A Biophysical Insight of Camptothecin Biodistribution: Towards a Molecular Understanding of Its Pharmacokinetic Issues
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Silico Predictors of Drug-Profiling
2.3. Membrane Models Preparation
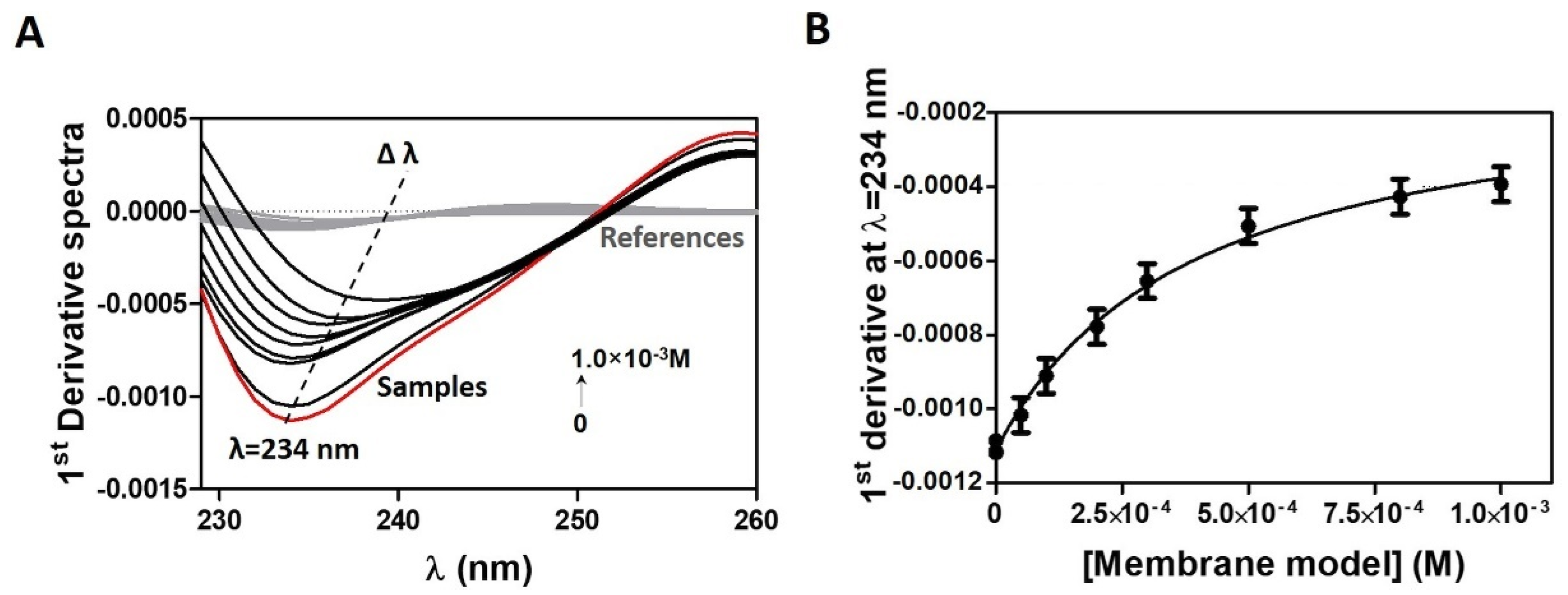
2.4. Drug Distribution Coefficient in Membrane/Aqueous Systems Studied by Derivative Spectroscopy
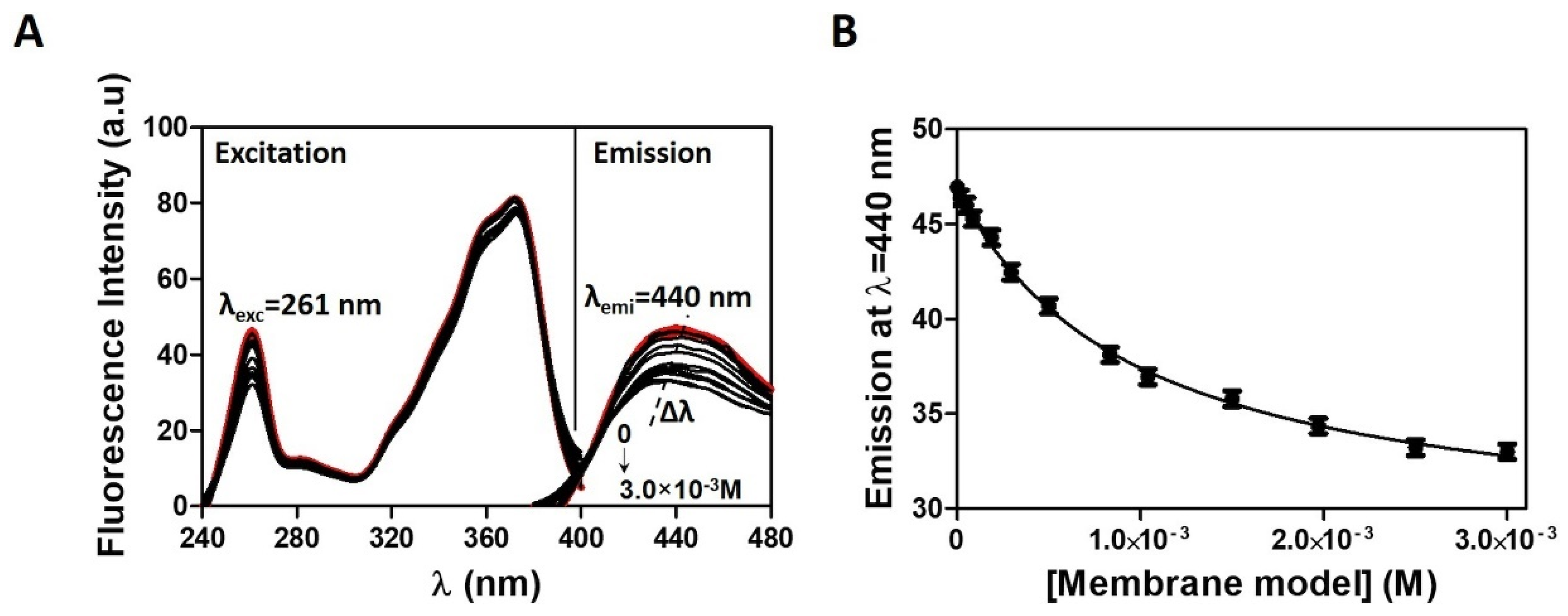
2.5. Drug Location in Membrane/Aqueous Systems Studied by Steady-State Fluorescence Quenching
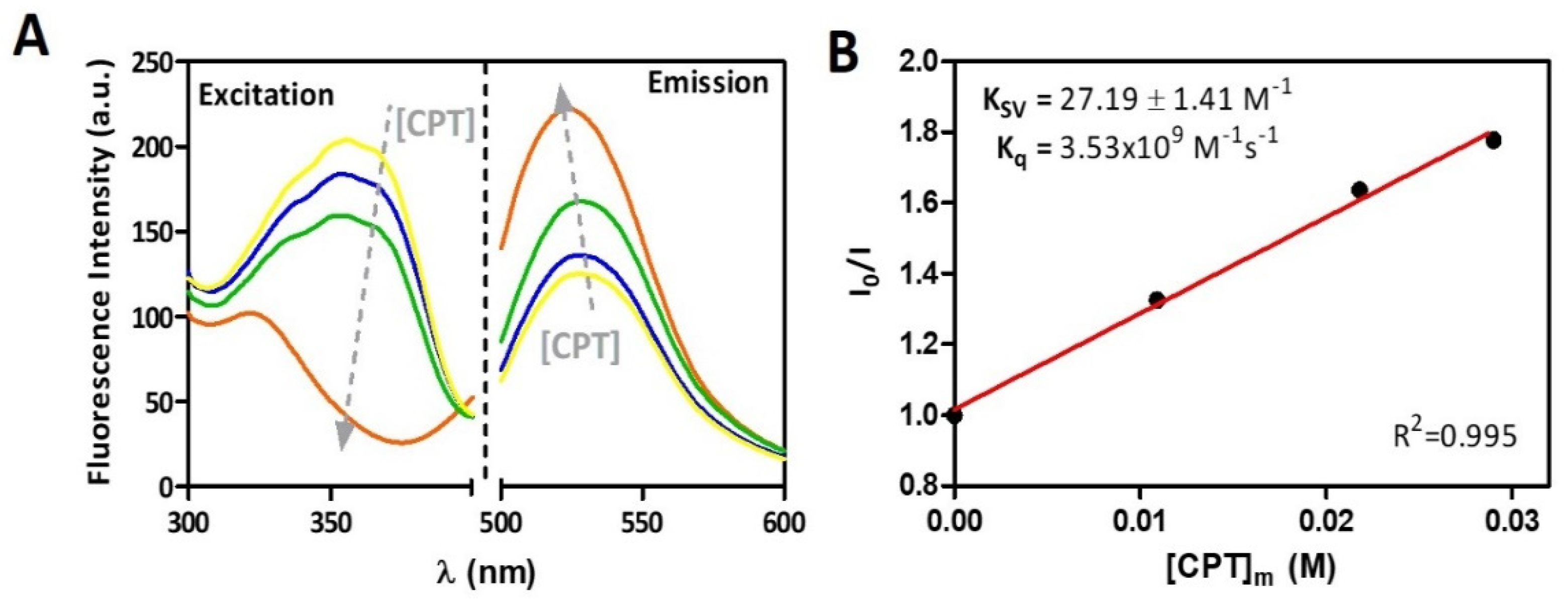
2.6. Plasma Protein Binding Evaluation Using Steady-State Fluorescence Quenching
2.7. Small- and Wide-Angle X-ray Scattering Studies at the Synchrotron
2.8. Studies on Dynamic and Electrophoretic Light Scattering
2.9. Modelling Biodistribution Using In Vitro Parameters
2.10. Statistical Analysis of Data
3. Results
3.1. In Silico Analysis of CPT Physicochemical Properties
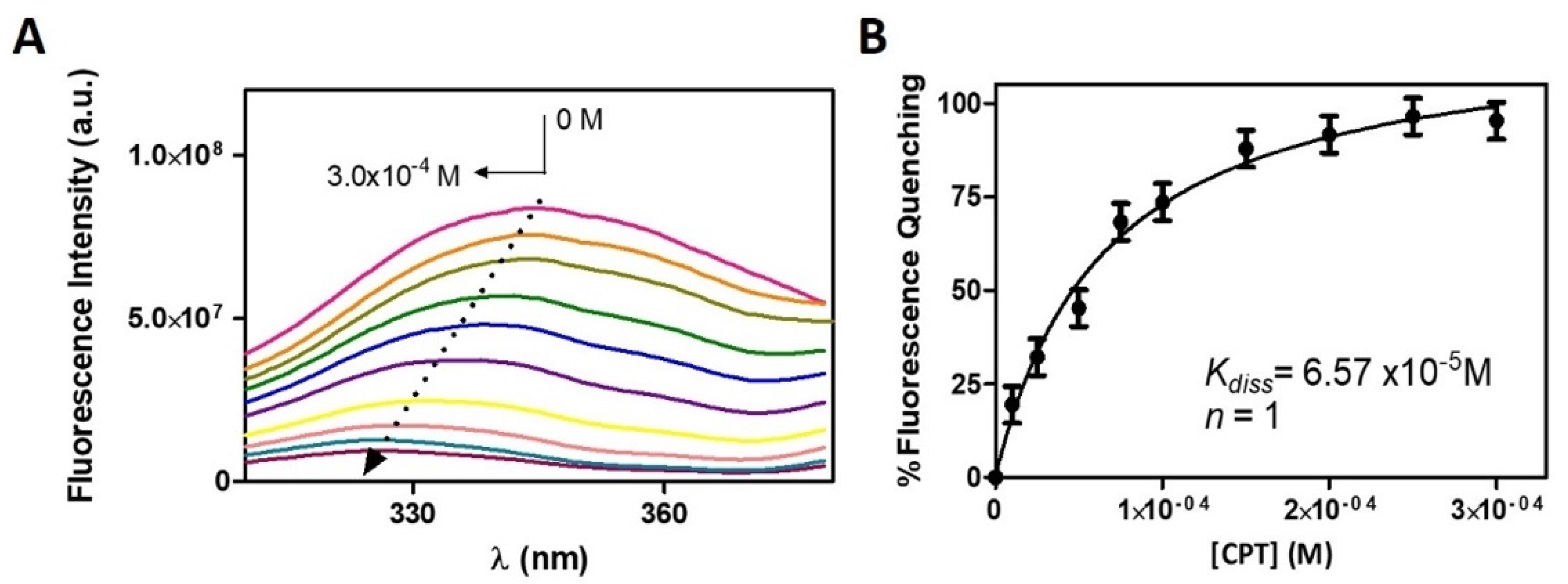
3.2. Distribution and Location of CPT in a Membrane/Aqueous System
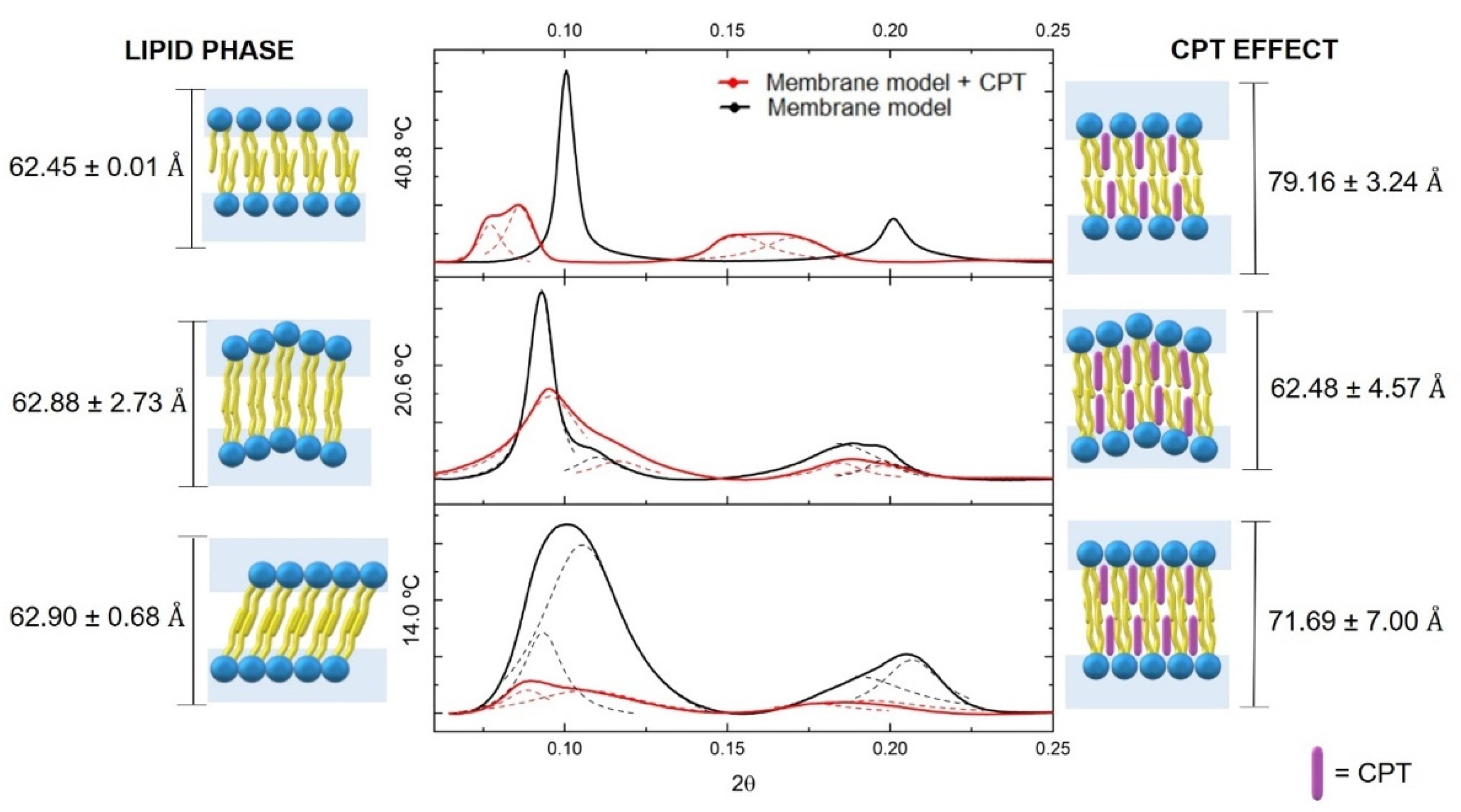
3.3. The CPT Effect on Membrane Biophysical Properties
3.4. Plasma Protein Binding of CPT and Prediction of Biodistribution Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wall, M.E.; Wani, M.C.; Cook, C.; Palmer, K.H.; McPhail, A.a.; Sim, G. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from camptotheca acuminata1, 2. J. Am. Chem. Soc. 1966, 88, 3888–3890. [Google Scholar] [CrossRef]
- Kohn, K.W.; Pommier, Y. Molecular and biological determinants of the cytotoxic actions of camptothecins: Perspective for the development of new topoisomerase I inhibitors. Ann. N. Y. Acad. Sci. 2000, 922, 11–26. [Google Scholar] [CrossRef]
- Moukharskaya, J.; Verschraegen, C. Topoisomerase 1 inhibitors and cancer therapy. Hematol. Oncol. Clin. North Am. 2012, 26, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Kruszewski, S.; Kruszewska, D. Fluorescence spectroscopy in Camptothecins studies. Acta Phys. Pol. B 2010, 118, 99–102. [Google Scholar] [CrossRef]
- Bom, D.; Curran, D.; Zhang, J.; Zimmer, S.; Bevins, R.; Kruszewski, S.; Howe, J.; Bingcang, A.; Latus, L.; Burke, T. The highly lipophilic DNA topoisomerase I inhibitor DB-67 displays elevated lactone levels in human blood and potent anticancer activity. J. Control. Release 2001, 74, 325–333. [Google Scholar] [CrossRef]
- Hu, Y.-J.; Liu, Y.; Sun, T.-Q.; Bai, A.-M.; Lü, J.-Q.; Pi, Z.-B. Binding of anti-inflammatory drug cromolyn sodium to bovine serum albumin. Int. J. Biol. Macromol. 2006, 39, 280–285. [Google Scholar] [CrossRef]
- Rösler, A.; Vandermeulen, G.W.; Klok, H.-A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 2012, 64, 270–279. [Google Scholar] [CrossRef]
- Lúcio, M.; Ferreira, H.; FC Lima, J.L.; Reis, S. Interactions between oxicams and membrane bilayers: An explanation for their different COX selectivity. Med. Chem. 2006, 2, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Lúcio, M.; Lima, J.; Reis, S. Drug-membrane interactions: Significance for medicinal chemistry. Curr. Med. Chem. 2010, 17, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Peetla, C.; Vijayaraghavalu, S.; Labhasetwar, V. Biophysics of cell membrane lipids in cancer drug resistance: Implications for drug transport and drug delivery with nanoparticles. Adv. Drug Deliv. Rev. 2013, 65, 1686–1698. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Li, Y.; Li, D.; Xu, L.; Hou, T. The application of in silico drug-likeness predictions in pharmaceutical research. Adv. Drug Deliv. Rev. 2015, 86, 2–10. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Liu, G.; Tang, Y. In silico ADMET prediction: Recent advances, current challenges and future trends. Curr. Top. Med. Chem. 2013, 13, 1273–1289. [Google Scholar] [CrossRef]
- Mi, Z.; Burke, T.G. Marked interspecies variations concerning the interactions of camptothecin with serum albumins: A frequency-domain fluorescence spectroscopic study. Biochemistry 1994, 33, 12540–12545. [Google Scholar] [CrossRef] [PubMed]
- Fleury, F.; Ianoul, A.; Berjot, M.; Feofanov, A.; Alix, A.J.; Nabiev, I. Camptothecin-binding site in human serum albumin and protein transformations induced by drug binding. FEBS letters 1997, 411, 215–220. [Google Scholar] [CrossRef]
- Burke, T.G.; Mi, Z. The structural basis of camptothecin interactions with human serum albumin: Impact on drug stability. J. Med. Chem. 1994, 37, 40–46. [Google Scholar] [CrossRef]
- Kruszweski, S.; Burke, T.G. Camptothecins affinity to HSA and membranes determined by fluorescence anisotropy measurements. Opt. Appl. 2002, 32, 721–730. [Google Scholar]
- Li, Q.; Zhu, Q.; Deng, X.; He, W.; Zhao, T.; Zhang, B. Binding interactions of water-soluble camptothecin derivatives with bovine serum albumin. Spectrochim. Acta A 2012, 86, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.K.; Chakraborty, K.; Hu, W.; Kang, M.; Loverde, S.M. Interaction of Camptothecin with Model Cellular Membranes. J. Chem. Theory Comput. 2020, 16, 3373–3384. [Google Scholar] [CrossRef]
- Lawrence, X.Y. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm. Res. 2008, 25, 781–791. [Google Scholar]
- Zhang, H. Thin-film hydration followed by extrusion method for liposome preparation. In Liposomes; Springer: Berlin/Heidelberg, Germany, 2017; pp. 17–22. [Google Scholar]
- Lúcio, M.; Ferreira, H.; Lima, J.L.; Matos, C.; de Castro, B.; Reis, S. Influence of some anti-inflammatory drugs in membrane fluidity studied by fluorescence anisotropy measurements. Phys. Chem. Chem. Phys. 2004, 6, 1493–1498. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Nunes, C.; Lúcio, M.; Segundo, M.A.; Reis, S.; Lima, J.L. High-throughput microplate assay for the determination of drug partition coefficients. Nat. Protoc. 2010, 5, 1823–1830. [Google Scholar] [CrossRef]
- Fernandes, E.; Soares, T.B.; Gonçalves, H.; Lúcio, M. Spectroscopic studies as a toolbox for biophysical and chemical characterization of lipid-based nanotherapeutics. Front. Chem. 2018, 6, 323. [Google Scholar] [CrossRef]
- Fernandes, E.; Soares, T.B.; Gonçalves, H.; Bernstorff, S.; Real Oliveira, M.E.C.; Lopes, C.M.; Lúcio, M. A molecular biophysical approach to diclofenac topical gastrointestinal damage. Int. J. Mol. Sci. 2018, 19, 3411. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of fluorescence spectroscopy; Springer science & business media: New York, NY, USA, 2013. [Google Scholar]
- Carvalho, A.M.; Fernandes, E.; Gonçalves, H.; Giner-Casares, J.J.; Bernstorff, S.; Nieder, J.B.; Oliveira, M.E.C.R.; Lúcio, M. Prediction of paclitaxel pharmacokinetic based on in vitro studies: Interaction with membrane models and human serum albumin. Int. J. Pharm. 2020, 580, 119222. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H. Drug-Like Properties: Concepts, Structure Design and Methods from ADME to Toxicity Optimization; Academic press: Cambridge, MA, USA, 2015. [Google Scholar]
- Seydel, J.K.; Wiese, M. Drug-Membrane Interactions: Analysis, Drug Distribution, Modeling; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 15. [Google Scholar]
- van de Waterbeemd, H.; Kansy, M. Hydrogen-bonding capacity and brain penetration. CHIMIA Int. J. Chem. 1992, 46, 299–303. [Google Scholar]
- Liu, X.; Tu, M.; Kelly, R.S.; Chen, C.; Smith, B.J. Development of a computational approach to predict blood-brain barrier permeability. Drug Metab. Dispos. 2004, 32, 132–139. [Google Scholar] [CrossRef]
- Korzekwa, K.; Nagar, S. Drug distribution part 2. Predicting volume of distribution from plasma protein binding and membrane partitioning. Pharm. Res. 2017, 34, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, F.; Obach, R.S.; Shalaeva, M.Y.; Gao, F. Prediction of volume of distribution values in humans for neutral and basic drugs using physicochemical measurements and plasma protein binding data. J. Med. Chem. 2002, 45, 2867–2876. [Google Scholar] [CrossRef] [PubMed]
- Musteata, F.M. Clinical utility of free drug monitoring. Ther. Drug Monit. Newer Drugs Biomarkers 2012, 75–101. [Google Scholar]
- Waters, N.J.; Jones, R.; Williams, G.; Sohal, B. Validation of a rapid equilibrium dialysis approach for the measurement of plasma protein binding. J. Pharm. Sci. 2008, 97, 4586–4595. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Clark, D.E. Rapid calculation of polar molecular surface area and its application to the prediction of transport phenomena. 1. Prediction of intestinal absorption. J. Pharm. Sci. 1999, 88, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Mandic, Z. Physico Chemical Methods in Drug Discovery and Development; IAPC Publishing: Zagreb, Croatia, 2012. [Google Scholar]
- Martínez-Martínez, M.; Rodríguez-Berna, G.; Bermejo, M.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M.; Merino, V. Covalently crosslinked organophosphorous derivatives-chitosan hydrogel as a drug delivery system for oral administration of camptothecin. Eur. J. Pharm. Biopharm. 2019, 136, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Benet, L.Z.; Broccatelli, F.; Oprea, T.I. BDDCS applied to over 900 drugs. AAPS J. 2011, 13, 519–547. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Lee, S.-K.; Lim, J.-L.; Shin, H.-J.; Hong, C.I. Preformulation studies of a novel camptothecin anticancer agent, CKD-602: Physicochemical characterization and hydrolytic equilibrium kinetics. Int. J. Pharm. 2002, 239, 207–211. [Google Scholar] [CrossRef]
- Rahman, S.; Patel, D.; Savva, M. Physicochemical Characterization of 9-Aminocamptothecin in Aqueous Solutions. AAPS PharmSciTech 2014, 15, 223–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dickins, M.; van de Waterbeemd, H. Simulation models for drug disposition and drug interactions. Drug Discov. Today: Biosilico 2004, 2, 38–45. [Google Scholar] [CrossRef]
- Choi, G.-W.; Lee, Y.-B.; Cho, H.-Y. Interpretation of non-clinical data for prediction of human pharmacokinetic parameters: In vitro-in vivo extrapolation and allometric scaling. Pharmaceutics 2019, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Poulin, P.; Theil, F.P. Prediction of pharmacokinetics prior to in vivo studies. 1. Mechanism-based prediction of volume of distribution. J. Pharm. Sci. 2002, 91, 129–156. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Chen, H.; Liang, D.; Lin, W.; Qi, X.; Liu, H.; Deng, X. Acidic pH and high-H2O2 dual tumor microenvironment-responsive nanocatalytic graphene oxide for cancer selective therapy and recognition. ACS Appl. Mater. Interfaces 2019, 11, 11157–11166. [Google Scholar] [CrossRef]
- Pignatello, R.; Musumeci, T.; Basile, L.; Carbone, C.; Puglisi, G. Biomembrane models and drug-biomembrane interaction studies: Involvement in drug design and development. J. Pharm. Bioallied Sci. 2011, 3, 4. [Google Scholar] [CrossRef]
- Bemporad, D.; Essex, J.W.; Luttmann, C. Permeation of Small Molecules through a Lipid Bilayer: A Computer Simulation Study. J. Phys. Chem. B 2004, 108, 4875–4884. [Google Scholar] [CrossRef]
- Oliveira, C.; Bagetta, D.; Cagide, F.; Teixeira, J.; Amorim, R.; Silva, T.; Garrido, J.; Remião, F.; Uriarte, E.; Oliveira, P.J. Benzoic acid-derived nitrones: A new class of potential acetylcholinesterase inhibitors and neuroprotective agents. Eur. J. Med. Chem. 2019, 174, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Leo, A.; Hansch, C.; Elkins, D. Partition coefficients and their uses. Chem. Rev. 1971, 71, 525–616. [Google Scholar] [CrossRef]
- Selvi, B.; Patel, S.; Savva, M. Physicochemical characterization and membrane binding properties of camptothecin. J. Pharm. Sci. 2008, 97, 4379–4390. [Google Scholar] [CrossRef]
- Chapeaux, A.; Simoni, L.D.; Stadtherr, M.A.; Brennecke, J.F. Liquid phase behavior of ionic liquids with water and 1-octanol and modeling of 1-octanol/water partition coefficients. J. Chem. Eng. Data 2007, 52, 2462–2467. [Google Scholar] [CrossRef]
- Giaginis, C.; Tsantili-Kakoulidou, A. Alternative measures of lipophilicity: From octanol–water partitioning to IAM retention. J. Pharm. Sci. 2008, 97, 2984–3004. [Google Scholar] [CrossRef]
- Azevedo, A.M.O.; Ribeiro, D.M.G.; Pinto, P.C.A.G.; Lúcio, M.; Reis, S.; Saraiva, M.L.M.F.S. Imidazolium ionic liquids as solvents of pharmaceuticals: Influence on HSA binding and partition coefficient of nimesulide. Int. J. Pharm. 2013, 443, 273–278. [Google Scholar] [CrossRef]
- Brittes, J.; Lúcio, M.; Nunes, C.; Lima, J.L.F.C.; Reis, S. Effects of resveratrol on membrane biophysical properties: Relevance for its pharmacological effects. Chem. Phys. Lipids 2010, 163, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Hodges, G.; Eadsforth, C.; Bossuyt, B.; Bouvy, A.; Enrici, M.-H.; Geurts, M.; Kotthoff, M.; Michie, E.; Miller, D.; Müller, J.; et al. A comparison of log Kow (n-octanol–water partition coefficient) values for non-ionic, anionic, cationic and amphoteric surfactants determined using predictions and experimental methods. Environ. Sci. Eur. 2019, 31, 1. [Google Scholar] [CrossRef]
- Pennington, E.R.; Fix, A.; Sullivan, E.M.; Brown, D.A.; Kennedy, A.; Shaikh, S.R. Distinct membrane properties are differentially influenced by cardiolipin content and acyl chain composition in biomimetic membranes. Biochim. Biophys. Acta Biomembr. 2017, 1859, 257–267. [Google Scholar] [CrossRef]
- Loura, L.; Prieto, M.J. FRET in membrane biophysics: An overview. Front. Physiol. 2011, 2, 82. [Google Scholar] [CrossRef] [PubMed]
- Raghuraman, H.; Shrivastava, S.; Chattopadhyay, A. Monitoring the looping up of acyl chain labeled NBD lipids in membranes as a function of membrane phase state. Biochim. Biophys. Acta Biomembr. 2007, 1768, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.; Charalambous, K.; Gee, A.; Law, R.V.; Ces, O. Amphiphilic drug interactions with model cellular membranes are influenced by lipid chain-melting temperature. J. R. Soc. Interface 2014, 11, 20131062. [Google Scholar] [CrossRef]
- Lúcio, M.; Nunes, C.; Gaspar, D.; Gołębska, K.; Wisniewski, M.; Lima, J.; Brezesinski, G.; Reis, S. Effect of anti-inflammatory drugs in phosphatidylcholine membranes: A fluorescence and calorimetric study. Chem. Phys. Lett. 2009, 471, 300–309. [Google Scholar] [CrossRef]
- Michel, N.; Fabiano, A.-S.; Polidori, A.; Jack, R.; Pucci, B. Determination of phase transition temperatures of lipids by light scattering. Chem. Phys. Lipids 2006, 139, 11–19. [Google Scholar] [CrossRef]
- Jain, M.K.; Wu, N.M. Effect of small molecules on the dipalmitoyl lecithin liposomal bilayer: III. Phase transition in lipid bilayer. J. Membr. Biol. 1977, 34, 157–201. [Google Scholar] [CrossRef]
- Tristram-Nagle, S.; Nagle, J.F. Lipid bilayers: Thermodynamics, structure, fluctuations, and interactions. Chem. Phys. Lipids 2004, 127, 3–14. [Google Scholar] [CrossRef]
- Martins, S.M.; Sarmento, B.; Nunes, C.; Lúcio, M.; Reis, S.; Ferreira, D.C. Brain targeting effect of camptothecin-loaded solid lipid nanoparticles in rat after intravenous administration. Eur. J. Pharm. Biopharm. 2013, 85, 488–502. [Google Scholar] [CrossRef]
- Schanker, L. Absorption of drugs from the gastrointestinal tract. In Concepts in Biochemical Pharmacology; Springer: Berlin/Heidelberg, Germany, 1971; pp. 9–24. [Google Scholar]
- Sigurdsson, H.H.; Kirch, J.; Lehr, C.-M. Mucus as a barrier to lipophilic drugs. Int. J. Pharm. 2013, 453, 56–64. [Google Scholar] [CrossRef]
- Norvaisas, P.; Ziemys, A. The role of payload hydrophobicity in nanotherapeutic pharmacokinetics. J. Pharm. Sci. 2014, 103, 2147–2156. [Google Scholar] [CrossRef]
- Lambrinidis, G.; Vallianatou, T.; Tsantili-Kakoulidou, A. In vitro, in silico and integrated strategies for the estimation of plasma protein binding. A review. Adv. Drug Deliv. Rev. 2015, 86, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Kerns, E. Pharmaceutical profiling in discovery research. Curr. Opin. Chem. Biol. 2003, 7, 402–408. [Google Scholar] [CrossRef]
- Ballard, P.; Brassil, P.; Bui, K.; Dolgos, H.; Petersson, C.; Tunek, A.; Webborn, P. Metabolism and pharmacokinetic optimization strategies in drug discovery. In Drug Discovery and Development-E-Book: Technology in Transition; Elsevier: Amsterdam, The Netherlands, 2012; p. 135. [Google Scholar]
- Gerrits, C.; Creemers, G.; Schellens, J.; Wissel, P.; Planting, A.; Kunka, R.; Selinger, K.; de Boer-Dennert, M.; Marijnen, Y.; Harteveld, M. Phase I and pharmacological study of the new topoisomerase I inhibitor GI147211, using a daily x 5 intravenous administration. Br. J. Cancer 1996, 73, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, D.J.; Abernethy, D.R.; Divoll, M. Is volume of distribution at steady state a meaningful kinetic variable? J. Clin. Pharmacol. 1983, 23, 391–400. [Google Scholar] [CrossRef]
- Venditto, V.J.; Simanek, E.E. Cancer therapies utilizing the camptothecins: A review of the in vivo literature. Mol. Pharm. 2010, 7, 307–349. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, Y.; Liu, X.; Zhang, W.; Xiong, X.; Han, Z.; Liang, X. Camptothecin-based nanodrug delivery systems. Cancer Biol. Med. 2017, 14, 363. [Google Scholar] [PubMed]
- Watanabe, M.; Kawano, K.; Yokoyama, M.; Opanasopit, P.; Okano, T.; Maitani, Y. Preparation of camptothecin-loaded polymeric micelles and evaluation of their incorporation and circulation stability. Int. J. Pharm. 2006, 308, 183–189. [Google Scholar] [CrossRef]
- Kawano, K.; Watanabe, M.; Yamamoto, T.; Yokoyama, M.; Opanasopit, P.; Okano, T.; Maitani, Y. Enhanced antitumor effect of camptothecin loaded in long-circulating polymeric micelles. J. Control. Release 2006, 112, 329–332. [Google Scholar] [CrossRef]
- Almeida, A.; Araújo, M.; Novoa-Carballal, R.; Andrade, F.; Gonçalves, H.; Reis, R.L.; Lúcio, M.; Schwartz Jr, S.; Sarmento, B. Novel amphiphilic chitosan micelles as carriers for hydrophobic anticancer drugs. Mater. Sci. Eng. C 2020, 112, 110920. [Google Scholar] [CrossRef]
- Burke, T.G.; Staubus, A.E.; Mishra, A.; Malak, H. Liposomal stabilization of camptothecin’s lactone ring. J. Am. Chem. Soc. 1992, 114, 8318–8319. [Google Scholar] [CrossRef]
- Watanabe, M.; Kawano, K.; Toma, K.; Hattori, Y.; Maitani, Y. In vivo antitumor activity of camptothecin incorporated in liposomes formulated with an artificial lipid and human serum albumin. J. Control. Release 2008, 127, 231–238. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Du, Y.; Zhou, W.; Yao, C.; Li, X. Redox-sensitive dimeric camptothecin phosphatidylcholines-based liposomes for improved anticancer efficacy. Nanomedicine 2019, 14, 3057–3074. [Google Scholar] [CrossRef]
- Lúcio, M.; Lopes, C.M.; Fernandes, E.; Gonẹalves, H.; Oliveira, M.E.C.R. Organic Nanocarriers for Brain Drug Delivery. In Nanoparticles for Brain Drug Delivery; Jenny Stanford Publishing: New York, NY, USA, 2021; pp. 75–160. [Google Scholar]
- Xia, M.; Zhang, Y.; Jin, K.; Lu, Z.; Zeng, Z.; Xiong, W. Communication between mitochondria and other organelles: A brand-new perspective on mitochondria in cancer. Cell Biosci. 2019, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
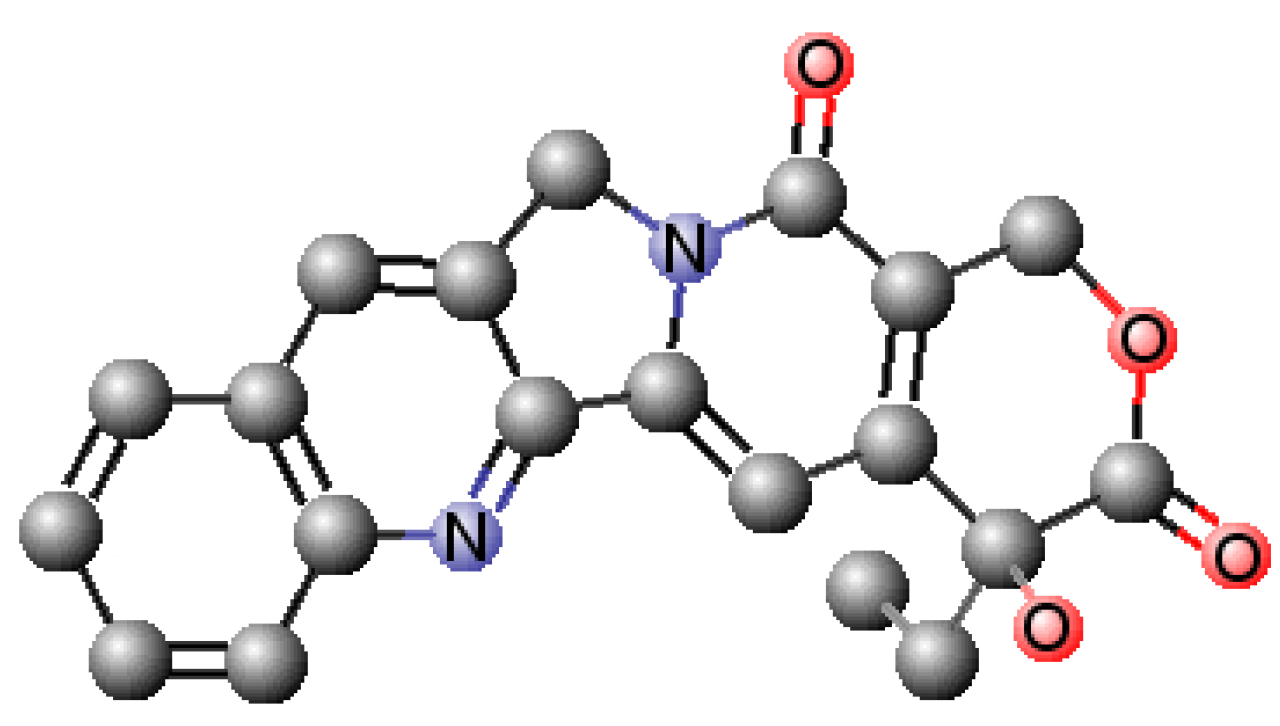


| MW (g∙mol−1) | PSA (Å2) | VWSA (Å2) | logP | S (mg∙mL−1) | pKa | H Donors | H Acceptors |
|---|---|---|---|---|---|---|---|
| 348.36 | 79.73 | 441.88 | 1.52 | 0.0559 | 3.07 11.71 | 1 | 4 |
| Membrane Model | pH | Composition | Method | LogD |
|---|---|---|---|---|
| Cancer cells membranes | 5.8 | DOPC (25%), CHOL (15%), EPC (31.8%), DOPS (17%), DOPE (8%), Cardiolipin (2.5%), SM (0.7%) | Derivative UV–Vis spectroscopy | 3.14 ± 0.13 ns |
| Fluorescence spectroscopy | 3.01 ± 0.31 ns | |||
| Normal cells membranes | 7.4 | DOPC (45%), DOPE (20%), DOPS (20%), CHOL (10%), SM (10%) | Derivative UV–Vis spectroscopy | 2.78 ± 0.28 ns |
| Fluorescence spectroscopy | 2.63 ± 0.15 ns | |||
| DMPC or DMPG [16,45,46] | Fluorescence anisotropy | 2.00 ± 0.16 *** | ||
| DOPC [47] | 1.55 ± 0.05 *** | |||
| DOPG [47] | 1.97 ± 0.05 *** | |||
| Octanol: water [18,47,48] | Fluorescence spectroscopy | 1.73 ± 0.08 *** | ||
| BBB endothelial membrane | 7.4 | PC (12.6%), PE (33.1%), PI (4.1%), PS (18.5%) and PA (0.8%) | Derivative spectroscopy | 3.64 ± 0.15 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, A.; Fernandes, E.; Sarmento, B.; Lúcio, M. A Biophysical Insight of Camptothecin Biodistribution: Towards a Molecular Understanding of Its Pharmacokinetic Issues. Pharmaceutics 2021, 13, 869. https://doi.org/10.3390/pharmaceutics13060869
Almeida A, Fernandes E, Sarmento B, Lúcio M. A Biophysical Insight of Camptothecin Biodistribution: Towards a Molecular Understanding of Its Pharmacokinetic Issues. Pharmaceutics. 2021; 13(6):869. https://doi.org/10.3390/pharmaceutics13060869
Chicago/Turabian StyleAlmeida, Andreia, Eduarda Fernandes, Bruno Sarmento, and Marlene Lúcio. 2021. "A Biophysical Insight of Camptothecin Biodistribution: Towards a Molecular Understanding of Its Pharmacokinetic Issues" Pharmaceutics 13, no. 6: 869. https://doi.org/10.3390/pharmaceutics13060869
APA StyleAlmeida, A., Fernandes, E., Sarmento, B., & Lúcio, M. (2021). A Biophysical Insight of Camptothecin Biodistribution: Towards a Molecular Understanding of Its Pharmacokinetic Issues. Pharmaceutics, 13(6), 869. https://doi.org/10.3390/pharmaceutics13060869









