A Radionuclide Generator of High-Purity Bi-213 for Instant Labeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Radionuclides
2.2. Batch Adsorption of Ra(II) and Bi(III) onto AG MP-50 Resin
2.3. Column Measurement of Capacity Factors of Fr(I) onto Actinide Resin
2.4. Column Measurement of Capacity Factors of Fr(I) and Bi(III) onto AG MP-50 Resin
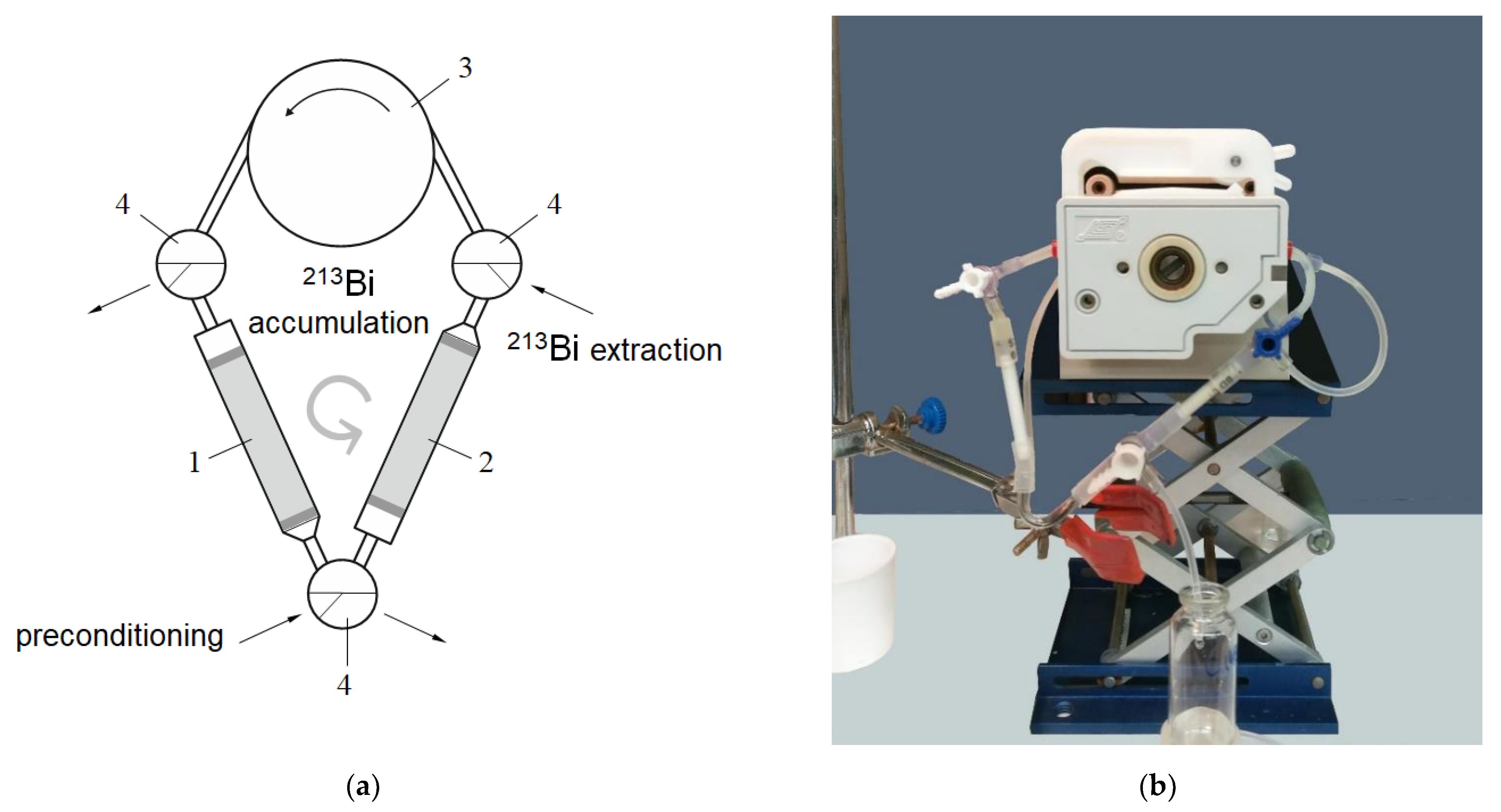
2.5. Preparation and Milking 225Ac/213Bi Generator “Afrabis”
- The first column was washed from the accumulated 223Ra with 15–20 mL of 0.25 M HNO3;
- Then, continuous separation of the intermediate daughter radionuclide 221Fr from 225Ac for ~4 h was occurring circularly (Figure 1) at flow rate 1–1.5 mL/min;
- 213Bi was desorbed from the AG MP-50 column with six 0.5 mL portions of a 0.1 M HCl/0.1 M KI solution at flow rate 1 mL/min.
2.6. Preparation and Milking A Single-Column 225Ac/213Bi Generator with AG MP-50
3. Results and Discussion
3.1. Elution of 221Fr from the Column Containing 225Ac
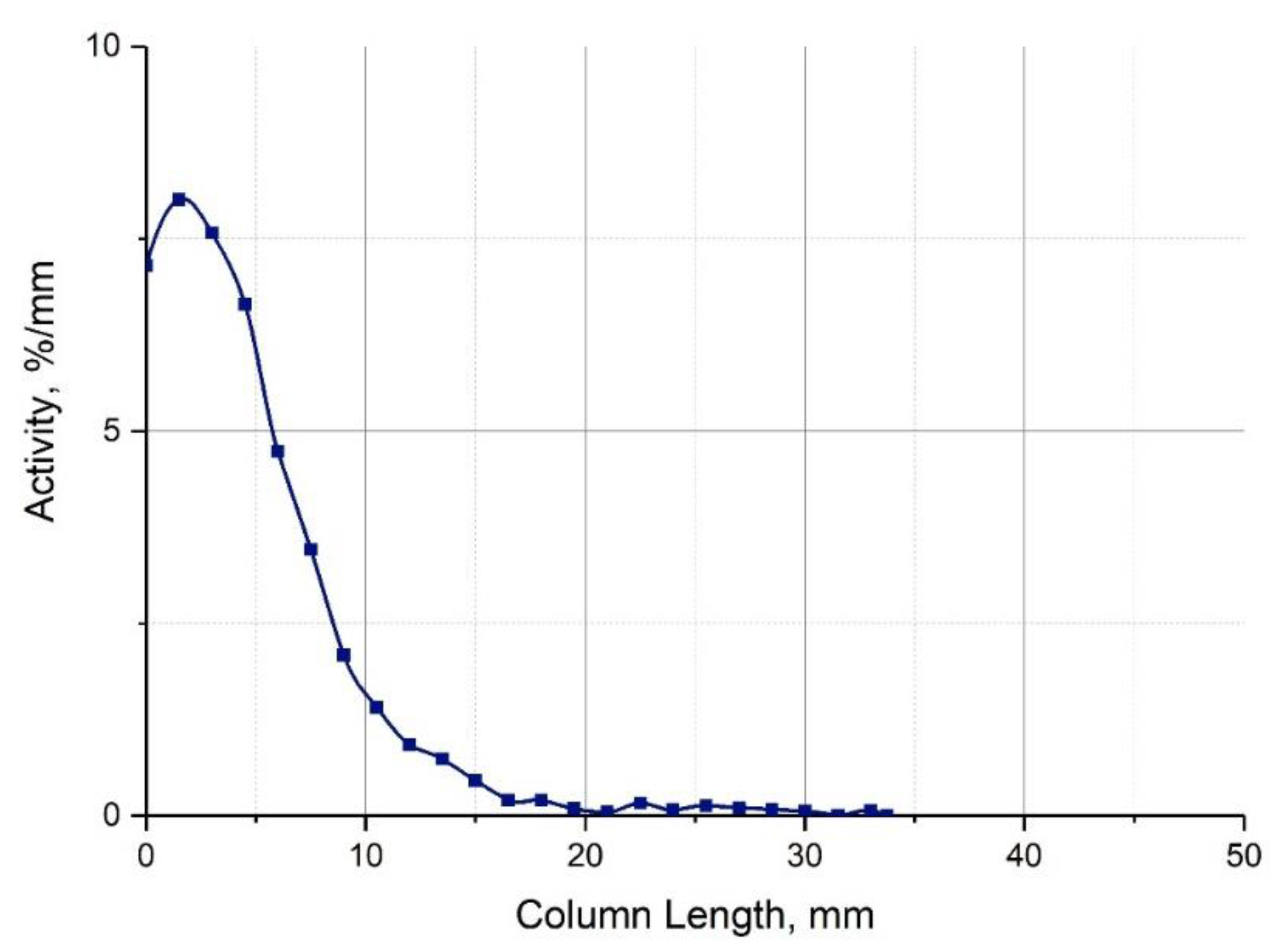
3.2. Concentrating 213Bi Apart from 225Ac
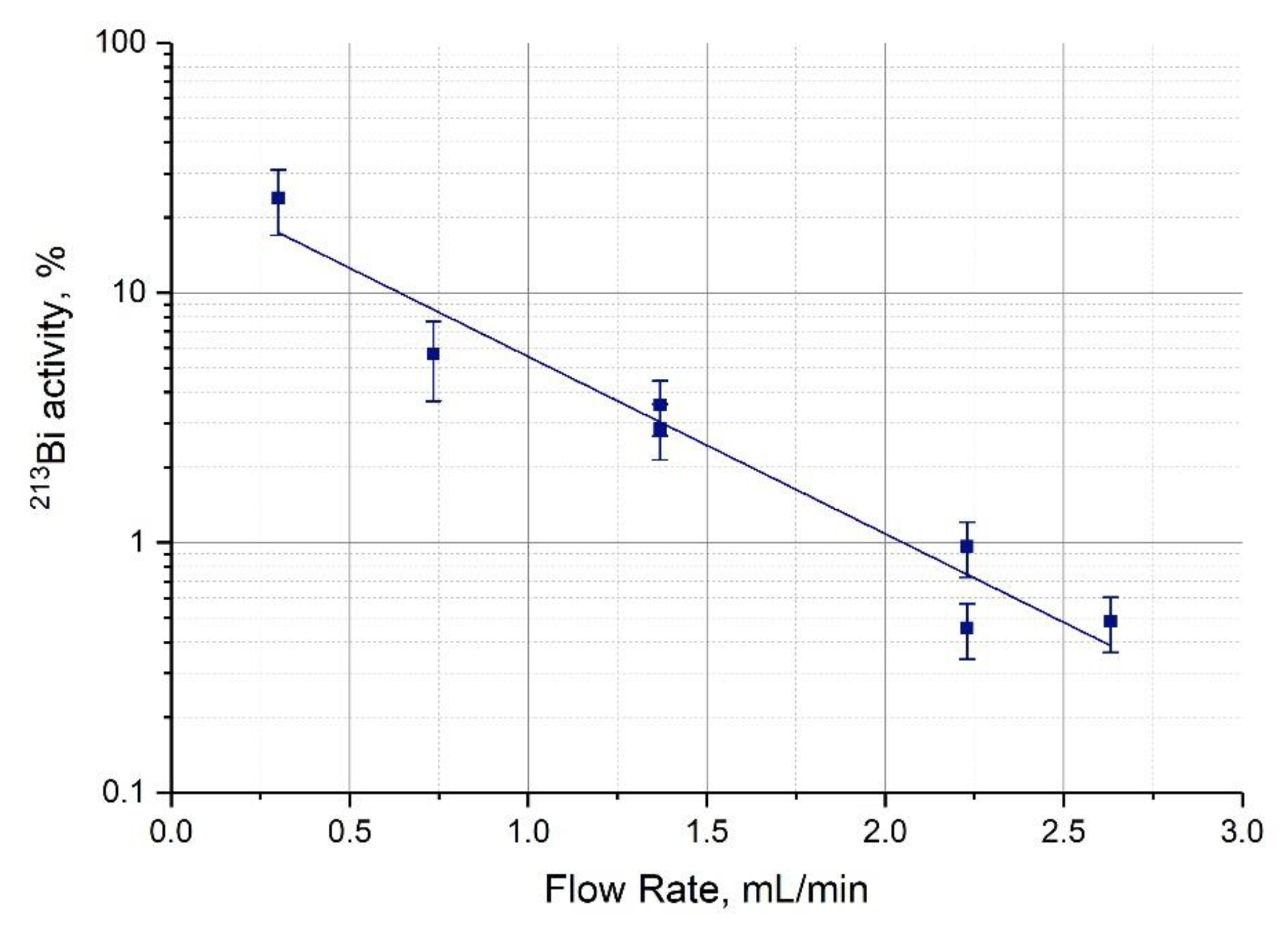
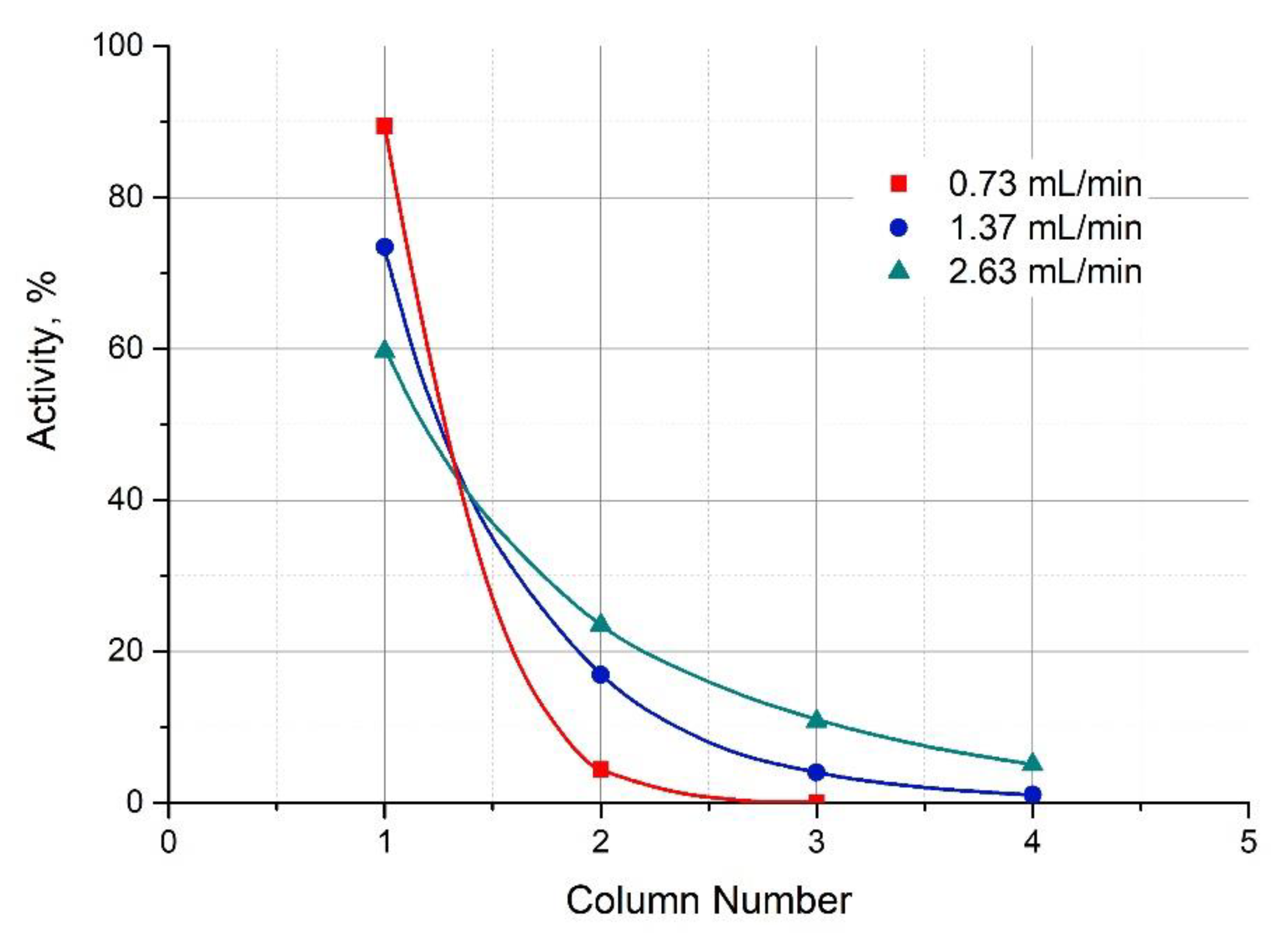
3.3. Extraction of 213Bi
3.4. Radionuclide Purity of 213Bi Extracted from the Generator “Afrabis”
3.5. Single-Column 225Ac/213Bi Generator with AG MP-50
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Ahenkorah, S.; Cassells, I.; Deroose, C.M.; Cardinaels, T.; Burgoyne, A.R.; Bormans, G.; Ooms, M.; Cleeren, F. Bismuth-213 for targeted radionuclide therapy: From atom to bedside. Pharmaceutics 2021, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, A.; Apostolidis, C.; Bruchertseifer, F. Supply and clinical application of actinium-225 and bismuth-213. Semin. Nucl. Med. 2020, 50, 119–123. [Google Scholar] [CrossRef]
- Rosenblat, T.L.; McDevitt, M.R.; Mulford, D.A.; Pandit-Taskar, N.; Divgi, C.R.; Panageas, K.S.; Heaney, M.L.; Chanel, S.; Morgenstern, A.; Sgouros, G.; et al. Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin. Cancer Res. 2010, 16, 5303–5311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, D.; Neumann, F.; Antke, C.; Apostolidis, C.; Martin, S.; Morgenstern, A.; Molinet, R.; Heeger, S.; Kronenwett, R.; Müller, H.W. Phase I clinical study on alphatherapy for non hodgkin lymphoma. In Proceedings of the 4th Alpha-Immunotherapy Symposium, ITU, Dusseldorf, Germany, 28–29 June 2004; Morgenstern, A., Ed.; ITU: Dusseldorf, Germany, 2004. [Google Scholar]
- Autenrieth, M.E.; Seidl, C.; Bruchertseifer, F.; Horn, T.; Kurtz, F.; Feuerecker, B.; D’Alessandria, C.; Pfob, C.; Nekolla, S.; Apostolidis, C.; et al. Treatment of carcinoma in situ of the urinary bladder with an alpha-emitter immunoconjugate targeting the epidermal growth factor receptor: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1364–1371. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. 213Bi-DOTATOC receptor- targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef] [Green Version]
- Krolicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Apostolidis, C.; Mirzadeh, S.; Rola, R.; et al. Safety and efficacy of targeted alpha therapy with 213Bi-DOTA-substance P in recurrent glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.J.; Singla, A.A.; Rizvi, S.M.A.; Graham, P.; Bruchertseifer, F.; Apostolidis, C.; Morgenstern, A. Analysis of patient survival in a Phase I trial of systemic targeted α-therapy for metastatic melanoma. Immunotherapy 2011, 3, 1041–1050. [Google Scholar] [CrossRef]
- Sathekge, M.; Knoesen, O.; Meckel, M.; Modiselle, M.; Vorster, M.; Marx, S. 213Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. 2017, 44, 1099–1100. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted a-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: Dosimetry estimate and empiric dose finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zacherl, M.J.; Gildehaus, F.J.; Mittlmeier, L.; Boening, G.; Gosewisch, A.; Wenter, V.; Unterrainer, M.; Schmidt-Hegemann, N.; Belka, C.; Kretschmer, A.; et al. First clinical results for PSMA targeted alpha therapy using 225 Ac-PSMA-I&T in advanced mCRPC patients. J. Nucl. Med. 2021, 62, 669–674. [Google Scholar] [CrossRef]
- Morgenstern, A.; Bruchertseifer, F.; Apostolidis, C. Bismuth-213 and actinium-225–generator performance and evolving therapeutic applications of two generator-derived alpha-emitting radioisotopes. Curr. Radiopharm. 2012, 5, 221–227. [Google Scholar] [CrossRef] [PubMed]
- McAlister, D.R.; Horwitz, E.P. Automated two column generator systems for medical radionuclides. Appl. Radiat. Isot. 2009, 67, 1985–1991. [Google Scholar] [CrossRef]
- Sinenko, I.L.; Kalmykova, T.P.; Likhosherstova, D.V.; Egorova, B.V.; Zubenko, A.D.; Vasiliev, A.N.; Ermolaev, S.V.; Lapshina, E.V.; Ostapenko, V.S.; Fedorova, O.A.; et al. 213Bi production and complexation with new picolinate containing ligands. J. Radioanal. Nucl. Chem. 2019, 321, 531–540. [Google Scholar] [CrossRef]
- Bray, L.A.; Tingey, J.M.; DesChane, J.R.; Egorov, O.B.; Tenforde, T.S.; Wilbur, D.S.; Hamlin, D.K.; Pathare, P.M. Development of a Unique Bismuth (Bi-213) automated generator for use in cancer therapy. Ind. Eng. Chem. Res. 2000, 39, 3189–3194. [Google Scholar] [CrossRef]
- Betenekov, N.D.; Denisov, E.I.; Vasiliev, A.N.; Ermolaev, S.V.; Zhuikov, B.L. Perspectives for Ac-225/Bi-213 generator developing using hydroxide inorganic sorbents. Radiochemistry 2019, 61, 211–219. [Google Scholar] [CrossRef]
- Vasiliev, A.N.; Ermolaev, S.V.; Lapshina, E.V.; Zhuikov, B.L.; Betenekov, N.D. 225Ac/213Bi generator based on inorganic sorbents. Radiochim. Acta 2019, 107, 1203–1211. [Google Scholar] [CrossRef]
- Zhuikov, B.L.; Kalmykov, S.N.; Ermolaev, S.V.; Aliev, R.A.; Kokhanyuk, V.M.; Matushko, V.L.; Tananaev, I.G.; Myasoedov, B.F. Production of 225Ac and 223Ra by irradiation of Th with accelerated protons. Radiochemistry 2011, 53, 73–80. [Google Scholar] [CrossRef]
- Ermolaev, S.V.; Zhuikov, B.L.; Kokhanyuk, V.M.; Matushko, V.L.; Kalmykov, S.N.; Aliev, R.A.; Tananaev, I.G.; Myasoedov, B.F. Production of actinium, thorium and radium isotopes from natural thorium irradiated with protons up to 141 MeV. Radiochim. Acta 2012, 100, 223–229. [Google Scholar] [CrossRef]
- Aliev, R.A.; Ermolaev, S.V.; Vasiliev, A.N.; Ostapenko, V.S.; Lapshina, E.V.; Zhuikov, B.L.; Zakharov, N.V.; Pozdeev, V.V.; Kokhanyuk, V.M.; Myasoedov, B.F.; et al. Isolation of medicine-applicable actinium-225 from thorium targets irradiated by medium-energy protons. Solvent Extr. Ion Exch. 2014, 32, 468. [Google Scholar] [CrossRef]
- Zhuikov, B.L.; Ermolaev, S.V. Radioisotope research and development at the linear accelerator of the Institute for Nuclear Research of RAS. Phys. Uspekhi 2021. to be published. [Google Scholar]
- Hoehr, C.; Bénard, F.; Buckley, K.; Crawford, J.; Gottberg, A.; Hanemaayer, V.; Kunz, P.; Ladouceur, K.; Radchenko, V.; Ramogida, C.; et al. Medical isotope production at TRIUMF-from imaging to treatment. Phys. Procedia 2017, 90, 200–208. [Google Scholar] [CrossRef]
- John, K. US DOE tri-lab research and production effort to provide accelerator- produced 225Ac for radiotherapy: 2019 update. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, S722. [Google Scholar] [CrossRef]
- Sgouros, G.; Frey, E.; He, B.; Ray, N.; Ludwig, D. Dosimetric Impact of Ac-227 in Accelerator-Produced Ac-225. J. Med. Imaging Radiat. Sci. 2019, 50, S37–S38. [Google Scholar] [CrossRef]
- Vasiliev, A.N.; Zobnin, V.A.; Pavlov, Y.S.; Chudakov, V.M. Radiation stability of sorbents in medical 225Ac/213Bi generators. Solvent Extr. Ion Exch. 2021, 39, 353–372. [Google Scholar] [CrossRef]
- Brookhaven National Laboratory. National Nuclear Data Center. Available online: http://www.nndc.bnl.gov/nudat2/ (accessed on 30 April 2021).
- Vasiliev, A.N.; Ostapenko, V.S.; Lapshina, E.V.; Ermolaev, S.V.; Danilov, S.S.; Zhuikov, B.L.; Kalmykov, S.N. Recovery of Ra-223 from natural thorium irradiated by protons. Radiochim. Acta 2016, 104, 539–547. [Google Scholar] [CrossRef]
- Strelow, F.W. Distribution coefficients and ion exchange behavior of 46 elements with a macroreticular cation exchange resin in hydrochloric acid. Anal. Chem. 1984, 56, 1053–1056. [Google Scholar] [CrossRef]
- Ostapenko, V.; Vasiliev, A.; Lapshina, E.; Ermolaev, S.; Aliev, R.; Totskiy, Y.; Zhuikov, B.; Kalmykov, S. Extraction chromatographic behavior of actinium and REE on DGA, Ln and TRU resins in nitric acid solutions. J. Radioanal. Nucl. Chem. 2015, 306, 707–711. [Google Scholar] [CrossRef]
- Ermolaev, S.V.; Skasyrskaya, A.K. Motion of genetically related 221Fr and 213Bi radionuclides in a chromatographic medium. Radiochemistry 2019, 61, 44–54. [Google Scholar] [CrossRef]
- Apostolidis, C.; Molinet, R.; Rasmussen, G.; Morgenstern, A. Production of Ac-225 from Th-229 for targeted α therapy. Anal. Chem. 2005, 77, 6288–6291. [Google Scholar] [CrossRef]
- Rösch, F.; Knapp, F.F. Radionuclide generators. In Radiochemistry and Radiopharmaceutical Chemistry in Life Sciences, 1st ed.; Vértes, A., Nagy, S., Klencsár, Z., Rösch, F., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 81–118. [Google Scholar]
- Horwitz, E.P.; Chiarizia, R.; Dietz, M.L. DIPEX: A new extraction chromatographic material for the separation and preconcentration of actinides from aqueous solution. React. Funct. Polym. 1997, 33, 25–36. [Google Scholar] [CrossRef]
- Wu, C.; Brechbiel, M.W.; Gansow, O.A. An improved generator for the production of 213Bi from 225Ac. Radiochim. Acta 1997, 79, 141–144. [Google Scholar] [CrossRef]
- Betenekov, N.D.; Ermolaev, S.V.; Skasyrskaya, A.K. Development of a 225Ac/213Bi generator with two inorganic sorbents. Processes involving radionuclides of the 225Ac subfamily. Radiochemistry 2020, 62, 524–531. [Google Scholar] [CrossRef]
- Strelow, F.W.E. Comparative distribution coefficients for some elements with a macroporous cation exchange resin in HNO3 and HCl. Solvent Extr. Ion Exch. 1988, 6, 323–334. [Google Scholar] [CrossRef]
- Strelow, F.W.E. An ion exchange selectivity scale of cations based on equilibrium distribution coefficients. Anal. Chem. 1960, 32, 1185–1188. [Google Scholar] [CrossRef]
- Strelow, F.W.; Rethemeyer, R.; Bothma, C.J.C. Ion exchange selectivity scales for cations in nitric acid and sulfuric acid media with a sulfonated polystyrene resin. Anal. Chem. 1965, 37, 106–111. [Google Scholar] [CrossRef]
- Marhol, M. Ion exchangers in analytical chemistry. Their properties and use in inorganic chemistry. In Wilson and Wilson’s Comprehensive Analytical Chemistry; Elsevier Scientific Pub. Co.: Amsterdam, The Netherlands, 1982; Volume 34. [Google Scholar]
- Spivakov, B.Y.; Stoyanov, E.S.; Gribov, L.A.; Zolotov, Y.A. Raman laser spectroscopic studies of bismuth (III) halide complexes in aqueous solutions. J. Inorg. Nucl. Chem. 1979, 41, 453–455. [Google Scholar] [CrossRef]
- Egorova, B.V.; Fedorova, O.A.; Kalmykov, S.N. Cationic radionuclides and ligands for targeted therapeutic radiopharmaceuticals. Russ. Chem. Rev. 2019, 88, 901. [Google Scholar] [CrossRef]
- Montavon, G.; Le Du, A.; Champion, J.; Rabung, T.; Morgenstern, A. DTPA complexation of bismuth in human blood serum. Dalton Trans. 2012, 41, 8615. [Google Scholar] [CrossRef]
- Nash, K.L.; Brigham, D.; Shehee, T.C.; Martin, A. The kinetics of lanthanide complexation by EDTA and DTPA in lactate media. Dalton Trans. 2012, 41, 14547–14556. [Google Scholar] [CrossRef]
- Csajbók, É.; Baranyai, Z.; Bányai, I.; Brücher, E.; Király, R.; Müller-Fahrnow, A.; Platzek, J.; Radüchel, B.; Schäfer, M. Equilibrium, 1H and 13C NMR spectroscopy, and X-ray diffraction studies on the complexes Bi (DOTA)-and Bi (DO3A-Bu). Inorg. Chem. 2003, 42, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Frischknecht, M.; Zhang, H.; Morgenstern, A.; Bruchertseifer, F.; Boisclair, J.; Provencher-Bolliger, A.; Reubi, J.-C.; Maecke, H.R. Alpha-versus beta-particle radiopeptide therapy in a human prostate cancer model (213Bi-DOTA-PESIN and 213Bi-AMBA versus177Lu-DOTA-PESIN). Cancer Res. 2011, 71, 1009–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ermolaev, S.V.; Skasyrskaya, A.K. Razrabotka tsirkuliruyushchey skhemy polucheniya 213Bi iz 221Fr, nepreryvno otdelyaemogo ot 225Ac. In Book of Abstracts, Proceedings of the IX Russian Conference “Radiochemistry 2018”; Saint-Petersburg, Russia, 17–21 September 2018, Myasoedov, B.F., Kalmykov, S.N., Petrov, V.G., Eds.; Rosatom: Saint-Petersburg, Russia, 2018. [Google Scholar]




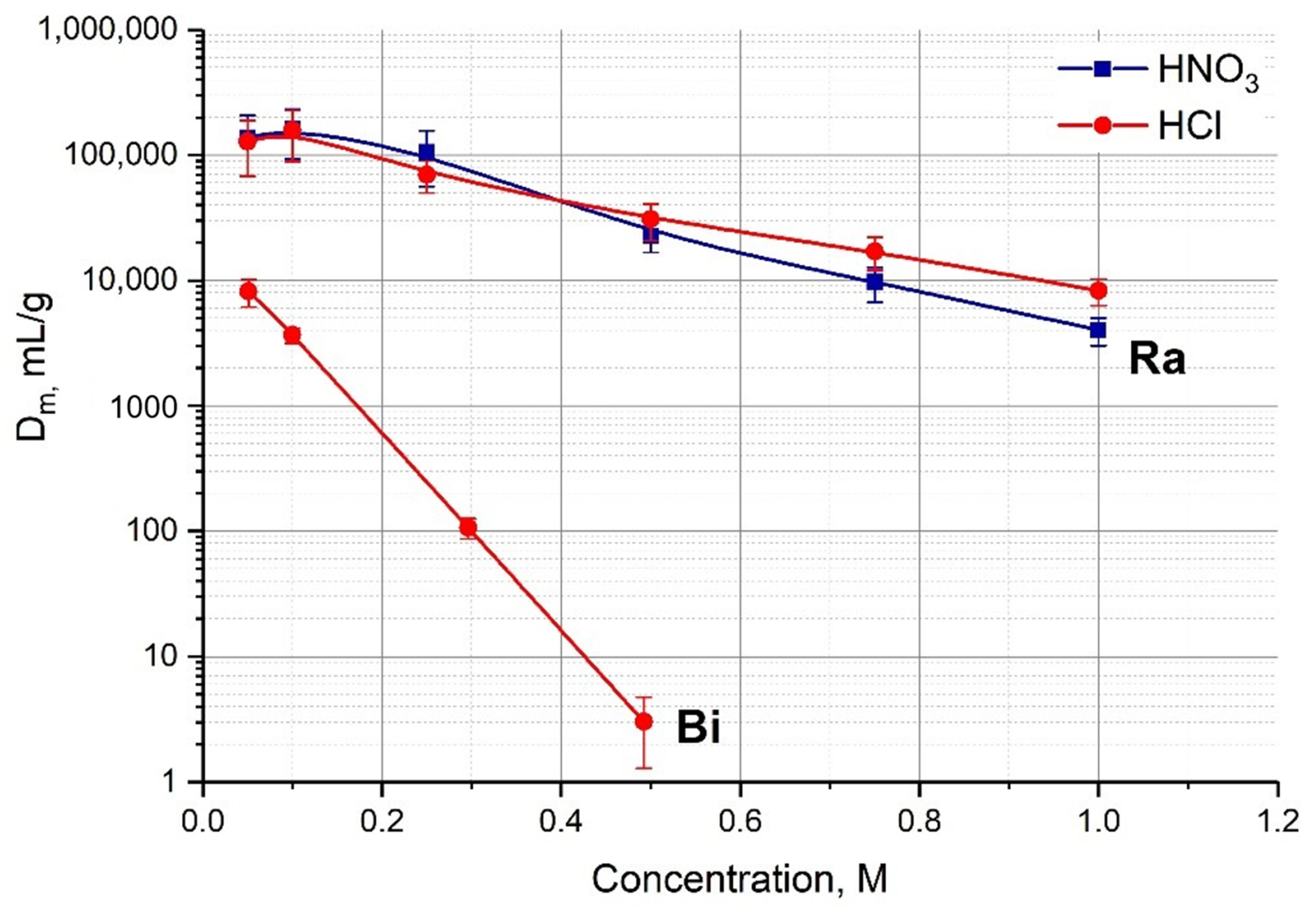

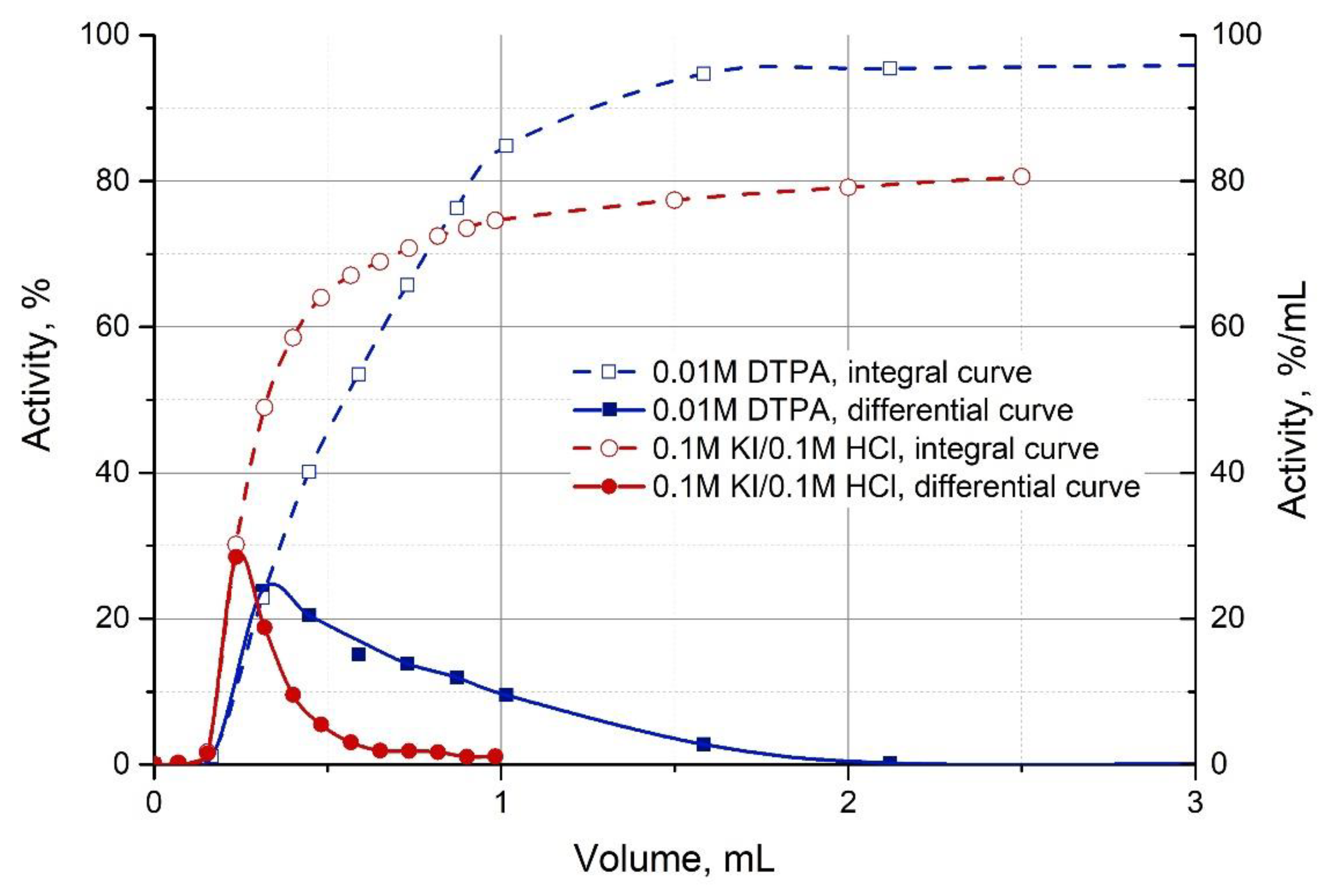


| Stationary Phase (Sorbent) | Mobile Phase (Solution) | k’ Fr(I) | k’ Bi(III) |
|---|---|---|---|
| AMP-PAN (Triskem) 1 | 0.25 M HNO3 | 2.5·102 | >3·104 |
| Dowex 50 × 8 (Dow) 1 | 0.25 M HNO3 | 102 | >6·104 |
| T-35 (Termoxid) 2 | 1 M NH4Cl, pH 6.8 | 5·102 | >5·104 |
| AG MP-50 (BioRad) | 0.25 M HNO3 | 4·102 | >5·104 |
| AG MP-50 (BioRad) | 0.017 M NaCl, pH ~6 | 103 | >5·104 |
| 225Ac/213Bi Generator | Efficiency of 213Bi Elution, %/in the First 0.5 mL of Eluate | Impurity in the First Eluate Portion of 0.5 mL, % | |||
|---|---|---|---|---|---|
| 225Ac | 227Ac | 227Th | 223Ra | ||
| “Afrabis” | 73 ± 2 | <10−6 | <10−8 ** | <10−6 | <10−6 |
| JRC (Karlsruhe, Germany) | 67 ± 2 76 ± 3 [10] * | <3.5·10−5 2·10−5 [10] * | <10−6 ** | <10−6 | 10−4–10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermolaev, S.; Skasyrskaya, A.; Vasiliev, A. A Radionuclide Generator of High-Purity Bi-213 for Instant Labeling. Pharmaceutics 2021, 13, 914. https://doi.org/10.3390/pharmaceutics13060914
Ermolaev S, Skasyrskaya A, Vasiliev A. A Radionuclide Generator of High-Purity Bi-213 for Instant Labeling. Pharmaceutics. 2021; 13(6):914. https://doi.org/10.3390/pharmaceutics13060914
Chicago/Turabian StyleErmolaev, Stanislav, Aino Skasyrskaya, and Aleksandr Vasiliev. 2021. "A Radionuclide Generator of High-Purity Bi-213 for Instant Labeling" Pharmaceutics 13, no. 6: 914. https://doi.org/10.3390/pharmaceutics13060914
APA StyleErmolaev, S., Skasyrskaya, A., & Vasiliev, A. (2021). A Radionuclide Generator of High-Purity Bi-213 for Instant Labeling. Pharmaceutics, 13(6), 914. https://doi.org/10.3390/pharmaceutics13060914






