Experimental Design Based Optimization and Ex Vivo Permeation of Desmopressin Acetate Loaded Elastic Liposomes Using Rat Skin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis Methodology
2.3. Preparation of Formulations
2.4. In Vitro Characterizations
2.4.1. Particle Size and Size Distribution, and Zeta Potential
2.4.2. Elasticity of the Formulations
2.4.3. Percent Entrapment Efficiency (% EE)
2.5. Optimization: Experimental Design Tool (Design Expert®)
2.6. Evaluation of Software Suggested Nine Formulations
2.6.1. Morphological Assessment
2.6.2. Drug Release Profile
2.6.3. Permeation Flux: Ex Vivo Study
2.7. Hemocompatibility Study: Biosafety Assessment
2.8. Ex Vivo Vesicle–Skin Interaction Study Using Scanning Electron Microscopy (SEM)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Prepared Elastic Liposomes and the Effect of Surfactants
3.2. Optimization Using Design Expert®
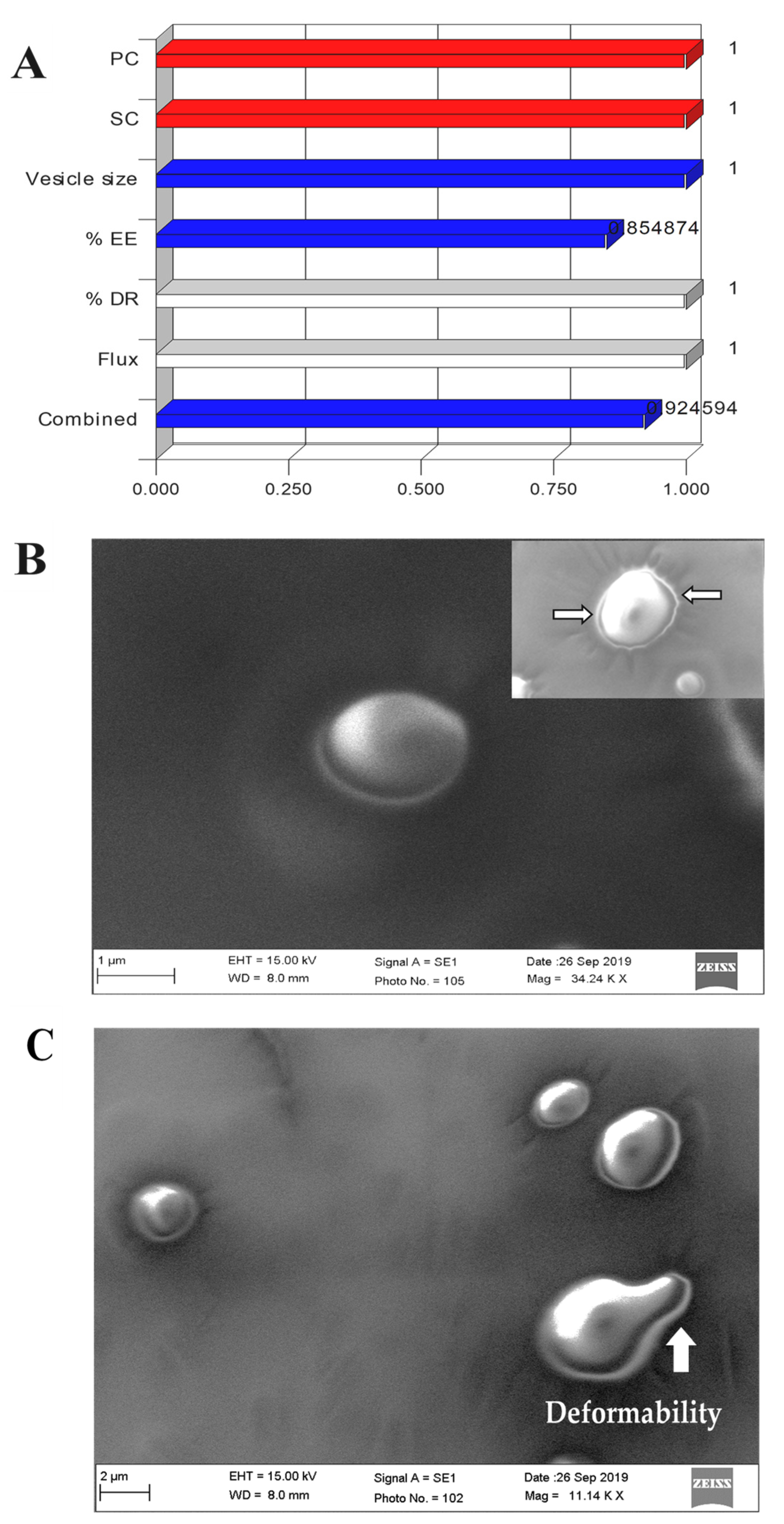
3.3. Desirability Function and Application
3.4. Post-Optimization
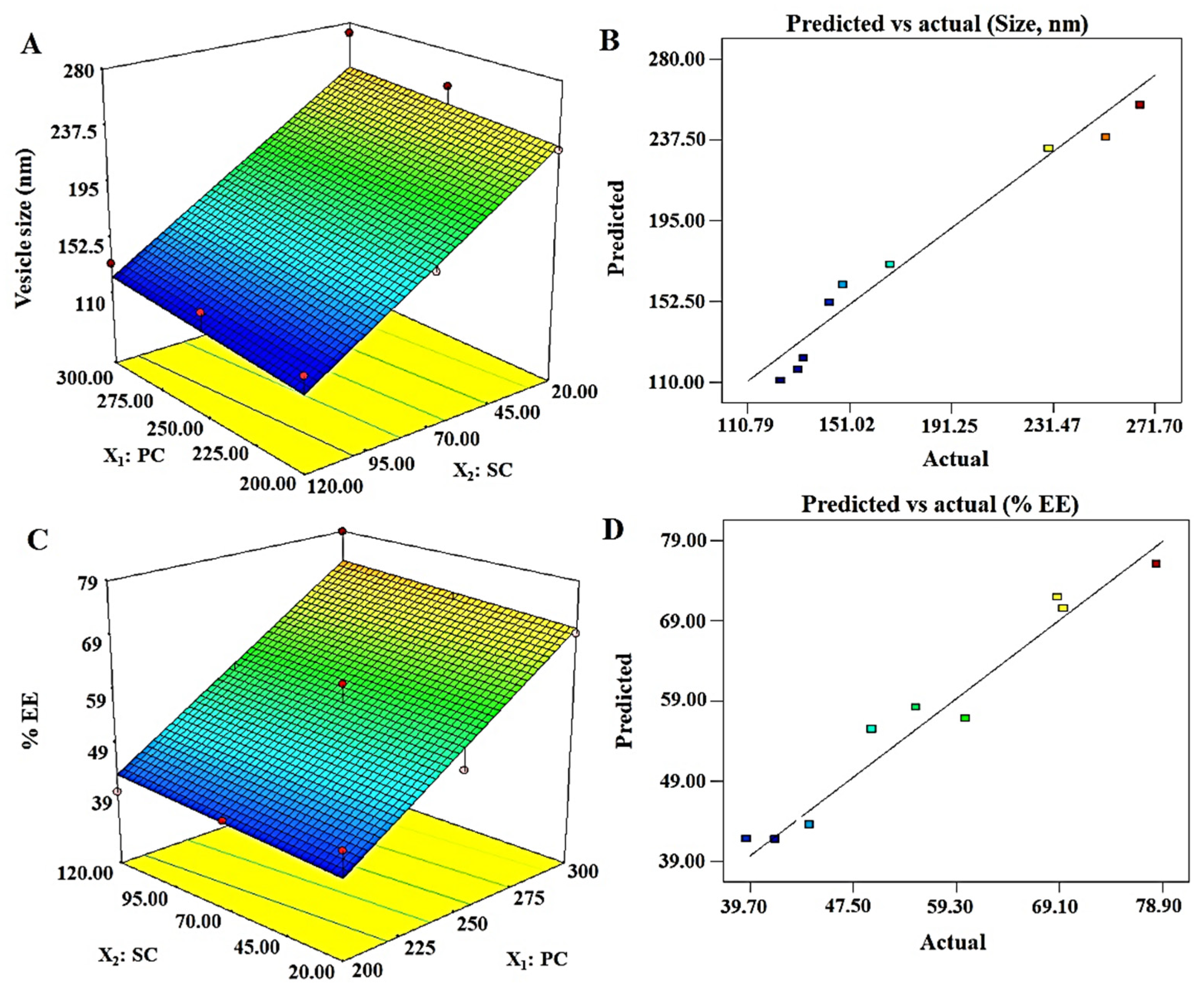
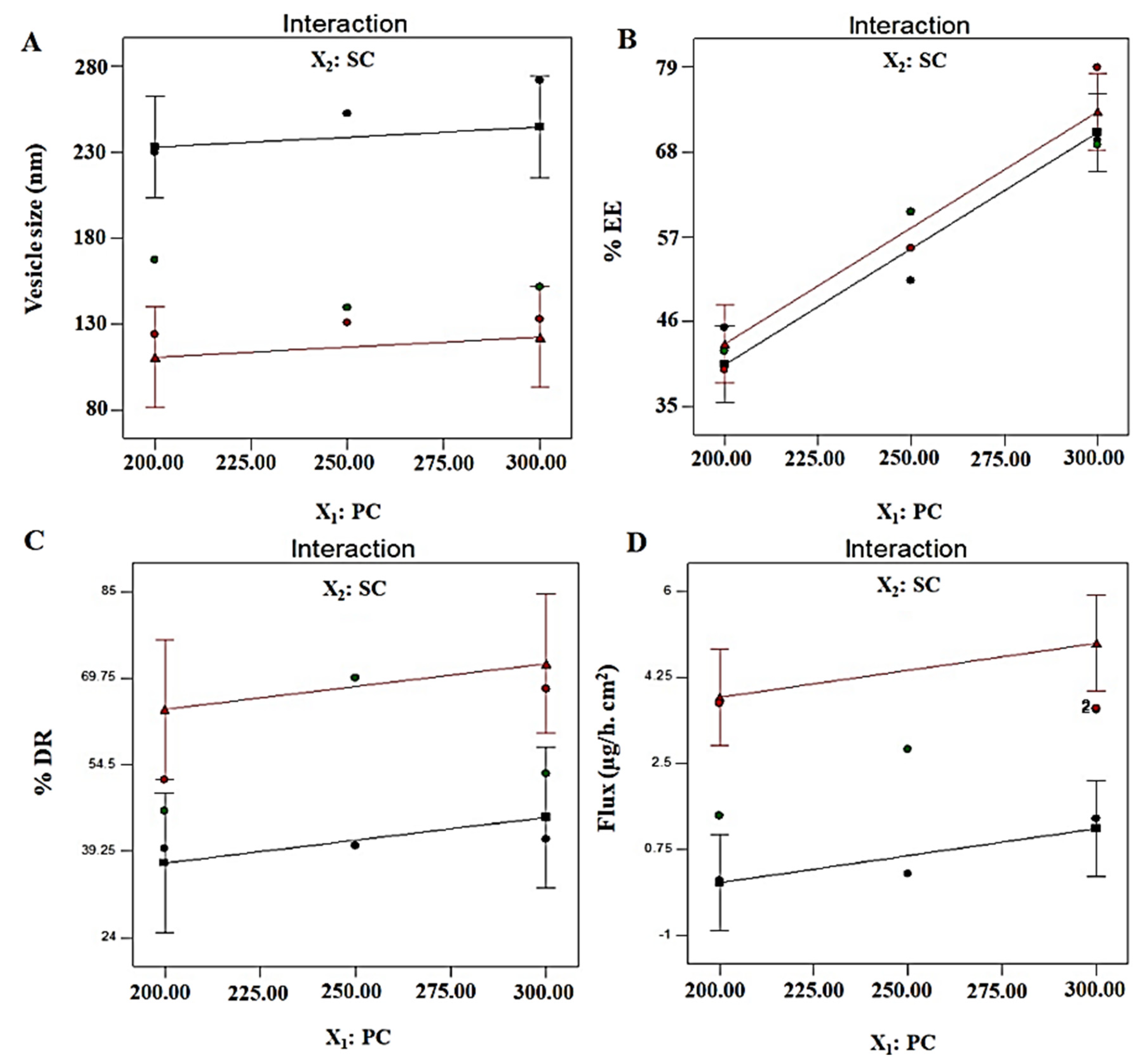
3.4.1. Vesicle Size (nm): Y1
3.4.2. % Entrapment Efficiency: Y2
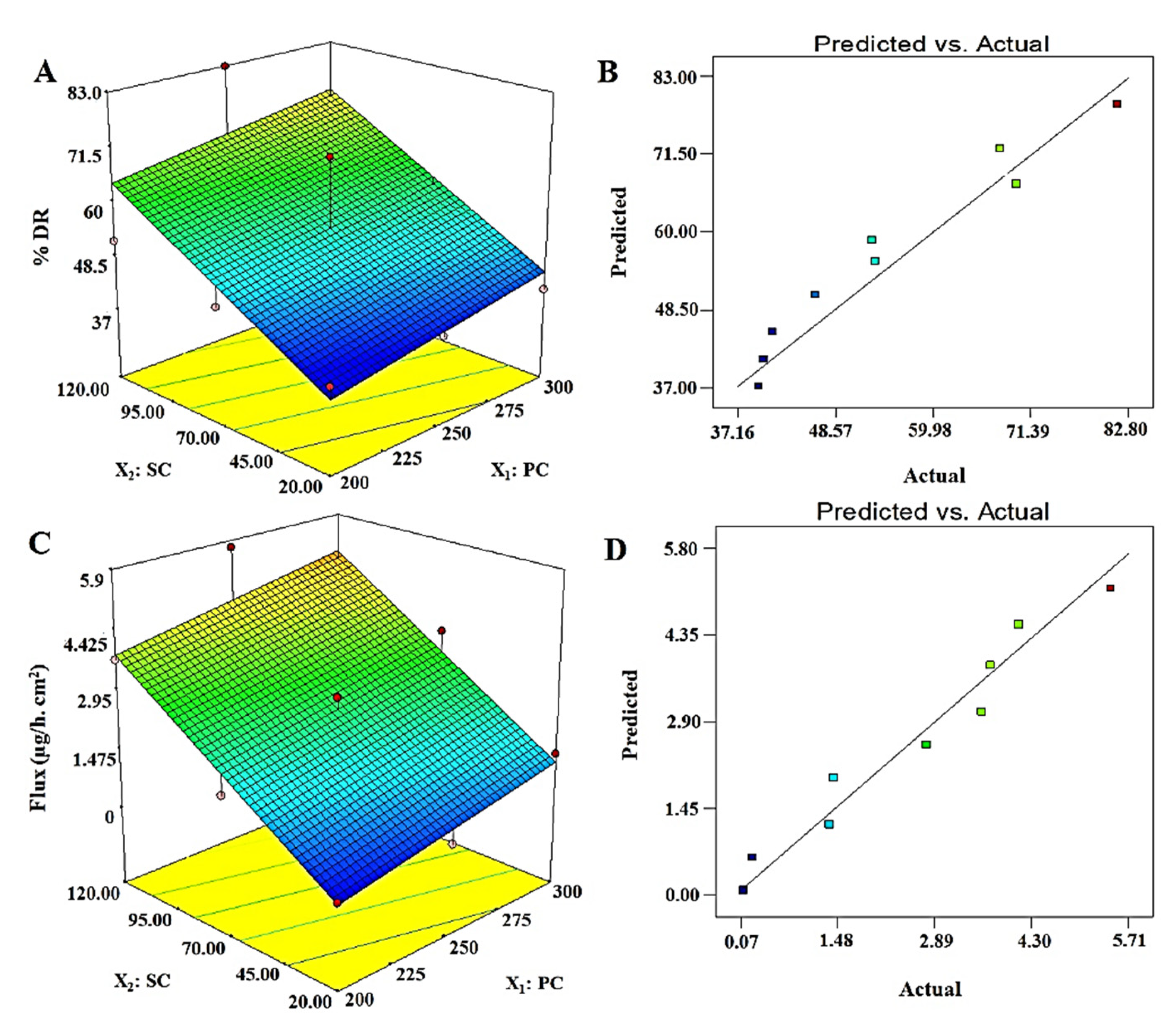
3.4.3. % Drug Release: Y3
3.4.4. Permeation Flux: Y4
3.5. Morphological Assessment
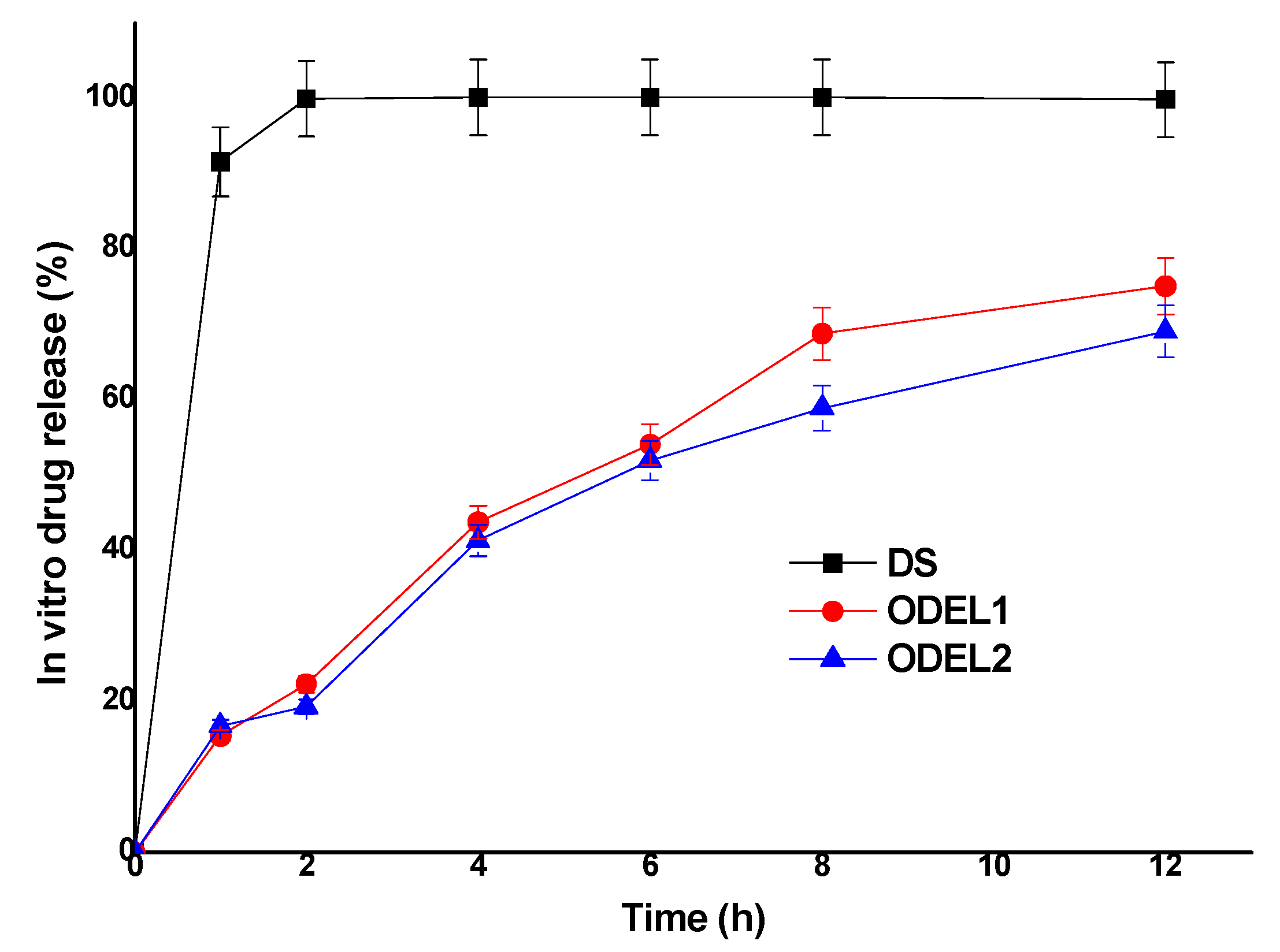
3.6. In Vitro Drug Release Kinetics and Comparison
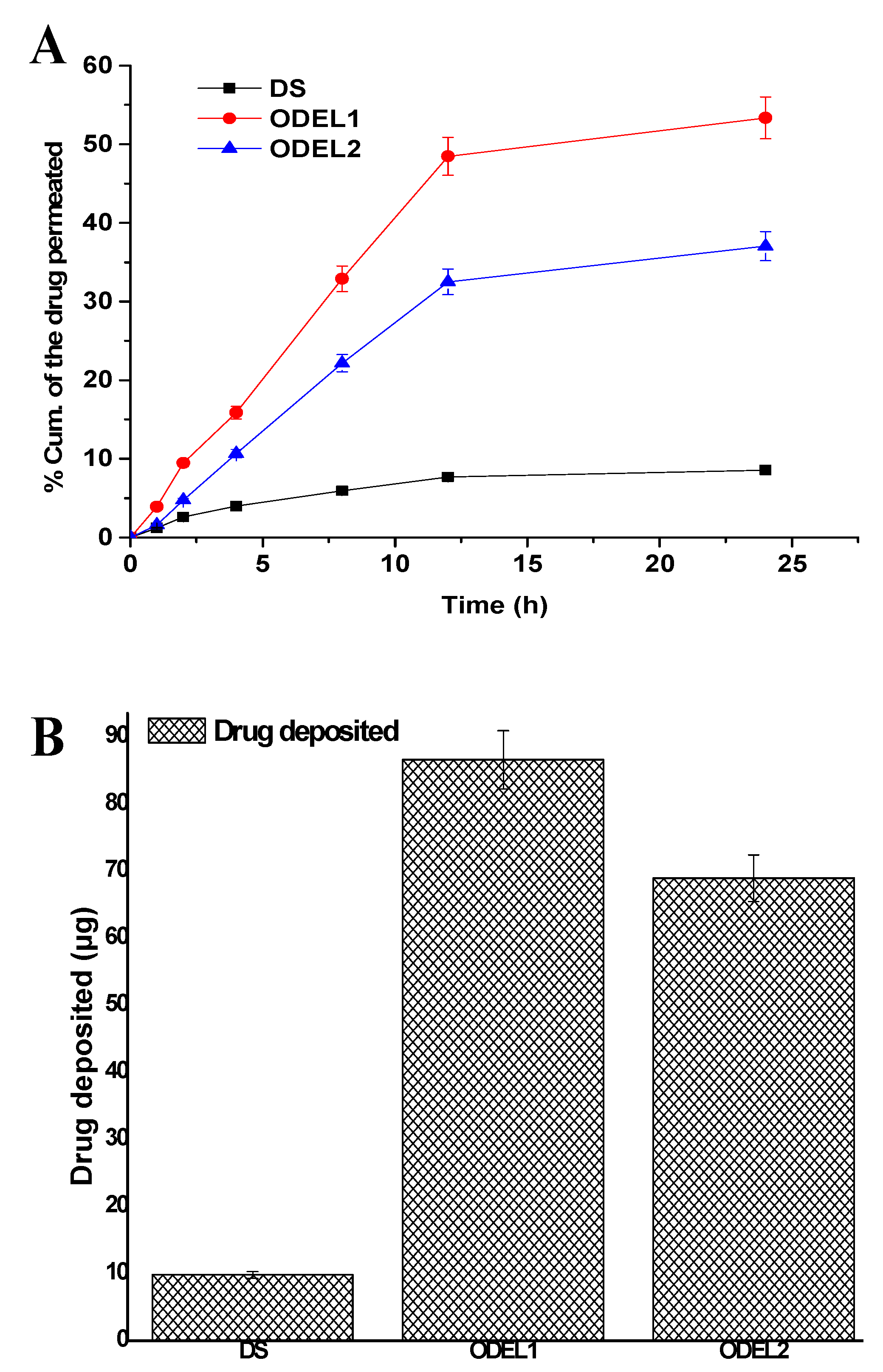
3.7. Skin Permeation Profiles and Drug Deposition
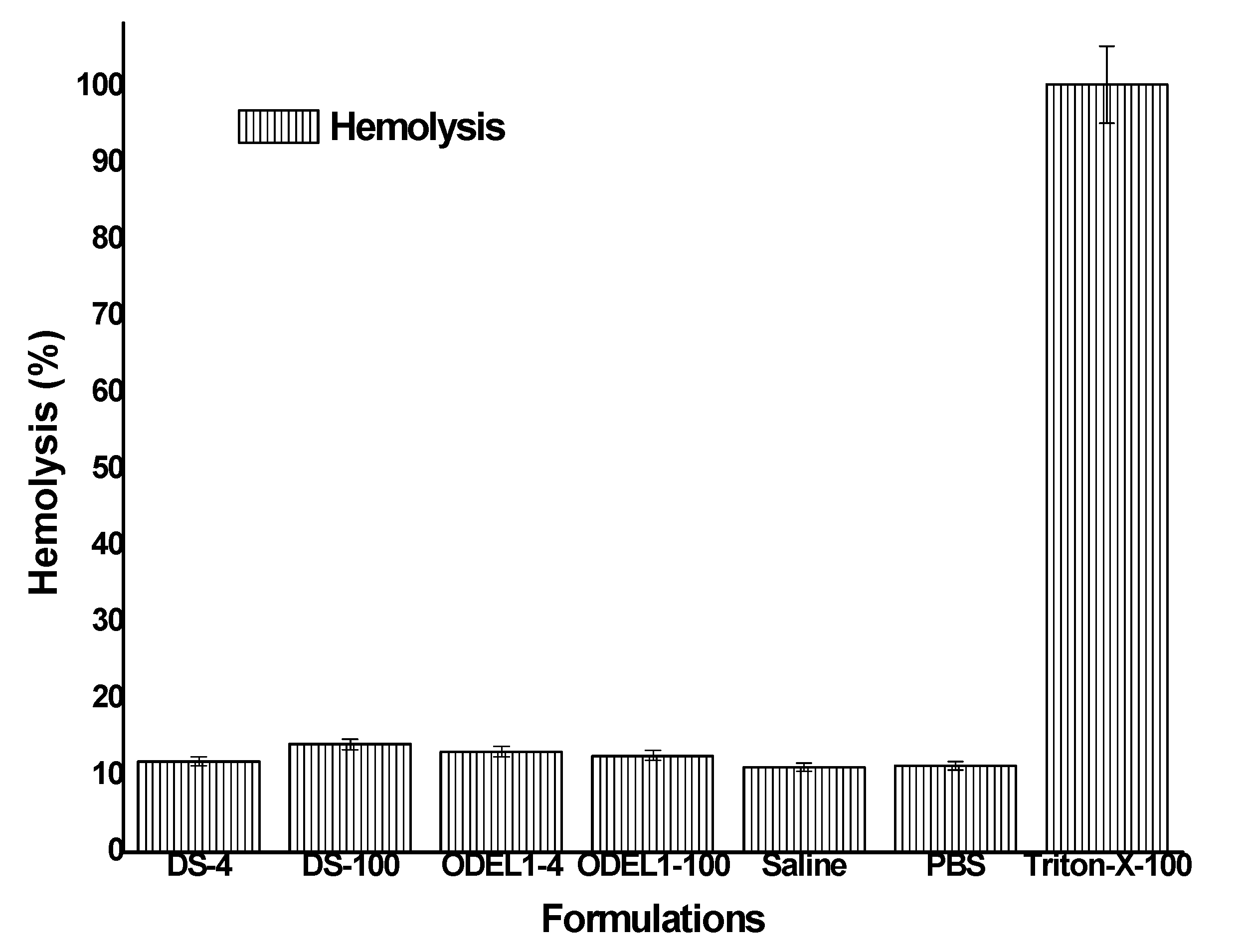
3.8. In Vitro Hemolysis Study
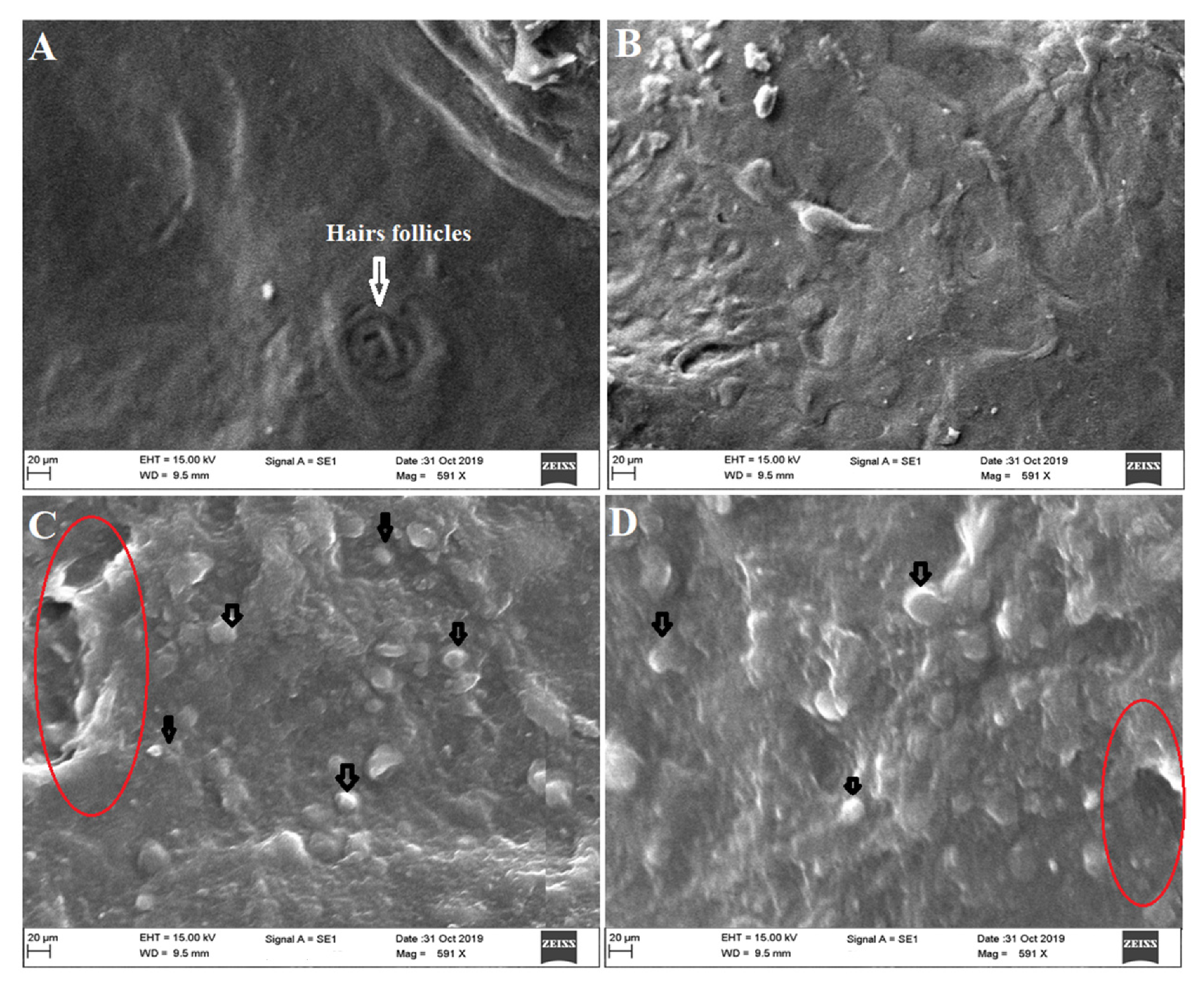
3.9. Ex Vivo Vesicles–Skin Interaction Study Using SEM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fjellestad-Paulsen, A.; Hoglund, P.; Lundin, S.; Paulsen, O. Pharmacokinetics of 1-deamino-8-D-arginine vasopressin after various routes of administration in healthy volunteers. Clin. Endocrinol. 1993, 38, 177–182. [Google Scholar] [CrossRef]
- Kahns, A.H.; Buur, A.; Bundgaard, H. Prodrugs of peptides. 18. Synthesis and evaluation of various esters of desmopressin (dDAVP). Pharm. Res. 1993, 10, 68–74. [Google Scholar] [CrossRef]
- Robson, W.L.; Leung, A.K.; Nørgaard, J.P. The comparative safety of oral versus intranasal desmopressin for the treatment of children with nocturnal enuresis. J. Urol. 2007, 178, 24–30. [Google Scholar] [CrossRef]
- Wang, J.; Shen, D.; Shen, W.C. Preparation, purification, and characterization of a reversibly lipidized desmopressin with potentiated anti-diuretic activity. Pharm. Res. 1999, 16, 1674–1679. [Google Scholar] [CrossRef]
- Wang, J.; Yadav, V.; Smart, A.L.; Tajiri, S.; Basit, A.W. Toward oral delivery of biopharmaceuticals: An assessment of the gastrointestinal stability of 17 peptide drugs. Mol. Pharm. 2015, 12, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Ilan, E.; Amselem, S.; Weisspapir, M.; Schwarz, J.; Yogev, A.; Zawoznik, E.; Friedman, D. Improved oral delivery of desmopressin via a novel vehicle: Mucoadhesive submicron emulsion. Pharm. Res. 1996, 13, 1083–1087. [Google Scholar] [CrossRef]
- Christophersen, P.C.; Zhang, L.; Mu¨llertz, A.; Neilsen, H.M.; Yang, M.; Mu, H. Solid lipid particles for oral delivery of peptide and protein drugs II–the digestion of trilaurin protects desmopressin from proteolytic degradation. Pharm. Res. 2014, 31, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, O.; Leonaviciute, G.; Lam, H.T.; Partenhauser, A.; Podričnik, S.; Bernkop-Schnürch, A. Development and in vitro evaluation of an oral SEDDS for desmopressin. Drug Deliv. 2016, 23, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Lelawongs, P.; Liu, J.-C.; Chien, Y.W. Transdermal iontophoretic delivery of arginine-vasopressin (I): Physicochemical considerations. Int. J. Pharm. 1989, 56, 13–22. [Google Scholar] [CrossRef]
- Lelawongs, P.; Liu, J.-C.; Chien, Y.W. Transdermal iontophoretic delivery of arginine-vasopressin (11): Evaluation of electrical and operational factors. Int. J. Pharm. 1990, 61, 179–188. [Google Scholar] [CrossRef]
- Morimoto, K.; Iwakura, Y.; Miyazaki, M.; Nakatani, E. Effects of proteolytic enzyme inhibitors of enhancement of transdermal iontophoretic delivery of vasopressin and an analogue in rats. Int. J. Pharm. 1992, 81, 119–125. [Google Scholar] [CrossRef]
- Nakakura, M.; Terajima, M.; Kato, Y.; Hayakawa, E.; Ito, K.; Kuroda, T. Effect of Iontophoretic Patterns on in vivo Antidiuretic Response to Desmopressin Acetate Administered Transdermally. J. Drug Target. 1995, 2, 487–492. [Google Scholar] [CrossRef]
- Nakakura, M.; Kato, Y.; Hayakawa, E.; Ito, K.; Kuroda, T. Effect of pulse on iontophoretic delivery of desmopressin acetate in rats. Biol. Pharm. Bull. 1996, 19, 738–740. [Google Scholar] [CrossRef][Green Version]
- Cormier, M.; Johnson, B.; Ameri, M.; Nyam, K.; Libiran, L.; Zhang, D.D.; Daddona, P. Transdermal delivery of desmopressin using a coated microneedle array patch system. J. Control. Release 2004, 97, 503–511. [Google Scholar] [CrossRef]
- Peralta, M.F.; Guzmán, M.L.; Pérez, A.P.; Apezteguia, G.A.; Fórmica, M.L.; Romero, E.L.; Olivera, M.E.; Carrer, D.C. Liposomes can both enhance or reduce drugs penetration through the skin. Sci. Rep. 2018, 8, 2074–2083. [Google Scholar] [CrossRef]
- Law, S.L.; Huang, K.J.; Chou, V.H.Y. Stability of Desmopressin Loaded in Liposomes. J. Liposome Res. 2003, 13, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Singh, S.; Sharma, D.; Webster, T.; Shafaat, K.; Faruk, A. Elastic liposomes as novel carriers: Recent advances in drug delivery. Int. J. Nanomed. 2017, 12, 5087–5108. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G.; Blume, G. New, highly efficient formulation of diclofenac for the topical, transdermal administration in ultradeformable drug carriers, Transfersomes. Biochim. Biophys. Acta (BBA)-Biomembr. 2001, 1514, 191–205. [Google Scholar] [CrossRef]
- Dubey, V.; Mishra, D.; Asthana, A.; Jain, N.K. Transdermal delivery of a pineal hormone: Melatonin via elastic liposomes. Biomaterials 2006, 27, 3491–3496. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Eum, J.Y.; Park, S.H.; Kang, M.H.; Park, K.H.; Choi, S.E.; Lee, M.W.; Kang, K.H.; Oh, C.H.; Choi, Y.W. Pep-1 peptide-conjugated elastic liposomal formulation of taxifolin glycoside for the treatment of atopic dermatitis in NC/Nga mice. Int. J. Pharm. 2010, 402, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Rothbard, J.B.; Garlington, S.; Lin, Q.; Kirschberg, T.; Kreider, E.; Mcgrane, P.L.; Wender, P.A.; Khavari, P.A. Conjugation of arginine oligomers to cyclosporine A facilitates topical delivery and inhibition of inflammation. Nat. Med. 2000, 6, 1253–1257. [Google Scholar] [CrossRef]
- Hou, Y.W.; Chan, M.H.; Hsu, H.R.; Liu, B.R.; Chen, C.P.; Chen, H.H.; Lee, H.J. Transdermal delivery of proteins mediated by non-covalently associated arginine-rich intracellular delivery peptides. Exp. Dermatol. 2007, 16, 999–1006. [Google Scholar] [CrossRef]
- Lopes, L.B.; Brophy, C.M.; Furnish, E.; Flynn, C.R.; Sparks, O.; Komalavilas, P.; Joshi, L.; Panitch, A.; Bentley, V.L.B. Comparative study of the skin penetration of protein transduction domains and a conjugated peptide. Pharm. Res. 2005, 22, 750–757. [Google Scholar] [CrossRef]
- El-Maghraby, G.M.M.; Williams, A.C.; Barry, A.W. Interactions of surfactants (edge activators) and skin penetration enhancers with liposomes. Int. J. Pharm. 2004, 276, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Takay, K.; Maitani, Y.; Machida, Y.; Nagai, T. Effect of pretreatment of skin with cyclic monoterpenes on permeation of diclofenac in hairless rat. Biol. Pharm. Bull. 1993, 16, 312–314. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, S.; Qin, M.; Han, Y.; Zhao, L.; Fu, Y.; Shang, Y.; Liu, Z.; Huang, Z. Assessment of the efficacy of drug transdermal delivery by electro-phonophoresis in treating tuberculous lymphadenitis. Drug Deliv. 2017, 23, 1588–1593. [Google Scholar] [CrossRef]
- Ahad, A.; Al-Saleh, A.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I.; Raish, M.; Yassin, A.E.B.; Alam, M.A. Formulation and characterization of novel soft nanovesicles for enhanced transdermal delivery of eprosartan mesylate. Saudi. Pharm. J. 2017, 25, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Dejaegher, B.; Heyden, Y.V. Experimental designs and their recent advances in set-up, data interpretation, and analytical applications. J. Pharm. Biomed. Anal. 2011, 56, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.W. LPP Theory of Skin Penetration Enhancement. J. Control. Release 1991, 15, 237–248. [Google Scholar] [CrossRef]
- Hussain, A.; Altamimi, M.A.; Alshehri, S.; Imam, S.A.; Shakeel, F.; Singh, S.K. Novel approach for transdermal delivery of rifampicin to induce synergistic anti-mycobacterial effects against cutaneous and systemic tuberculosis using a cationic nanoemulsion gel. Int. J. Nanomed. 2020, 15, 1073–1094. [Google Scholar] [CrossRef]
- Bnyan, R.; Khan, I.; Ehtezazi, T.; Saleem, I.; Gordon, S.; O’Neill, F.; Roberts, M. Surfactant effects on lipid-based vesicles properties. J. Pharmacol. Sci. 2018, 107, 1237–1246. [Google Scholar] [CrossRef]
- Jain, S.K.; Gupta, Y.; Jain, A.; Rai, K. Enhanced transdermal delivery of acyclovir sodium via elastic liposomes. Drug Deliv. 2008, 15, 141–147. [Google Scholar] [CrossRef]
- van den Bergh, B.A.; Vroom, J.; Gerritsen, H.; Junginger, H.E.; Bouwstra, J.A. Interactions of elastic and rigid vesicles with human skin in vitro: Electron microscopy and two-photon excitation microscopy. Biochim. Biophys. Acta 1999, 1461, 155–173. [Google Scholar] [CrossRef]
- Duangjit, S.; Pamornpathomkul, B.; Opanasopit, P.; Rojanarata, T.; Obata, Y.; Takayama, K.; Ngawhirunpat, T. Role of the charge, carbon chain length, and content of surfactant on the skin penetration of meloxicamloaded liposomes. Int. J. Nanomed. 2014, 9, 2005–2017. [Google Scholar] [CrossRef]
- Lopez, C.; Maza, A.; Coderch, L.; Iglesias, L.L.; Wehli, E.; Parra, J.L. Direct formation of mixed micelles in the solubilization of phospholipids liposome by Triton X-100. FEBS Lett. 1998, 426, 314–318. [Google Scholar] [CrossRef]
- Lasch, J.; Hoffman, J.; Amelyaneenka, W.G.; Klibanov, A.A.; Torchilin, V.P.; Binder, H.; Gawrisch, K. Interaction of Triton X-100 and Octylglycoside with liposomal membranes at sublytic and lytic concentration: Spectroscopic studies. Biochim. Biophys. Acta Biomembr. 1990, 1022, 171–180. [Google Scholar] [CrossRef]
- van Hal, D.A.; Bouwstra, J.A.; van Rensen, A.; Jeremiasse, E.; de Vringer, T.; Junginger, H.E. Preparation and Characterization of Nonionic Surfactant Vesicles. J. Colloid Interface Sci. 1994, 178, 263–273. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous optimization of several response variables. J. Qual. Tech. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Shaji, J.; Lal, M. Preparation, optimization and evaluation of transferosomal formulation for enhanced transdermal delivery of a COX-2 inhibitor. Int. J. Pharm. Pharm. Sci. 2014, 6, 467–477. [Google Scholar]
- Cevc, G.; Gebauer, D.; Stieber, J.; Schatzlein, A.; Blume, G. Ultraflexible vesicles. Transfersomes have an extremely low permeation resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim. Biophys. Acta 1998, 136, 8201–8215. [Google Scholar]
- Boinpally, R.R.; Zhou, S.-L.; Poondru, S.; Delran, G.; Jasti, B.R. Lecithin vesicles for topical delivery of diclofenac. Eur. J. Pharm. Biopharm. 2003, 56, 389–392. [Google Scholar] [CrossRef]
- El Maghraby, G.M.M.; Williams, A.C.; Barry, B.W. Skin delivery of 5-fluorouracil from ultradeformable and standard liposomes in-vitro. J. Pharm. Pharm. 2001, 53, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Pardakhty, A.; Val-iollah, H.; Najafabadi, A.R. Development and Physical Characterization of Sorbitan Monoester Niosomes for Insulin Oral Delivery. Drug Deliv. 2003, 10, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Nakakura, M.; Kato, Y.; Ito, K. Prolongation of antidiuretic response to desmopressin acetate by iontophoretic transdermal delivery in rats. Biol. Pharm. Bull. 1997, 20, 537–540. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakakura, M.; Kato, Y.; Ito, K. Safe and efficient transdermal delivery of desmopressin acetate by iontophoresis in rats. Biol. Pharm. Bull. 1998, 21, 268–271. [Google Scholar] [CrossRef] [PubMed][Green Version]
- FDA Label, minirin®. Available online: https://www.ferring.com/launch-of-minirin-2/ (accessed on 2 June 2021).
- Van’t Hoff, W.; Veerman, E.; Helmerhorst, E.J.; Amerongen, A. Antimicrobial peptides: Properties and applicability. Biol. Chem. 2001, 382, 597–619. [Google Scholar]
- Rowe, G.E.; Welch, R.A. Assays of hemolytic toxins. Methods Enzym. 1994, 235, 657–667. [Google Scholar]
- de Lima, J.M.; Sarmento, R.R.; de Souza, J.R.; Brayner, F.A.; Feitosa, A.P.S.; Padilha, R.; Alves, L.C.; Porto, I.J.; Batista, R.F.B.D.; de Oliveira, J.E.; et al. Evaluation of hemagglutination activity of chitosan nanoparticles using human erythrocytes. BioMed Res. Int. 2015, 2015, 247965. [Google Scholar] [CrossRef]
- Kirjavainen, M.; Urtti, A.; Jaaskelainen, I.; Suhonen, T.M.; Paronen, P.; Valjakka-Koskela, R.; Kiesvaara, J.; Mönkkönen, J. Interaction of liposomes with human skin in vitro—The influence of lipid composition and structure. Biochim. Biophys. Acta 1996, 1304, 179–189. [Google Scholar] [CrossRef]
| Formulation Code | * PC: S Ratio (%) | Surfactant Type | Hydrating Media | Mean Size (nm) | ** PDI | Elasticity | % EE |
|---|---|---|---|---|---|---|---|
| DELT90 | 90 (90:10) | Tween 80 | PBS | 335.6 ± 22.81 | 0.47 | 15.2 ± 2.1 | 23.6 ± 2.8 |
| DELT70 | 70 (70:30) | Tween 80 | PBS | 203.9 ± 10.02 | 0.36 | 27.1 ± 2.7 | 32.6 ± 3.1 |
| DELT60 | 60 (60:40) | Tween 80 | PBS | 178.1 ± 9.54 | 0.28 | 36.3 ± 4.2 | 40.2 ± 4.8 |
| DELS90 | 90 (90:10) | Span 80 | PBS | 212.7 ± 23.22 | 0.47 | 25.8 ± 2.2 | 28.8 ± 5.1 |
| DELS70 | 70 (70:30) | Span 80 | PBS | 169.5 ± 11.34 | 0.41 | 39.9 ± 3.7 | 53.8 ± 5.9 |
| DELS60 | 60 (60:40) | Span 80 | PBS | 156.6 ± 10.54 | 0.23 | 58.0 ± 2.7 | 24.7 ± 6.2 |
| DSC90 | 90 (90:10) | SC | PBS | 209.6 ± 18.5 | 0.21 | 35.2 ± 5.7 | 47.8 ± 4.6 |
| DSC70 | 70 (70:30) | SC | PBS | 149.8 ± 14.8 | 0.21 | 58.2 ± 6.8 | 73.9 ± 7.1 |
| DSC60 | 60 (60:40) | SC | PBS | 111.7 ± 9.09 | 0.11 | 66.7 ± 8.1 | 77.4 ± 6.9 |
| Liposome | PC: C: Span 80 (75: 10: 15) | PBS | 276.8 ± 20.7 | 0.29 | 11.1 ± 0.5 | 32.0 ± 1.1 | |
| Independent Variables | Levels | |||||
|---|---|---|---|---|---|---|
| Low | Middle | High | ||||
| Coded | Actual | Coded | Actual | Coded | Actual | |
| X1: PC (mg) | −1 | 200 | 0 | 250 | +1 | 300 |
| X2: SC (mg) | −1 | 20 | 0 | 70 | +1 | 120 |
| Dependent Variables | Constraints | |||||
| Low | High | Goal | Model | |||
| Y1: Vesicle size (nm) | 123.9 | 271.7 | Minimum | Linear | ||
| Y2: % EE | 39.7 | 78.9 | Maximum | Linear | ||
| Y3: % DR | 39.7 | 82.8 | In range | Linear | ||
| Y4: Permeation flux (µg/h·cm2) | 0.111 | 5.71 | In range | Linear | ||
| Combination levels of independent variables and their responses | ||||||
| Formulation Code | X1 | X2 | Y1 | Y2 | Y3 | Y4 |
| DEL1 | 200 | 20 | 229.8 | 45.2 | 39.7 | 0.111 |
| DEL2 | 200 | 70 | 167.2 | 42.1 | 46.3 | 1.43 |
| DEL3 | 200 | 120 | 123.9 | 39.7 | 51.8 | 3.71 |
| DEL4 | 250 | 20 | 252.3 | 51.3 | 40.2 | 0.247 |
| DEL5 | 250 | 70 | 139.5 | 60.2 | 69.7 | 2.78 |
| DEL6 | 250 | 120 | 130.7 | 55.4 | 82.8 | 5.71 |
| DEL7 | 300 | 20 | 271.7 | 69.5 | 41.3 | 1.37 |
| DEL8 | 300 | 70 | 151.5 | 68.9 | 52.9 | 3.58 |
| DEL9 | 300 | 120 | 132.8 | 78.9 | 67.8 | 3.61 |
| Model Coefficients | Responses | |||
|---|---|---|---|---|
| Y1 | Y2 | Y3 | Y4 | |
| B0 | 177.71 | 54.81 | 54.72 | 2.50 |
| B1 | 5.85 | 0.301 | 4.03 | +0.5515 |
| p-value | 0.5949 | 0.0001 | 0.3914 | 0.1631 |
| F-value | 0.32 | 72.34 | 0.8528 | 2.52 |
| B2 | −61.07 | 0.027 | 13.53 | 1.88 |
| p-value | 0.0011 | 0.4744 | 0.02 | 0.0016 |
| F-value | 34.33 | 0.5821 | 9.6 | 29.49 |
| Model Statistics | ||||
| r2 | 0.9898 | 0.9972 | 0.9963 | 0.9927 |
| Adjusted r2 | 0.9613 | 0.9891 | 0.9813 | 0.989 |
| Predicted r2 | 0.9601 | 0.9809 | 0.9706 | 0.981 |
| Model F-value | 17.32 | 36.46 | 5.22 | 15.99 |
| Model p-value | 0.0032 | 0.0004 | 0.048 | 0.0039 |
| Observed/(predicted) Values | ||||
| Optimized | Y1 | Y2 | Y3 | Y4 |
| ODEL1 (X1 = 285 and X2 = 115 mg) | 118.7 (122.49) | 78.9 (73.21) | 75.1 (72.28) | 5.3 (0.58) |
| ODEL 2 (X1 = 272 and X2 = 100 mg) | 131.9 (127.85) | 74.13 (73.09) | 69.0 (71.02) | 3.1 (0.29) |
| Polynomial Equations for Each Response | ||||
| Y1 = 177.71 + 5.85X1 − 61.07X2 | ||||
| Y2 = 56.81 + 15.05X1 + 1.35X2 | ||||
| Y3 = 15.61 + 0.081X1 + 0.27X2 | ||||
| Y4 = 2.51 + 0.55X1 + 1.88X2 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altamimi, M.A.; Hussain, A.; Alshehri, S.; Imam, S.S. Experimental Design Based Optimization and Ex Vivo Permeation of Desmopressin Acetate Loaded Elastic Liposomes Using Rat Skin. Pharmaceutics 2021, 13, 1047. https://doi.org/10.3390/pharmaceutics13071047
Altamimi MA, Hussain A, Alshehri S, Imam SS. Experimental Design Based Optimization and Ex Vivo Permeation of Desmopressin Acetate Loaded Elastic Liposomes Using Rat Skin. Pharmaceutics. 2021; 13(7):1047. https://doi.org/10.3390/pharmaceutics13071047
Chicago/Turabian StyleAltamimi, Mohammad A., Afzal Hussain, Sultan Alshehri, and Syed Sarim Imam. 2021. "Experimental Design Based Optimization and Ex Vivo Permeation of Desmopressin Acetate Loaded Elastic Liposomes Using Rat Skin" Pharmaceutics 13, no. 7: 1047. https://doi.org/10.3390/pharmaceutics13071047
APA StyleAltamimi, M. A., Hussain, A., Alshehri, S., & Imam, S. S. (2021). Experimental Design Based Optimization and Ex Vivo Permeation of Desmopressin Acetate Loaded Elastic Liposomes Using Rat Skin. Pharmaceutics, 13(7), 1047. https://doi.org/10.3390/pharmaceutics13071047









