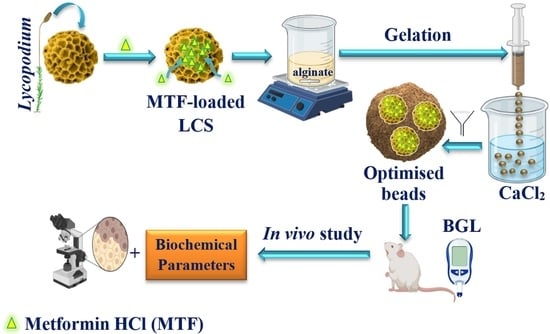
Sustained In Vitro and In Vivo Delivery of Metformin from Plant Pollen-Derived Composite Microcapsules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
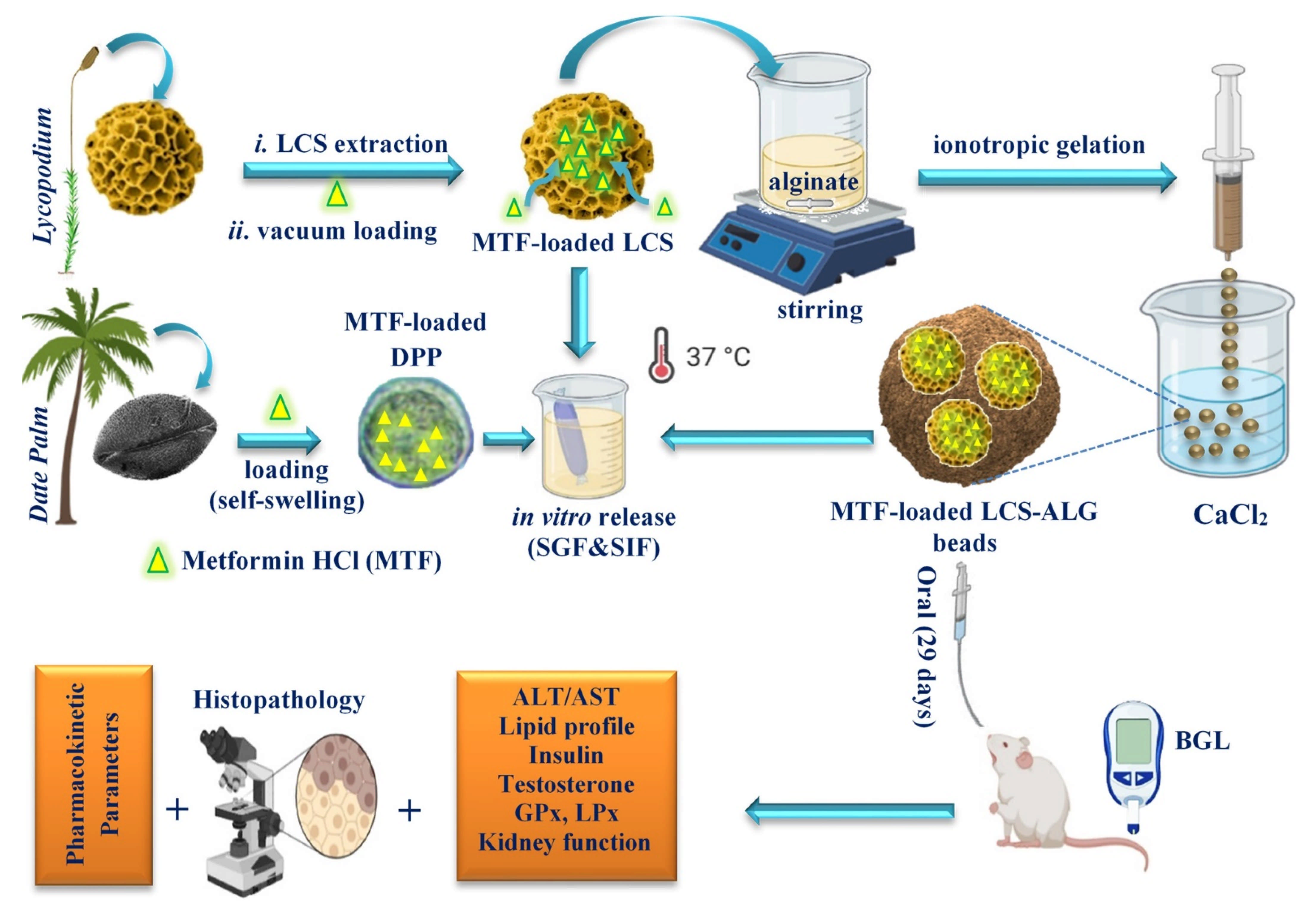
2.2. Encapsulation of MTF into LCS Sporopollenin Microcapsules
2.3. Encapsulation of MTF into Raw DPP Pollens
2.4. Encapsulation of MTF-Loaded LCS into Calcium Alginate (ALG) Beads
2.5. Loading Capacity, Encapsulation Efficiency, and Production Yield
2.6. In Vitro MTF Release from MTF-Loaded Microcapsules and Microbeads
2.7. In Vitro Drug Release Kinetics and Mechanism
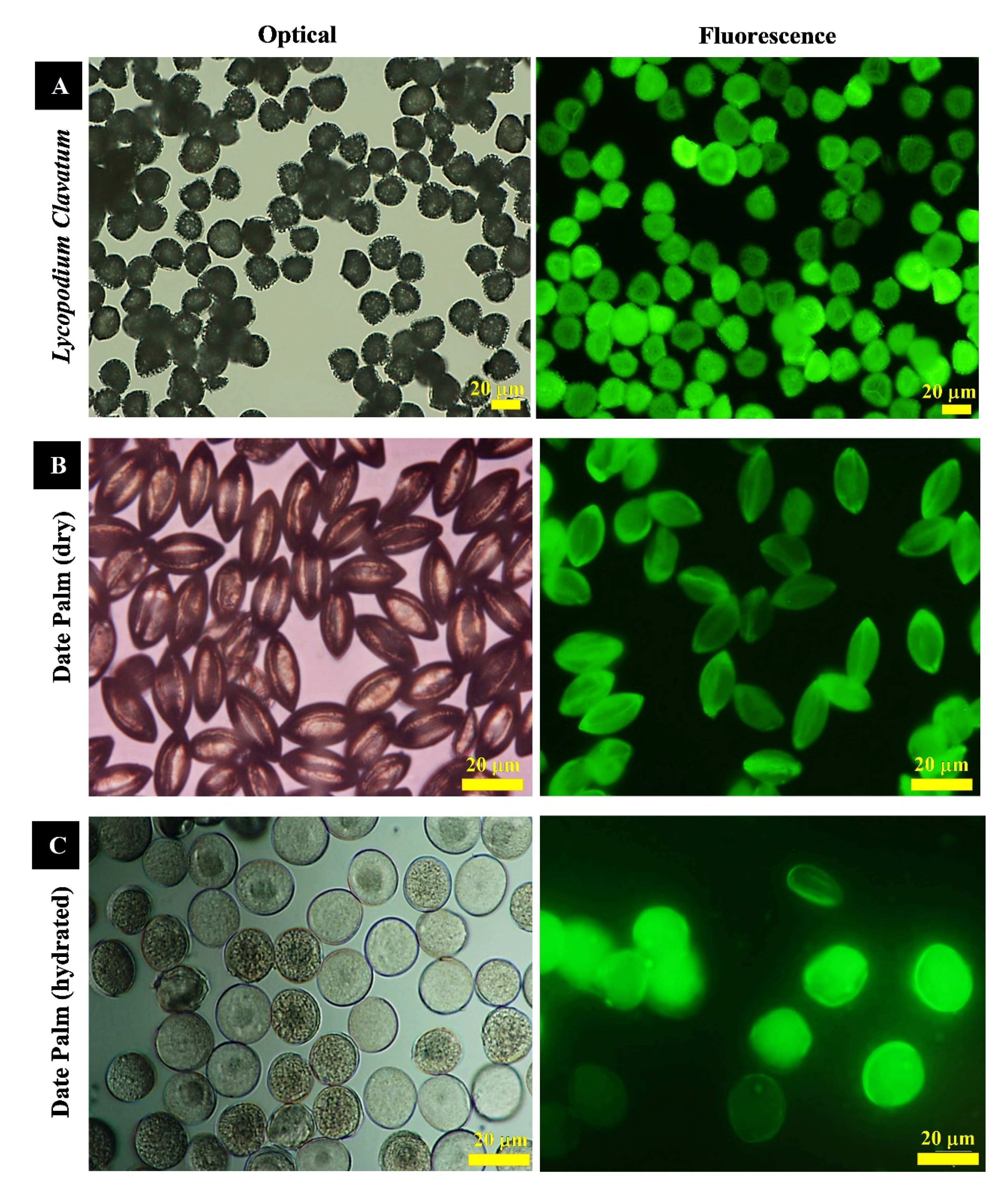
2.8. Optical, Fluorescence Microscopy and Digital Imagining
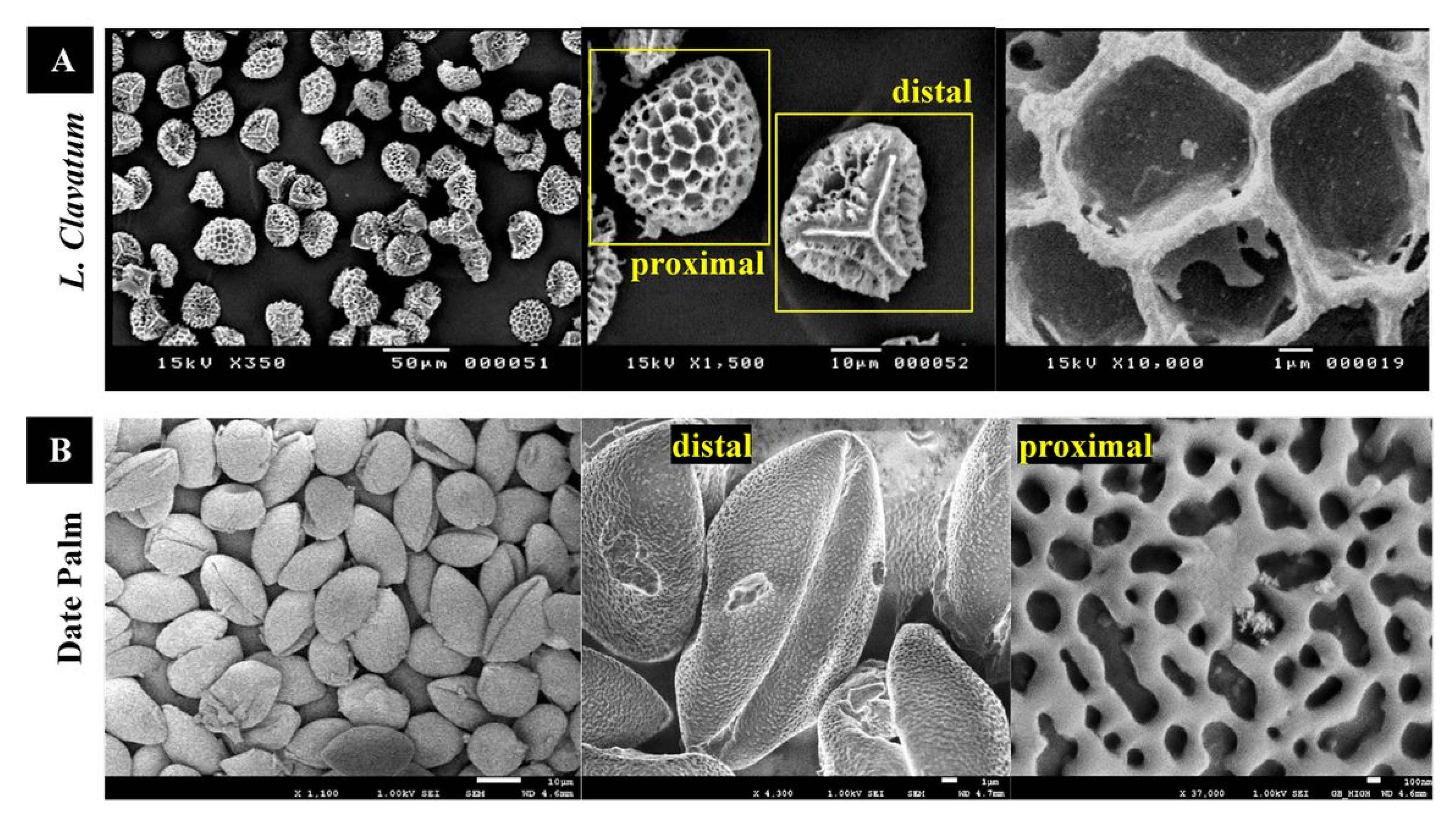
2.9. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
2.10. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
2.11. Thermogravimetric Analysis (TGA)
2.12. In Vivo Studies
2.12.1. Animals
2.12.2. Induction of Type 2 Diabetes Mellitus (T2DM)
2.12.3. Experimental Design
- Group 1: Normal control rats.
- Group 2: Vehicle group treated with LCS-ALG (250 mg/kg/day) dispersed in pure water.
- Group 3: Untreaded diabetic control group (Dia group).
- Group 4: Diabetic group treated with MTF (25 mg/kg/day) [70] dissolved in pure water (Dia + MTF group).
- Group 5: Diabetic group treated with MTF-loaded LCS-ALG beads (250 mg/kg/day), equivalent to 25 mg/kg bw MTF, dispersed in pure water (Dia+MTF-loaded LCS-ALG beads group).
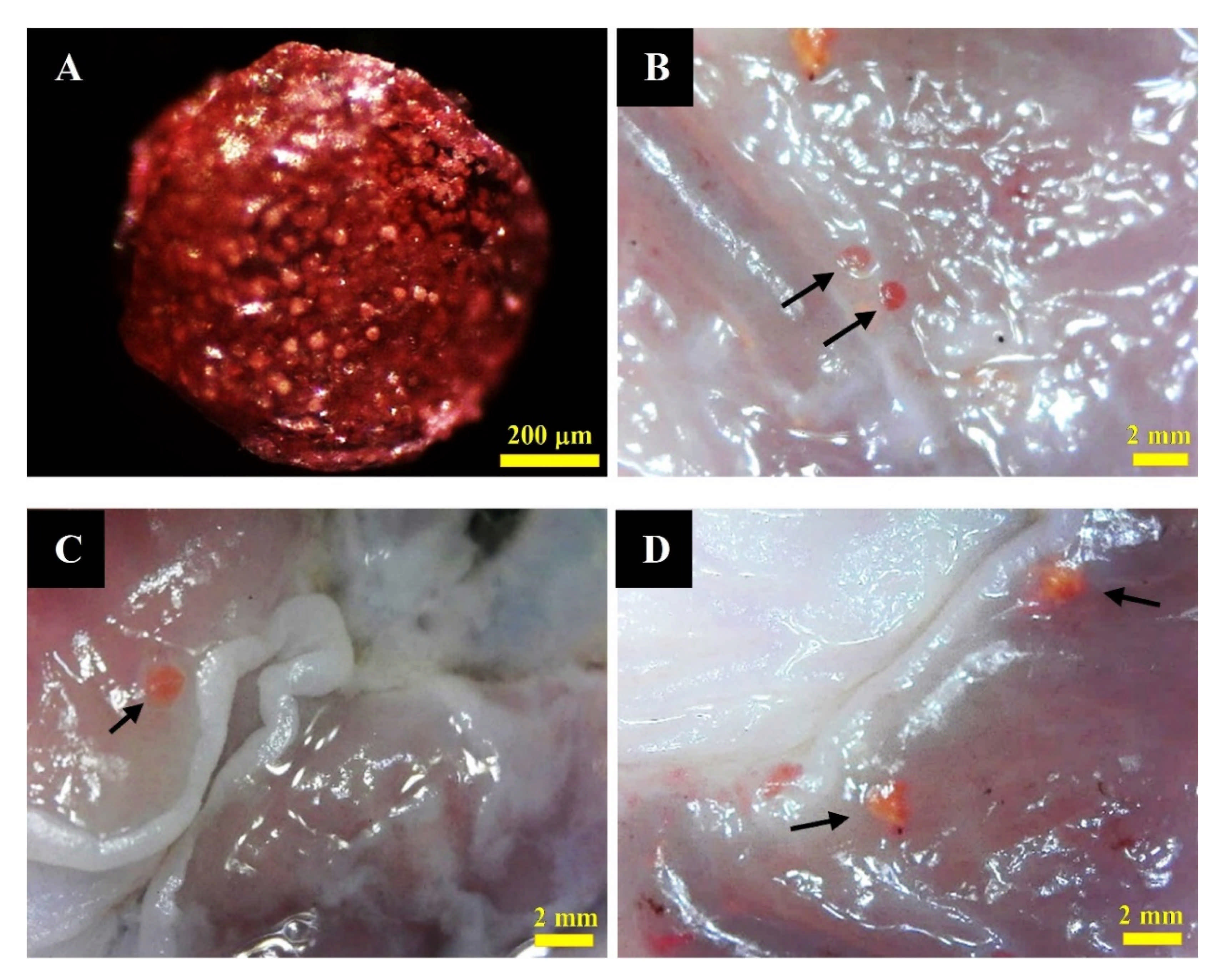
Detection of MTF-Loaded LCS-ALG Beads in Rats’ Stomach
2.12.4. Body Weight
2.12.5. Blood Glucose Level (BGL)
2.12.6. High Performance Liquid Chromatography (HPLC) MTF Assay
2.12.7. Pharmacokinetic Analysis
2.12.8. Blood Sampling
2.12.9. Biochemical Profile Assays
2.12.10. Histological Procedures
2.12.11. Statistical Analyses
3. Results and Discussion
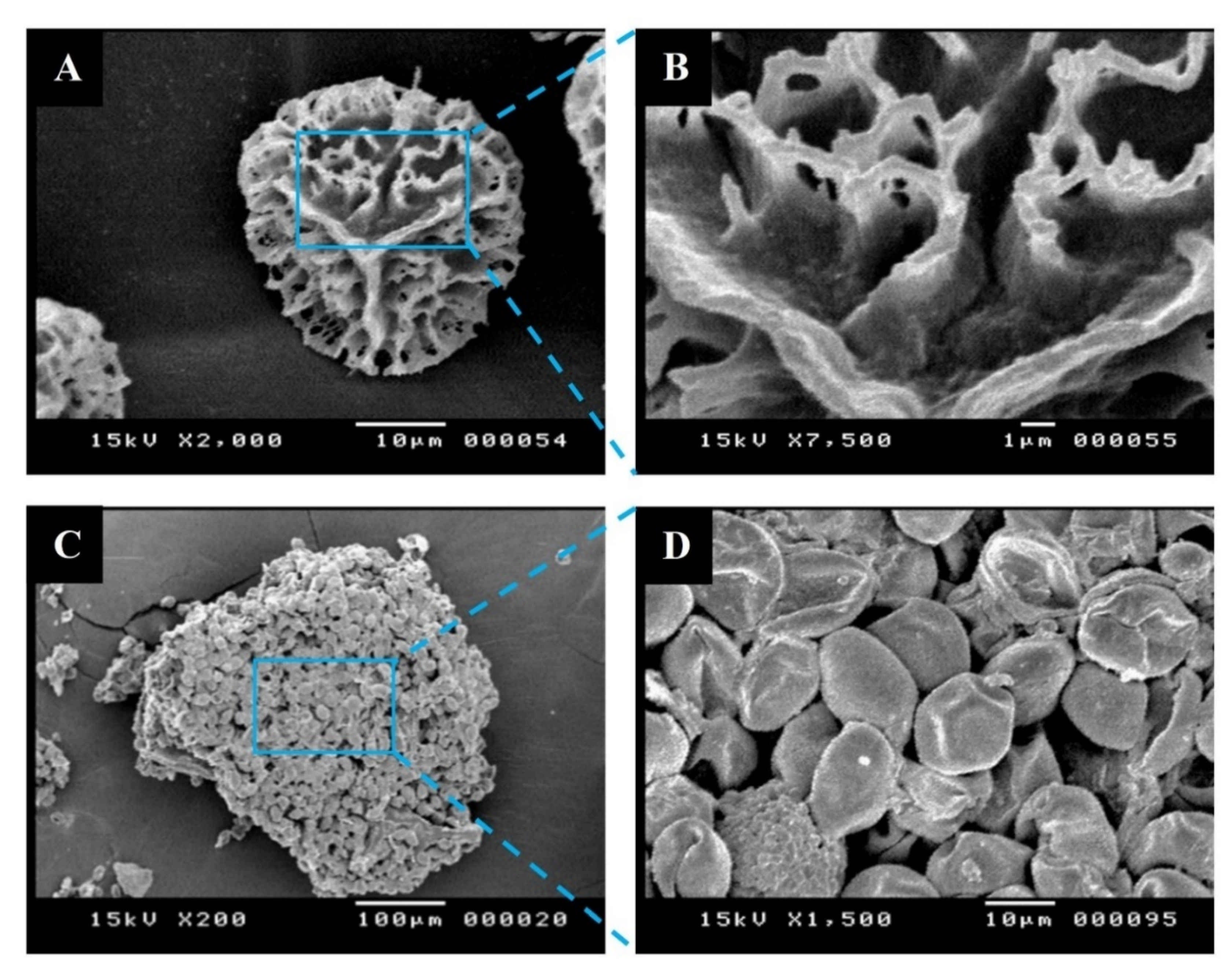
3.1. Characterization of Raw Lycopodium Clavatum and Date Palm Pollens
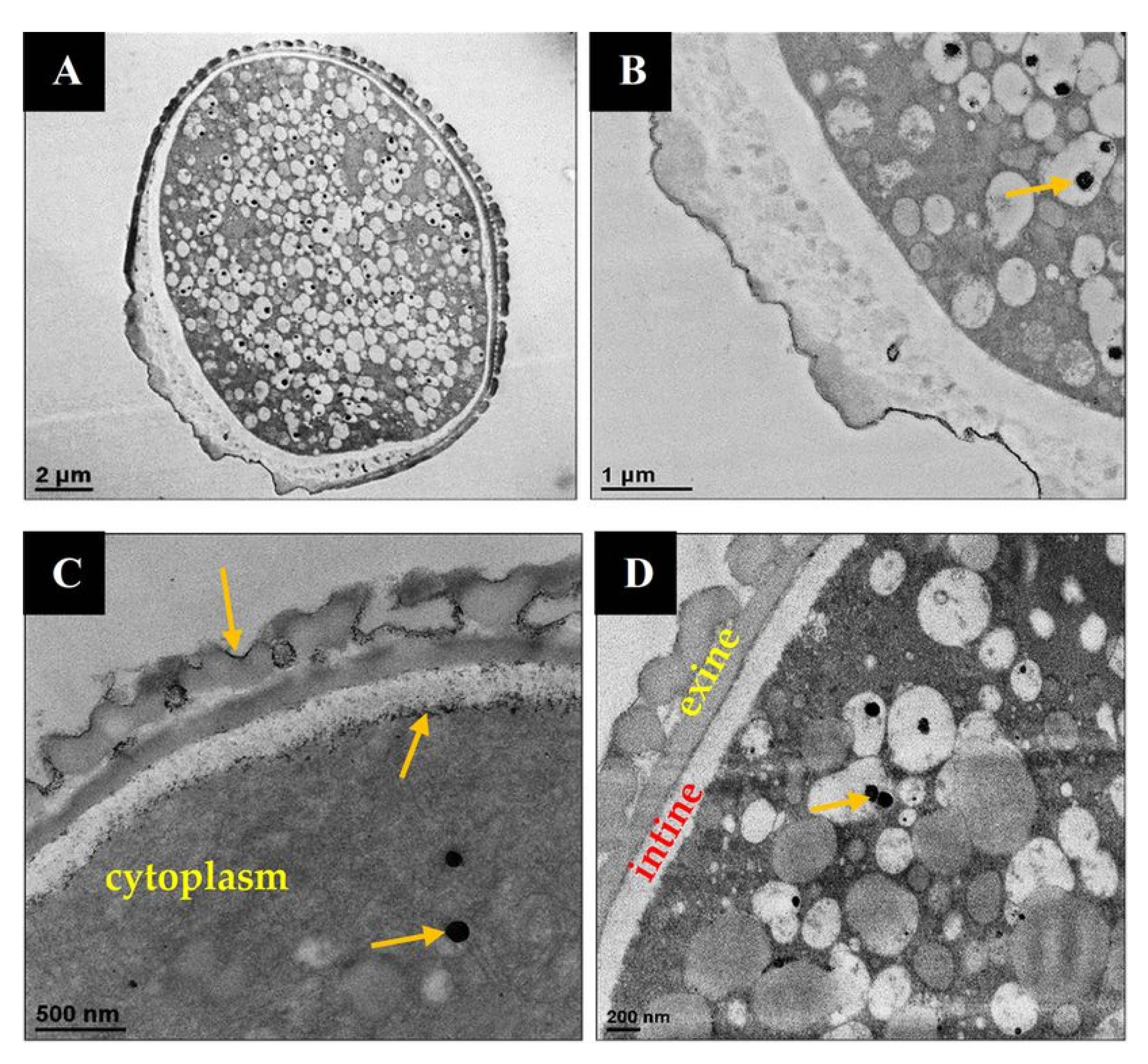
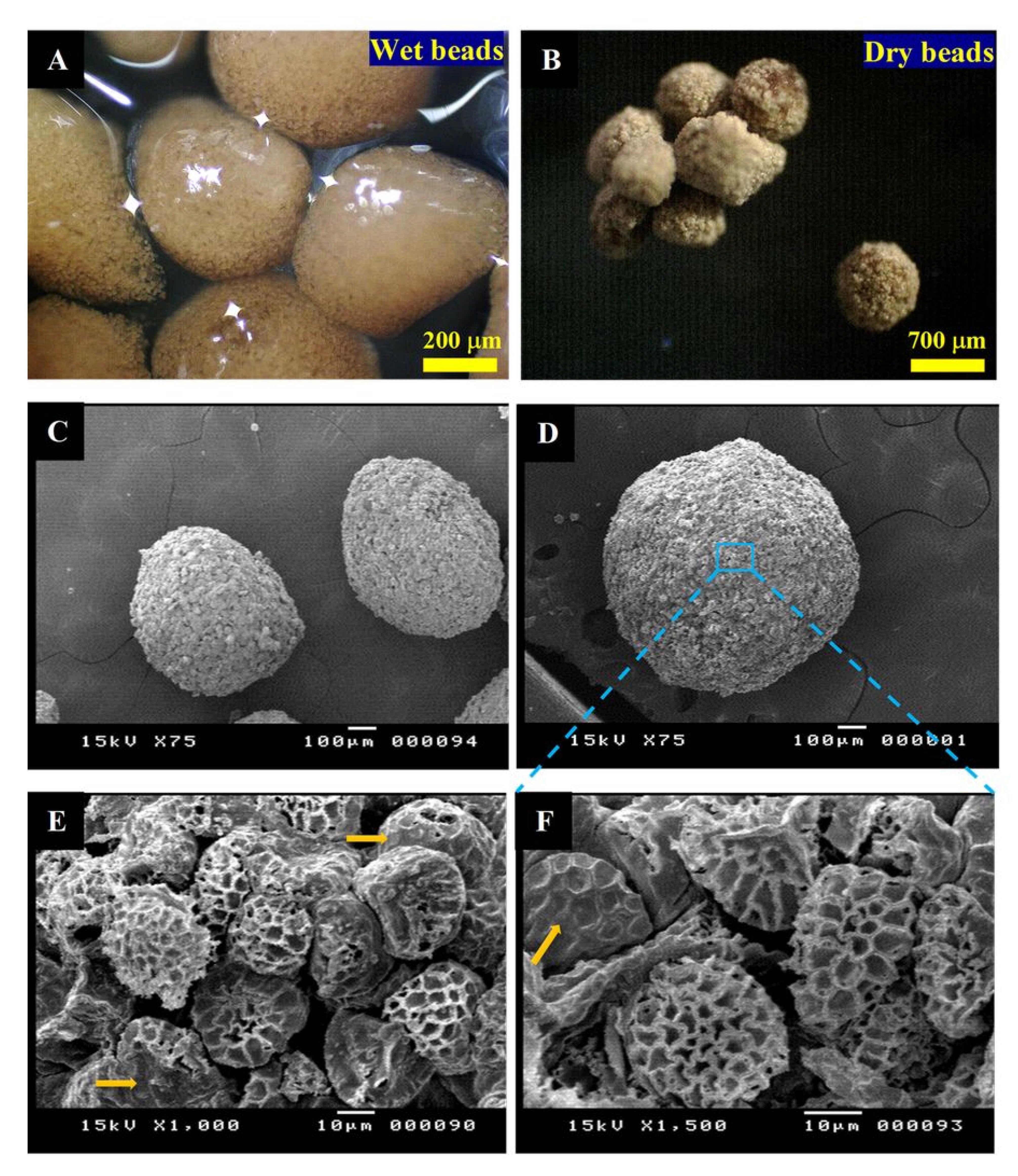
3.2. MTF-Loaded LCS, MTF-Loaded DPP Microcapsules, and MTF-Loaded LCS-ALG Beads
3.3. FTIR and Thermogravimetric Analyses (TGA)
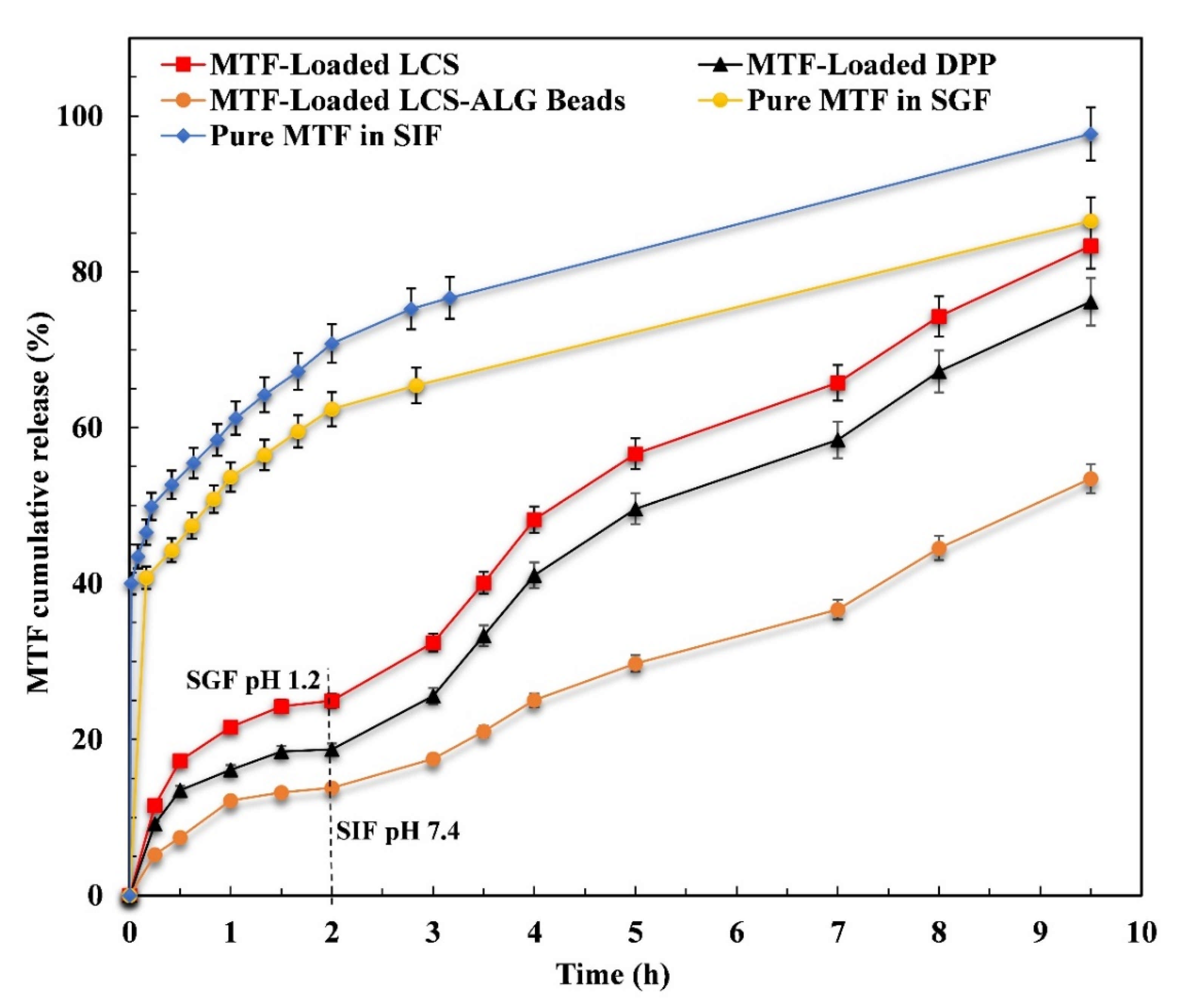
3.4. In Vitro MTF Release Study
3.5. In Vivo Study
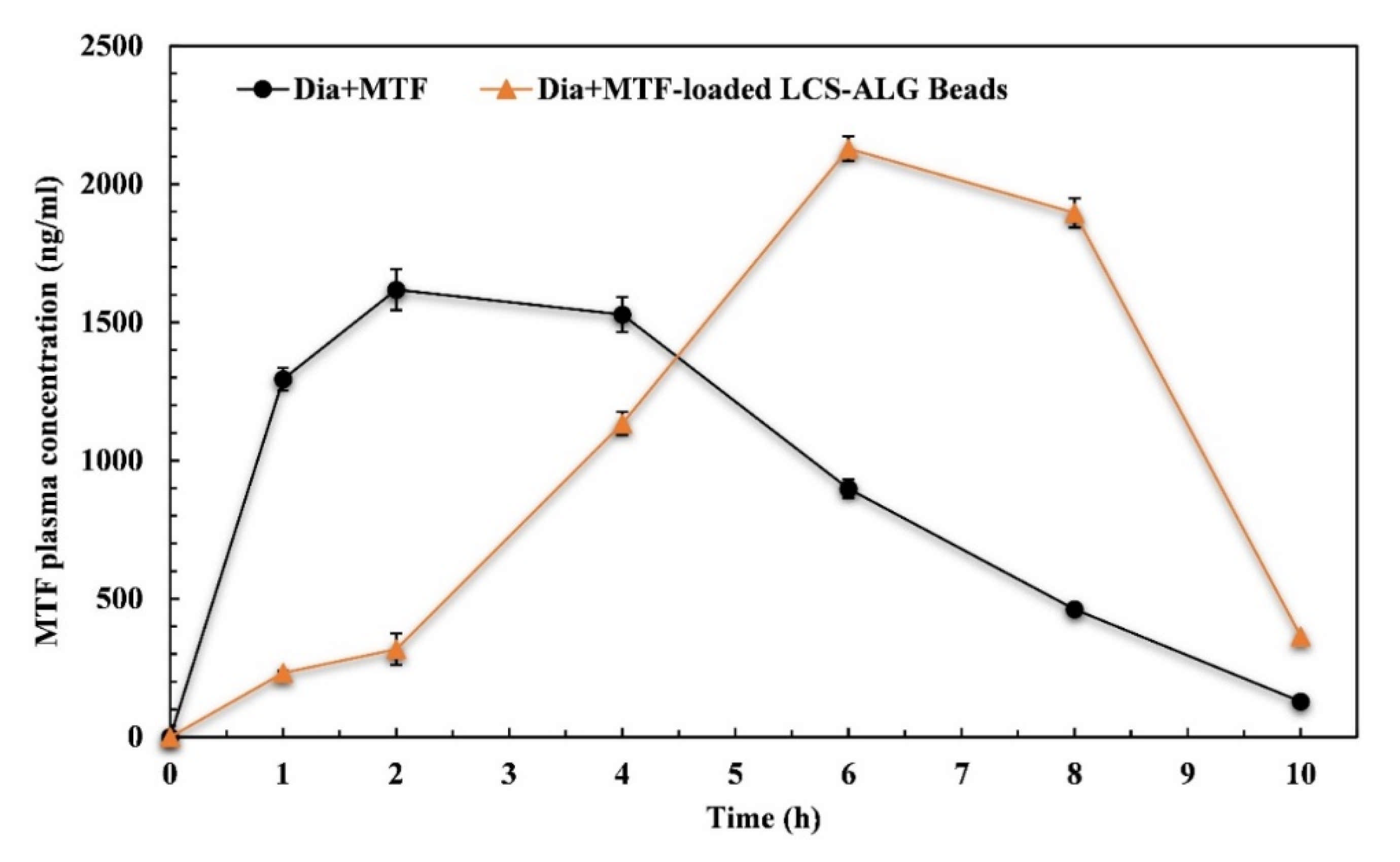
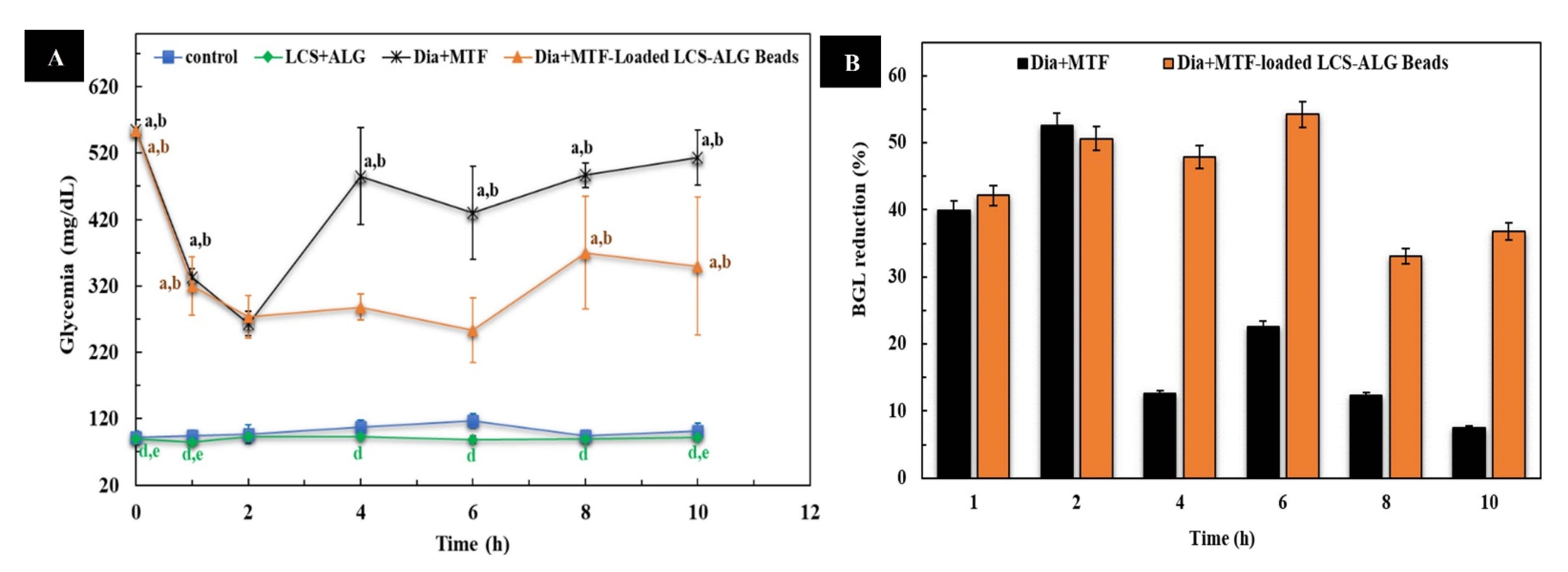
3.5.1. Pharmacokinetics (PK) Evaluation
3.5.2. Detection of MTF-Loaded LCS-ALG Beads in Rats’ Stomach
3.5.3. Changes in Body Weight
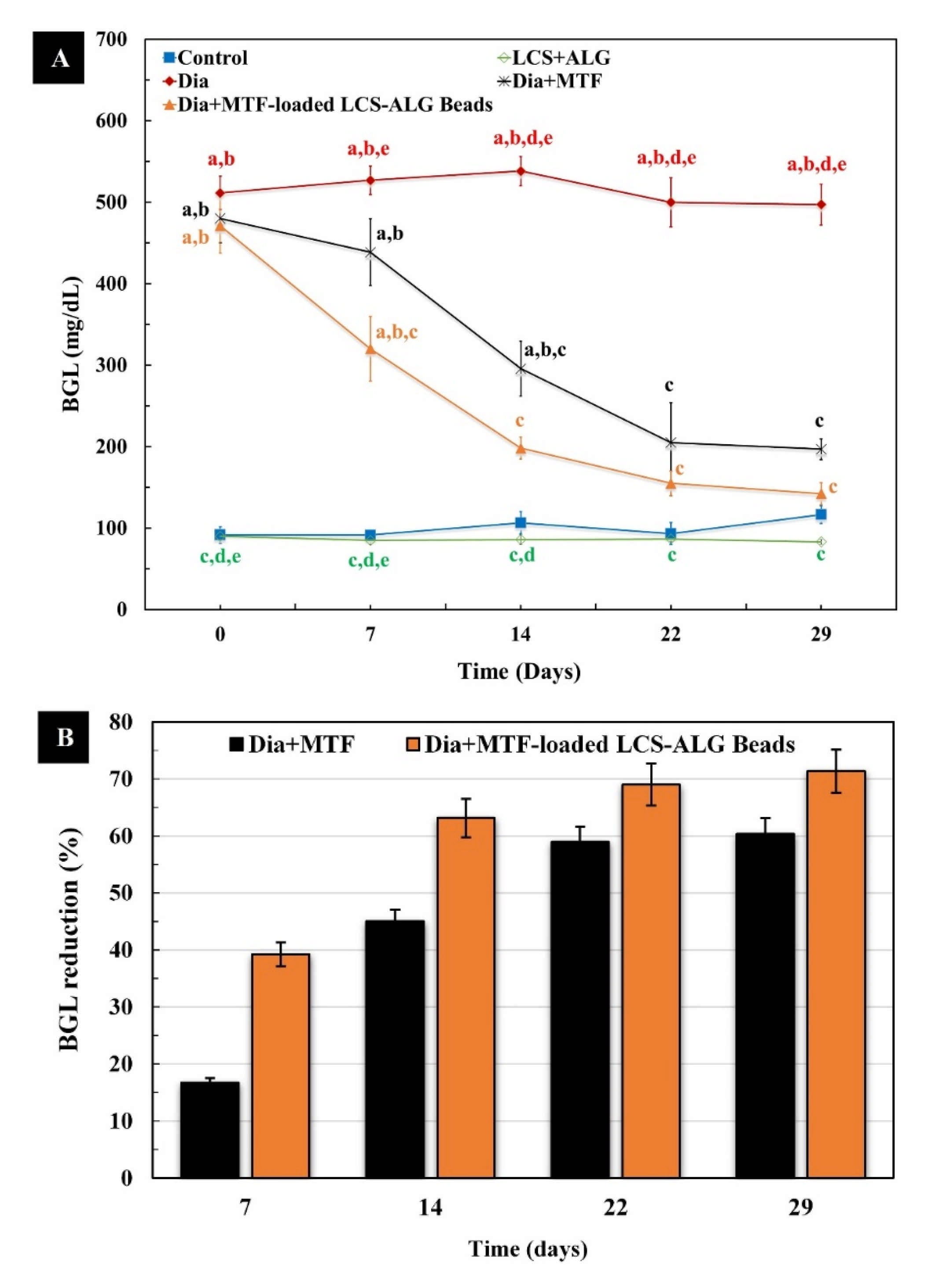
3.5.4. Blood Glucose Level (BGL)
3.5.5. Biochemical Studies
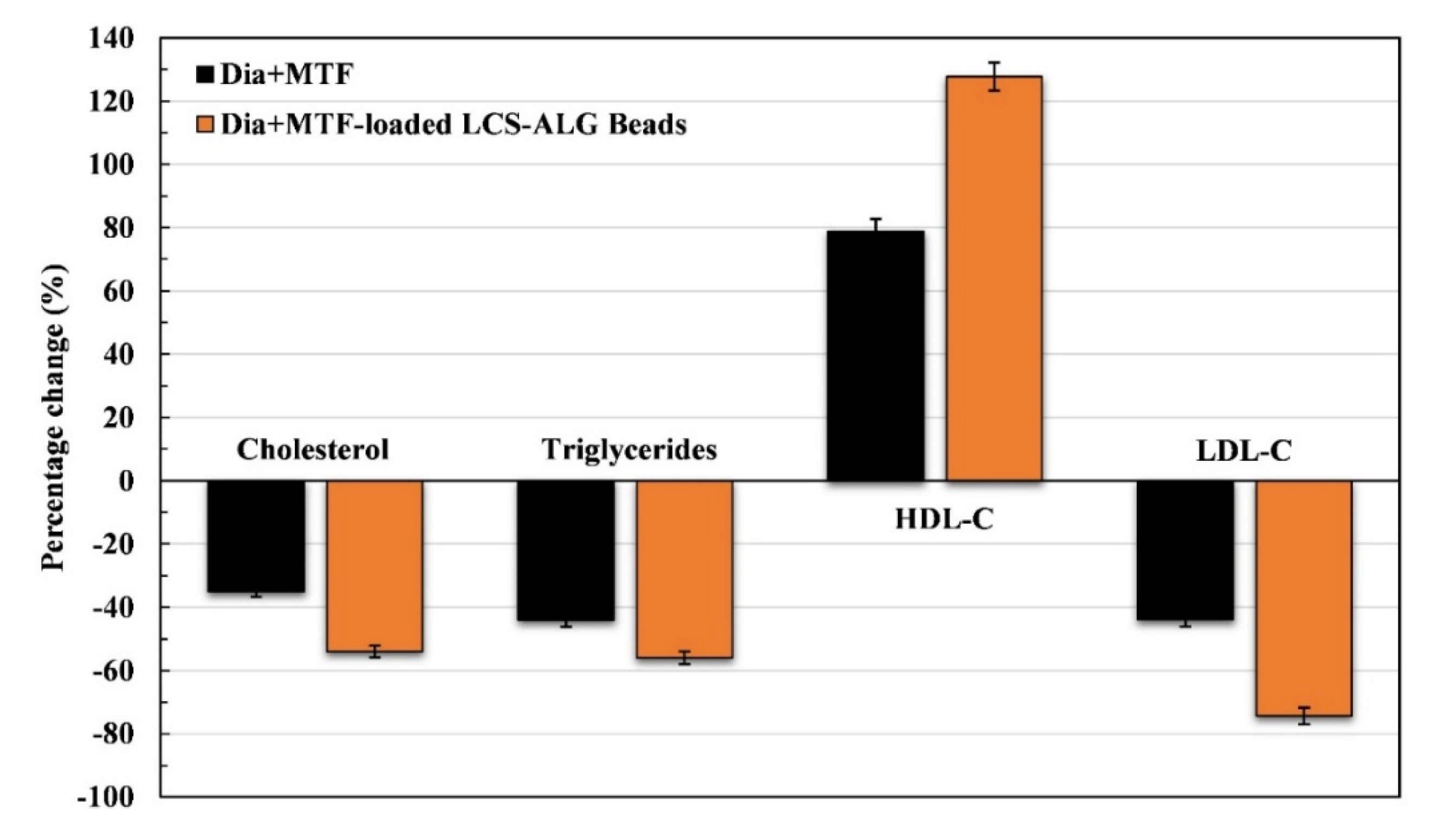
Lipid Profile
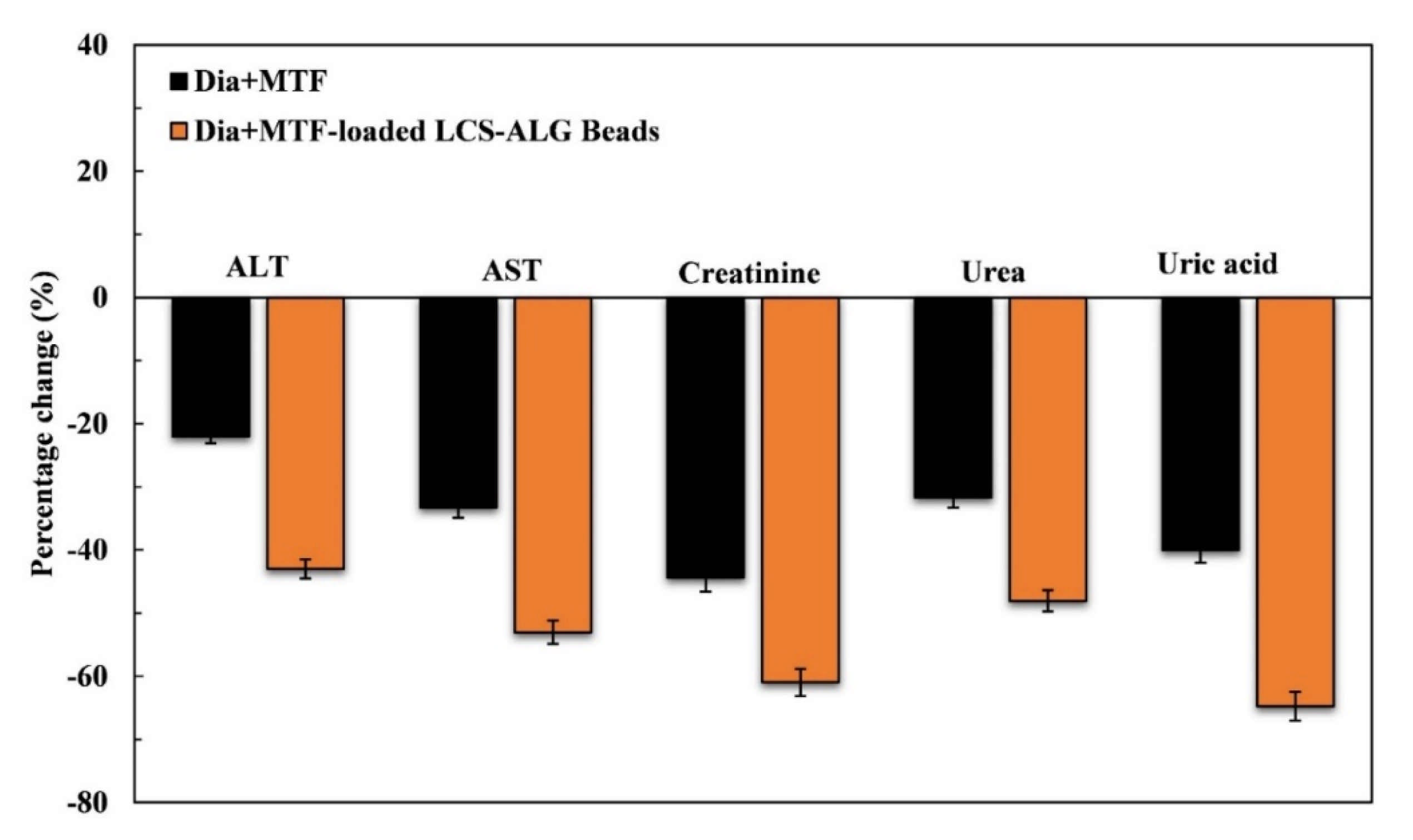
Liver Enzymes (ALT and AST)
Kidney Function
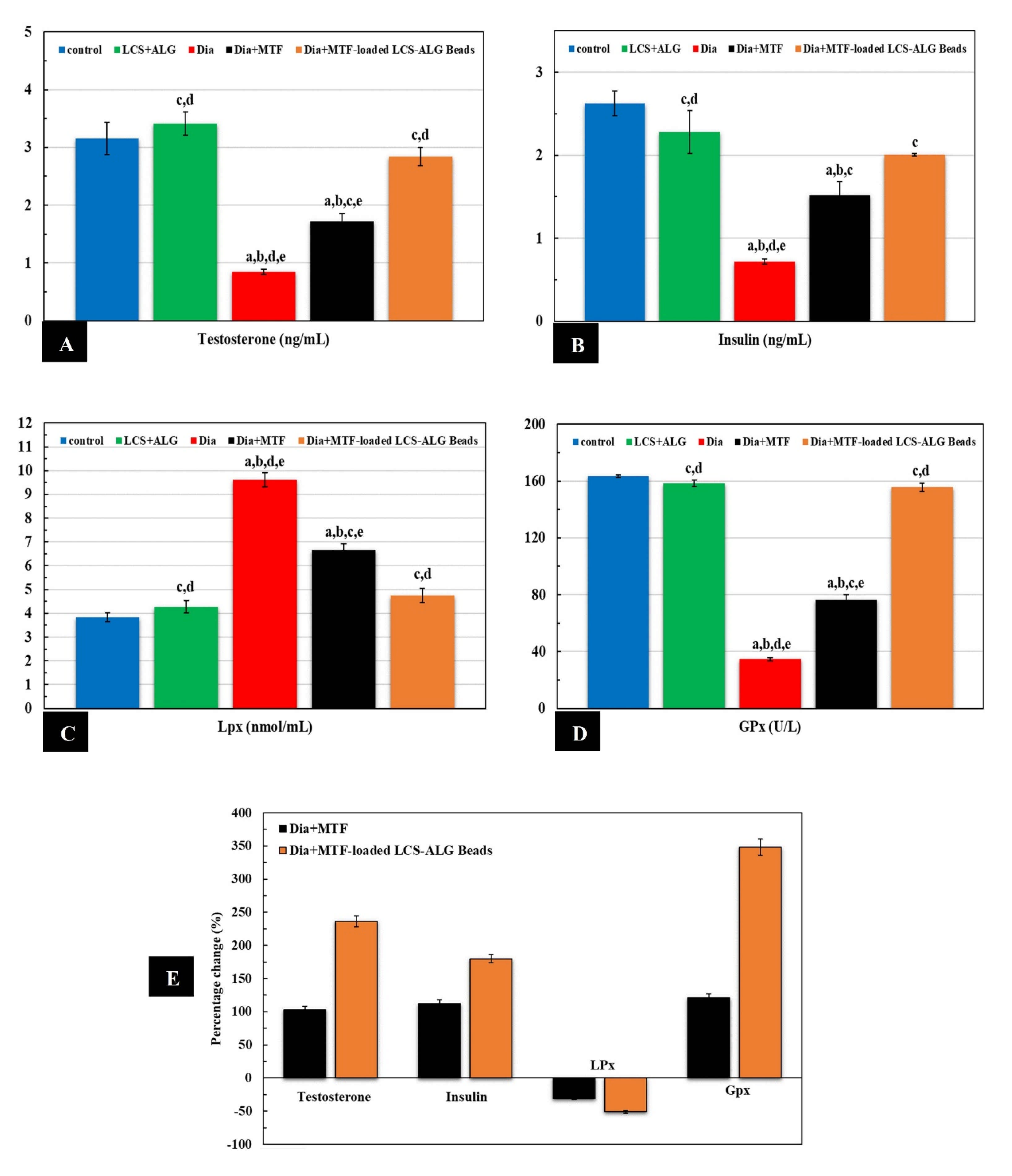
Testosterone Level
Insulin Level
Oxidative Stress
3.5.6. Histological Studies
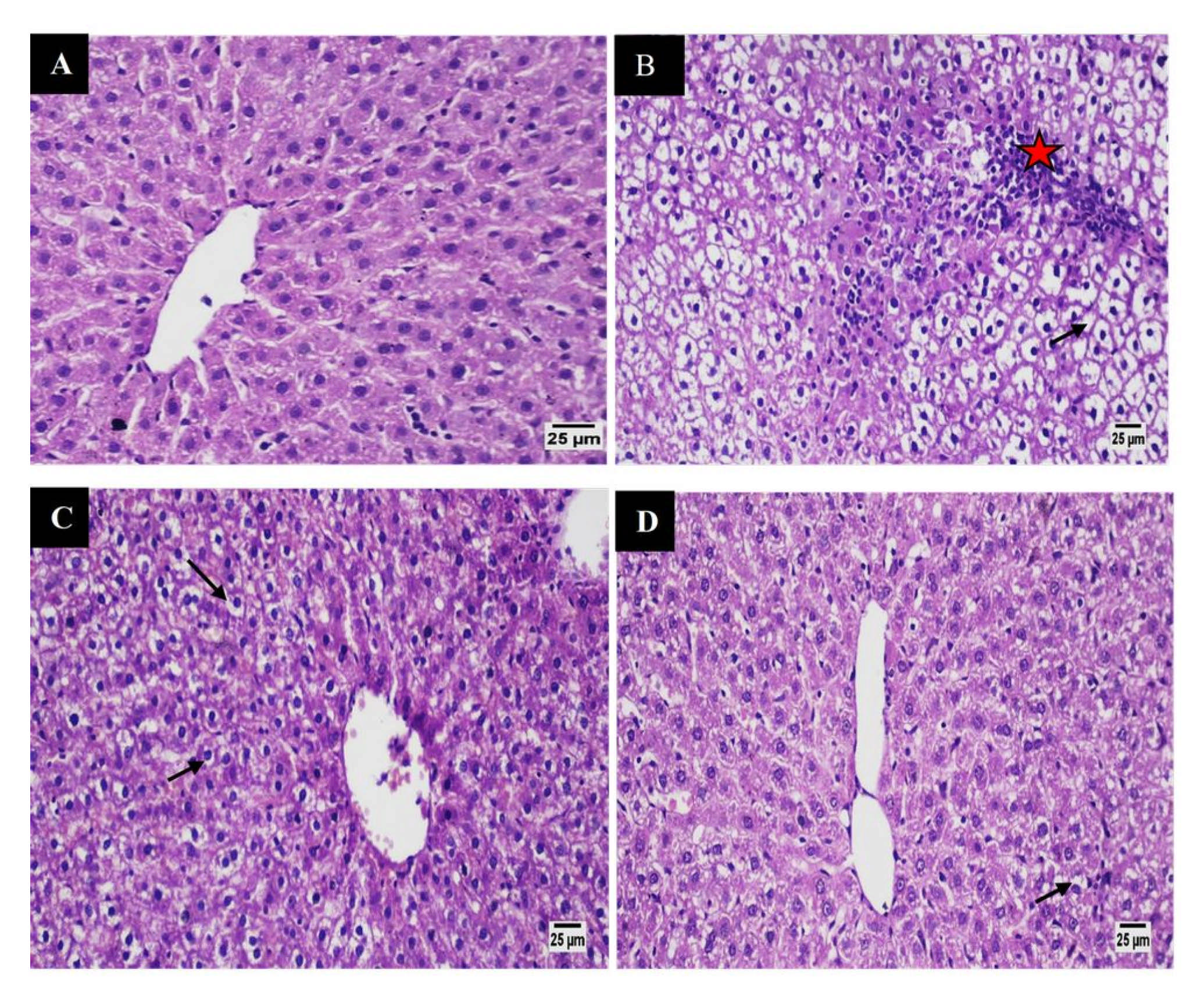
Liver
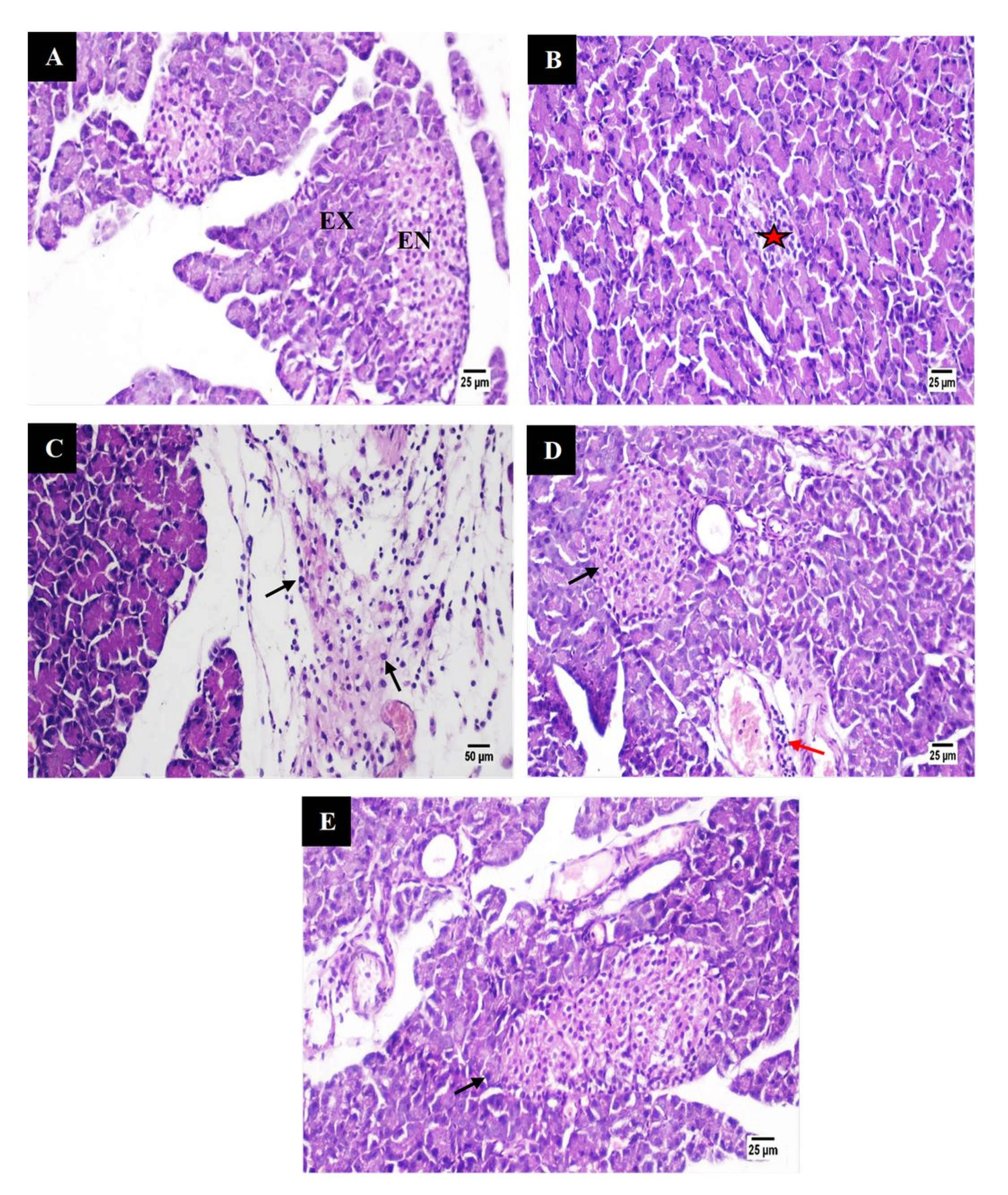
Pancreas
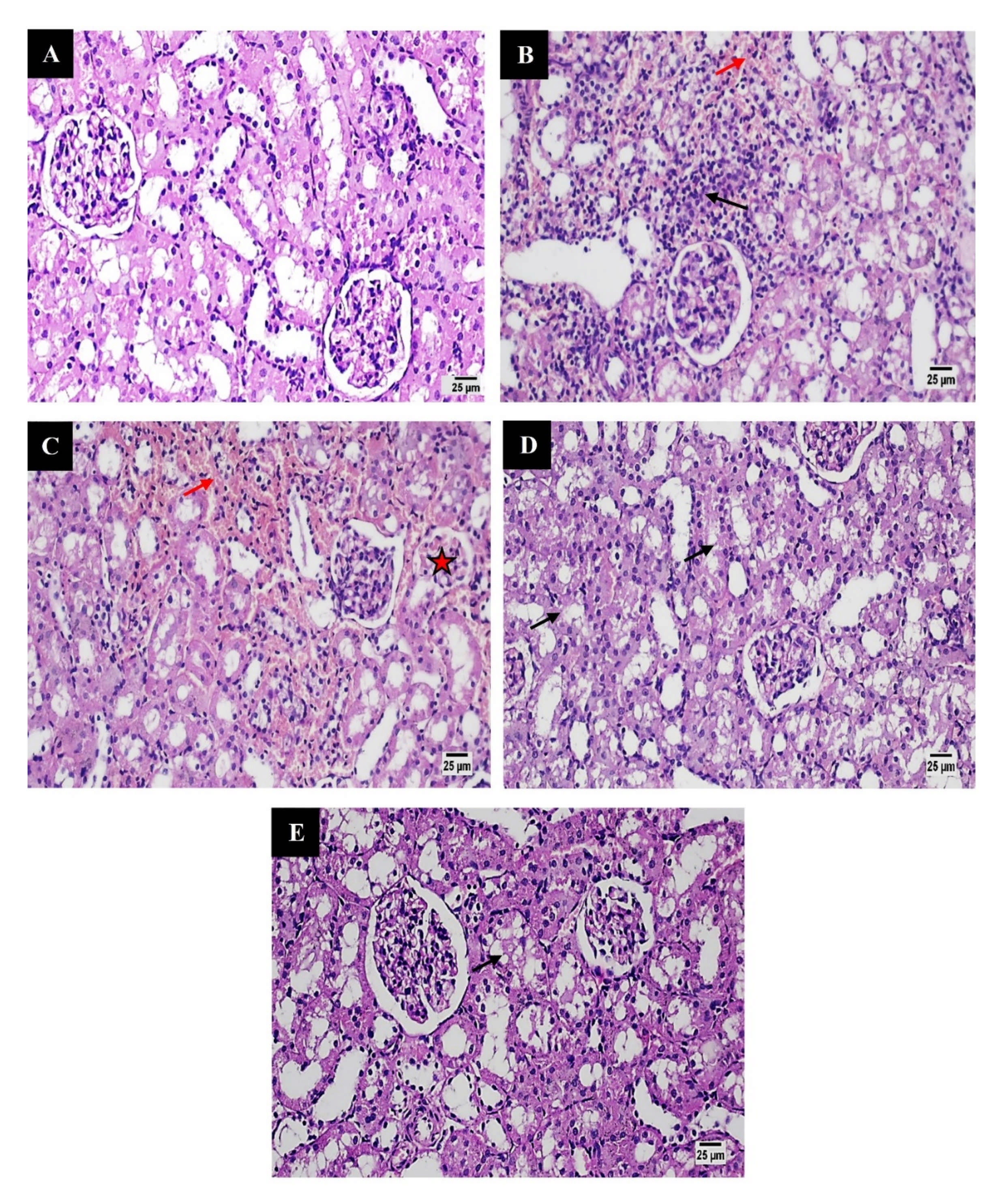
Kidneys
Testes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashcroft, F.M.; Rorsman, P. Diabetes mellitus and the β cell: The last ten years. Cell 2012, 148, 1160–1171. [Google Scholar] [CrossRef] [Green Version]
- Nolan, C.J.; Damm, P.; Prentki, M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet 2011, 378, 169–181. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A.W. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, R. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drzewoski, J.; Hanefeld, M. The current and potential therapeutic use of metformin—the good old drug. Pharmaceuticals 2021, 14, 122. [Google Scholar] [CrossRef]
- Gedawy, A.; Al-Salami, H.; Dass, C.R. Role of metformin in various pathologies: State-of-the-art microcapsules for improving its pharmacokinetics. Ther. Deliv. 2020, 11, 733–753. [Google Scholar] [CrossRef] [PubMed]
- Markowicz-Piasecka, M.; Huttunen, K.M.; Mateusiak, L.; Mikiciuk-Olasik, E.; Sikora, J. Is Metformin a Perfect Drug? Updates in Pharmacokinetics and Pharmacodynamics. Curr. Pharm. Des. 2016, 23, 2532–2550. [Google Scholar] [CrossRef]
- Szunerits, S.; Melinte, S.; Barras, A.; Pagneux, Q.; Voronova, A.; Abderrahmani, A.; Boukherroub, R. The impact of chemical engineering and technological advances on managing diabetes: Present and future concepts. Chem. Soc. Rev. 2021, 50, 2102–2146. [Google Scholar] [CrossRef]
- Romero, R.; Erez, O.; Hüttemann, M.; Maymon, E.; Panaitescu, B.; Conde-Agudelo, A.; Pacora, P.; Yoon, B.H.; Grossman, L.I. Metformin, the aspirin of the 21st century: Its role in gestational diabetes mellitus, prevention of preeclampsia and cancer, and the promotion of longevity. Am. J. Obstet. Gynecol. 2017, 217, 282–302. [Google Scholar] [CrossRef] [PubMed]
- Antoszczak, M.; Markowska, A.; Markowska, J.; Huczyński, A. Old wine in new bottles: Drug repurposing in oncology. Eur. J. Pharmacol. 2020, 866, 172784. [Google Scholar] [CrossRef] [PubMed]
- Mallik, R.; Chowdhury, T.A. Metformin in cancer. Diabetes Res. Clin. Pract. 2018, 143, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.S.; Gubbi, S.; Barzilai, N. Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab. 2020, 32, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y. Metformin and Inflammation: Its Potential Beyond Glucose-lowering Effect. Endocr. Metab. Immune Disord. Targets 2015, 15, 196–205. [Google Scholar] [CrossRef]
- Mahmood, K.; Naeem, M.; Rahimnajjad, N.A. Metformin: The hidden chronicles of a magic drug. Eur. J. Intern. Med. 2013, 24, 20–26. [Google Scholar] [CrossRef]
- Bramante, C.T.; Ingraham, N.E.; Murray, T.A.; Marmor, S.; Hovertsen, S.; Gronski, J.; McNeil, C.; Feng, R.; Guzman, G.; Abdelwahab, N.; et al. Metformin and risk of mortality in patients hospitalised with COVID-19: A retrospective cohort analysis. Lancet Health Longev. 2021, 2, e34–e41. [Google Scholar] [CrossRef]
- Sharma, S.; Ray, A.; Sadasivam, B. Metformin in COVID-19: A possible role beyond diabetes. Diabetes Res. Clin. Pract. 2020, 164, 108183. [Google Scholar] [CrossRef]
- Cheng, C.L.; Yu, L.X.; Lee, H.L.; Yang, C.Y.; Lue, C.S.; Chou, C.H. Biowaiver extension potential to BCS Class III high solubility-low permeability drugs: Bridging evidence for metformin immediate-release tablet. Eur. J. Pharm. Sci. 2004, 22, 297–304. [Google Scholar] [CrossRef]
- Glossmann, H.H.; Lutz, O.M.D. Pharmacology of metformin—An update. Eur. J. Pharmacol. 2019, 865, 172782. [Google Scholar] [CrossRef] [PubMed]
- Boldhane, S.; Kuchekar, B. Gastroretentive Drug Delivery of Metformin Hydrochloride: Formulation and In Vitro Evaluation Using 32 Full Factorial Design. Curr. Drug Deliv. 2009, 6, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Bristol-Myers Squibb Company. Glucophage (Metformin Hydrochloride) Tablets. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020357s037s039,021202s021s023lbl.pdf (accessed on 15 April 2021).
- Song, R. Mechanism of metformin: A tale of two sites. Diabetes Care 2016, 39, 187–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proctor, W.R.; Bourdet, D.L.; Thakker, D.R. Mechanisms underlying saturable intestinal absorption of metformin. Drug Metab. Dispos. 2008, 36, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Robert, F.; Fendri, S.; Hary, L.; Lacroix, C.; Andréjak, M.; Lalau, J. Kinetics of plasma and erythrocyte metformin after acute administration in healthy subjects. Diabetes Metab. 2003, 29, 279–283. [Google Scholar] [CrossRef]
- Scheen, A.J. Clinical Pharmacokinetics of Metformin. Clin. Pharmacokinet. 1996, 30, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Shurrab, N.T.; Arafa, E.S.A. Metformin: A review of its therapeutic efficacy and adverse effects. Obes. Med. 2020, 17, 100186. [Google Scholar] [CrossRef]
- Salpeter, S.R.; Greyber, E.; Pasternak, G.A.; Salpeter, E.E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2010, CD002967. [Google Scholar] [CrossRef]
- Chen, Y.; Shan, X.; Luo, C.; He, Z. Emerging nanoparticulate drug delivery systems of metformin. J. Pharm. Investig. 2020, 50, 219–230. [Google Scholar] [CrossRef]
- Cetin, M.; Sahin, S. Microparticulate and nanoparticulate drug delivery systems for metformin hydrochloride. Drug Deliv. 2016, 23, 2796–2805. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.N.; Hemant, K.S.Y.; Ram, M.; Shivakumar, H.G. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar] [PubMed]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, microspheres, and microcapsules for advanced drug delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef] [Green Version]
- Manconi, M.; Nácher, A.; Merino, V.; Merino-Sanjuan, M.; Manca, M.L.; Mura, C.; Mura, S.; Fadda, A.M.; Diez-Sales, O. Improving oral bioavailability and pharmacokinetics of liposomal metformin by glycerolphosphate-chitosan microcomplexation. AAPS PharmSciTech 2013, 14, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Sosnowska, K.; Czajkowska-Kósnik, A.; Winnicka, K. Calcium chloride modified alginate microparticles formulated by the spray drying process: A strategy to prolong the release of freely soluble drugs. Materials 2018, 11, 1522. [Google Scholar] [CrossRef] [Green Version]
- Yari, K.; Akbari, I.; Yazdi, S.A.V. Development and evaluation of sodium alginate-basil seeds mucilage beads as a suitable carrier for controlled release of metformin. Int. J. Biol. Macromol. 2020, 159, 1–10. [Google Scholar] [CrossRef]
- Szekalska, M.; Wroblewska, M.; Sosnowska, K.; Winnicka, K. Influence of Sodium Alginate on Hypoglycemic Activity of Metformin Hydrochloride in the Microspheres Obtained by the Spray Drying. Int. J. Polym. Sci. 2016, 2016, 8635408. [Google Scholar] [CrossRef] [Green Version]
- Nayak, A.; Jain, S.K.; Pandey, R.S. Controlling release of metformin HCl through incorporation into stomach specific floating alginate beads. Mol. Pharm. 2011, 8, 2273–2281. [Google Scholar] [CrossRef]
- Raza, H.; Javeria, S.; Rashid, Z. Sustained released Metformin microparticles for better management of type II diabetes mellitus: In-vitro studies. Mater. Res. Express 2020, 7, 015343. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D.; Santra, K. Swelling and drug release behavior of metformin HCl-loaded tamarind seed polysaccharide-alginate beads. Int. J. Biol. Macromol. 2016, 82, 1023–1027. [Google Scholar] [CrossRef]
- Paques, J.P.; Van Der Linden, E.; Van Rijn, C.J.M.; Sagis, L.M.C. Preparation methods of alginate nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D.; Pradhan, J.; Hasnain, M.S. Fenugreek seed mucilage-alginate mucoadhesive beads of metformin HCl: Design, optimization and evaluation. Int. J. Biol. Macromol. 2013, 54, 144–154. [Google Scholar] [CrossRef]
- Braccini, I.; Pérez, S. Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef]
- Patel, M.A.; AbouGhaly, M.H.H.; Schryer-Praga, J.V.; Chadwick, K. The effect of ionotropic gelation residence time on alginate cross-linking and properties. Carbohydr. Polym. 2017, 155, 362–371. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D. Ionotropically-gelled mucoadhesive beads for oral metformin HCl delivery: Formulation, optimization and antidiabetic evaluation. J. Sci. Ind. Res. 2013, 72, 15–22. [Google Scholar]
- Maestrelli, F.; Mura, P.; González-Rodríguez, M.L.; Cózar-Bernal, M.J.; Rabasco, A.M.; Di Cesare Mannelli, L.; Ghelardini, C. Calcium alginate microspheres containing metformin hydrochloride niosomes and chitosomes aimed for oral therapy of type 2 diabetes mellitus. Int. J. Pharm. 2017, 530, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Ubaidulla, U.; Nayak, A.K. Okra (Hibiscus esculentus) gum-alginate blend mucoadhesive beads for controlled glibenclamide release. Int. J. Biol. Macromol. 2015, 72, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, G.; Bouropoulos, N. Swelling studies and in vitro release of verapamil from calcium alginate and calcium alginate-chitosan beads. Int. J. Pharm. 2006, 323, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, G.; Boa, A.N.; Diego-Taboada, A.; Atkin, S.L.; Sathyapalan, T. Sporopollenin, the least known yet toughest natural biopolymer. Front. Mater. 2015, 2, 66. [Google Scholar] [CrossRef] [Green Version]
- Paunov, V.N.; Mackenzie, G.; Stoyanov, S.D. Sporopollenin micro-reactors for in-situ preparation, encapsulation and targeted delivery of active components. J. Mater. Chem. 2007, 17, 609. [Google Scholar] [CrossRef]
- Uddin, M.J.; Liyanage, S.; Abidi, N.; Gill, H.S. Physical and Biochemical Characterization of Chemically Treated Pollen Shells for Potential Use in Oral Delivery of Therapeutics. J. Pharm. Sci. 2018, 107, 3047–3059. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Plant Pollen Grains: A Move Towards Green Drug and Vaccine Delivery Systems. Nano Micro Lett. 2021, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Hamad, S.A.; Dyab, A.F.K.; Stoyanov, S.D.; Paunov, V.N. Encapsulation of living cells into sporopollenin microcapsules. J. Mater. Chem. 2011, 21, 18018–18023. [Google Scholar] [CrossRef]
- Stamatopoulos, K.; Kafourou, V.; Batchelor, H.K.; Konteles, S.J. Sporopollenin Exine Microcapsules as Potential Intestinal Delivery System of Probiotics. Small 2021, 17, 2004573. [Google Scholar] [CrossRef]
- Fan, T.F.; Park, S.; Shi, Q.; Zhang, X.; Liu, Q.; Song, Y.; Chin, H.; Ibrahim, M.S.B.; Mokrzecka, N.; Yang, Y.; et al. Transformation of hard pollen into soft matter. Nat. Commun. 2020, 11, 1449. [Google Scholar] [CrossRef] [Green Version]
- Sudareva, N.; Suvorova, O.; Saprykina, N.; Vilesov, A.; Bel’Tiukov, P.; Petunov, S.; Radilov, A. Two-level delivery systems for oral administration of peptides and proteins based on spore capsules of Lycopodium clavatum. J. Mater. Chem. B 2017, 5, 7711–7720. [Google Scholar] [CrossRef]
- Uddin, M.J.; Gonzalez-Cruz, P.; Warzywoda, J.; Gill, H.S. Sporopollenin Spikes Augment Antigen-Specific Immune Response and Generate Long-Lived Humoral Immunity. Adv. Ther. 2020, 3, 2000102. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Ahmed, O.M.; Hozayen, W.G.; Ahmed, M.A. Ameliorative effects of bee pollen and date palm pollen on the glycemic state and male sexual dysfunctions in streptozotocin-Induced diabetic wistar rats. Biomed. Pharmacother. 2018, 97, 9–18. [Google Scholar] [CrossRef]
- El-Kashlan, A.M.; Nooh, M.M.; Hassan, W.A.; Rizk, S.M. Therapeutic potential of date palm pollen for testicular dysfunction induced by thyroid disorders in male rats. PLoS ONE 2015, 10, e0139493. [Google Scholar] [CrossRef] [Green Version]
- Dyab, A.K.F.; Sadek, K.U. Microwave assisted one-pot green synthesis of cinnoline derivatives inside natural sporopollenin microcapsules. RSC Adv. 2018, 8, 23241–23251. [Google Scholar] [CrossRef] [Green Version]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Wójcik-Pastuszka, D.; Krzak, J.; Macikowski, B.; Berkowski, R.; Osiński, B.; Musiał, W. Evaluation of the release kinetics of a pharmacologically active substance from model intra-articular implants replacing the cruciate ligaments of the knee. Materials 2019, 12, 1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baishya, H. Application of Mathematical Models in Drug Release Kinetics of Carbidopa and Levodopa ER Tablets. J. Dev. Drugs 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef]
- Al-Awady, M.J.; Greenway, G.M.; Paunov, V.N. Nanotoxicity of polyelectrolyte-functionalized titania nanoparticles towards microalgae and yeast: Role of the particle concentration, size and surface charge. RSC Adv. 2015, 5, 37044–37059. [Google Scholar] [CrossRef] [Green Version]
- Jayaprasad, B.; Sharavanan, P.S.; Sivaraj, R. Antidiabetic effect of Chloroxylon swietenia bark extracts on streptozotocin induced diabetic rats. Beni Suef Univ. J. Basic Appl. Sci. 2016, 5, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Sankrityayan, H.; Dixit, D.; Jadhav, P. Cocos nucifera and metformin combination for modulation of diabetic symptoms in streptozotocin induced diabetic rats. J. Ayurveda Integr. Med. 2020, 11, 3–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Halbritter, H.; Ulrich, S.; Grímsson, F.; Weber, M.; Zetter, R.; Hesse, M.; Buchner, R.; Svojtka, M.; Frosch-Radivo, A. Pollen Morphology and Ultrastructure. In Illustrated Pollen Terminology; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 37–65. [Google Scholar]
- Katifori, E.; Alben, S.; Cerda, E.; Nelson, D.R.; Dumais, J. Foldable structures and the natural design of pollen grains. Proc. Natl. Acad. Sci. USA 2010, 107, 7635–7639. [Google Scholar] [CrossRef] [Green Version]
- Diego-Taboada, A.; Beckett, S.T.; Atkin, S.L.; Mackenzie, G. Hollow pollen shells to enhance drug delivery. Pharmaceutics 2014, 6, 80–96. [Google Scholar] [CrossRef] [Green Version]
- Mundargi, R.C.; Potroz, M.G.; Park, S.; Park, J.H.; Shirahama, H.; Lee, J.H.; Seo, J.; Cho, N.J. Lycopodium Spores: A Naturally Manufactured, Superrobust Biomaterial for Drug Delivery. Adv. Funct. Mater. 2016, 26, 487–497. [Google Scholar] [CrossRef]
- Atwe, S.U.; Ma, Y.; Gill, H.S. Pollen grains for oral vaccination. J. Control. Release 2014, 194, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Dyab, A.K.F.; Mohamed, M.A.; Meligi, N.M.; Mohamed, S.K. Encapsulation of erythromycin and bacitracin antibiotics into natural sporopollenin microcapsules: Antibacterial, cytotoxicity, in vitro and in vivo release studies for enhanced bioavailability. RSC Adv. 2018, 8, 33432–33444. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, C.; Gazeau, F.; Roger, J.; Pons, J.N.; Bacri, J.C. Interaction of anionic superparamagnetic nanoparticles with cells: Kinetic analyses of membrane adsorption and subsequent internalization. Langmuir 2002, 18, 8148–8155. [Google Scholar] [CrossRef]
- Potroz, M.G.; Mundargi, R.C.; Gillissen, J.J.; Tan, E.L.; Meker, S.; Park, J.H.; Jung, H.; Park, S.; Cho, D.; Bang, S.I.; et al. Plant-Based Hollow Microcapsules for Oral Delivery Applications: Toward Optimized Loading and Controlled Release. Adv. Funct. Mater. 2017, 27, 1700270. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Tan, E.L.; Seo, J.; Cho, N.J. Encapsulation and controlled release formulations of 5-fluorouracil from natural Lycopodium clavatum spores. J. Ind. Eng. Chem. 2016, 36, 102–108. [Google Scholar] [CrossRef]
- Voo, W.P.; Lee, B.B.; Idris, A.; Islam, A.; Tey, B.T.; Chan, E.S. Production of ultra-high concentration calcium alginate beads with prolonged dissolution profile. RSC Adv. 2015, 5, 36687–36695. [Google Scholar] [CrossRef]
- Adzmi, F.; Meon, S.; Musa, M.H.; Yusuf, N.A. Preparation, characterisation and viability of encapsulated Trichoderma harzianum UPM40 in alginate-montmorillonite clay. J. Microencapsul. 2012, 29, 205–210. [Google Scholar] [CrossRef]
- Mostafavi, S.A.; Salavati, S.; Dizaji, H.B.; Mehdi, B. Pyrolysis and combustion kinetics of lycopodium particles in thermogravimetric analysis. J. Cent. South Univ. 2015, 22, 3409–3417. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D. Trigonella foenum-graecum L. seed mucilage-gellan mucoadhesive beads for controlled release of metformin HCl. Carbohydr. Polym. 2014, 107, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Pandit, V.; Pai, R.S.; Yadav, V.; Devi, K.; Surekha, B.B.; Inamdar, M.N.; Suresh, S. Pharmacokinetic and pharmacodynamic evaluation of floating microspheres of metformin hydrochloride. Drug Dev. Ind. Pharm. 2013, 39, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Zake, D.M.; Kurlovics, J.; Zaharenko, L.; Komasilovs, V.; Klovins, J.; Stalidzans, E. Physiologically based metformin pharmacokinetics model of mice and scale-up to humans for the estimation of concentrations in various tissues. PLoS ONE 2021, 16, e0249594. [Google Scholar] [CrossRef]
- Schwartz, S.L.; Gordi, T.; Hou, E.; Cramer, M.; Heritier, M.; Cowles, V.E. Clinical development of metformin extended-release tablets for type 2 diabetes: An overview. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1235–1243. [Google Scholar] [CrossRef]
- Gusler, G.; Gorsline, J.; Levy, G.; Zhang, S.Z.; Weston, I.E.; Naret, D.; Berner, B. Pharmacokinetics of Metformin Gastric-Retentive Tablets in Healthy Volunteers. J. Clin. Pharmacol. 2001, 41, 655–661. [Google Scholar] [CrossRef]
- Buse, J.B.; DeFronzo, R.A.; Rosenstock, J.; Kim, T.; Burns, C.; Skare, S.; Baron, A.; Fineman, M. The Primary Glucose-Lowering Effect of Metformin Resides in the Gut, Not the Circulation. Results From Short-term Pharmacokinetic and 12-Week Dose-Ranging Studies. Diabetes Care 2015, 39, dc150488. [Google Scholar] [CrossRef] [Green Version]
- Shirwaikar, A.; Rajendran, K.; Kumar, C.D.; Bodla, R. Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin-nicotinamide type 2 diabetic rats. J. Ethnopharmacol. 2004, 91, 171–175. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Andrikopoulos, S.; Filippis, C.; Thorburn, A.W.; Khan, D.; Proietto, J. Mechanism of fat-induced hepatic gluconeogenesis: Effect of metformin. Am. J. Physiol. Metab. 2001, 281, E275–E282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasri, H.; Rafieian-Kopaei, M. Metformin: Current Knowledge. J. Res. Med. Sci. 2014, 19, 658–664. [Google Scholar]
- Petersen, M.C.; Vatner, D.F.; Shulman, G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef] [Green Version]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minamii, T.; Nogami, M.; Ogawa, W. Mechanisms of metformin action: In and out of the gut. J. Diabetes Investig. 2018, 9, 701–703. [Google Scholar] [CrossRef]
- Shao, Y.; Yu, Y.; Li, C.; Yu, J.; Zong, R.; Pei, C. Synergistic effect of quercetin and 6-gingerol treatment in streptozotocin induced type 2 diabetic rats and poloxamer P-407 induced hyperlipidemia. RSC Adv. 2016, 6, 12235–12242. [Google Scholar] [CrossRef]
- Yanardag, R.; Ozsoy-Sacan, O.; Bolkent, S.; Orak, H.; Karabulut-Bulan, O. Protective effects of metformin treatment on the liver injury of streptozotocin-diabetic rats. Hum. Exp. Toxicol. 2005, 24, 129–135. [Google Scholar] [CrossRef]
- Moree, S.S.; Kavishankar, G.B.; Rajesha, J. Antidiabetic effect of secoisolariciresinol diglucoside in streptozotocin-induced diabetic rats. Phytomedicine 2013, 20, 237–245. [Google Scholar] [CrossRef]
- Shawky, L.M.; Morsi, A.A.; El Bana, E.; Hanafy, S.M. The biological impacts of sitagliptin on the pancreas of a rat model of type 2 diabetes mellitus: Drug interactions with metformin. Biology 2020, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Ismail, T.A.; Soliman, M.M.; Nassan, M.A. Molecular and immunohistochemical effects of metformin in a rat model of type 2 diabetes mellitus. Exp. Ther. Med. 2015, 9, 1921–1930. [Google Scholar] [CrossRef] [Green Version]
- Van Stee, M.F.; de Graaf, A.A.; Groen, A.K. Actions of metformin and statins on lipid and glucose metabolism and possible benefit of combination therapy. Cardiovasc. Diabetol. 2018, 17, 94. [Google Scholar] [CrossRef] [Green Version]
- Westerbacka, J.; Cornér, A.; Tiikkainen, M.; Tamminen, M.; Vehkavaara, S.; Häkkinen, A.M.; Fredriksson, J.; Yki-Järvinen, H. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: Implications for sex differences in markers of cardiovascular risk. Diabetologia 2004, 47, 1360–1369. [Google Scholar] [CrossRef] [Green Version]
- Schindhelm, R.K.; Diamant, M.; Dekker, J.M.; Tushuizen, M.E.; Teerlink, T.; Heine, R.J. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab. Res. Rev. 2006, 22, 437–443. [Google Scholar] [CrossRef]
- Pinhas-Hamiel, O.; Zeitler, P. Clinical presentation and treatment of type 2 diabetes in children. Pediatr. Diabetes 2007, 8, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Alshathly, M. Efficacy of Ginger (Zingiber officinale) in ameliorating streptozotocin-induced diabetic liver injury in rats: Histological and biochemical studies. J. Microsc. Ultrastruct. 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Kabil, S. Metformin Attenuates Thioacetamide Induced Hepatotoxic Effects in Rats. Al-Azhar J. Pharm. Sci. 2015, 52, 270–282. [Google Scholar] [CrossRef]
- Jarald, E.E.; Joshi, S.B.; Jain, D.C. Antidiabetic activity of aqueous extract and non polysaccharide fraction of Cynodon dactylon Pers. Indian J. Exp. Biol. 2008, 46, 660–667. [Google Scholar] [PubMed]
- Alhaider, A.A.; Korashy, H.M.; Sayed-Ahmed, M.M.; Mobark, M.; Kfoury, H.; Mansour, M.A. Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem. Biol. Interact. 2011, 192, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Cavaglieri, R.C.; Day, R.T.; Feliers, D.; Abboud, H.E. Metformin prevents renal interstitial fibrosis in mice with unilateral ureteral obstruction. Mol. Cell. Endocrinol. 2015, 412, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Baradaran, A.; Ardalan, M.R.; Mardani, S.; Momeni, A.; Rafieian-Kopaei, M. Bright renoprotective properties of metformin: Beyond blood glucose regulatory effects. Iran. J. Kidney Dis. 2013, 7, 423–428. [Google Scholar]
- Reddy, K.P.; Narayana Rao, M.; Murthy, J.S.R.; Reddy, P.S. Lead aggravates the diabetic-induced reproductive toxicity in male Wistar rats. Toxicol. Res. 2016, 5, 1465–1476. [Google Scholar] [CrossRef] [Green Version]
- Nna, V.U.; Bakar, A.B.A.; Ahmad, A.; Umar, U.Z.; Suleiman, J.B.; Zakaria, Z.; Othman, Z.A.; Mohamed, M. Malaysian propolis and metformin mitigate subfertility in streptozotocin-induced diabetic male rats by targeting steroidogenesis, testicular lactate transport, spermatogenesis and mating behaviour. Andrology 2020, 8, 731–746. [Google Scholar] [CrossRef]
- Nna, V.U.; Bakar, A.B.A.; Ahmad, A.; Mohamed, M. Down-regulation of steroidogenesis-related genes and its accompanying fertility decline in streptozotocin-induced diabetic male rats: Ameliorative effect of metformin. Andrology 2019, 7, 110–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derkach, K.V.; Bakhtyukov, A.A.; Bayunova, L.V.; Zorina, I.I.; Shpakov, A.O. Normalization of Testicular Steroidogenesis and Spermatogenesis in Male Rats with Type 2 Diabetes Mellitus under the Conditions of Metformin Therapy. Dokl. Biol. Sci. 2020, 493, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Shimon, I.; Lubina, A.; Gorfine, M.; Ilany, J. Feedback inhibition of gonadotropins by testosterone in men with hypogonadotropic hypogonadism: Comparison to the intact pituitary-testicular axis in primary hypogonadism. J. Androl. 2006, 27, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Gregg, B.; Elghazi, L.; Alejandro, E.U.; Smith, M.R.; Blandino-Rosano, M.; El-Gabri, D.; Cras-Méneur, C.; Bernal-Mizrachi, E. Exposure of mouse embryonic pancreas to metformin enhances the number of pancreatic progenitors. Diabetologia 2014, 57, 2566–2575. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Massey, S.; Story, D.; Li, L. Molecular Sciences Metformin: An Old Drug with New Applications. Int. J. Mol. Sci. 2018, 19, 2863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telci, A.; Çakatay, U.; Salman, S.; Satman, I.; Sivas, A. Oxidative protein damage in early stage Type 1 diabetic patients. Diabetes Res. Clin. Pract. 2000, 50, 213–223. [Google Scholar] [CrossRef]
- Turk, H.M.; Sevinc, A.; Camci, C.; Cigli, A.; Buyukberber, S.; Savli, H.; Bayraktar, N. Plasma lipid peroxidation products and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Acta Diabetol. 2002, 39, 117–122. [Google Scholar] [CrossRef]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 2020. [Google Scholar] [CrossRef]
- Faure, P.; Rossini, E.; Wiernsperger, N.; Richard, M.J.; Favier, A.; Halimi, S. An insulin sensitizer improves the free radical defense system potential and insulin sensitivity in high fructose-fed rats. Diabetes 1999, 48, 353–357. [Google Scholar] [CrossRef]
- Singh, R.K.; Gupta, B.; Tripathi, K.; Singh, S.K. Anti oxidant potential of Metformin and Pioglitazone in Type 2 Diabetes Mellitus: Beyond their anti glycemic effect. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, 102–104. [Google Scholar] [CrossRef]
- Jemai, H.; Feki, A.E.L.; Sayadi, S. Antidiabetic and antioxidant effects of hydroxytyrosol and oleuropein from olive leaves in alloxan-diabetic rats. J. Agric. Food Chem. 2009, 57, 8798–8804. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halban, P.A.; Polonsky, K.S.; Bowden, D.W.; Hawkins, M.A.; Ling, C.; Mather, K.J.; Powers, A.C.; Rhodes, C.J.; Sussel, L.; Weir, G.C. β-Cell failure in type 2 diabetes: Postulated mechanisms and prospects for prevention and treatment. J. Clin. Endocrinol. Metab. 2014, 99, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Nna, V.U.; Abu Bakar, A.B.; Md Lazin, M.R.M.L.; Mohamed, M. Antioxidant, anti-inflammatory and synergistic anti-hyperglycemic effects of Malaysian propolis and metformin in streptozotocin–induced diabetic rats. Food Chem. Toxicol. 2018, 120, 305–320. [Google Scholar] [CrossRef]
- Ahmed, D.; Kumar, V.; Verma, A.; Gupta, P.S.; Kumar, H.; Dhingra, V.; Mishra, V.; Sharma, M. Antidiabetic, renal/hepatic/pancreas/cardiac protective and antioxidant potential of methanol/dichloromethane extract of Albizzia Lebbeck Benth. stem bark (ALEx) on streptozotocin induced diabetic rats. BMC Complement. Altern. Med. 2014, 14, 243. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.S.; Karunakaran, U.; Elumalai, S.; Lee, I.K.; Lee, H.W.; Kim, Y.W.; Won, K.C. Metformin prevents glucotoxicity by alleviating oxidative and ER stress–induced CD36 expression in pancreatic beta cells. J. Diabetes Complicat. 2017, 31, 21–30. [Google Scholar] [CrossRef]
- Gheissari, A.; Hemmatzadeh, S.; Merrikhi, A.; Fadaei Tehrani, S.; Madihi, Y. Chronic kidney disease in children: A report from a tertiary care center over 11 years. J. Nephropathol. 2012, 1, 177–182. [Google Scholar] [CrossRef] [Green Version]
- De Broe, M.E.; Kajbaf, F.; Lalau, J.D. Renoprotective Effects of Metformin. Nephron 2018, 138, 261–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Moon, S.Y.; Kim, J.S.; Baek, C.H.; Kim, M.; Min, J.Y.; Lee, S.K. Activation of AMP-activated protein kinase inhibits ER stress and renal fibrosis. Am. J. Physiol. Ren. Physiol. 2015, 308, F226–F236. [Google Scholar] [CrossRef] [PubMed]
- Guneli, E.; Tugyan, K.; Ozturk, H.; Gumustekin, M.; Cilaker, S.; Uysal, N. Effect of Melatonin on Testicular Damage in Streptozotocin-Induced Diabetes Rats. Eur. Surg. Res. 2008, 40, 354–360. [Google Scholar] [CrossRef]
- Gholizadeh, F.; Dastghaib, S.; Koohpeyma, F.; Bayat, E.; Mokarram, P. The protective effect of Stevia rebaudiana Bertoni on serum hormone levels, key steroidogenesis enzymes, and testicular damage in testes of diabetic rats. Acta Histochem. 2019, 121, 833–840. [Google Scholar] [CrossRef]
- Faure, M.; Bertoldo, M.J.; Khoueiry, R.; Bongrani, A.; Brion, F.; Giulivi, C.; Dupont, J.; Froment, P. Metformin in Reproductive Biology. Front. Endocrinol. 2018, 9, 675. [Google Scholar] [CrossRef] [Green Version]
| MTF-Loaded LCS | MTF-Loaded DPP | MTF-Loaded LCS-ALG Beads | |
|---|---|---|---|
| %LC | 14.91 ± 0.65 | 15.17 ± 0.72 | 10.00 ± 0.80 |
| %EE | 29.83 ± 0.83 | 30.34 ± 1.02 | 24.45 ± 1.95 |
| %Yield | 68.50 ± 5.21 | 66.10 ± 4.68 | 74.30 ± 3.24 |
| Pharmacokinetic Parameter | Pure MTF | MTF-Loaded LCS-ALG Beads |
|---|---|---|
| AUC0–10 (ng.h/mL) | 9453.85 | 10982.46 |
| AUC0–∞ (ng.h/mL) | 9718.15 | 11808.68 |
| Cmax (ng/mL) | 1618.00 | 2128.50 |
| t1/2 (h) | 1.425 | 1.571 |
| Kel (h−1) | 0.49 | 0.44 |
| Tmax (h) | 2.00 | 6.00 |
| MRT0–10 (h) | 3.97 | 6.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meligi, N.M.; Dyab, A.K.F.; Paunov, V.N. Sustained In Vitro and In Vivo Delivery of Metformin from Plant Pollen-Derived Composite Microcapsules. Pharmaceutics 2021, 13, 1048. https://doi.org/10.3390/pharmaceutics13071048
Meligi NM, Dyab AKF, Paunov VN. Sustained In Vitro and In Vivo Delivery of Metformin from Plant Pollen-Derived Composite Microcapsules. Pharmaceutics. 2021; 13(7):1048. https://doi.org/10.3390/pharmaceutics13071048
Chicago/Turabian StyleMeligi, Noha M., Amro K. F. Dyab, and Vesselin N. Paunov. 2021. "Sustained In Vitro and In Vivo Delivery of Metformin from Plant Pollen-Derived Composite Microcapsules" Pharmaceutics 13, no. 7: 1048. https://doi.org/10.3390/pharmaceutics13071048
APA StyleMeligi, N. M., Dyab, A. K. F., & Paunov, V. N. (2021). Sustained In Vitro and In Vivo Delivery of Metformin from Plant Pollen-Derived Composite Microcapsules. Pharmaceutics, 13(7), 1048. https://doi.org/10.3390/pharmaceutics13071048