Modelling and Simulation of the Drug Release from a Solid Dosage Form in the Human Ascending Colon: The Influence of Different Motility Patterns and Fluid Viscosities
Abstract
1. Introduction
2. Methodology
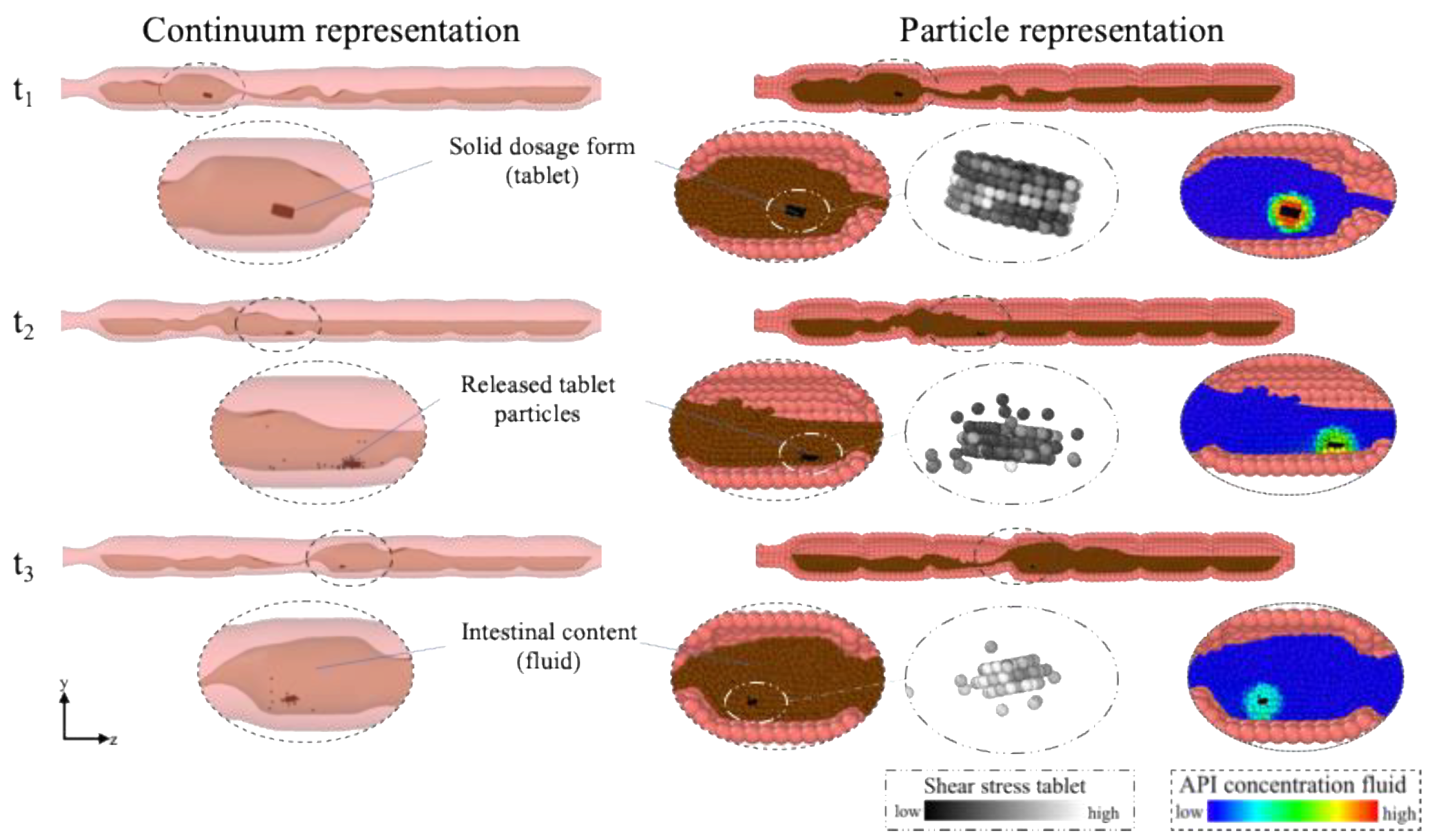
2.1. Modelling Approach
2.2. Colon-Model Geometry
2.3. Colon-Model Geometry
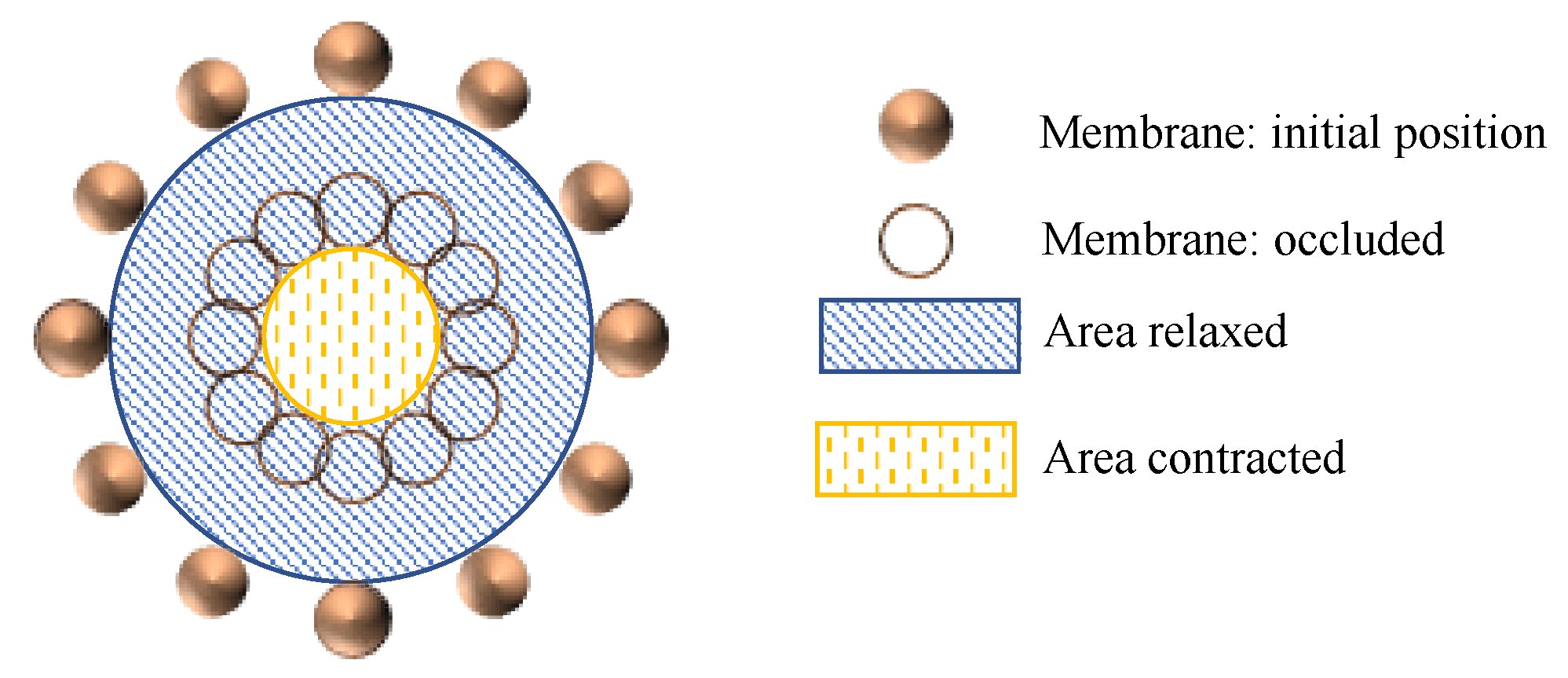
2.3.1. Membrane
2.3.2. Fluid
2.3.3. Fluid-Structure and Global Boundary Conditions
2.3.4. Tablet and Tablet Disintegration
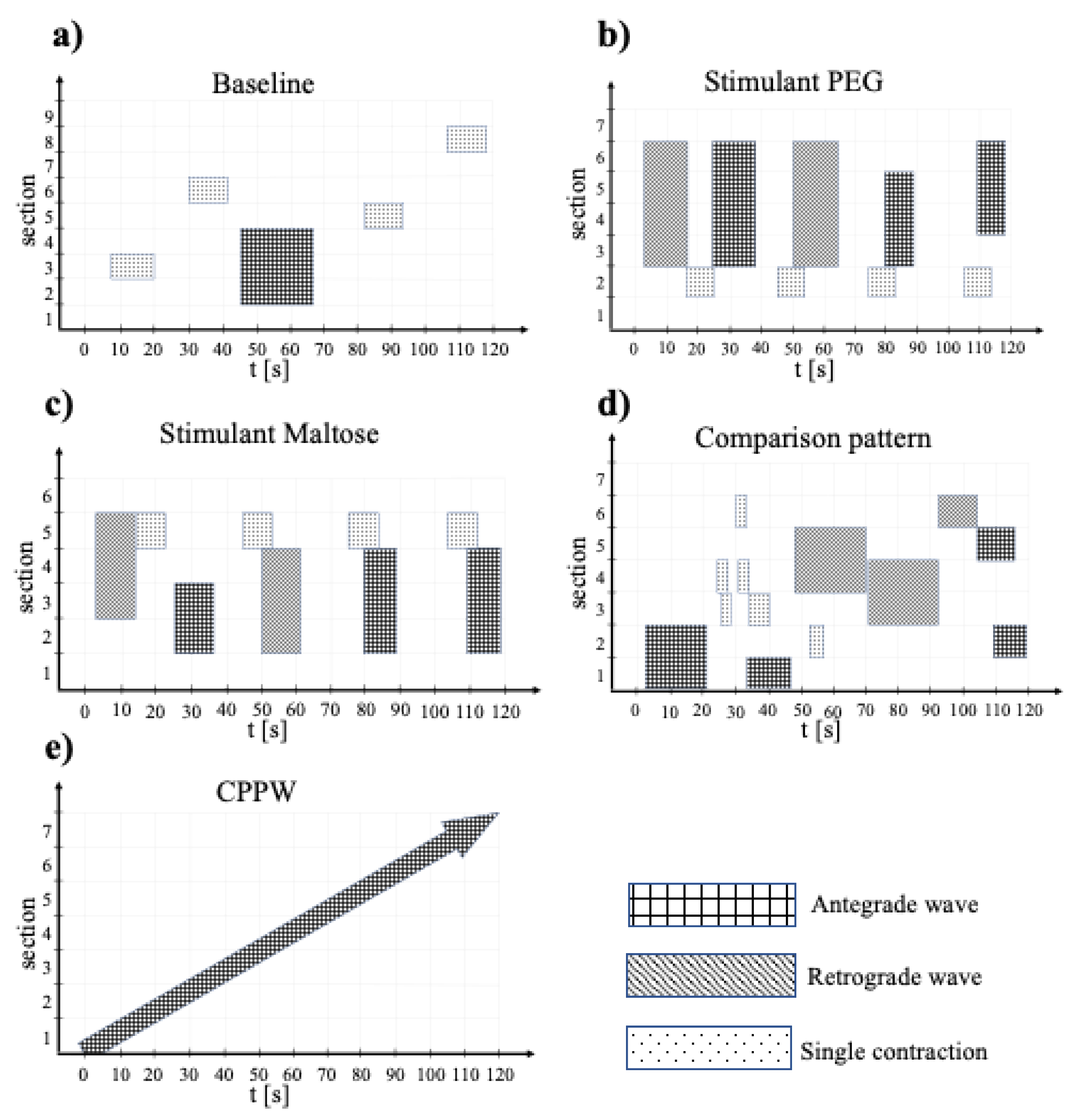
2.4. Model Motility Patterns
2.5. Method of Analysis
2.6. Software
3. Results and Discussion
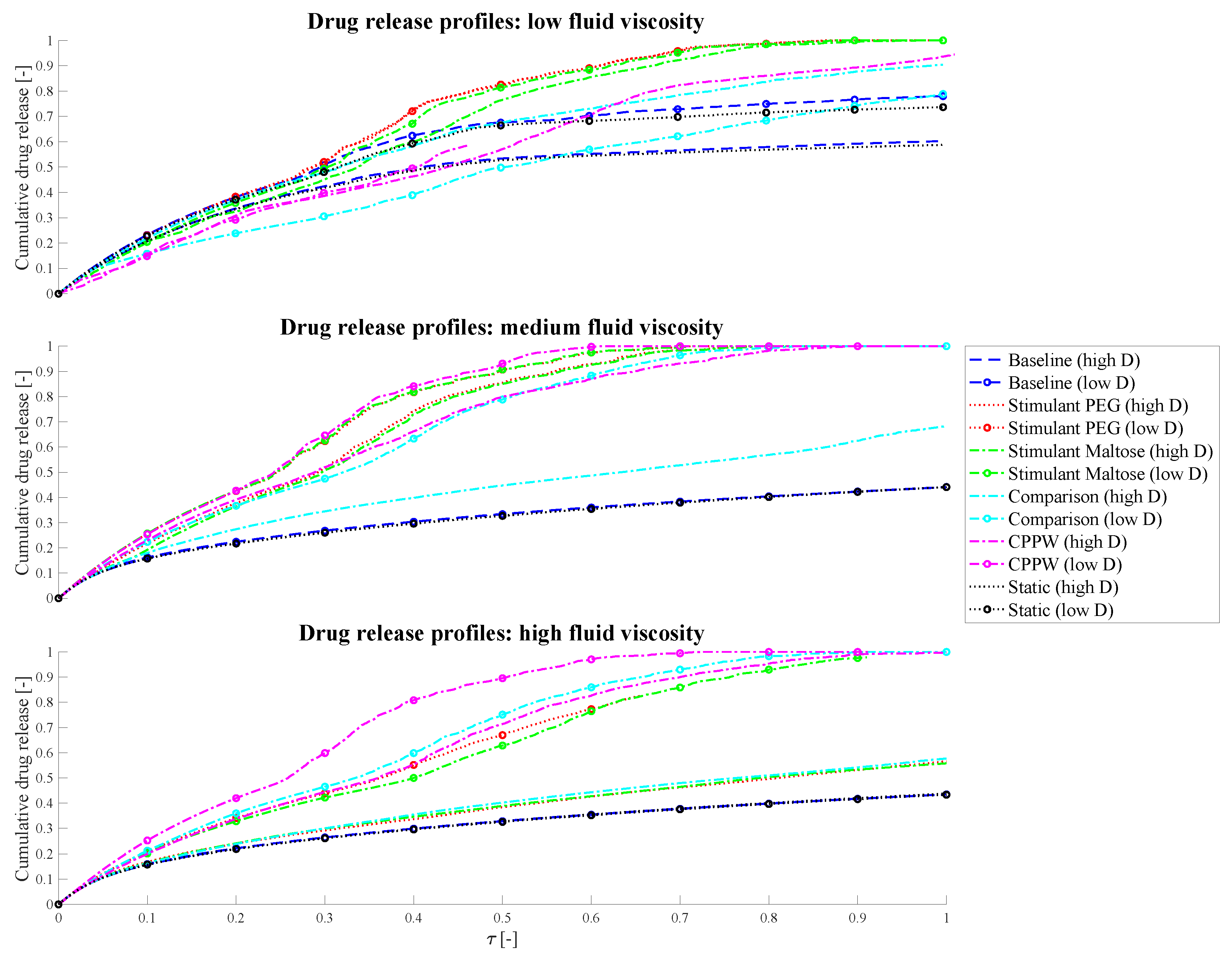
3.1. Comparison of Different Motility Pattern on the Drug Release/Disintegration of the Tablet at Different Fluid Viscosities
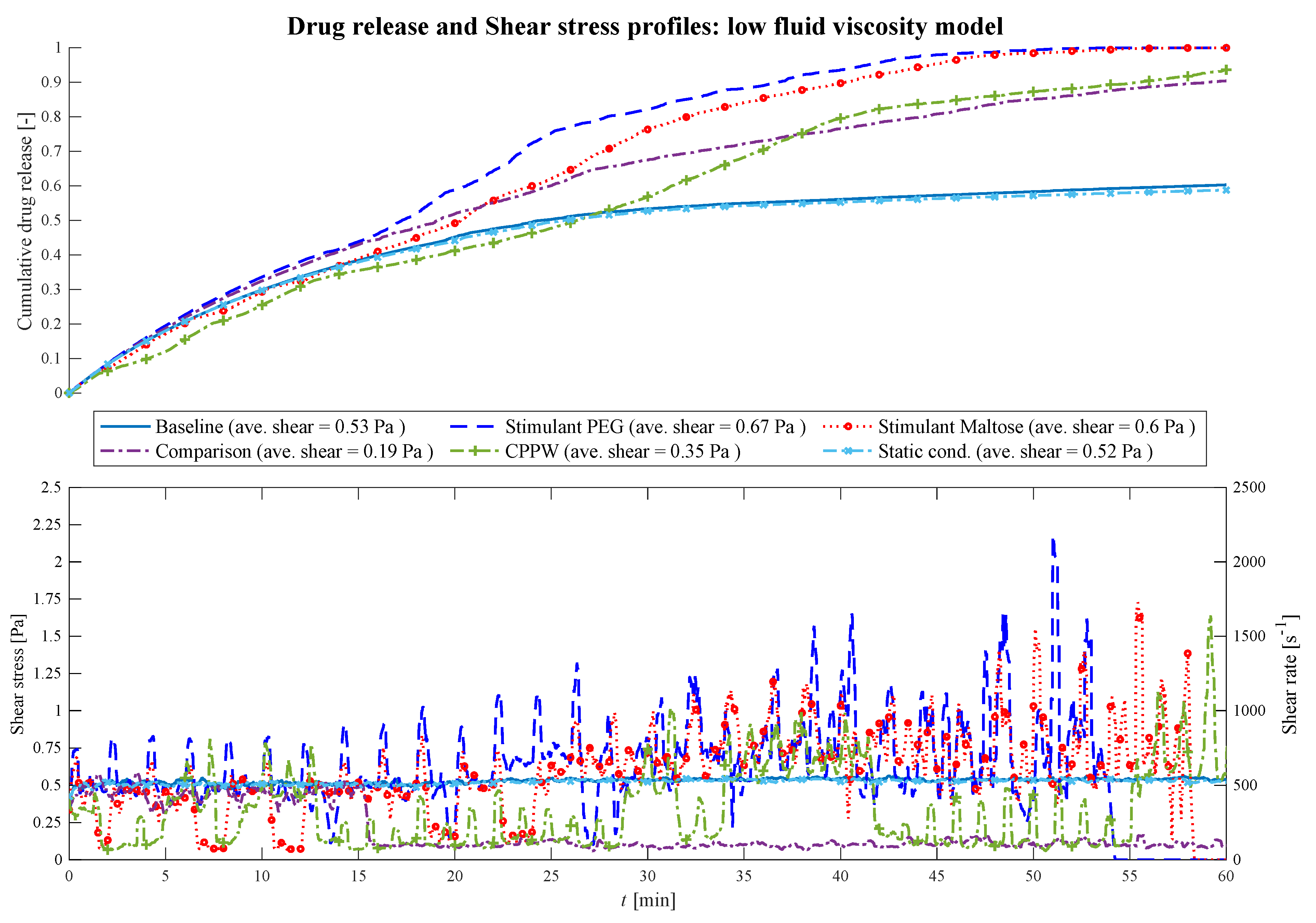
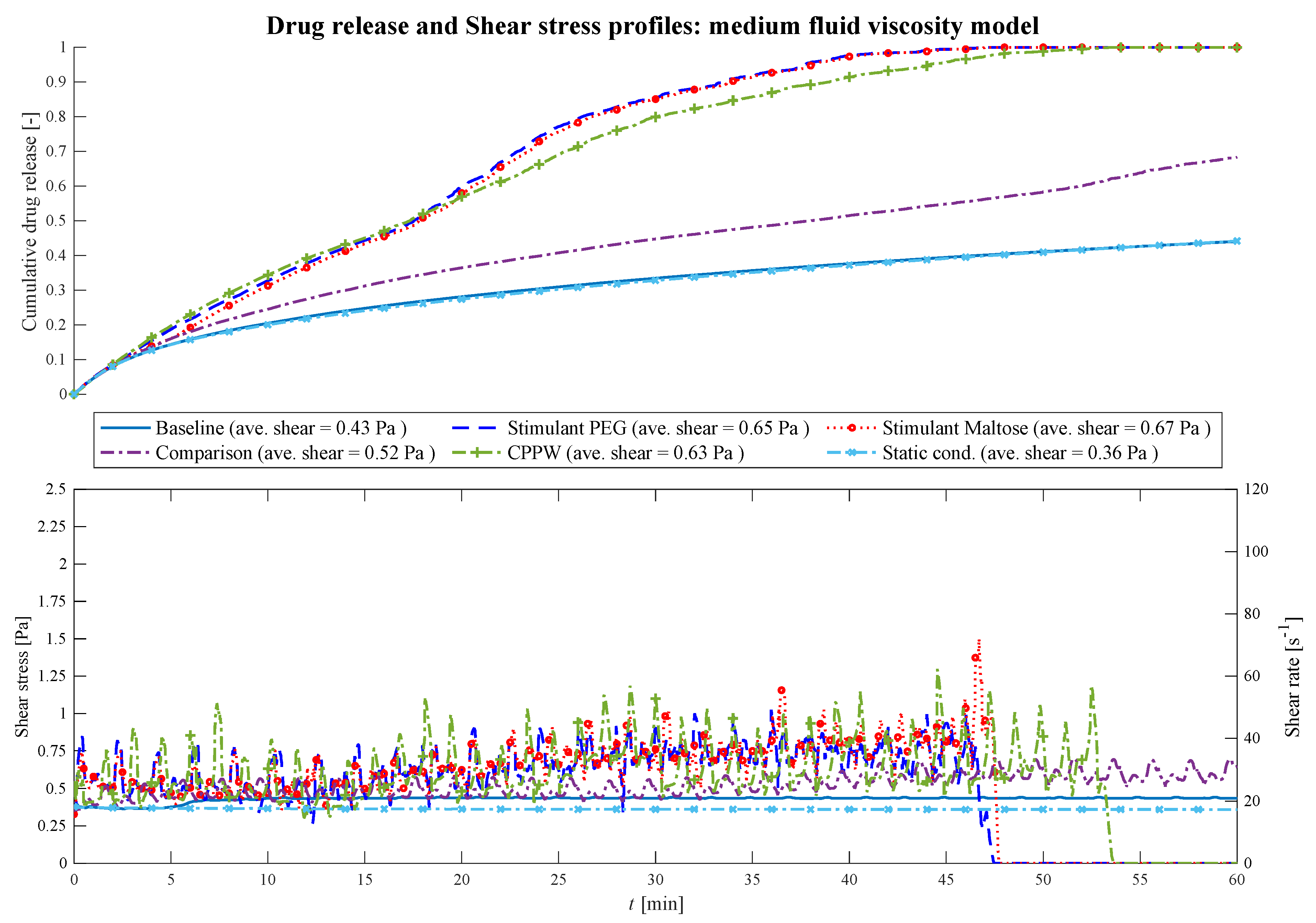

3.2. Comparison of the API Distribution along the Colon
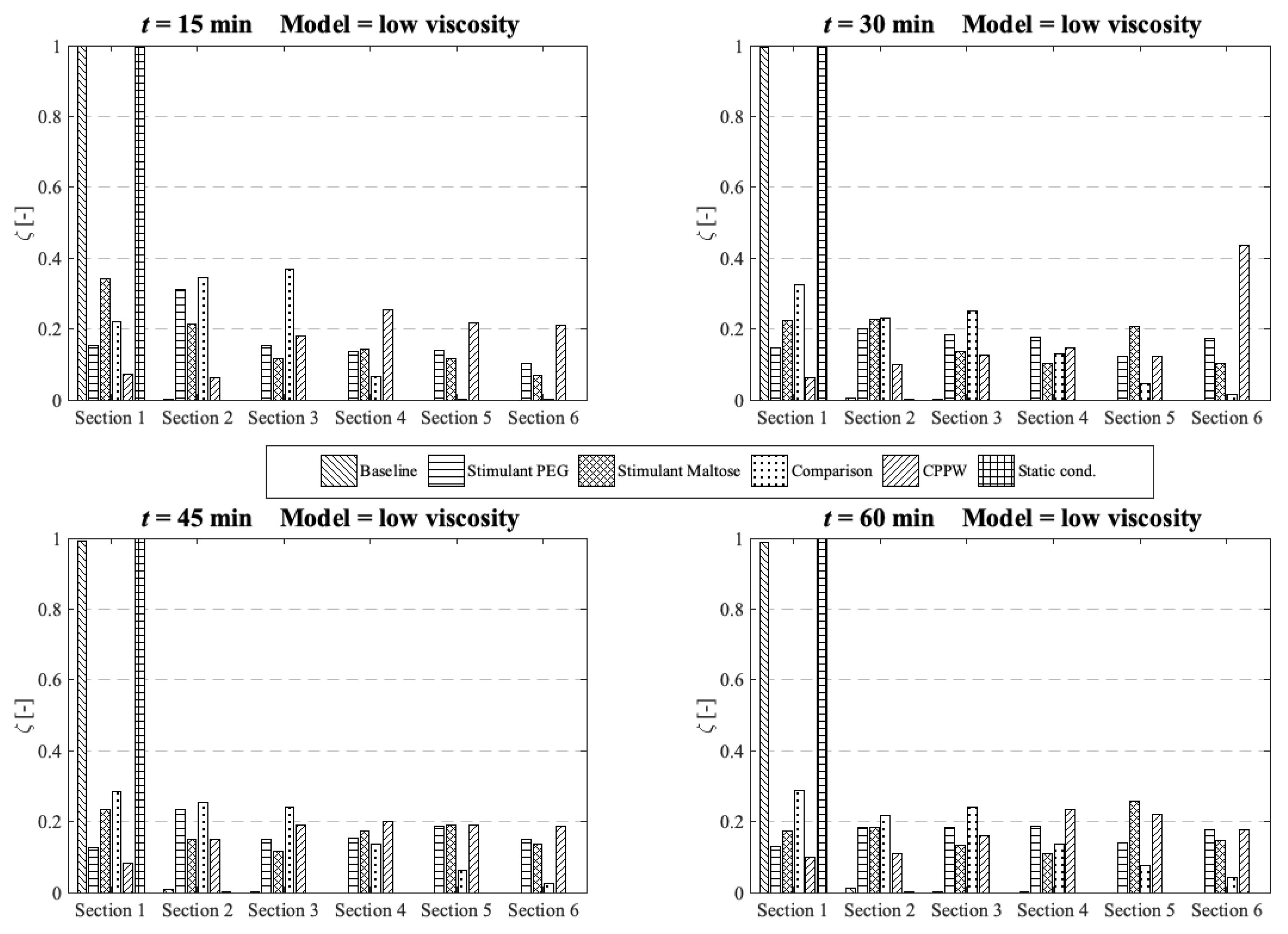
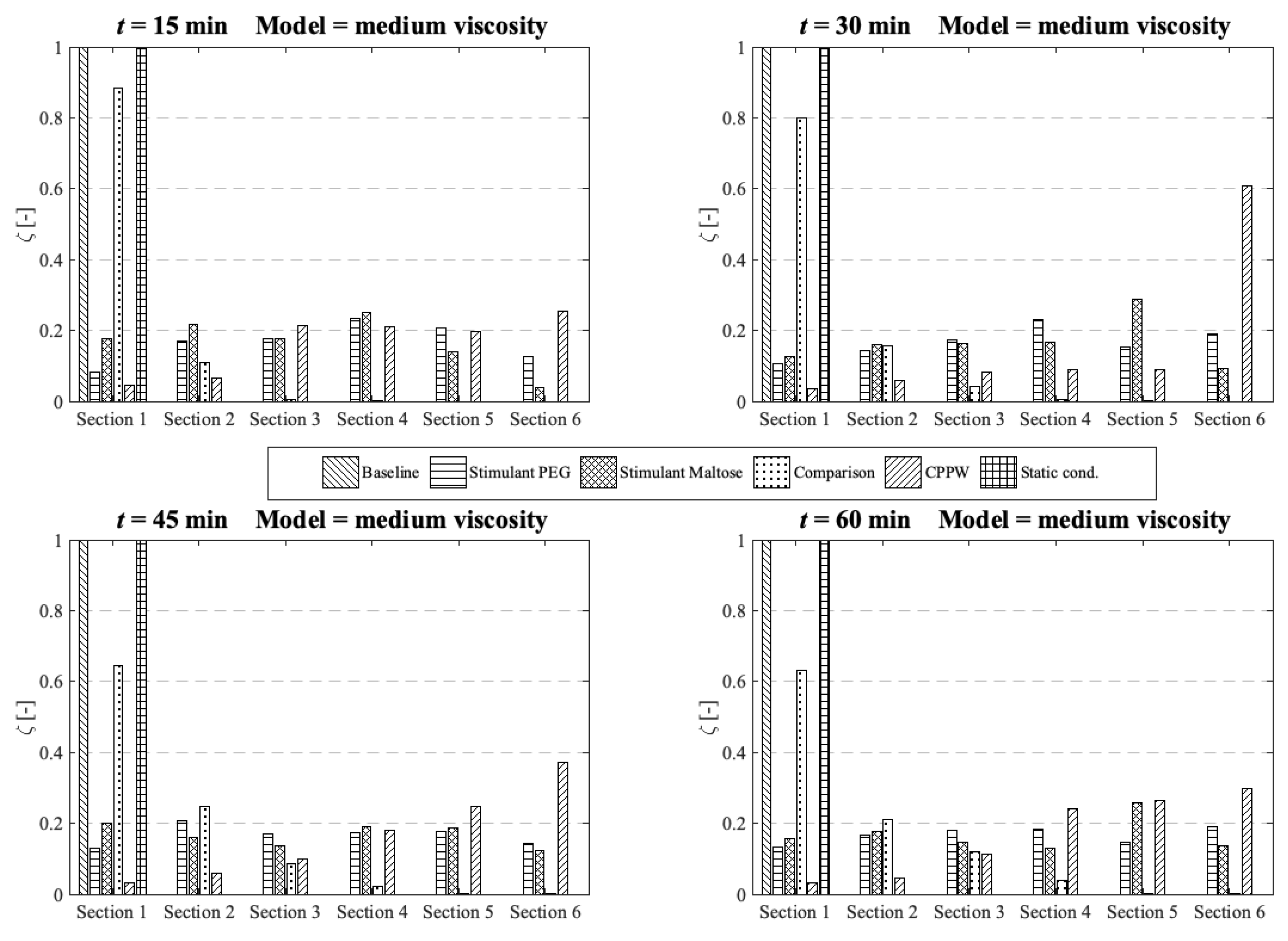

3.3. Influence of the Diffusion Coefficient on the Drug Release from Tablet
3.4. Strengths and Limitations of the Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
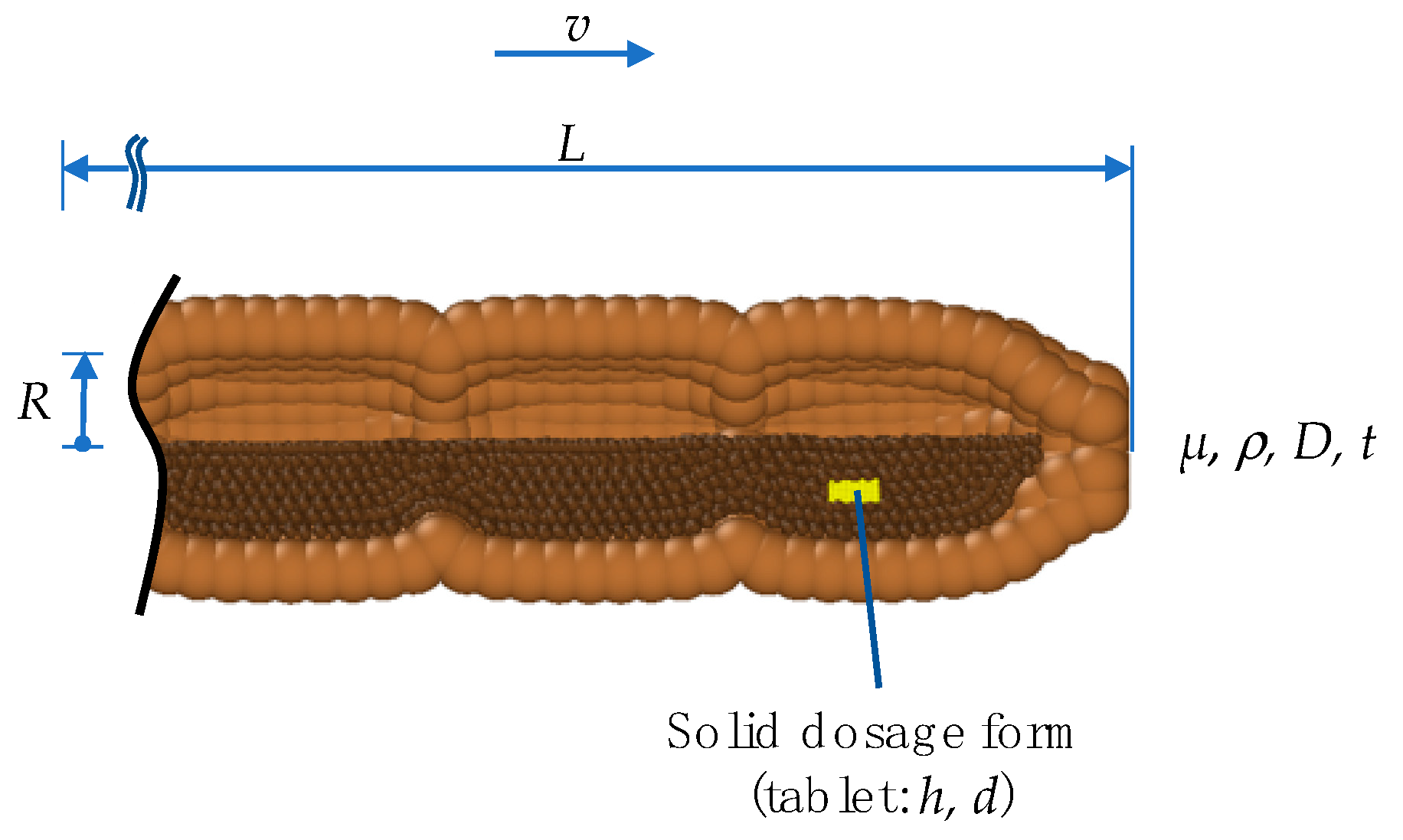
| Variable | Unit | Description | |
|---|---|---|---|
| (1) | t | s | dissolution time |
| (2) | v | m s−1 | velocity of wave |
| (3) | h | m | thickness of tablet |
| (4) | d | m | diameter of tablet |
| (5) | µ | kg m−1 s−1 | dynamic viscosity |
| (6) | ρ | kg m−3 | density |
| (7) | D | m2 s−1 | diffusion coefficient |
| (8) | R | m | radius of colon |
| (9) | L | m | length of colon |
References
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Goffredo, R.; Pecora, A.; Maiolo, L.; Ferrone, A.; Guglielmelli, E.; Accoto, D. A swallowable smart pill for local drug delivery. J. Microelectromech. Syst. 2016, 25, 362–370. [Google Scholar] [CrossRef]
- Teruel, A.H.; Gonzalez-Alvarez, I.; Bermejo, M.; Merino, V.; Marcos, M.D.; Sancenon, F.; Gonzalez-Alvarez, M.; Martinez-Manez, R. New insights of oral colonic drug delivery systems for inflammatory bowel disease therapy. Int. J. Mol. Sci. 2020, 21, 6502. [Google Scholar] [CrossRef] [PubMed]
- Amidon, S.; Brown, J.E.; Dave, V.S. Colon-targeted oral drug delivery systems: Design trends and approaches. AAPS PharmSciTech 2015, 16, 731–741. [Google Scholar] [CrossRef]
- Christensen, J. Physiology of the Gastrointestinal Tract, 3rd ed.; Johnson, L.R., Ed.; Raven Press: New York, NY, USA, 1994. [Google Scholar]
- Kumar, S.P.; Prathibha, D.; Parthibarajan, R.; Reichal, C.R. Novel colon specific drug delivery system: A review. Int. J. Pharm. Pharmaceut. Sci. 2012, 4, 22–29. [Google Scholar]
- Murray, K.; Hoad, C.L.; Mudie, D.M.; Wright, J.; Heissam, K.; Abrehart, N.; Pritchard, S.E.; Al Atwah, S.; Gowland, P.A.; Garnett, M.C.; et al. Magnetic resonance imaging quantification of fasted state colonic liquid pockets in healthy humans. Mol. Pharm. 2017, 14, 2629–2638. [Google Scholar] [CrossRef] [PubMed]
- Watts, P.J.; Illum, L. Colonic drug delivery. Drug Dev. Ind. Pharm. 1997, 23, 893–913. [Google Scholar] [CrossRef]
- Long, M.; Chen, Y. Developing Solid Oral Dosage Forms; Qiu, Y., Chen, Y., Zhang, G.G.Z., Liu, L., Porter, W.R., Eds.; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Tenjarla, S. Dissolution of commercially available mesalamine formulations at various pH levels. Drugs RD 2015, 15, 211–215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stamatopoulos, K.; Batchelor, H.K.; Simmons, M.J.H. Dissolution profile of theophylline modified release tablets, using a biorelevant Dynamic Colon Model (DCM). Eur. J. Pharm. Biopharm. 2016, 108, 9–17. [Google Scholar] [CrossRef]
- Schütt, M.; Stamatopoulos, K.; Simmons, M.J.H.; Batchelor, H.K.; Alexiadis, A. Modelling and simulation of the hydrodynamics and mixing profiles in the human proximal colon using Discrete Multiphysics. Comput. Biol. Med. 2020, 121. [Google Scholar] [CrossRef]
- Stamatopoulos, K.; Karandikar, S.; Goldstein, M.; O’Farrell, C.; Marciani, L.; Sulaiman, S.; Hoad, C.L.; Simmons, M.J.H.; Batchelor, H.K. Dynamic colon model (DCM): A cine-MRI informed biorelevant in vitro model of the human proximal large intestine characterized by positron imaging techniques. Pharmaceutics 2020, 12, 659. [Google Scholar] [CrossRef]
- Marciani, L.; Garsed, K.C.; Hoad, C.L.; Fields, A.; Fordham, I.; Pritchard, S.E.; Placidi, E.; Murray, K.; Chaddock, G.; Costigan, C.; et al. Stimulation of colonic motility by oral PEG electrolyte bowel preparation assessed by MRI: Comparison of split vs single dose. Neurogastroenterol. Motil. 2014, 26, 1426–1436. [Google Scholar] [CrossRef]
- Dinning, P.G.; Wiklendt, L.; Maslen, L.; Gibbins, I.; Patton, V.; Arkwright, J.W.; Lubowski, D.Z.; O’Grady, G.; Bampton, P.A.; Brookes, S.J.; et al. Quantification of in vivo colonic motor patterns in healthy humans before and after a meal revealed by high-resolution fiber-optic manometry. Neurogastroenterol. Motil. 2014, 26, 1443–1457. [Google Scholar] [CrossRef]
- Sarna, S.K. Colonic Motility: From Bench Side to Bedside; Morgan & Claypool Publishers: Williston, VT, USA, 2010. [Google Scholar]
- Alexiadis, A. The discrete multi-hybrid system for the simulation of solid-liquid flows. PLoS ONE 2015, 10, e0124678. [Google Scholar] [CrossRef]
- Alexiadis, A. A new framework for modelling the dynamics and the breakage of capsules, vesicles and cells in fluid flow. Procedia IUTAM 2015, 16, 80–88. [Google Scholar] [CrossRef]
- Alexiadis, A.; Stamatopoulos, K.; Wen, W.; Batchelor, H.K.; Bakalis, S.; Barigou, M.; Simmons, M.J. Using discrete multi-physics for detailed exploration of hydrodynamics in an in vitro colon system. Comput. Biol. Med. 2017, 81, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Ariane, M.; Allouche, M.H.; Bussone, M.; Giacosa, F.; Bernard, F.; Barigou, M.; Alexiadis, A. Discrete multi-physics: A mesh-free model of blood flow in flexible biological valve including solid aggregate formation. PLoS ONE 2017, 12, e1002047. [Google Scholar] [CrossRef]
- Ariane, M.; Kassinos, S.; Velaga, S.; Alexiadis, A. Discrete multi-physics simulations of diffusive and convective mass transfer in boundary layers containing motile cilia in lungs. Comput. Biol. Med. 2018, 95, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Ariane, M.; Wen, W.; Vigolo, D.; Brill, A.; Nash, F.G.B.; Barigou, M.; Alexiadis, A. Modelling and simulation of flow and agglomeration in deep veins valves using discrete multi physics. Comput. Biol. Med. 2017, 89, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.M.; Ariane, M.; Alexiadis, A. Using discrete multiphysics modelling to assess the effect of calcification on hemodynamic and mechanical deformation of aortic valve. ChemEngineering 2020, 4, 48. [Google Scholar] [CrossRef]
- Liu, G.R.; Liu, M.B. Smoothed Particle Hydrodynamics: A Meshfree Particle Method; World Scientific: Singapore, 2003. [Google Scholar]
- Kot, M.; Nagahashi, H.; Szymczak, P. Elastic moduli of simple mass spring models. Vis. Comput. 2015, 31, 1339–1350. [Google Scholar] [CrossRef]
- Lloyd, B.A.; Szekely, G.; Harders, M. Identification of spring parameters for deformable object simulation. IEEE Trans. Visualizat. Comput. Graph. 2007, 13, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Pazdniakou, A.; Adler, P.M. Lattice spring models. Trans. Porous Media 2012, 93, 243–262. [Google Scholar] [CrossRef]
- Rahmat, A.; Barigou, M.; Alexiadis, A. Numerical simulation of dissolution of solid particles in fluid flow using the SPH method. Int. J. Numer. Methods Heat Fluid Flow 2020, 30, 290–307. [Google Scholar] [CrossRef]
- Bampton, P.A.; Dinning, P.G.; Kennedy, M.L.; Lubowski, D.Z.; deCarle, D.; Cook, I.J. Spatial and temporal organization of pressure patterns throughout the unprepared colon during spontaneous defecation. Am. J. Gastroenterol. 2000, 95, 1027–1035. [Google Scholar] [CrossRef]
- Dinning, P.G.; Szczesniak, M.M.; Cook, I.J. Proximal colonic propagating pressure waves sequences and their relationship with movements of content in the proximal human colon. Neurogastroenterol. Motil. 2008, 20, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Badley, A.D.; Camilleri, M.; Oconnor, M.K. Noninvasive measurement of human ascending colon volume. Nucl. Med. Commun. 1993, 14, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, V.V.; Jayaprakas, R.; Mathew, S.T. Colon specific drug delivery systems: A review on various pharmaceutical approaches. J. Appl. Pharm. Sci. 2012, 2, 163–169. [Google Scholar]
- Lucy, L.B. A numerical approach to the testing of the fission hypothesis. Astronom. J. 1977, 82, 1013–1024. [Google Scholar] [CrossRef]
- Monaghan, J.J.; Gingold, R.A. Shock simulation by the particle method SPH. J. Comput. Phys. 1983, 52, 374–389. [Google Scholar] [CrossRef]
- Monaghan, J.J. Smoothed particle hydrodynamics. Rep. Prog. Phys. 2005, 68, 1703–1759. [Google Scholar] [CrossRef]
- Iacucci, M.; de Silva, S.; Ghosh, S. Mesalazine in inflammatory bowel disease: A trendy topic once again? Can. J. Gastroenterol. 2010, 24, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; van Langenberg, D.R. Mesalazine preparations for the treatment of ulcerative colitis: Are all created equal? World J. Gastrointest. Pharmacol. Ther. 2015, 6, 137–144. [Google Scholar] [CrossRef] [PubMed]
- French, D.L.; Mauger, J.W. Evaluation of the physicochemical properties and dissolution characteristics of mesalamine—Relevance to controlled intestinal drug-delivery. Pharm. Res. 1993, 10, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Hoad, C.L.; Menys, A.; Garsed, K.; Marciani, L.; Hamy, V.; Murray, K.; Costigan, C.; Atkinson, D.; Major, G.; Spiller, R.C.; et al. Colon wall motility: Comparison of novel quantitative semi-automatic measurements using cine MRI. Neurogastroenterol. Motil. 2016, 28, 327–335. [Google Scholar] [CrossRef]
- Moore, J.W.; Flanner, H.H. Mathematical comparison of curves with an emphasis on in vitro dissolution profiles. Pharmaceut. Technol. 1996, 20, 64–74. [Google Scholar]
- Shah, V.P.; Lesko, L.J.; Fan, J.; Fleischer, N.; Handerson, J.; Malinowski, H.; Makary, M.; Duderkirk, L.; Roy, S.; Sathe, P.; et al. FDA Guidance for industry dissolution testing of immediate release solid oral dosage forms. Dissolut. Technol. 1997, 15–22. [Google Scholar] [CrossRef]
- Shah, V.P.; Tsong, Y.; Sathe, P.; Liu, J.P. In vitro dissolution profile comparison—Statistics and analysis of the similarity factor, f2. Pharm. Res. 1998, 15, 889–896. [Google Scholar] [CrossRef]
- Birmingham, U. University of Birmingham’s BlueBEAR HPC Service. Available online: http://www.birmingham.ac.uk.bear (accessed on 12 December 2020).
- Ganzenmüller, G.C.; Steinhauser, M.O.; Van Liedekerke, P. The Implementation of Smoothed Particle Hydrodynamics in LAMMPS. 2011. Available online: lammps.sandia.gov/doc/PDF/SPH_LAMMPS_userguide.pdf (accessed on 17 October 2019).
- Plimpton, S. Fast parallel algorithms for short-range molecular-dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO-the Open Visualization Tool. Modell. Simulat. Mater. Sci. Eng. 2010, 18. [Google Scholar] [CrossRef]
- Hinkle, B. Hatched fill patterns. In MATLAB Central File Exchange; The MathWorks Inc.: Natick, MA, USA, 2020. [Google Scholar]
- MATLAB. MATLAB 9.9.0.1495850 (R2020b); The MathWorks Inc.: Natick, MA, USA, 2020. [Google Scholar]
- Abrahamsson, B.; Pal, A.; Sjoberg, M.; Carlsson, M.; Laurell, E.; Brasseur, J.G. A novel in vitro and numerical analysis of shear-induced drug release from extended-release tablets in the fed stomach. Pharm. Res. 2005, 22, 1215–1226. [Google Scholar] [CrossRef]
- Kindgen, S.; Wachtel, H.; Abrahamsson, B.; Langguth, P. Computational fluid dynamics simulation of hydrodynamics and stresses in the PhEur/USP disintegration tester under fed and fasted fluid characteristics. J. Pharm. Sci. 2015, 104, 2956–2968. [Google Scholar] [CrossRef][Green Version]
- Salehi, N.; Al-Gousous, J.; Mudie, D.M.; Amidon, G.L.; Ziff, R.M.; Amidon, G.E. Hierarchical mass transfer analysis of drug particle dissolution, highlighting the hydrodynamics, pH, particle size, and buffer effects for the dissolution of ionizable and nonionizable drugs in a compendial dissolution vessel. Mol. Pharm. 2020, 17, 3870–3884. [Google Scholar] [CrossRef]
- Hopgood, M.; Reynolds, G.; Barker, R. Using computational fluid dynamics to compare shear rate and turbulence in the TIM-automated gastric compartment with USP apparatus II. J. Pharm. Sci. 2018, 107, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Kukura, J.; Baxter, J.L.; Muzzio, F.J. Shear distribution and variability in the USP Apparatus 2 under turbulent conditions. Int. J. Pharm. 2004, 279, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Baxter, J.L.; Kukura, J.; Muzzio, F.J. Hydrodynamics-induced variability in the USP apparatus II dissolution test. Int. J. Pharm. 2005, 292, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Norlander, B.; Gotthard, R.; Strom, M. Pharmacokinetics of a 5-aminosalicylic acid enteric-coated tablet in patients with crohns-disease or ulcerative-colitis and in healthy-volunteers. Aliment. Pharmacol. Ther. 1990, 4, 497–505. [Google Scholar] [CrossRef]
- Effinger, A.; O’Driscoll, C.M.; McAllister, M.; Fotaki, N. Gastrointestinal diseases and their impact on drug solubility: Crohn’s disease. Eur. J. Pharm. Sci. 2020, 152. [Google Scholar] [CrossRef]
- Effinger, A.; O’Driscoll, C.M.; McAllister, M.; Fotaki, N. Gastrointestinal diseases and their impact on drug solubility: Ulcerative colitis. Eur. J. Pharm. Sci. 2020, 152. [Google Scholar] [CrossRef]
- Hebden, J.M.; Blackshaw, P.E.; Perkins, A.C.; Wilson, C.G.; Spiller, R.C. Limited exposure of the healthy distal colon to orally-dosed formulation is further exaggerated in active left-sided ulcerative colitis. Aliment. Pharmacol. Ther. 2000, 14, 155–161. [Google Scholar] [CrossRef]
- Sinha, A.; Ball, D.J.; Connor, A.; Nightingale, J.; Wilding, I. Intestinal performance of two mesalamine formulations in patients with active ulcerative colitis as assessed by gamma scintigraphy. Pract. Gastroenterol. 2003, 27, 56–69. [Google Scholar]
- Thorpe, M.P.; Ehrenpreis, E.D.; Putt, K.S.; Hannon, B. A dynamic model of colonic concentrations of delayed-release 5-aminosalicylic acid (Asacol). Aliment. Pharmacol. Ther. 2009, 29, 1193–1201. [Google Scholar] [CrossRef] [PubMed]



| Parameter Membrane | Value |
|---|---|
| SPH | |
| Number of SPH particles (1 layer) | 2500 |
| Mass of each particle mM,0 | 3.89 × 10−4 kg |
| LSM | |
| Hookean coefficient (bonds) kM,b | 0.2 J m−2 |
| Hookean coefficient (position anchor) kM,p | 0.012 J m−2 |
| Viscous damping coefficient kM,v | 1.0 × 10−2 kg s−1 |
| Equilibrium distance rM,0 | 6.28 × 10−3 m |
| SPH Parameter Fluid | Value |
|---|---|
| Number of SPH fluid particles | 25,758 |
| Mass of each particle mF,low viscosity | 1.19 × 10−5 kg |
| Mass of each particle mF,medium viscosity | 1.21 × 10−5 kg |
| Mass of each particle mF,high viscosity | 1.22 × 10−5 kg |
| Density (fluid) ρF,low viscosity | 1000 kg m−3 |
| Density (fluid) ρF,medium viscosity | 1017 kg m−3 |
| Density (fluid) ρF,high viscosity | 1020 kg m−3 |
| Dynamic viscosity (fluid) ηL,low viscosity | 1 mPa s |
| Dynamic viscosity (fluid) ηL,medium viscosity | 13 mPa s |
| Dynamic viscosity (fluid) ηL,high viscosity | 98 mPa s |
| Parameter | Value |
|---|---|
| SPH | |
| Artificial speed of sound c0 | 0.1 m s−1 |
| Time-step Δt | 5.0 × 10−4 s |
| Smoothing length membrane h | 9.42 × 10−3 m |
| Momentum–Smoothing length (fluid) hM,F | 4.71 × 10−3 m |
| Momentum–Smoothing length (tablet) hM,T | 1.41 × 10−3 m |
| Momentum–Smoothing length (fluid/tablet) hM,F/T | 4.71 × 10−3 m |
| Diffusion–Smoothing length (fluid/tablet) hD,F/T | 3.35 × 10−3 m |
| Diffusion–Smoothing length (fluid) hD,F | 4.71 × 10−3 m |
| Diffusion–Smoothing length (tablet) hD,T | 1.41 × 10−3 m |
| Diffusion coefficient (tablet) DT | 1.0 × 10−30 m2s−1 |
| Diffusion coefficient (fluid/tablet) DF/T | 7.46 × 10−9 m2s−1 |
| Parameter Tablet | Value |
|---|---|
| SPH | |
| Number of SPH tablet particles | 445 |
| Mass of each particle mT,low viscosity | 8.82 × 10−7 kg |
| Mass of each particle mT,medium viscosity | 8.97 × 10−7 kg |
| Mass of each particle mT, high viscosity | 9.00 × 10−7 kg |
| Density (Tablet) ρT,low viscosity | 1000 kg m−3 |
| Density (Tablet) ρT,medium viscosity | 1017 kg m−3 |
| Density (Tablet) ρT,high viscosity | 1020 kg m−3 |
| LSM | |
| Hookean coefficient kT,b | 0.2 J m−2 |
| Equilibrium distance (linear bonds) rTL,0 | 0.012 J m−2 |
| Equilibrium distance (diagonal bonds) rTD,0 | 6.28 × 10−3 m |
| Parameter | Baseline | PEG | Maltose | Comparison | CPPW |
|---|---|---|---|---|---|
| No. of sections | 9 | 7 | 6 | 7 | 7 |
| Motility index [segment × s] | 140 | 460 | 380 | 300 | 280 |
| Specific motility index [segment × s × stotal−1] | 16 | 66 | 63 | 43 | 40 |
| Occlusion velocity ‘wave’ [cm/s] | 0.1 | 2.3 | 2.3 | 0.8 | 0.1 |
| Occlusion velocity ‘single contraction’ [cm/s] | 0.1 | 0.1 | 0.1 | 0.25 | - |
| Wave travel velocity [cm/s] | 0.9 | 2.5 | 2.5 | 0.85 | 1.0 |
| Occlusion degree OD [%] | |||||
| Single contraction 1 | 25 | 60 | 60 | 30 | - |
| Single contraction 2 | 25 | 60 | 60 | 50 | - |
| Single contraction 3 | 25 | 60 | 60 | 60 | - |
| Single contraction 4 | 25 | 60 | 60 | 40 | - |
| Single contraction 5 | - | - | - | 50 | - |
| Single contraction 6 | - | - | - | 30 | - |
| Antegrade wave 1 | 20 | 75 | 75 | 40 | - |
| Antegrade wave 2 | - | 55 | 55 | 55 | 40 |
| Antegrade wave 3 | - | 40 | 40 | 75 | - |
| Antegrade wave 4 | - | - | - | 40 | - |
| Retrograde wave 1 | - | 75 | 75 | 40 | - |
| Retrograde wave 2 | - | 40 | 40 | 75 | - |
| Retrograde wave 3 | - | - | - | 40 | - |
| Model/Motility Pattern | Low Viscosity | Medium Viscosity | High Viscosity |
|---|---|---|---|
| Static | 15% | 0% | 0% |
| Baseline | 16% | 0% | 0% |
| PEG | 54.3 min | 45.6 min | 16% |
| Maltose | 58.4 min | 46.1 min | 13% |
| Comparison | 81% | 47% | 33% |
| CPPW | 93% | 53.7 min | 57.8 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schütt, M.; Stamatopoulos, K.; Batchelor, H.K.; Simmons, M.J.H.; Alexiadis, A. Modelling and Simulation of the Drug Release from a Solid Dosage Form in the Human Ascending Colon: The Influence of Different Motility Patterns and Fluid Viscosities. Pharmaceutics 2021, 13, 859. https://doi.org/10.3390/pharmaceutics13060859
Schütt M, Stamatopoulos K, Batchelor HK, Simmons MJH, Alexiadis A. Modelling and Simulation of the Drug Release from a Solid Dosage Form in the Human Ascending Colon: The Influence of Different Motility Patterns and Fluid Viscosities. Pharmaceutics. 2021; 13(6):859. https://doi.org/10.3390/pharmaceutics13060859
Chicago/Turabian StyleSchütt, Michael, Konstantinos Stamatopoulos, Hannah K. Batchelor, Mark J. H. Simmons, and Alessio Alexiadis. 2021. "Modelling and Simulation of the Drug Release from a Solid Dosage Form in the Human Ascending Colon: The Influence of Different Motility Patterns and Fluid Viscosities" Pharmaceutics 13, no. 6: 859. https://doi.org/10.3390/pharmaceutics13060859
APA StyleSchütt, M., Stamatopoulos, K., Batchelor, H. K., Simmons, M. J. H., & Alexiadis, A. (2021). Modelling and Simulation of the Drug Release from a Solid Dosage Form in the Human Ascending Colon: The Influence of Different Motility Patterns and Fluid Viscosities. Pharmaceutics, 13(6), 859. https://doi.org/10.3390/pharmaceutics13060859







