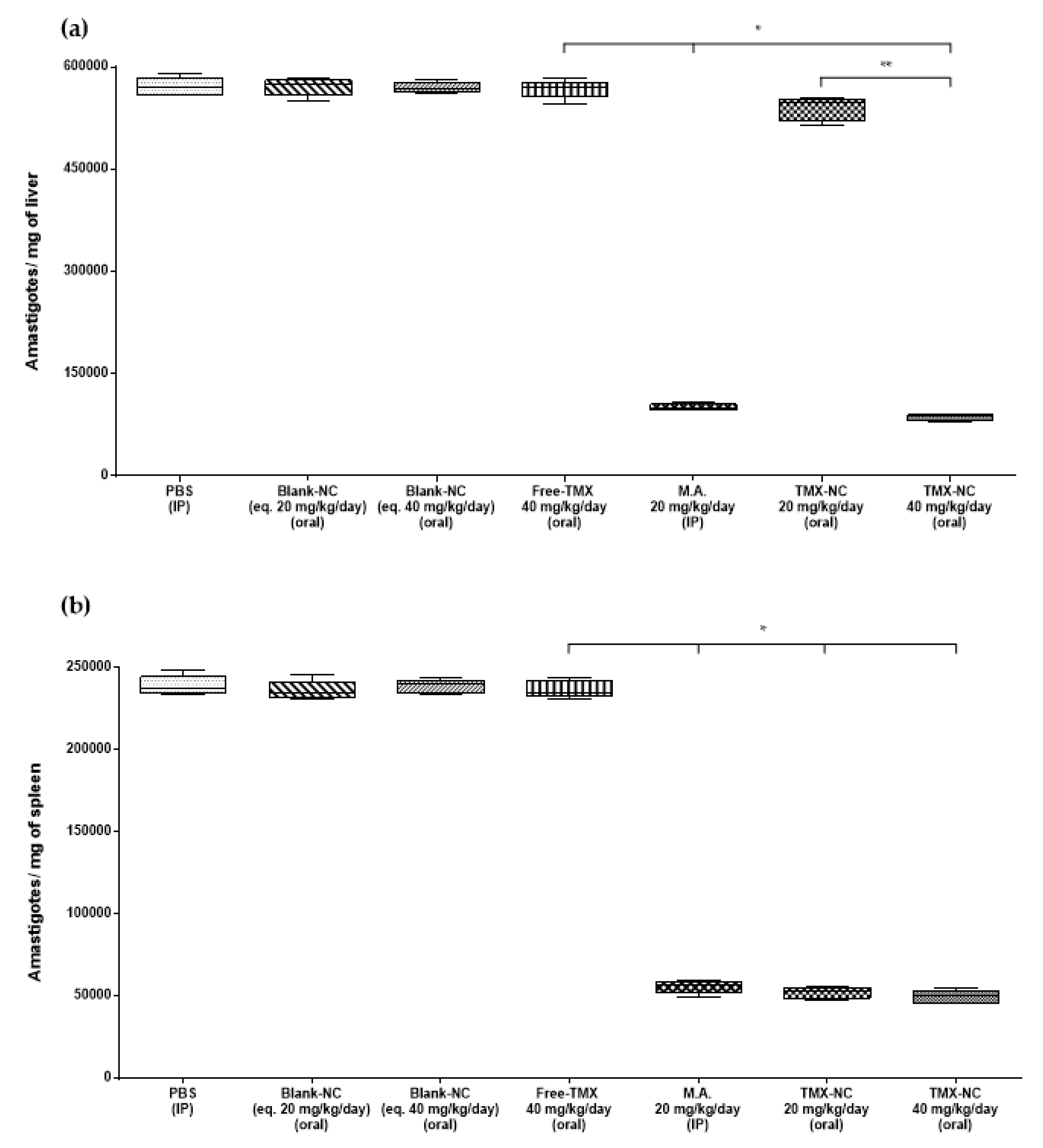
3.4. Effect of Tamoxifen Nanocapsules on Tissue Parasite Burden
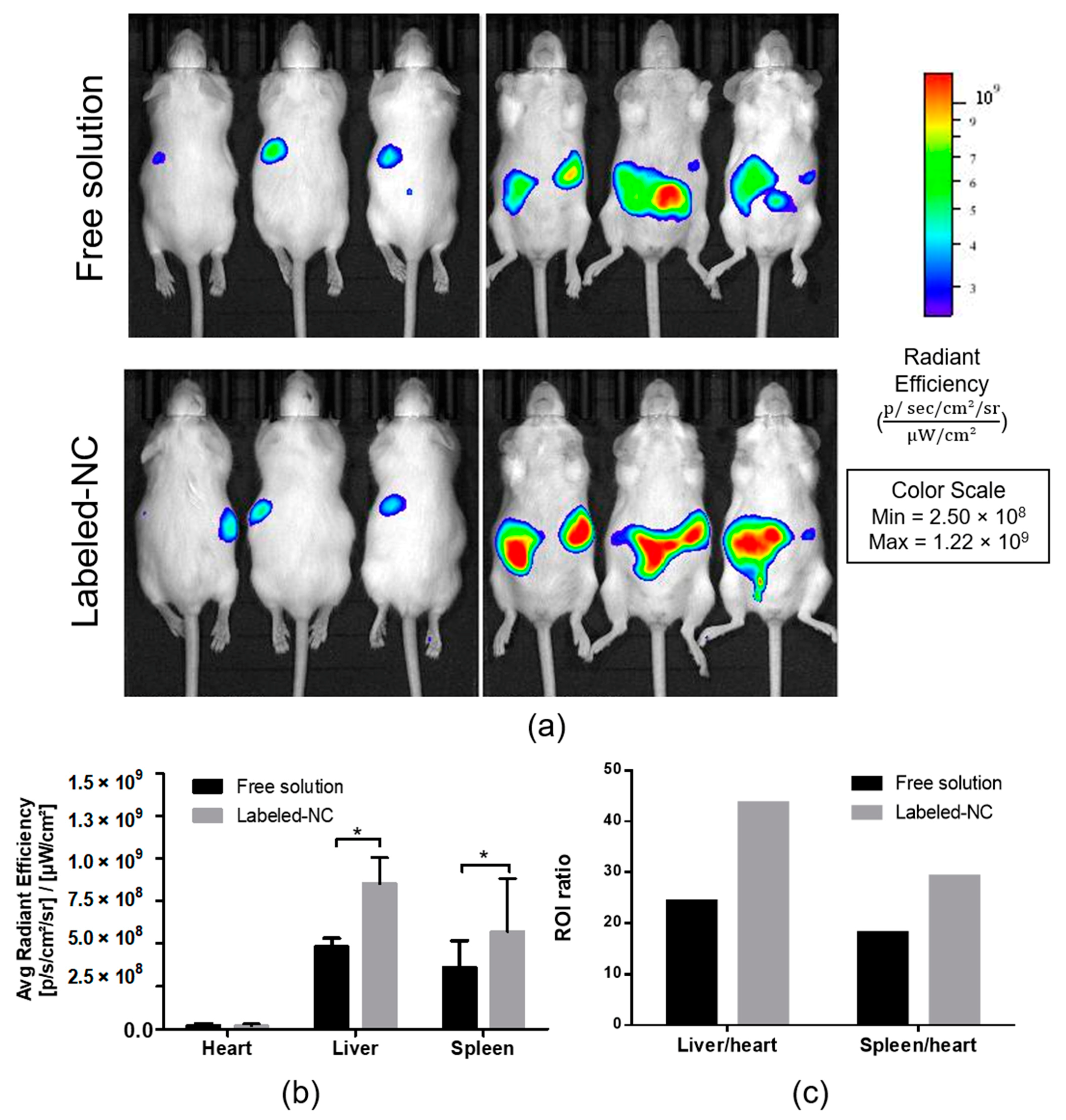
3.5. Biodistribution Analysis of Labeled Nanocapsules
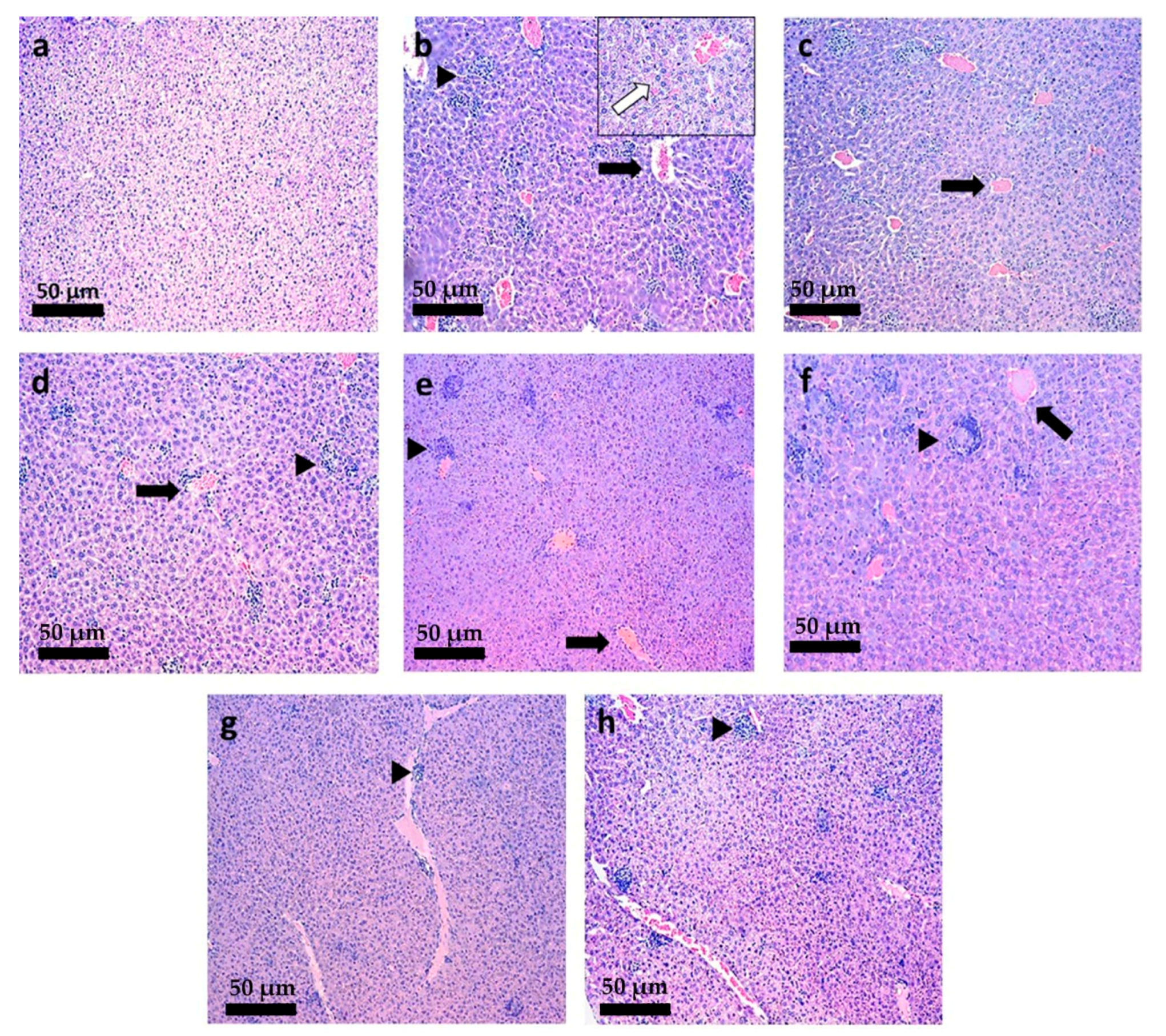
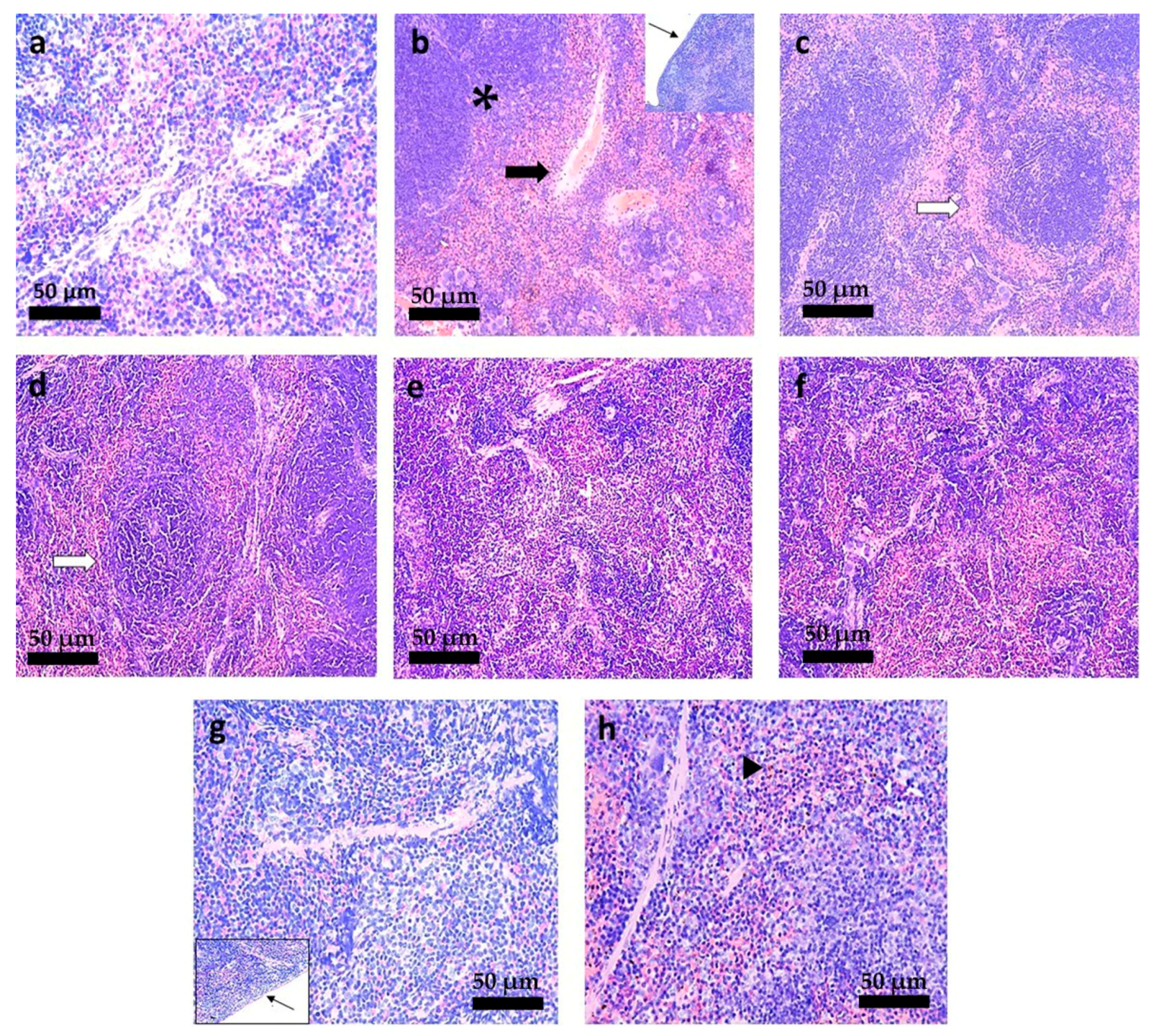
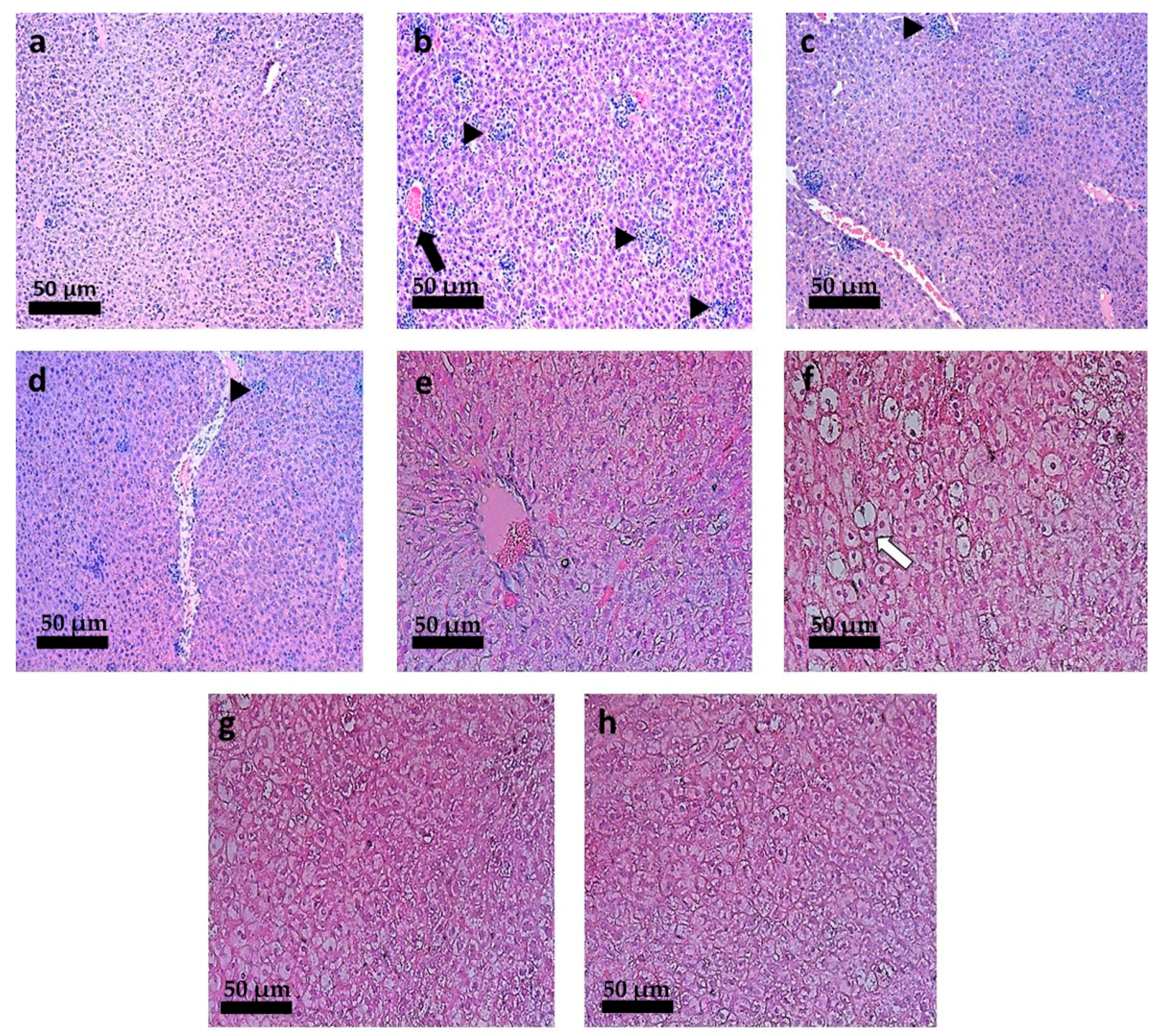
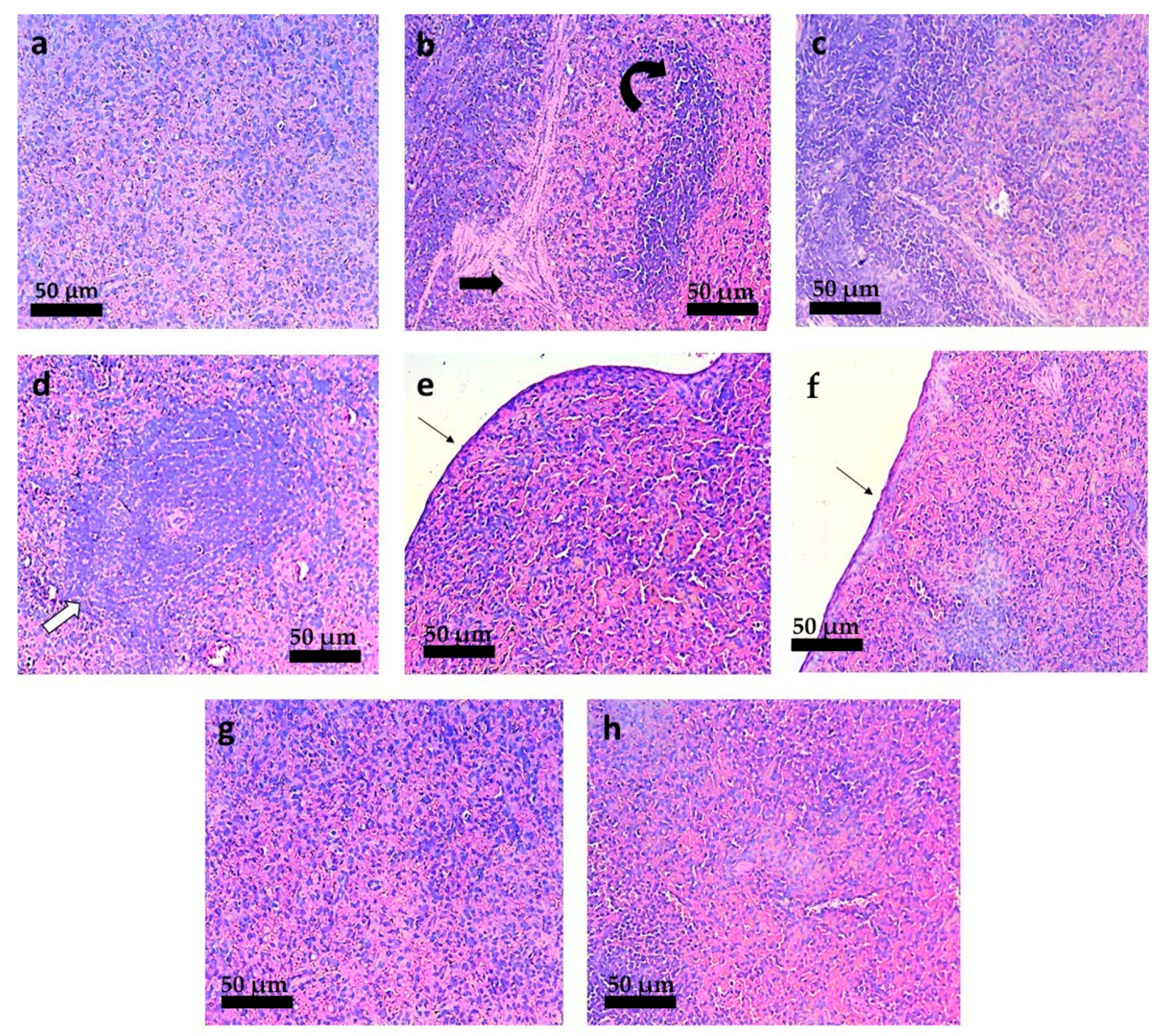
3.6. Histopathological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2019, 393, 951–970. [Google Scholar] [CrossRef]
- Espada, C.R.; Magalhães, R.M.; Cruz, M.C.; Machado, P.R.; Schriefer, A.; Carvalho, E.M.; Hornillos, V.; Alves, J.M.; Cruz, A.K.; Coelho, A.C.; et al. Investigation of the pathways related to intrinsic miltefosine tolerance in Leishmania (Viannia) braziliensis clinical isolates reveals differences in drug uptake. Int. J. Parasitol. Drugs Drug Resist. 2019, 11, 139–147. [Google Scholar] [CrossRef]
- Castro, M.D.M.; Cossio, A.; Velasco, C.; Osorio, L. Risk factors for therapeutic failure to meglumine antimoniate and miltefosine in adults and children with cutaneous leishmaniasis in Colombia: A cohort study. PLoS Negl. Trop. Dis. 2017, 11, e0005515. [Google Scholar] [CrossRef]
- Uliana, S.R.B.; Barcinski, M.A.J.S. Repurposing for neglected diseases. Science 2009, 326, 935. [Google Scholar] [CrossRef]
- Pinto, E.G.; Tempone, A.G. Activity of the antiarrhythmic drug amiodarone against Leishmania (L.) infantum: An in vitro and in vivo approach. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 1–6. [Google Scholar] [CrossRef]
- Charlton, R.L.; Rossi-Bergmann, B.; Denny, P.W.; Steel, P.G. Repurposing as a strategy for the discovery of new anti- leishmanials: The-state-of-the-art. Parasitology 2018, 145, 219–236. [Google Scholar] [CrossRef]
- Montoya, M.C.; Krysan, D.J. Repurposing estrogen receptor antagonists for the treatment of infectious disease. mBio 2018, 9, e02272-18. [Google Scholar] [CrossRef] [PubMed]
- Miguel, D.C.; Zauli-Nascimento, R.C.; Yokoyama-Yasunaka, J.K.; Katz, S.; Barbiéri, C.L.; Uliana, S.R.B. Tamoxifen as a potential antileishmanial agent: Efficacy in the treatment of Leishmania braziliensis and Leishmania chagasi infections. J. Antimicrob. Chemother. 2008, 63, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Domínguez-Bernal, G.; Orden, J.A.; De La Fuente, R.; Madrid-Elena, N.; Carrión, J. Mechanisms of resistance and susceptibility to experimental visceral leishmaniosis: BALB/c mouse versus syrian hamster model. Vet. Res. 2011, 42, 39. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Chard, L.S.; Wang, Z.; Wang, Y. Syrian hamster as an animal model for the study on infectious diseases. Front. Immunol. 2019, 10, 2329. [Google Scholar] [CrossRef]
- Jensen, E.V.; Block, G.E.; Smith, S.; Kyser, K.; DeSombre, E.R. Estrogen receptors and breast cancer response to adrenalectomy. Natl. Cancer Inst. Monogr. 1971, 34, 55–70. [Google Scholar]
- Costa Filho, A.V.; Lucas, Í.C.; Sampaio, R.N.R. Estudo comparativo entre miltefosina oral e antimoniato de N-metil glucamina parenteral no tratamento da leishmaniose experimental causada por Leishmania (Leishmania) amazonensis. Rev. Soc. Bras. Med. Trop. 2008, 41, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Trinconi, C.T.; Miguel, D.C.; Silber, A.M.; Brown, C.; Mina, J.G.; Denny, P.W.; Heise, N.; Uliana, S.R.B. Tamoxifen inhibits the biosynthesis of inositolphosphorylceramide in Leishmania. Int. J. Parasitol. Drug Drug Resist. 2018, 8, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Reimão, J.Q.; Uliana, S.R.B. Tamoxifen alters cell membrane properties in Leishmania amazonensis promastigotes. Parasitol. Open 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Bergström, C.A.; Wassvik, C.M.; Johansson, K.; Hubatsch, I. Poorly soluble marketed drugs display solvation limited solubility. J. Med. Chem. 2007, 50, 5858–5862. [Google Scholar] [CrossRef] [PubMed]
- Santana, D.P.; Braga, R.M.C.; Strattmman, R.; Albuquerque, M.M.; Bedor, D.C.G.; Leal, L.B.; Silva, J.A. Reversed phase HPLC determination of tamoxifen in dog plasma and its pharmaco-kinetics after a single oral dose administration. Quím. Nova 2008, 31, 47–52. [Google Scholar] [CrossRef]
- Buchanan, C.M.; Buchanan, N.L.; Edgar, K.J.; Little, J.L.; Malcolm, M.O.; Ruble, K.M.; Wacher, V.J.; Wempe, M.F. Pharmacokinetics of tamoxifen after intravenous and oral dosing of tamoxifen–hydroxybutenyl-β-cyclodextrin formulations. J. Pharm. Sci. 2007, 96, 644–660. [Google Scholar] [CrossRef]
- Dickschen, K.; Willmann, S.; Thelen, K.; Lippert, J.; Hempel, G.; Eissing, T. Physiologically-based pharmacokinetic modeling of tamoxifen and its metabolites in women of different CYP2D6 phenotypes provides new insight into the tamoxifen mass balance. Front. Pharmacol. 2012, 3, 92. [Google Scholar] [CrossRef]
- Shin, S.C.; Choi, J.S.; Li, X. Enhanced bioavailability of tamoxifen after oral administration of tamoxifen with quercetin in rats. Int. J. Pharm. 2006, 313, 144–149. [Google Scholar] [CrossRef]
- Trinconi, C.T.; Reimão, J.Q.; Coelho, A.C.; Uliana, S.R.B. Efficacy of tamoxifen and miltefosine combined therapy for cutaneous leishmaniasis in the murine model of infection with Leishmania amazonensis. J. Antimicrob. Chemoter. 2016, 71, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.C.; Trinconi, C.T.; Senra, L.; Yokoyama-Yasunaka, J.K.; Uliana, S.R.B. Leishmania is not prone to develop resistance to tamoxifen. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 77–83. [Google Scholar] [CrossRef]
- Espuelas, S.; Plano, D.; Nguewa, P.; Font, M.; Palop, J.; Irache, J.; Sanmartín, C. Innovative lead compounds and formulation strategies as newer kinetoplastid therapies. Curr. Med. Chem. 2012, 19, 4259–4288. [Google Scholar] [CrossRef]
- Formiga, R.F.; Inamuddin; Severino, P. Applications of Nanobiotechnology for Neglected Tropical Diseases, 1st ed.; Academic Press: Cambridge, UK, 2021; p. 614. [Google Scholar]
- Lanza, J.S.; Pomel, S.; Loiseau, P.M.; Frézard, F. Recent advances in amphotericin B delivery strategies for the treatment of leishmaniases. Expert Opin. Drug Deliv. 2019, 16, 1063–1079. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Attili-Qadri, S.; Karra, N.; Nemirovski, A.; Schwob, O.; Talmon, Y.; Nassar, T.; Benita, S. Oral delivery system prolongs blood circulation of docetaxel nanocapsules via lymphatic absorption. Proc. Natl. Acad. Sci. USA 2013, 110, 17498–17503. [Google Scholar] [CrossRef]
- Hickey, J.W.; Santos, J.L.; Williford, J.M.; Mao, H.Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control. Rel. 2015, 219, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Karabasz, A.; Bzowska, M.; Szczepanowicz, K. Biomedical applications of multifunctional polymeric nanocarriers: A review of current literature. Int. J. Nanomed. 2020, 15, 8673–8696. [Google Scholar] [CrossRef] [PubMed]
- Branquinho, R.T.; Mosqueira, V.C.F.; Oliveira-Silva, J.C.V.; Simões-Silva, M.R.; Lana, M. Sesquiterpene lactone in nanostructured parenteral dosage form is efficacious in experimental Chagas disease. Antimicrob. Agents Chemoter. 2014, 58, 2067–2075. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mello, C.G.C.; Branquinho, R.T.; Oliveira, M.T.; Milagre, M.M.; Mosqueira, V.C.F.; Lana, M. Efficacy of lychnopholide polymeric nanocapsules after oral and intravenous administration in murine experimental Chagas disease. Antimicrob. Agents Chemoter. 2016, 60, 5215–5222. [Google Scholar] [CrossRef] [PubMed]
- Branquinho, R.T.; Roy, J.; Farah, C.; Garcia, G.M.; Aimond, F.; Le Guennec, J.Y.; Saude-Guimaraes, D.A.; Grabe-Guimaraes, A.; Mosqueira, V.C.; Lana, M.; et al. Biodegradable polymeric nanocapsules prevent cardiotoxicity of anti-trypanosomal lychnopholide. Sci. Rep. 2017, 7, 44998. [Google Scholar] [CrossRef]
- Branquinho, R.T.; Mello, C.G.C.; Oliveira, M.T.; Reis, L.E.S.; Vieira, P.M.A.; Mosqueira, V.C.F.; Lana, M. Lychnopholide in poly (d, l-Lactide)-block-polyethylene glycol nanocapsules cures infection with a drug-resistant Trypanosoma cruzi strain at acute and chronic phases. Antimicrob. Agents Chemoter. 2020, 64, e01937-19. [Google Scholar] [CrossRef]
- Souza, A.C.M.; Mosqueira, V.C.F.; Silveira, A.P.A.; Antunes, L.R.; Richard, S.; Guimarães, H.N.; Grabe-Guimarães, A. Reduced cardiotoxicity and increased oral efficacy of artemether polymeric nanocapsules in Plasmodium berghei-infected mice. Parasitology 2018, 145, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Pound-Lana, G.; Rabanel, J.M.; Hildgen, P.; Mosqueira, V.C.F. Functional polylactide via ring-opening copolymerisation with allyl, benzyl and propargyl glycidyl ethers. Eur. Polym. J. 2017, 90, 344–353. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Galia, E.; Nicolaides, E.; Hörter, D.; Löbenberg, R.; Reppas, C.; Dressman, J.B. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm. Res. 1998, 15, 698–705. [Google Scholar] [CrossRef]
- Reis, L.E.S.; de Brito, R.C.F.; de Oliveira Cardoso, J.M.; Mathias, F.A.S.; Soares, R.D.O.A.; Carneiro, C.M.; Vieira, P.M.A.; Ramos, G.S.; Frézard, F.J.G.; Roatt, B.M.; et al. Mixed formulation of conventional and pegylated meglumine antimoniate-containing liposomes reduces inflammatory process and parasite burden in Leishmania infantum-infected BALB/c mice. Antimicrob. Agents Chemoter. 2017, 61, e00962-17. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.D.; Vitoriano-Souza, J.; Roatt, B.M.; Vieira, P.M.A.; Ker, H.G.; Cardoso, J.M.O.; Giunchetti, R.C.; Carneiro, C.M.; Lana, M.; Reis, A.B. Parasite burden in hamsters infected with two different strains of Leishmania (Leishmania) infantum: “Leishman Donovan units” versus real-time PCR. PLoS ONE 2012, 7, e47907. [Google Scholar] [CrossRef]
- Cummings, K.L.; Tarleton, R.L.J.M. Rapid quantitation of Trypanosoma cruzi in host tissue by real-time PCR. Mol. Biochem. Parasitol. 2003, 129, 53–59. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Pound-Lana, G.; Capelari-Oliveira, P.; Pontífice, T.G.; Silva, S.E.D.; Machado, M.G.C.; Postacchini, B.B.; Mosqueira, V.C.F. Release, transfer and partition of fluorescent dyes from polymeric nanocarriers to serum proteins monitored by asymmetric flow field-flow fractionation. J. Cromatogr. A 2021, 1641, 461959. [Google Scholar] [CrossRef]
- DrugBank. Available online: https://go.drugbank.com/drugs/DB00675 (accessed on 5 April 2021).
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tamoxifen#section=DSSTox-Substance-ID (accessed on 4 April 2021).
- Saini, S.; Rai, A.K. Hamster, a close model for visceral leishmaniasis: Opportunities and challenges. Paras. Immunol. 2020, 42, e12768. [Google Scholar] [CrossRef]
- Natera, S.; Machuca, C.; Padrón-Nieves, M.; Romero, A.; Díaz, E.; Ponte-Sucre, A. Leishmania spp.: Proficiency of drug-resistant parasites. Int. J. Antimicrob. Agents 2007, 29, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Carrión, J.; Folgueira, C.; Alonso, C. Development of immunization strategies against leishmaniosis based on the Leishmania histones pathoantigens. J. Pro. Vac. 2009, 1, 101–103. [Google Scholar] [CrossRef]
- Mazzeti, A.L.; Capelari-Oliveira, P.; Bahia, M.T.; Mosqueira, V.C.F. Review on experimental treatment strategies against Trypanosoma cruzi. J. Exp. Pharm. 2021, 13, 409–432. [Google Scholar] [CrossRef] [PubMed]
- Maji, R.; Dey, N.S.; Satapathy, B.S.; Mukherjee, B.; Mondal, S. Preparation and characterization of Tamoxifen citrate loaded nanoparticles for breast cancer therapy. Int. J. Nanomedicine 2014, 9, 3107–3118. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sun, L.; Tan, L.; Wang, M.; Ren, X.; Pi, J.; Jiang, M.; Li, N. Preparation and characterization of PLGA–PEG–PLGA nanoparticles containing salidroside and tamoxifen for breast cancer therapy. AAPS Pharm. Sci. Tech. 2020, 21, 85. [Google Scholar] [CrossRef] [PubMed]
- Chawla, J.S.; Amiji, M.M. Cellular uptake and concentrations of tamoxifen upon administration in poly (ε-caprolactone) nanoparticles. AAPS Pharm. Sci. 2003, 5, 28–34. [Google Scholar] [CrossRef]
- Ravikumara, N.; Bharadwaj, M.; Madhusudhan, B. Tamoxifen citrate-loaded poly (d, l) lactic acid nanoparticles: Evaluation for their anticancer activity in vitro and in vivo. J. Biomater. Appl. 2016, 31, 755–772. [Google Scholar] [CrossRef]
- Mosqueira, V.C.F.; Legrand, P.; Pinto-Alphandary, H.; Puisieux, F.; Barratt, G. Poly (D, L-lactide) nanocapsules prepared by a solvent displacement process: Influence of the composition on physicochemical and structural properties. J. Pharm. Sci. 2000, 89, 614–626. [Google Scholar] [CrossRef]
- Mosqueira, V.C.F.; Legrand, P.; Morgat, J.L.; Vert, M.; Mysiakine, E.; Gref, R.; Devissaguet, J.P.; Barratt, G. Biodistribution of long-circulating PEG-grafted nanocapsules in mice: Effects of PEG chain length and density. Pharm. Res. 2001, 18, 1411–1419. [Google Scholar] [CrossRef]
- Mosqueira, V.C.F.; Legrand, P.; Gulik, A.; Bourdon, O.; Gref, R.; Labarre, D.; Barratt, G. Relationship between complement activation, cellular uptake and surface physicochemical aspects of novel PEG-modified nanocapsules. Biomaterials 2001, 22, 2967–2979. [Google Scholar] [CrossRef]
- Mosqueira, V.C.F.; Legrand, P.; Barratt, G. Surface-modified and conventional nanocapsules as novel formulations for parenteral delivery of halofantrine. J. Nanosci. Nanotechnol. 2006, 6, 3193–3202. [Google Scholar] [CrossRef] [PubMed]
- Jena, S.K.; Singh, C.; Dora, C.P.; Suresh, S. Development of tamoxifen-phospholipid complex: Novel approach for improving solubility and bioavailability. Int. J. Pharm. 2014, 473, 1–9. [Google Scholar] [CrossRef]
- Oliveira, L.T.; de Paula, M.A.; Roatt, B.M.; Garcia, G.M.; Silva, L.S.B.; Reis, A.B.; de Paula, C.S.; Vilela, J.M.C.; Andrade, M.S.; Pound-Lana, G. Impact of dose and surface features on plasmatic and liver concentrations of biodegradable polymeric nanocapsules. Eur. J. Pharm. Sci. 2017, 105, 19–32. [Google Scholar] [CrossRef]
- Machado, P.R.; Ribeiro, C.S.; França-Costa, J.; Dourado, M.E.; Trinconi, C.T.; Yokoyama-Yasunaka, J.K.; Malta-Santos, H.; Borges, V.M.; Carvalho, E.M.; Uliana, S.R. Tamoxifen and meglumine antimoniate combined therapy in cutaneous leishmaniasis patients: A randomised trial. Trop. Med. Int. Health 2018, 23, 936–942. [Google Scholar] [CrossRef]
- Bekele, R.T.; Venkatraman, G.; Liu, R.-Z.; Tang, X.; Mi, S.; Benesch, M.G.; Mackey, J.R.; Godbout, R.; Curtis, J.M.; McMullen, T.P. Oxidative stress contributes to the tamoxifen-induced killing of breast cancer cells: Implications for tamoxifen therapy and resistance. Sci. Rep. 2016, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Caffa, I.; Spagnolo, V.; Vernieri, C.; Valdemarin, F.; Becherini, P.; Wei, M.; Brandhorst, S.; Zucal, C.; Driehuis, E.; Ferrando, L. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 2020, 583, 620–624. [Google Scholar] [CrossRef]
- Eissa, M.M.; Amer, E.I.; El Sawy, S.M. Leishmania major: Activity of tamoxifen against experimental cutaneous leishmaniasis. Exp. Parasitol. 2011, 128, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.D.; Vitoriano-Souza, J.; Roatt, B.M.; Vieira, P.M.A.; Coura-Vital, W.; Cardoso, J.M.O.; Rezende, M.T.; Ker, H.G.; Giunchetti, R.C.; Carneiro, C.M.; et al. Clinical, hematological and biochemical alterations in hamster (Mesocricetus auratus) experimentally infected with Leishmania infantum through different routes of inoculation. Parasit. Vectors 2016, 9, 1–13. [Google Scholar] [CrossRef]
- Espuelas, S. Conventional formulations and emerging delivery systems for the topical treatment of cutaneous leishmaniasis. Ther. Deliv. 2015, 6, 101–103. [Google Scholar] [CrossRef]
| Formulation | TMX (mg/mL) | Ethanol/Acetone Ratio (v/v) | Oil Phase (mL) | Aqueous Phase (mL) | Oil Phase/Aqueous Phase Ratio (v/v) | Z-Average Dh ± SD | PdI ± SD |
|---|---|---|---|---|---|---|---|
| F1 | 0.0 | 1:1 | 2 | 4 | 1:2 | 174.5 ± 11 | 0.157 ± 0.09 |
| F2 | 2.5 | 1:1 | 2 | 4 | 1:2 | 215.4 ± 13 | 0.231 ± 0.10 |
| F3 | 5.0 | 1:1 | 2 | 4 | 1:2 | 235.5 ± 9 | 0.262 ± 0.09 |
| F4 | 0.0 | 2:2 | 4 | 8 | 1:2 | 136.9 ± 7 | 0.145 ± 0.05 |
| F5 | 2.5 | 2:2 | 4 | 8 | 1:2 | 185.7 ± 4 | 0.118 ± 0.08 |
| F6 | 5.0 | 2:2 | 4 | 8 | 1:2 | 191.5 ± 5 | 0.131 ± 0.09 |
| F7 | 0.0 | 3:1 | 4 | 8 | 1:2 | 154.7 ± 8 | 0.156 ± 0.05 |
| F8 | 2.5 | 3:1 | 4 | 8 | 1:2 | 203.1 ± 11 | 0.242 ± 0.06 |
| F9 | 5.0 | 3:1 | 4 | 8 | 1:2 | 215.1 ± 12 | 0.261 ± 0.12 |
| F10 | 0.0 | 3:1 | 4 | 16 | 1:4 | 121.7 ± 7 | 0.171 ± 0.08 |
| F11 | 2.5 | 3:1 | 4 | 16 | 1:4 | 356.6 ± 14 1 | 0.392 ± 0.09 1 |
| F12 | 5.0 | 3:1 | 4 | 16 | 1:4 | 405.8 ± 12 2 | 0.42 ± 0.13 2 |
| TMX (mg/mL) | Z-Average Dh ± SD | PdI ± SD | Geometric Diameter a (nm) (AFM) | pH ± SD | Loading Yield (%) ± SD | Encapsulation Efficiency (%) ± SD | TMX Payload (µg/mg) |
|---|---|---|---|---|---|---|---|
| 0.0 | 136.9 ± 10 | 0.157 ± 0.07 | 136.5 ± 7.5 | 6.10 ± 0.05 | - | - | - |
| 0.5 | 152.9 ± 11 | 0.163 ± 0.05 | ND | 5.82 ± 0.05 | 98.19 ± 0.07 | 96.48 ± 0.05 | 11,11 |
| 1.5 | 163.9 ± 8.5 1 | 0.198 ± 0.03 | ND | 5.53 ± 0.08 | 98.54 ± 0.07 | 96.24 ± 0.07 | 32.61 |
| 2.5 | 185.7 ± 6 1 | 0.103 ± 0.05 | ND | 5.14 ± 0.03 1 | 98.85 ± 0.09 | 96.84 ± 0.06 | 53.19 |
| 5.0 | 191.5 ± 7 1 | 0.128 ± 0.06 | 180.17 ± 5.7 1 | 4.77 ± 0.02 1 | 98.68 ± 0.11 | 93.90 ± 0.14 | 101.01 |
| 7.5 | 255.8 ± 9 1 | 0.261 ± 0.04 1 | ND | 4.25 ± 0.06 1 | 95.21 ± 0.18 | 90.11 ± 0.19 | 144.23 |
| 10.0 | 306.2 ± 9.5 1 | 0.374 ± 0.8 1 | ND | 3.82 ± 0.09 1 | 93.54 ± 0.09 | 88.45 ± 0.17 1 | 183.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, D.F.; Reis, L.E.S.; Machado, M.G.C.; Dophine, D.D.; de Andrade, V.R.; de Lima, W.G.; Andrade, M.S.; Vilela, J.M.C.; Reis, A.B.; Pound-Lana, G.; et al. Repositioning of Tamoxifen in Surface-Modified Nanocapsules as a Promising Oral Treatment for Visceral Leishmaniasis. Pharmaceutics 2021, 13, 1061. https://doi.org/10.3390/pharmaceutics13071061
Silva DF, Reis LES, Machado MGC, Dophine DD, de Andrade VR, de Lima WG, Andrade MS, Vilela JMC, Reis AB, Pound-Lana G, et al. Repositioning of Tamoxifen in Surface-Modified Nanocapsules as a Promising Oral Treatment for Visceral Leishmaniasis. Pharmaceutics. 2021; 13(7):1061. https://doi.org/10.3390/pharmaceutics13071061
Chicago/Turabian StyleSilva, Débora Faria, Levi Eduardo Soares Reis, Marina Guimarães Carvalho Machado, Douglas Daniel Dophine, Vinicius Roberto de Andrade, Wanderson Geraldo de Lima, Margareth Spangler Andrade, José Mário Carneiro Vilela, Alexandre Barbosa Reis, Gwenaelle Pound-Lana, and et al. 2021. "Repositioning of Tamoxifen in Surface-Modified Nanocapsules as a Promising Oral Treatment for Visceral Leishmaniasis" Pharmaceutics 13, no. 7: 1061. https://doi.org/10.3390/pharmaceutics13071061
APA StyleSilva, D. F., Reis, L. E. S., Machado, M. G. C., Dophine, D. D., de Andrade, V. R., de Lima, W. G., Andrade, M. S., Vilela, J. M. C., Reis, A. B., Pound-Lana, G., Rezende, S. A., & Mosqueira, V. C. F. (2021). Repositioning of Tamoxifen in Surface-Modified Nanocapsules as a Promising Oral Treatment for Visceral Leishmaniasis. Pharmaceutics, 13(7), 1061. https://doi.org/10.3390/pharmaceutics13071061