Thiolated-Polymer-Based Nanoparticles as an Avant-Garde Approach for Anticancer Therapies—Reviewing Thiomers from Chitosan and Hyaluronic Acid
Abstract
1. Introduction
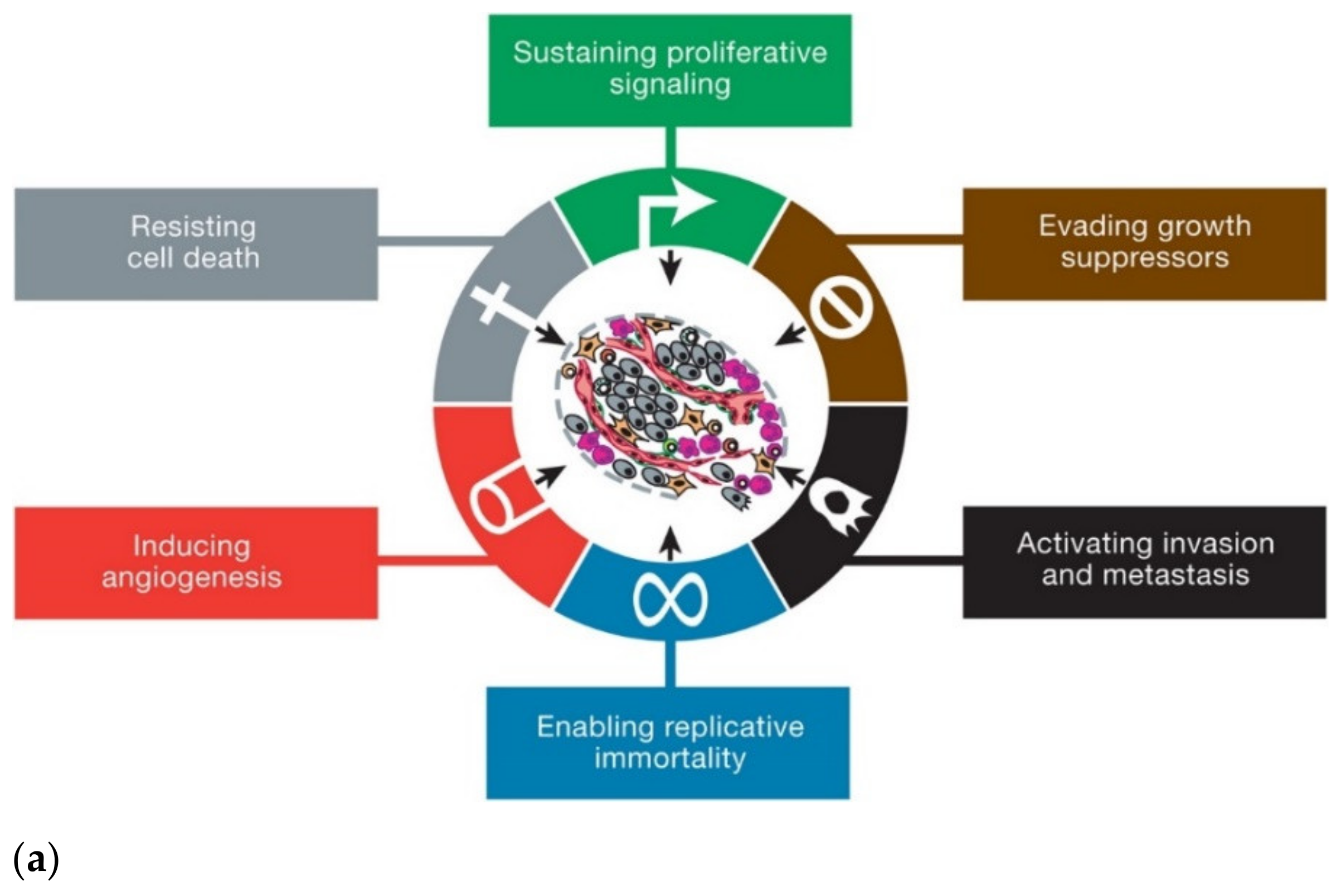
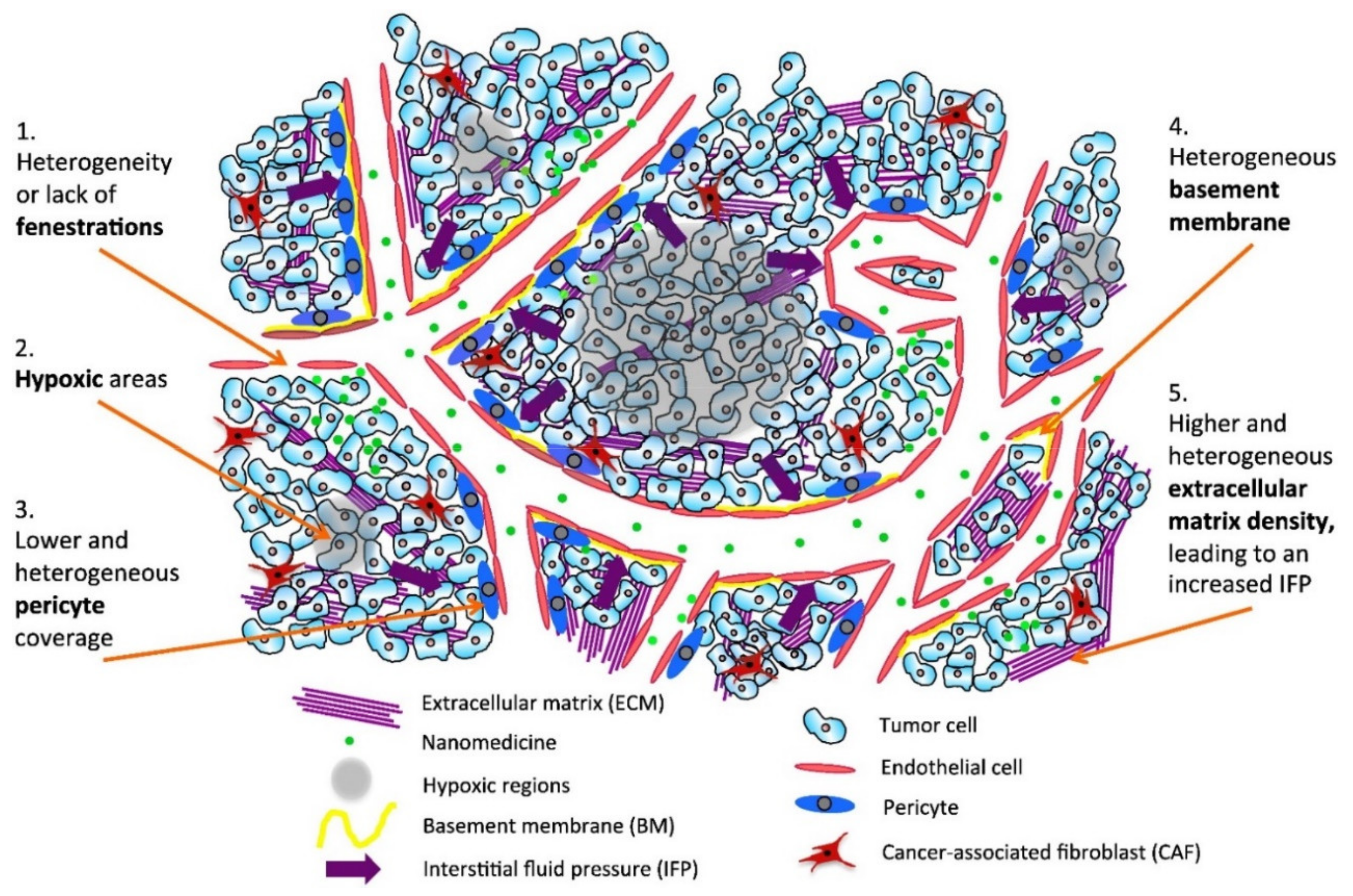
2. Cancer and Nanotechnology
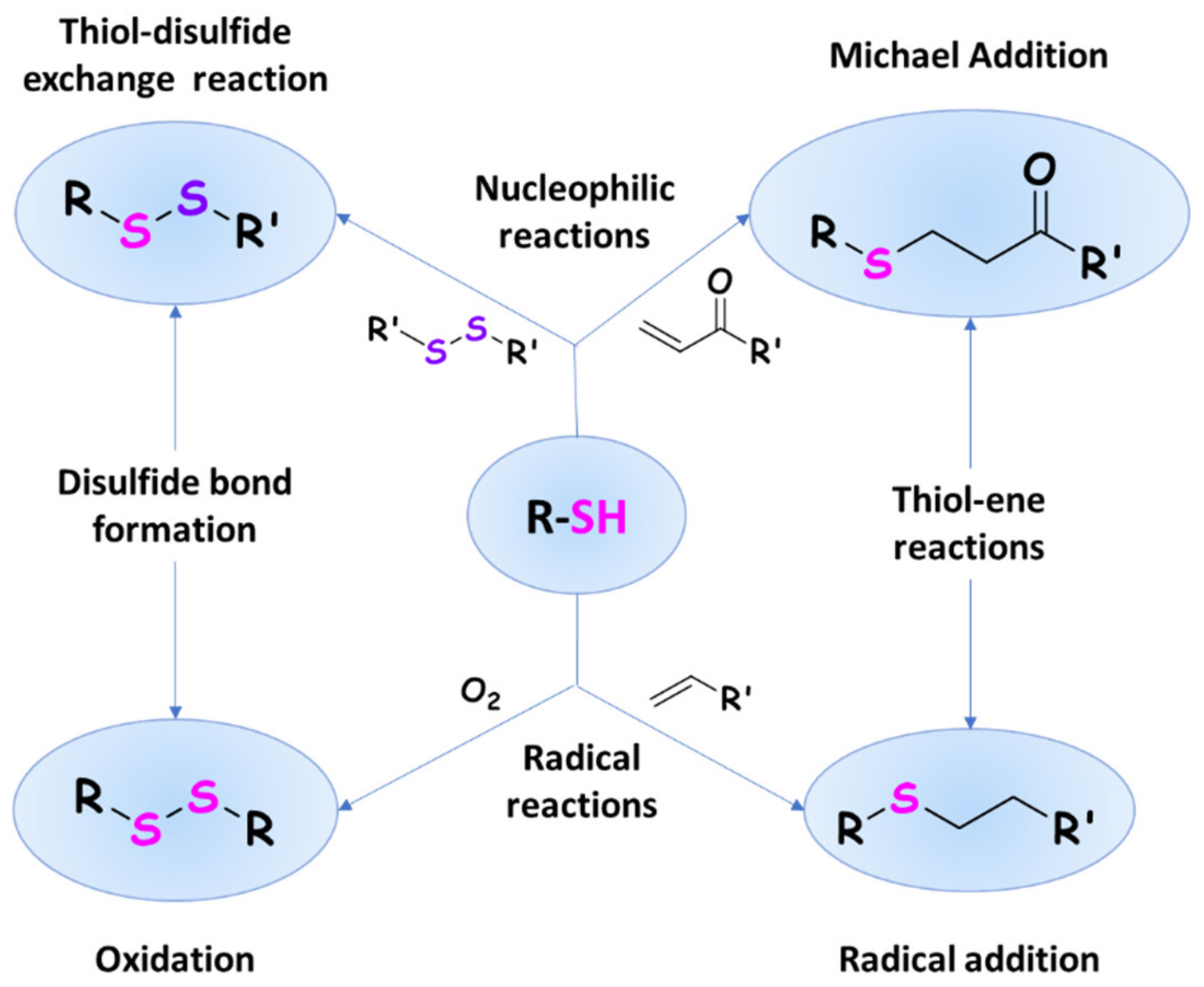
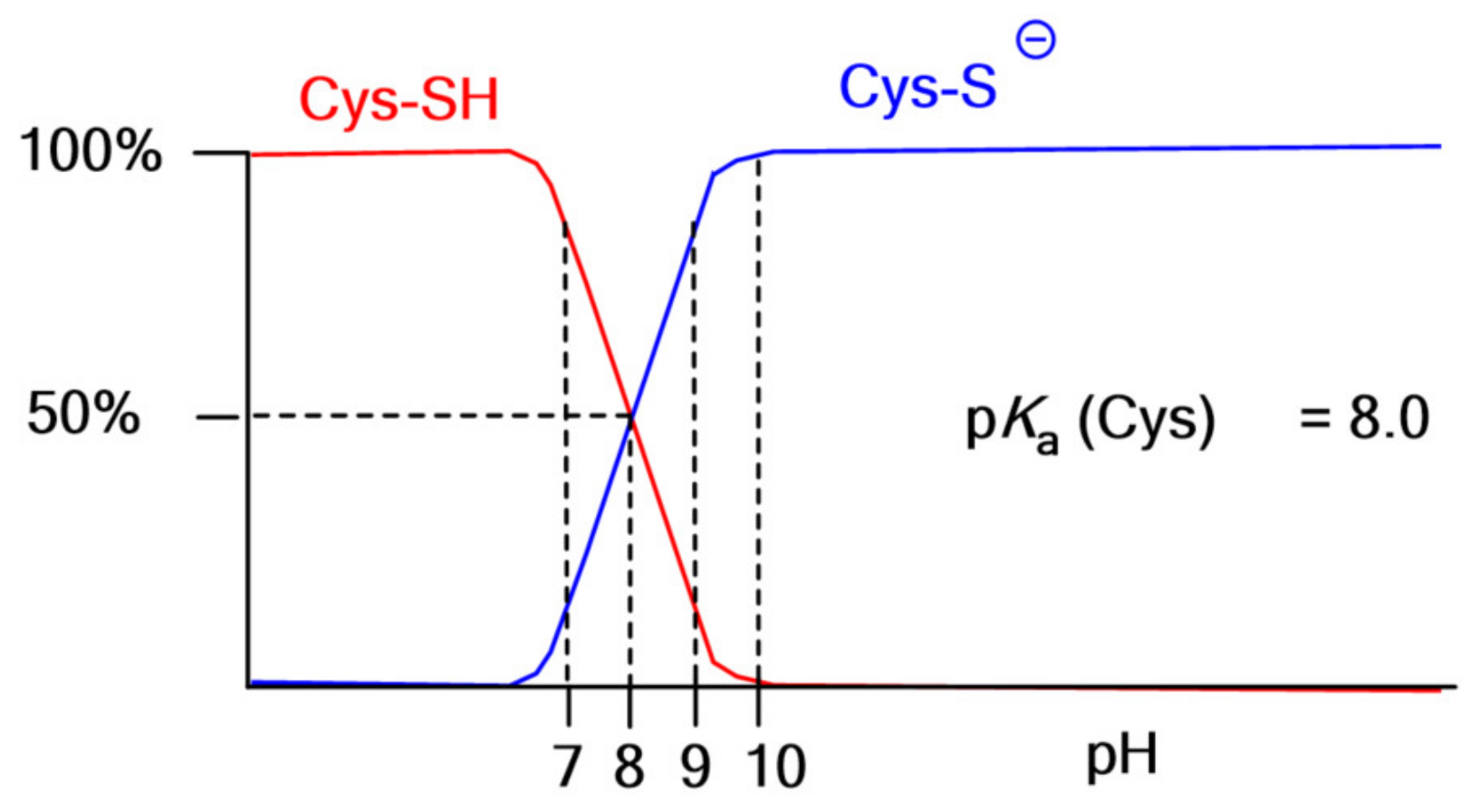
3. Thiomers: Reactivity and Distinguished Properties
3.1. Thiol Oxidation and Disulfide Bond Formation: In Situ Gelling Properties
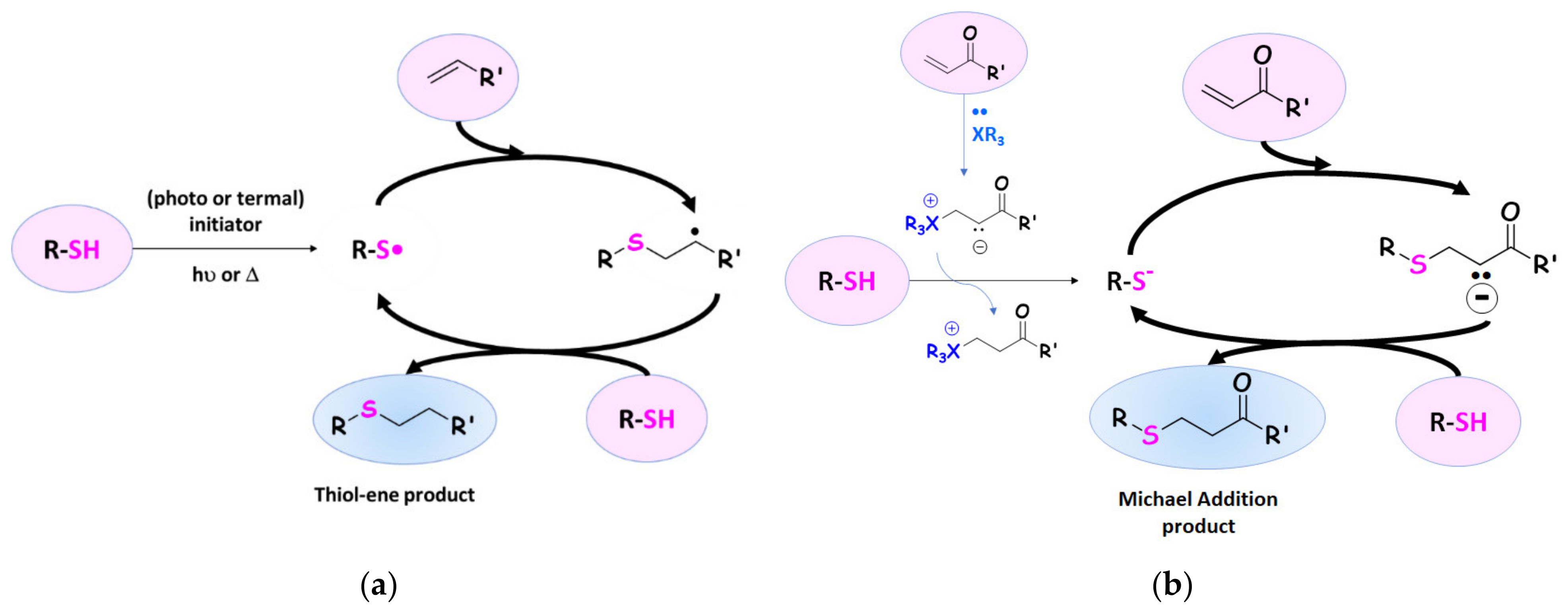
3.2. Thiol–Ene Reactions
3.3. Properties for Biomedical Applications
4. Thiolation of (Semi)Natural-Occurring Polysaccharides
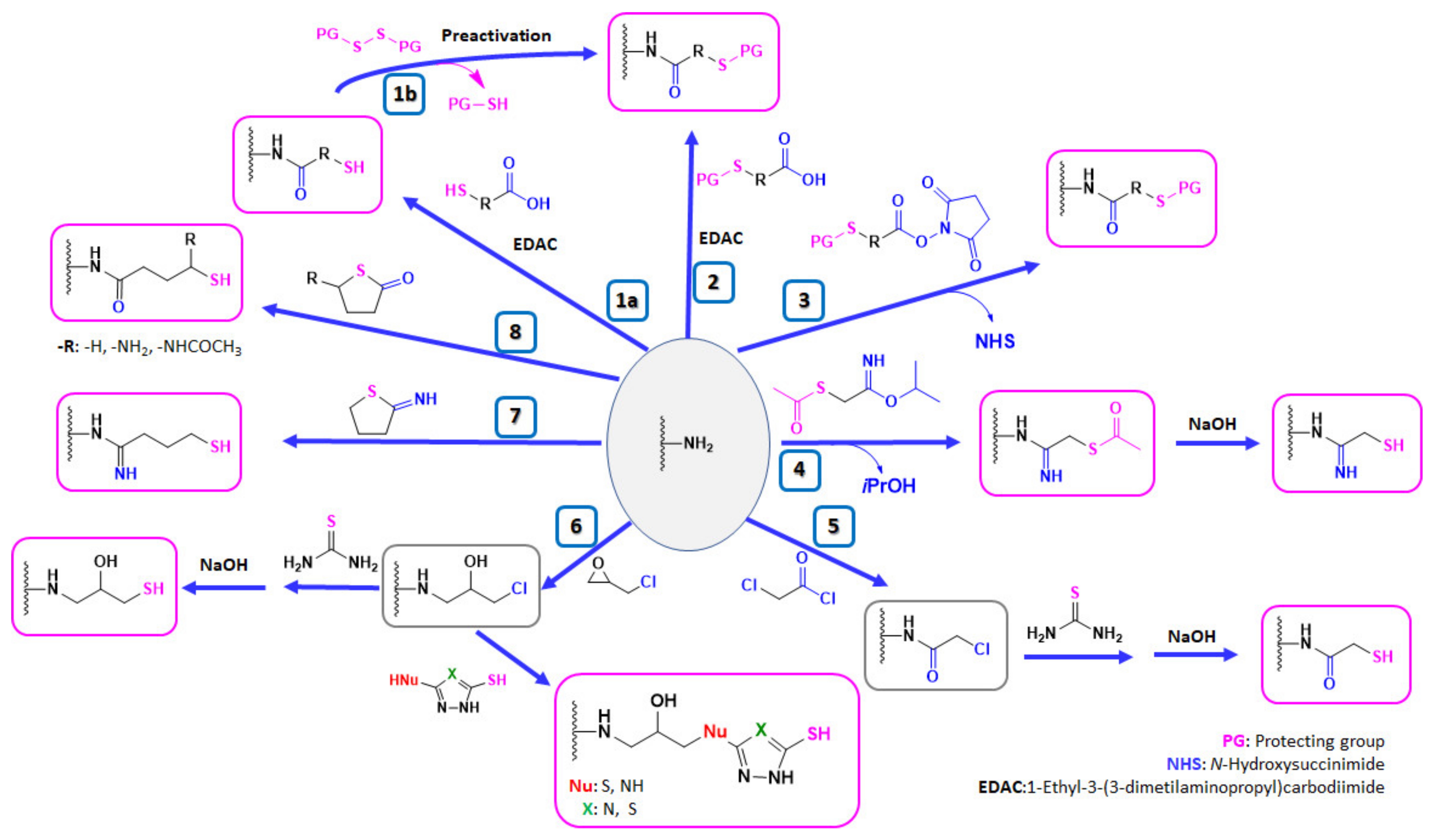
4.1. Strategies for the Thiolation of Multiamino Polymers
4.1.1. Coupling Reaction of Multiamino Polymers and Mercaptocarboxylic Acids
| Reaction Code | Compound | Ref. | Reaction Code | Compound | Ref. | ||
|---|---|---|---|---|---|---|---|
| Formula | Abbrev. | Formula | Abbrev. | ||||
| 1a | 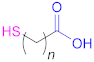 n = 1, 2, 3, 5, 7, 10 n = 1: Thioglycolic acid | n = 1: TGA n = 2: MPA n = 3: MBA n = 5: MHA n = 7: MOA n = 10: MUA | n = 1: [18,23,29,70] n = 2: [18,113] n = 3: [18] n = 5: [18] n = 7: [18] n = 10: [19,62] | 1a |  Reduced Glutathione | GSH | [26,70,110] |
| 1a | 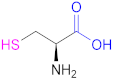 Cysteine | Cys | [62] | 1a | 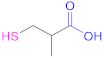 2-Methyl-3-sulfanyl propanoic acid | MSPA | [18] |
| 1a | 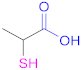 Thiolactic acid | TLA | [18] | 1a | 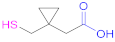 1-(1-(Mercaptomethyl) cyclopropane) acetic acid | MCA | [20] |
| 1a | 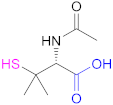 N-Acetylpenicillamine | NAP | [114] | 1a | 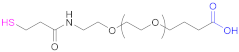 O-(3-Carboxypropyl)-O’-(2-(3-mercaptopropionyl amino)ethyl) polyethyleneglycol | -- | [111] |
| 1a | 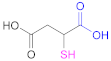 Mercaptosuccinic acid (Thiomalic acid) | -- | [24] | 1a | 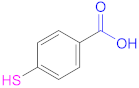 4-Mercaptobenzoic acid | 4-MBA | [25,70] |
| 1a | 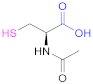 N-Acetylcysteine | NAC | [26,64,70,110,114] | 1a | 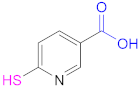 6-Mercaptonicotinic acid | 6-MNA | [70,115] |
| Reaction Code | Compound | Ref. | Reaction Code | Compound | Ref. | ||
|---|---|---|---|---|---|---|---|
| Formula | Abbrev. | Formula | Abbrev. | ||||
| 1b | 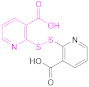 2,2′-Dithiodinicotinic acid | 2,2′-DTNA | [26,48] | 1b | 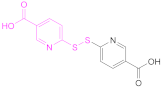 6,6′-Dithiodinicotinic acid | 6,6′-DTNA | [92] |
| 1b | 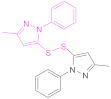 Dimer of 3-Methyl-1-phenylpyrazole-5-thiol | MPPT | [83] | 1b | 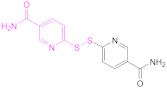 6,6′-Dithiodinicotiamide | 6,6′-DTNAm | [22,116,119] |
4.1.2. Reaction of Multiamino Polymers with Highly Reactive Carboxylic Acid Derivatives
Reaction with N-hydroxysuccinimide Esters
Reaction with Acetimidates
4.1.3. Thiolation in Two Steps: Nucleophilic Reactions of Multiamino Polymers with Chloro-Derivatives and Incorporation of Thiol Moiety
Reaction with Chloroacetyl Chloride
Reaction with Epichlorohydrin
4.1.4. Ring Opening of Reactive Thiolating Cycles: 2-Iminothiolane and Thiolactones
| Reaction Code | Compound | Ref. | Reaction Code | Compound | Ref. | ||
|---|---|---|---|---|---|---|---|
| Formula | Abbrev. | Formula | Abbrev. | ||||
| 7 |  2-Iminothiolane | -- | [30,69,70] | 8 |  γ-Thiobutyrolactone | -- | [53] |
| 8 |  Homocysteine thiolactone | HT | [130] | 8 |  N-Acetylhomocysteine thiolactone | -- | [67,129] |
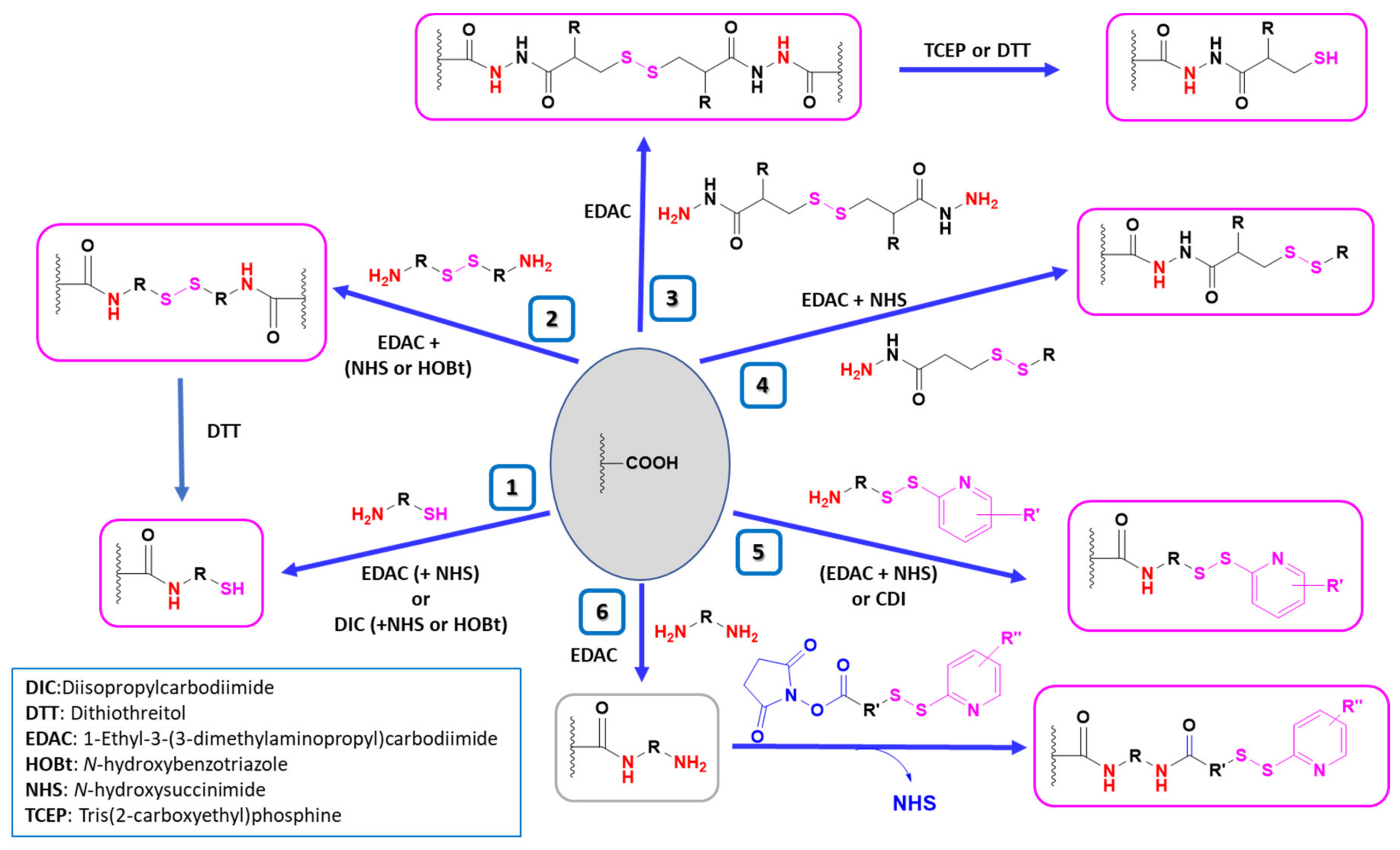
4.2. Strategies for the Thiolation of Multicarboxyl Polymers
| Reaction Code | Polymer | Compound | Coupling Reagents Used and Other Reaction Details | Ref. | |
|---|---|---|---|---|---|
| Formula | Abbrev. | ||||
| 1 | HA | 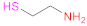 Cysteamine | CSA | 1.- EDAC + NHS (2 h) 2.- NH2-R-SH | [16,78] |
| 1 | PAA | 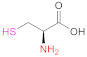 Cysteine | Cys | EDAC (in H2O) or DIC + (NHS or HOBt) (in DMF-DCM mixtures) | [85] |
| 1 | Pec | 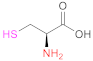 Cysteine | Cys | EDAC | [29,71] |
| 2 | HA | 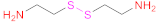 Cystamine | 1.- EDAC + NHS 2.- DTT | [15] | |
| 2 | HA | 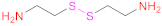 Cystamine | 1.- EDAC + HOBt 2.- DTT | [31,136] | |
| 3 | HA | 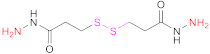 3,3’-Dithiobis(propanoic hydrazide) | DTPH | 1. EDAC 2. TCEP | [14,79] |
| 3 | HA | 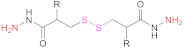 R: H: 3,3’-Dithiobis(propanoic hydrazide) R: NHAc: 3,3’-Dithiobis(2-acetamidopropanoic hydrazide) R: NH2 HCl: 3,3’-Dithiobis(2-aminopropanoic hydrazide) | R:H: DTPH | 1. EDAC 2. DTT | [63,137] |
| 4 | HA | 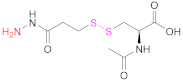 N-acetyl-S-[(3-hydrazineyl-3-oxopropyl)thio]-cysteine | NAC-TPH | EDAC + NHS | [17] |
| 5 | PAA | 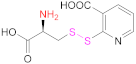 Cysteine 2-mercaptonicotinic acid adduct | Cys-2-MNA | EDAC + NHS (in H2O) or CDI (in DMF-DCM mixtures) | [85] |
| 5 | Pec | 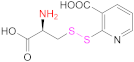 Cysteine 2-mercaptonicotinic acid adduct | Cys-2-MNA | EDAC + NHS | [71] |
| 6 | HA | 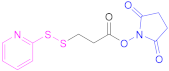 N-Succinimidyl 3-(2-pyridyldithio)propionate | SPDP | 1.- Diamine(C6) + EDAC 2.- SPDP 3.- SH-PEG-SH | [138] |
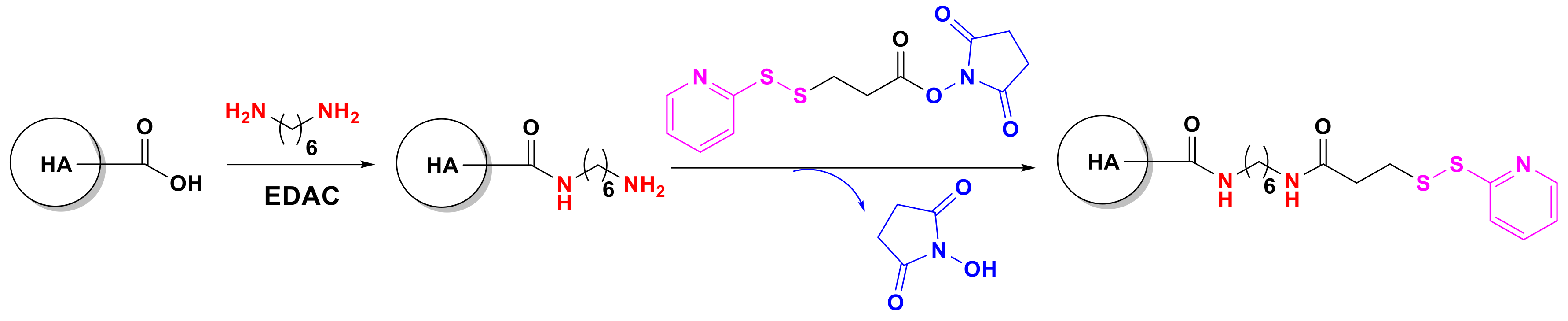
4.3. Nanostructures from Chitosan and Hyaluronic Acid-Based Thiomers
5. Thiomer Conjugates in Anticancer Therapy
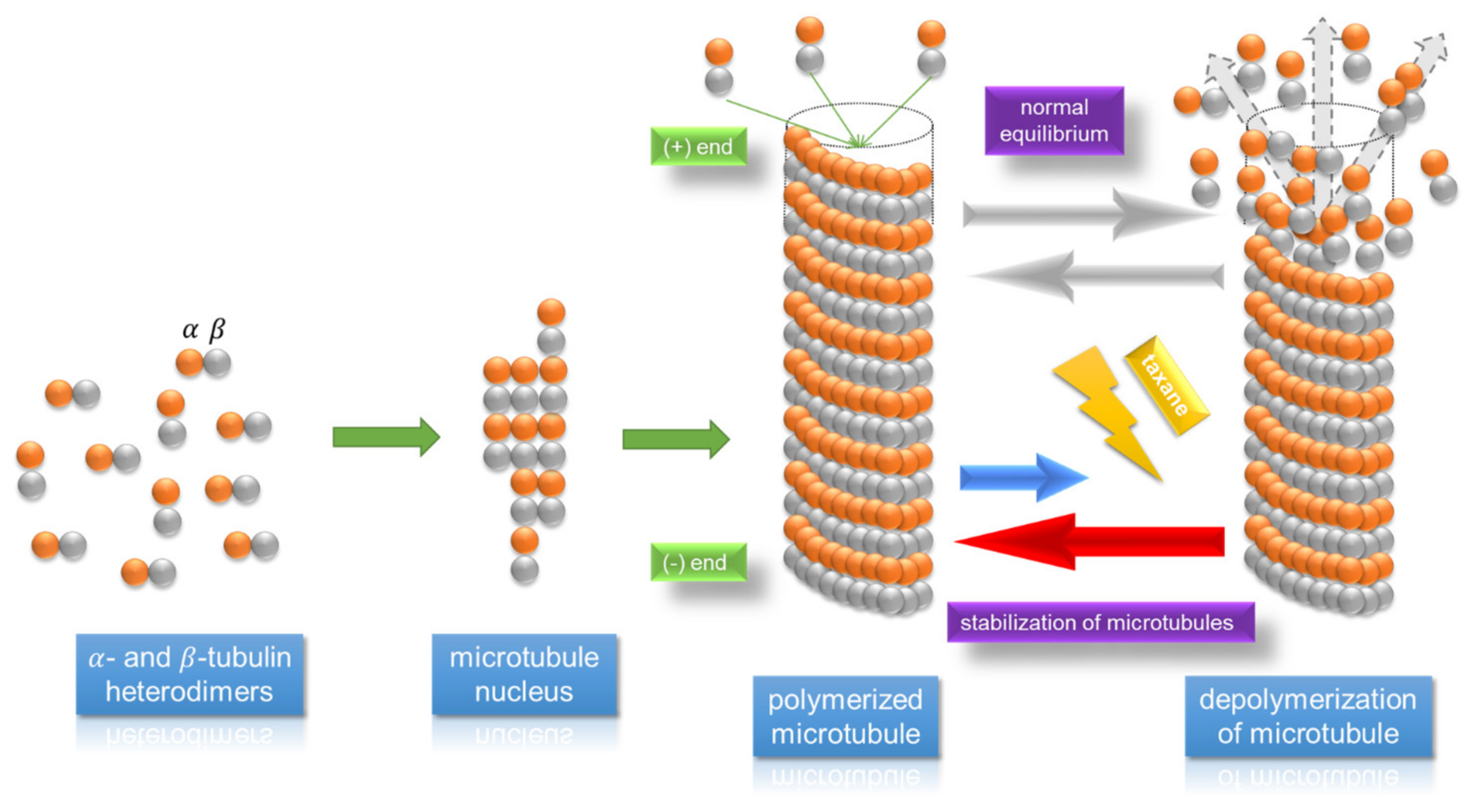
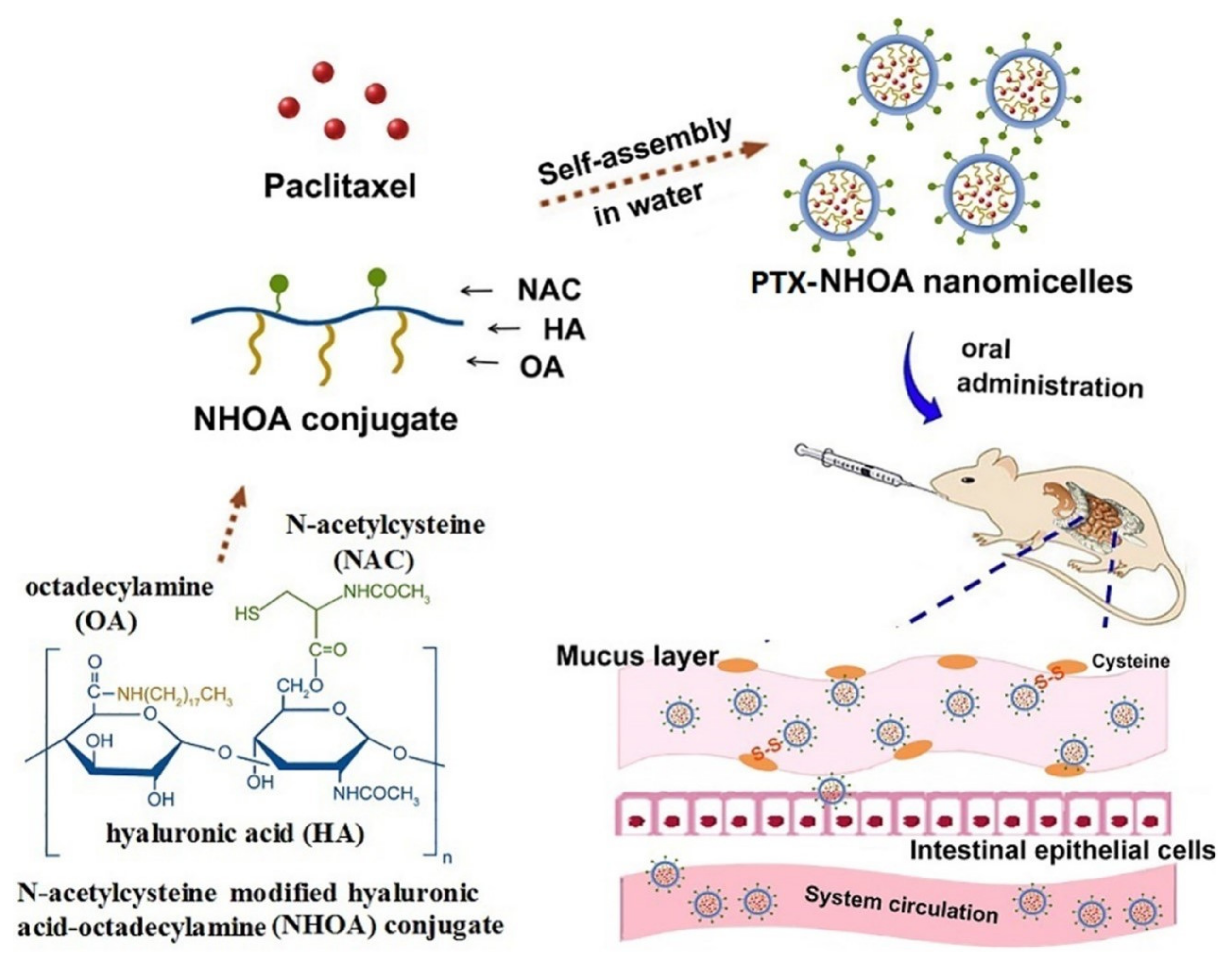
5.1. Thiomer-Based Conjugates for Taxanes and Other Anticancer API Administration
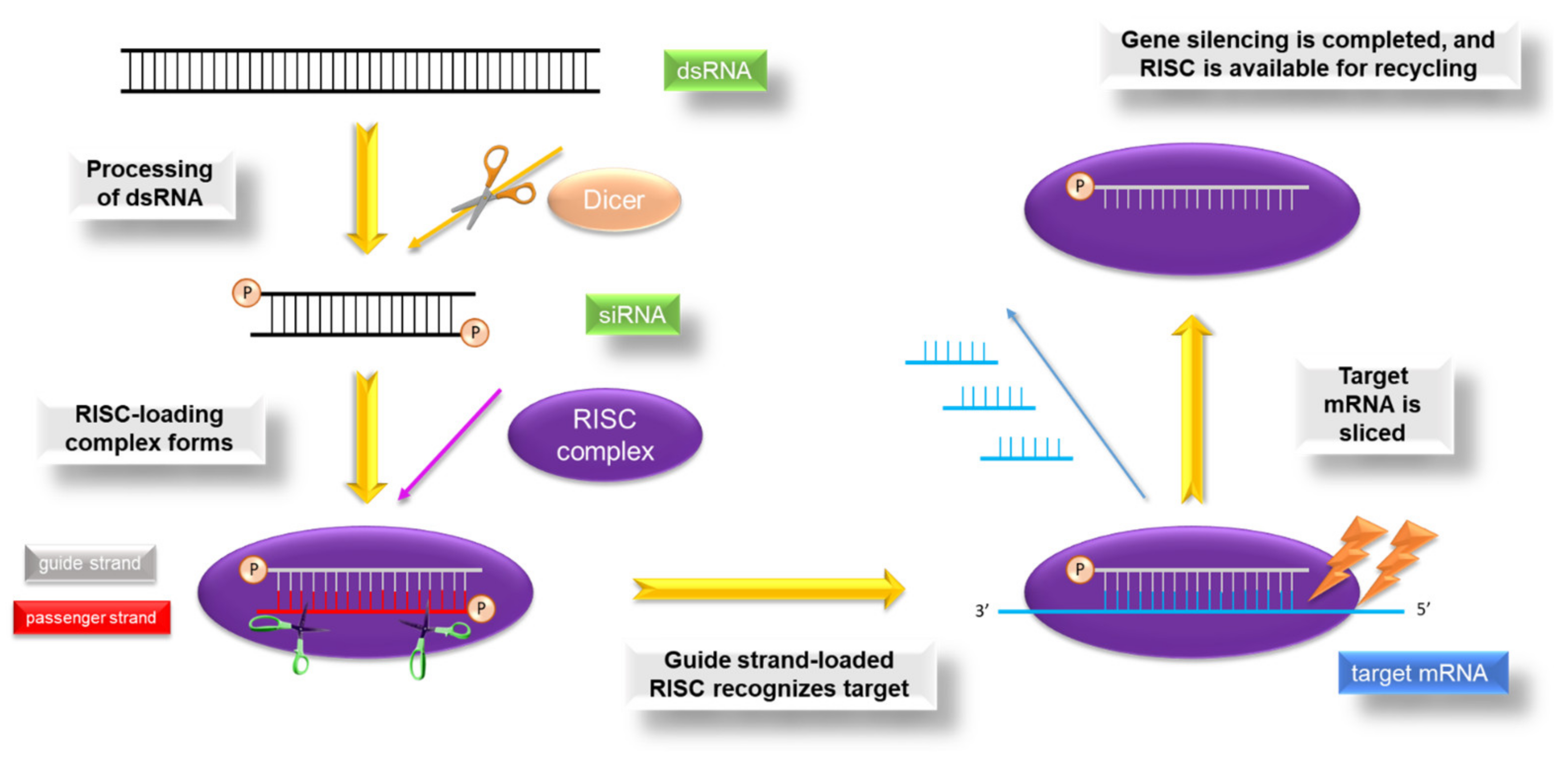
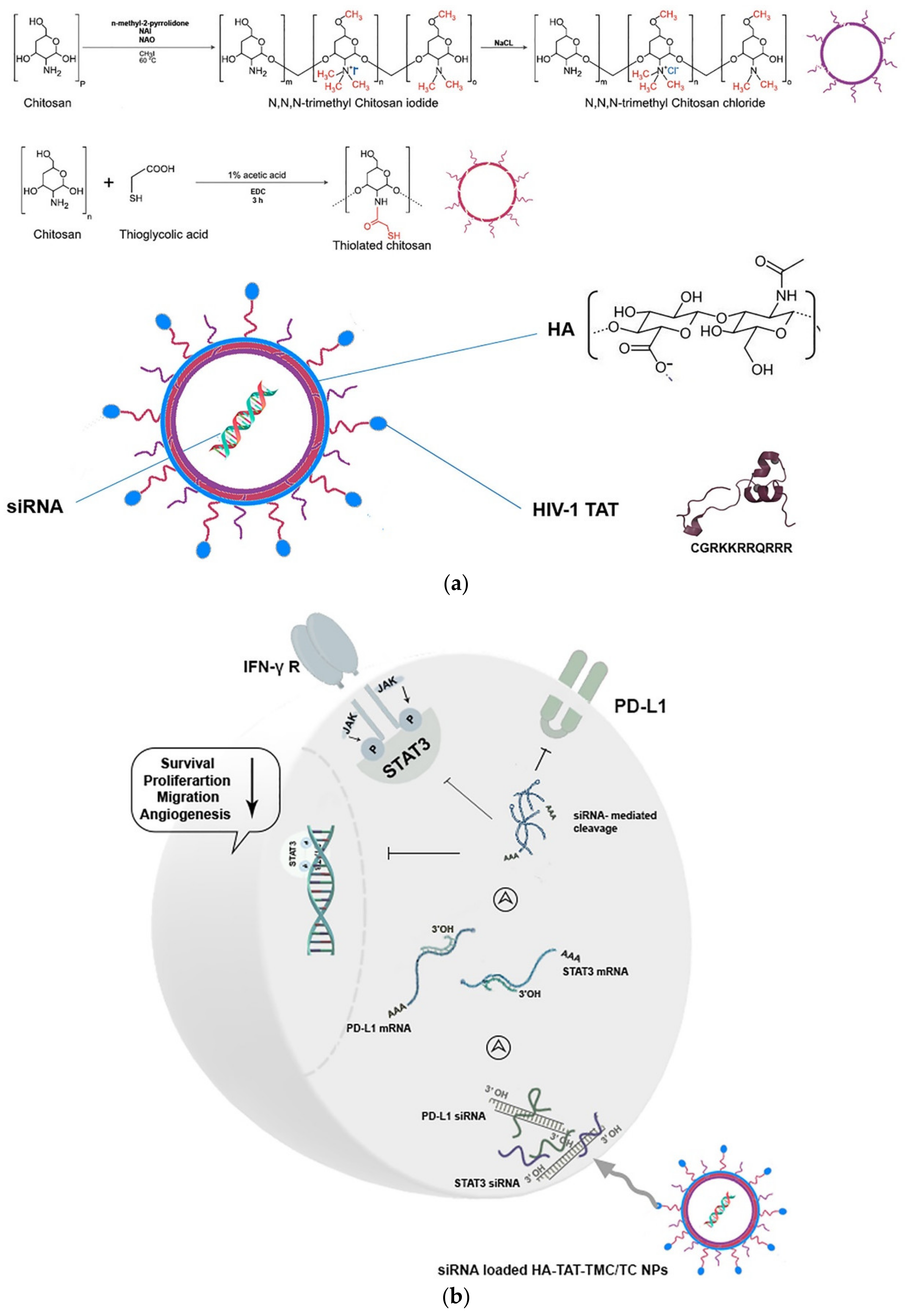
5.2. Thiomer-Based Conjugates for siRNA Administration
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 2,2′-DTNA | 2,2′-Dithiodinicotinic acid |
| 2-MNA | 2-Mercaptonicotinic acid |
| 4-MBA | 4-Mercaptobenzoic acid |
| 6,6′-DTNA | 6,6′-Dithiodinicotinic acid |
| 6,6′-DTNAm | 6,6′-Dithiodinicotinic amide |
| 6-MNA | 6-Mercaptonicotinic acid |
| 6-MNAm | 6 Mercaptonicotinamide |
| APIs | Active pharmaceutical ingredients |
| ASOND | Anti-sense oligonucleotides |
| Bcl-2 | B-cell lymphoma 2 |
| CD44 | Cluster of differentiation 44 |
| CDI | 1,1′ Carbonyldiimidazole |
| CSA | Cysteamine |
| CTS | Chitosan |
| CTX | Cetuximab |
| Cys | L-Cysteine |
| DCM | Dichloromethane |
| DDS | Drug delivery system |
| DIC | N,N′-Diisopropylcarbodiimide |
| DMAP | 4-(Dimethylamino)pyridine |
| DMF | N,N-Dimethylformamide |
| DOX | Doxorubicin |
| DTNB | 5,5′-Dithiobis-(2-nitrobenzoic acid) |
| DTPH | 3,3′ Dithiobis(propanoic hydrazide) |
| DTT | Dithiothreitol |
| DTX | Docet-axel |
| ECM | Extracellular matrix |
| EDAC | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| EGFR | Epidermal growth factor re-ceptor |
| EPR | Enhanced permeability and retention |
| FD4 | Fluorescein isothiocyanate–dextran |
| FDA | Fluorescein diacetate |
| GSH | Reduced Glutathione |
| GSSG | Oxidized Glutathione |
| HA | Hyaluronic acid |
| HAase | Hyaluronidase |
| HOBt | N Hydroxybenzotriazole |
| HPMAM-s-AMPAM | N (2 Hydroxypropyl)methacrylamide-s-N-(3-aminopropyl)methacrylamide |
| HSA | Human serum albumin |
| i-PATAI | Isopropyl (acetylthio)acetimidate |
| iRNAs | Interfer-ing RNAs |
| LDC | Lipid drug conjugate |
| MBA | 4-Mercaptobutanoic acid |
| MDR | Multi-drug resistance |
| MHA | 6-Mercaptohexanoic acid |
| MOA | 8-Mercaptooctanoic acid |
| MPA | Mercaptopropanoic acid |
| MPA | 3-Mercaptopropanoic acid |
| MPPT | 3-Methyl-1-phenylpyrazole-5-thiol |
| mRNA | Messenger RNA |
| MUA | 11 Mercaptoundecanoic acid |
| NAC | N-Acetylcysteine |
| NAP | N-Acetylpenicillamine |
| NHS | N-hydroxysuccinimide |
| NLC | Nanostructured lipid carriers |
| NPs | Nanoparticles |
| OA | Octadecylamine |
| PAA | Poly(acrylic acid) |
| PD-L1 | Programmed cell death-ligand 1 |
| Pec | Pectin |
| PEG | Polyethyleneglycol |
| PEI | Polyethylenimine |
| P-gp | P-glycoprotein |
| pIL-12 | Plasmid encoding IL-12 gene |
| PLA-PCL-TPGS | Poly(lactide-co-ε-caprolactone)-D-α-tocopheryl polyethylene glycol 1000 succinate |
| PLGA | Poly(lactic-co-glycolic) acid |
| PMAA | Poly(methacrylic acid) |
| PMMA | Poly(methyl methacrylate) |
| Poly-siRNA | polymeric siRNA |
| PTX | Paclitaxel |
| QA-CTS-TGA-SH | Quaternary ammonium CTS thiomers |
| RISC | RNA-induced si-lencing complex |
| ROS | Reactive oxygen species |
| SATA | N-succinimidyl-S-(acetyl) thi-oglycolate |
| siRNA | Small interfering RNA |
| SLN | Solid lipid nanoparticles |
| STAT-3 | Signal transducer and activator of transcription-3 |
| Sulfo-NHS | N-Hydroxysulfosuccinimide sodium salt |
| TCEP | Tris(2 carboxyethyl)phosphine |
| TGA | Thioglycolic acid |
| TJs | Tight junctions |
| TMC | N,N,N-trimethylated chitosan |
| TPP | Tripolyphosphate |
| VEGF | Vascular endothelial growth factor |
| XrL | Crosslinked |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef]
- Gavhane, Y.; Shete, A.; Bhagat, A.; Shinde, V.; Bhong, K.; Khairnar, G.; Yadav, A. Solid Tumors: Facts, Challenges and Solutions. Int. J. Pharma Sci. Res. 2011, 2, 1–12. [Google Scholar]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Cocheiro, A. Smart drug delivery systems: From fundamentals to the clinic. Chem. Commun. 2014, 50, 7743–7765. [Google Scholar] [CrossRef]
- Muller, R.H.; Keck, C.M. Challenges and solutions for the delivery of biotech drugs—A review of drug nanocrystal technology and lipid nanoparticles. J. Biotechnol. 2004, 113, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, N.; Galbis, E.; Díaz-Blanco, M.J.; de-Paz, M.V.; Galbis, J.A. Loading studies of the anticancer drug camptothecin into dual stimuli-sensitive nanoparticles. Stability scrutiny. Int. J. Pharm. 2018, 550, 429–438. [Google Scholar] [CrossRef]
- Galbis, E.; De-Paz, M.-V.; Iglesias, N.; Lacroix, B.; Alcudia, A.; Galbis, J.A. Core cross-linked nanoparticles from self-assembling polyfma-based micelles. Encapsulation of lipophilic molecules. Eur. Polym. J. 2017, 89, 406–418. [Google Scholar] [CrossRef]
- Romero-Azogil, L.; Benito, E.; Iglesias, N.; Galbis, E.; De-Paz, M.-V.; García-Martín, M.G. Chapter 11. Redox polymers for drug delivery. In Redox Polymers for Energy and Nanomedicine; Casado, N., Mecerreyes, D., Eds.; Polymer Chemistry Series; The Royal Society of Chemistry: London, UK, 2020; ISBN 978-1-78801-871-5. [Google Scholar]
- Bernkop-Schnürch, A.; Schwarz, V.; Steininger, S. Polymers with thiol groups: A new generation of mucoadhesive polymers? Pharm. Res. 1999, 16, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Summonte, S.; Racaniello, G.F.; Lopedota, A.; Denora, N.; Bernkop-Schnürch, A. Thiolated polymeric hydrogels for biomedical application: Cross-linking mechanisms. J. Control. Release 2021, 330, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Federer, C.; Kurpiers, M.; Bernkop-Schnürch, A. Thiolated Chitosans: A Multi-talented Class of Polymers for Various Applications. Biomacromolecules 2021, 22, 24–56. [Google Scholar] [CrossRef]
- Leichner, C.; Jelkmann, M.; Bernkop-Schnürch, A. Thiolated polymers: Bioinspired polymers utilizing one of the most important bridging structures in nature. Adv. Drug Deliv. Rev. 2019, 151–152, 191–221. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Madrigal, M.M.; Shaw, J.E.; Arno, M.C.; Hoyland, J.A.; Richardson, S.M.; Dove, A.P. Robust alginate/hyaluronic acid thiol-yne click-hydrogel scaffolds with superior mechanical performance and stability for load-bearing soft tissue engineering. Biomater. Sci. 2020, 8, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Teixeira, L.S.M.; Krouwels, A.; Dijkstra, P.J.; Van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Synthesis and characterization of hyaluronic acid-poly(ethylene glycol) hydrogels via Michael addition: An injectable biomaterial for cartilage repair. Acta Biomater. 2010, 6, 1968–1977. [Google Scholar] [CrossRef]
- Bian, S.; He, M.; Sui, J.; Cai, H.; Sun, Y.; Liang, J.; Fan, Y.; Zhang, X. The self-crosslinking smart hyaluronic acid hydrogels as injectable three-dimensional scaffolds for cells culture. Colloids Surf. B Biointerfaces 2016, 140, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Asim, M.H.; Silberhumer, S.; Shahzadi, I.; Jalil, A.; Matuszczak, B.; Bernkop-Schnürch, A. S-protected thiolated hyaluronic acid: In-situ crosslinking hydrogels for 3D cell culture scaffold. Carbohydr. Polym. 2020, 237. [Google Scholar] [CrossRef] [PubMed]
- Croce, M.; Conti, S.; Maake, C.; Patzke, G.R. Synthesis and screening of N-acyl thiolated chitosans for antibacterial applications. Carbohydr. Polym. 2016, 151, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.C.; Medeiros Borsagli, F.G.L.; Mansur, A.A.P.; Caldeira, C.L.; Haas, D.J.; Lage, A.P.; Ciminelli, V.S.T.; Mansur, H.S. 3D sponges of chemically functionalized chitosan for potential environmental pollution remediation: Biosorbents for anionic dye adsorption and ‘antibiotic-free’ antibacterial activity. Environ. Technol. 2021, 42, 2046–2066. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Han, Q.; Wang, Y.; Zhang, H.; Fei, Z.; Wang, Y. The thiolated chitosan: Synthesis, gelling and antibacterial capability. Int. J. Biol. Macromol. 2019, 139, 521–530. [Google Scholar] [CrossRef]
- Geisberger, G.; Gyenge, E.B.; Hinger, D.; Käch, A.; Maake, C.; Patzke, G.R. Chitosan-thioglycolic acid as a versatile antimicrobial agent. Biomacromolecules 2013, 14, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F. Evaluation of chemical modified hydrogel formulation for topical suitability. Int. J. Biol. Macromol. 2017, 105, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, M.; Pallagi, E.; Csóka, I.; Benke, E.; Farkas, Á.; Zeeshan, M.; Burián, K.; Kókai, D.; Ambrus, R. Aerodynamic properties and in silico deposition of isoniazid loaded chitosan/thiolated chitosan and hyaluronic acid hybrid nanoplex DPIs as a potential TB treatment. Int. J. Biol. Macromol. 2020, 165, 3007–3019. [Google Scholar] [CrossRef] [PubMed]
- Rekha, M.R.; Sharma, C.P. Simultaneous Effect of Thiolation and Carboxylation of Chitosan Particles towards Mucoadhesive Oral Insulin Delivery Applications: An In Vitro and In Vivo Evaluation. J. Biomed. Nanotechnol. 2015, 11, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Liu, T.; Dang, Y.; Yu, Z.; Wang, W.; Guo, J.; Zhang, X.; He, G.; Zheng, H.; Yin, Y.; et al. PH/redox responsive core cross-linked nanoparticles from thiolated carboxymethyl chitosan for in vitro release study of methotrexate. Carbohydr. Polym. 2014, 111, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.; Lopalco, A.; Lopedota, A.; Cutrignelli, A.; Laquintana, V.; Franco, M.; Bernkop-Schnürch, A.; Denora, N. S-preactivated thiolated glycol chitosan useful to combine mucoadhesion and drug delivery. Eur. J. Pharm. Biopharm. 2018, 132, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Saremi, S.; Atyabi, F.; Akhlaghi, S.P.; Ostad, S.N.; Dinarvand, R. Thiolated chitosan nanoparticles for enhancing oral absorption of docetaxel: Preparation, in vitro and ex vivo evaluation. Int. J. Nanomed. 2011, 6, 119–128. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, R.; Zhou, L.; Zhao, H.; Lv, F.; Liu, L.; Huang, Y.; Zhang, H.W.; Yu, C.; Wang, S. Blood-brain-barrier penetrable thiolated paclitaxel-oligo (p-phenylene vinylene) nanomedicine with increased drug efficiency for glioblastoma treatment. Nano Today 2020, 35, 100969. [Google Scholar] [CrossRef]
- Dünnhaupt, S.; Barthelmes, J.; Köllner, S.; Sakloetsakun, D.; Shahnaz, G.; Düregger, A.; Bernkop-Schnürch, A. Thiolated nanocarriers for oral delivery of hydrophilic macromolecular drugs. Carbohydr. Polym. 2015, 117, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.C.; Wu, S.J.; Mi, F.L.; Chiu, Y.L.; Yu, S.H.; Panda, N.; Sung, H.W. Thiol-modified chitosan sulfate nanoparticles for protection and release of basic fibroblast growth factor. Bioconjug. Chem. 2010, 21, 28–38. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, H.; Wang, K.; Guo, Q.; Li, C.; Jiang, H.; Hu, Y.; Oupicky, D.; Sun, M. Bioreducible Cross-Linked Hyaluronic Acid/Calcium Phosphate Hybrid Nanoparticles for Specific Delivery of siRNA in Melanoma Tumor Therapy. ACS Appl. Mater. Interfaces 2017, 9, 14576–14589. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P.; Alam, C.; Bendayan, R. Efflux Transporters in Cancer Resistance: Molecular and Functional Characterization of P-glycoprotein; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128164341. [Google Scholar]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Benito, E.; Romero-Azogil, L.; Galbis, E.; De-Paz, M.V.; García-Martín, M.G. Structurally simple redox polymersomes for doxorubicin delivery. Eur. Polym. J. 2020, 137, 109952. [Google Scholar] [CrossRef]
- Akhlaghi, S.P.; Saremi, S.; Ostad, S.N.; Dinarvand, R.; Atyabi, F. Discriminated effects of thiolated chitosan-coated pMMA paclitaxel-loaded nanoparticles on different normal and cancer cell lines. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 689–697. [Google Scholar] [CrossRef]
- Vivek, R.; Nipun Babu, V.; Thangam, R.; Subramanian, K.S.; Kannan, S. PH-responsive drug delivery of chitosan nanoparticles as Tamoxifen carriers for effective anti-tumor activity in breast cancer cells. Colloids Surf. B Biointerfaces 2013, 111, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Manouras, T.; Vamvakaki, M. Field responsive materials: Photo-, electro-, magnetic- and ultrasound-sensitive polymers. Polym. Chem. 2017, 8, 74–96. [Google Scholar] [CrossRef]
- Wong, P.T.; Choi, S.K. Mechanisms of Drug Release in Nanotherapeutic Delivery Systems. Chem. Rev. 2015, 115, 3388–3432. [Google Scholar] [CrossRef]
- Zhou, L.; Qiu, T.; Lv, F.; Liu, L.; Ying, J.; Wang, S. Self-Assembled Nanomedicines for Anticancer and Antibacterial Applications. Adv. Healthc. Mater. 2018, 1800670, 1–29. [Google Scholar] [CrossRef]
- Friedl, P.; Alexander, S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. Release 2014, 190, 485–499. [Google Scholar] [CrossRef]
- Ortega, A.L.; Mena, S.; Estrela, J.M. Glutathione in Cancer Cell Death. Cancers 2011, 3, 1285–1310. [Google Scholar] [CrossRef]
- Balendiran, G.K.; Dabur, R.; Fraser, D. The role of glutathione in cancer. Cell Biochem. Funct. 2004, 22, 343–352. [Google Scholar] [CrossRef] [PubMed]
- López-Mirabal, H.R.; Winther, J.R. Redox characteristics of the eukaryotic cytosol. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2008, 1783, 629–640. [Google Scholar] [CrossRef]
- Netsomboon, K.; Suchaoin, W.; Laffleur, F.; Prüfert, F.; Bernkop-Schnürch, A. Multifunctional adhesive polymers: Preactivated thiolated chitosan-EDTA conjugates. Eur. J. Pharm. Biopharm. 2017, 111, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, G.; Orlando, P.; Edelio, T. Synthesis and applications of chitosan mercaptanes as heavy metal retention agent. Int. J. Biol. Macromol. 2001, 28, 167–174. [Google Scholar] [CrossRef]
- Fei, L.; Bao, C.; Zhang, J.; Sun, Q.; Kong, W.; Han, X.; Wang, Y. Synthesis of Chemically Modified Chitosan with 2,5-Dimercapto-1,3,4-thiodiazole and Its Adsorption Abilities for Au(III), Pd(II), and Pt(IV). J. Appl. Polym. Sci. 2009, 113, 1604–1610. [Google Scholar] [CrossRef]
- Singh, P.; Chauhan, K.; Priya, V.; Singhal, R.K. A greener approach for impressive removal of As(III)/As(v) from an ultra-low concentration using a highly efficient chitosan thiomer as a new adsorbent. RSC Adv. 2016, 6, 64946–64961. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y.; Liu, Z.; Huang, Q. Characteristics of equilibrium, kinetics studies for adsorption of Hg(II), Cu(II), and Ni(II) ions by thiourea-modified magnetic chitosan microspheres. J. Hazard. Mater. 2009, 161, 995–1002. [Google Scholar] [CrossRef]
- Frindy, S.; Primo, A.; Lahcini, M.; Bousmina, M.; Garcia, H.; El Kadib, A. Pd embedded in chitosan microspheres as tunable soft-materials for Sonogashira cross-coupling in water-ethanol mixture. Green Chem. 2015, 17, 1893–1898. [Google Scholar] [CrossRef]
- Chauhan, K.; Singh, P.; Singhal, R.K. New Chitosan-Thiomer: An Efficient Colorimetric Sensor and Effective Sorbent for Mercury at Ultralow Concentration. ACS Appl. Mater. Interfaces 2015, 7, 26069–26078. [Google Scholar] [CrossRef]
- Cheng, B.; Thapa, B.; Remant, K.C.; Xu, P. Dual secured nano-melittin for the safe and effective eradication of cancer cells. J. Mater. Chem. B 2015, 3, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, H.; Ceper, E.B.; Li, X.; Yang, J.; Dong, W.; Ozmen, M.M.; Theato, P. Sulfur Chemistry in Polymer and Materials Science. Macromol. Rapid Commun. 2019, 40, 1800650. [Google Scholar] [CrossRef]
- Su, J. Thiol-Mediated Chemoselective Strategies for In Situ Formation of Hydrogels. Gels 2018, 4, 72. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Rehmann, M.S.; Skeens, K.M.; Maverakis, E.; Kloxin, A.M. Thiol–ene Click Hydrogels for Therapeutic Delivery. ACS Biomater. Sci. Eng. 2016, 2, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Sarti, F.; Bernkop-Schnürch, A. Chitosan and Thiolated Chitosan. In Chitosan for Biomaterials I; Jayakumar, R., Prabaharan, M., Muzzarelli, R.A.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 93–110. ISBN 978-3-642-23113-1. [Google Scholar]
- Petrin, T.H.C.; Fadel, V.; Martins, D.B.; Dias, S.A.; Cruz, A.; Sergio, L.M.; Arcisio-Miranda, M.; Castanho, M.A.R.B.; Dos Santos Cabrera, M.P. Synthesis and Characterization of Peptide-Chitosan Conjugates (PepChis) with Lipid Bilayer Affinity and Antibacterial Activity. Biomacromolecules 2019, 20, 2743–2753. [Google Scholar] [CrossRef]
- Gradauer, K.; Barthelmes, J.; Vonach, C.; Almer, G.; Mangge, H.; Teubl, B.; Roblegg, E.; Dünnhaupt, S.; Fröhlich, E.; Bernkop-Schnürch, A.; et al. Liposomes coated with thiolated chitosan enhance oral peptide delivery to rats. J. Control. Release 2013, 172, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Medeiros Borsagli, F.G.L.; Carvalho, I.C.; Mansur, H.S. Amino acid-grafted and N-acylated chitosan thiomers: Construction of 3D bio-scaffolds for potential cartilage repair applications. Int. J. Biol. Macromol. 2018, 114, 270–282. [Google Scholar] [CrossRef]
- Bermejo-Velasco, D.; Azémar, A.; Oommen, O.P.; Hilborn, J.; Varghese, O.P. Modulating Thiol pKa Promotes Disulfide Formation at Physiological pH: An Elegant Strategy to Design Disulfide Cross-Linked Hyaluronic Acid Hydrogels. Biomacromolecules 2019, 20, 1412–1420. [Google Scholar] [CrossRef]
- Miles, K.B.; Ball, R.L.; Matthew, H.W.T. Chitosan films with improved tensile strength and toughness from N-acetyl-cysteine mediated disulfide bonds. Carbohydr. Polym. 2016, 139, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.-P.R.; Pratt, D.A. On the Reactions of Thiols, Sulfenic Acids, and Sulfinic Acids with Hydrogen Peroxide. Angew. Chem. Int. Ed. 2017, 56, 6255–6259. [Google Scholar] [CrossRef]
- Kirihara, M.; Asai, Y.; Ogawa, S.; Noguchi, T.; Hatano, A.; Hirai, Y. A Mild and Environmentally Benign Oxidation of Thiols to Disulfides. Synthesis 2007, 2007, 3286–3289. [Google Scholar] [CrossRef]
- Lucero, M.J.; Ferris, C.; Sanchez-Gutierrez, C.A.; Jimenez-Castellanos, M.R.; de-Paz, M.-V. Novel aqueous chitosan-based dispersions as efficient drug delivery systems for topical use. Rheological, textural and release studies. Carbohydr. Polym. 2016, 151, 692–699. [Google Scholar] [CrossRef]
- Trombino, S.; Servidio, C.; Curcio, F.; Cassano, R. Strategies for hyaluronic acid-based hydrogel design in drug delivery. Pharmaceutics 2019, 11, 407. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Hornof, M.; Zoidl, T. Thiolated polymers—Thiomers: Synthesis and in vitro evaluation of chitosan-2-iminothiolane conjugates. Int. J. Pharm. 2003, 260, 229–237. [Google Scholar] [CrossRef]
- Mueller, C.; Verroken, A.; Iqbal, J.; Bernkop-Schnuerch, A. Thiolated Chitosans: In Vitro Comparison of Mucoadhesive Properties. J. Appl. Polym. Sci. 2012, 124, 5046–5055. [Google Scholar] [CrossRef]
- Hintzen, F.; Hauptstein, S.; Perera, G.; Bernkop-Schnürch, A. Synthesis and in vitro characterization of entirely S-protected thiolated pectin for drug delivery. Eur. J. Pharm. Biopharm. 2013, 85, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
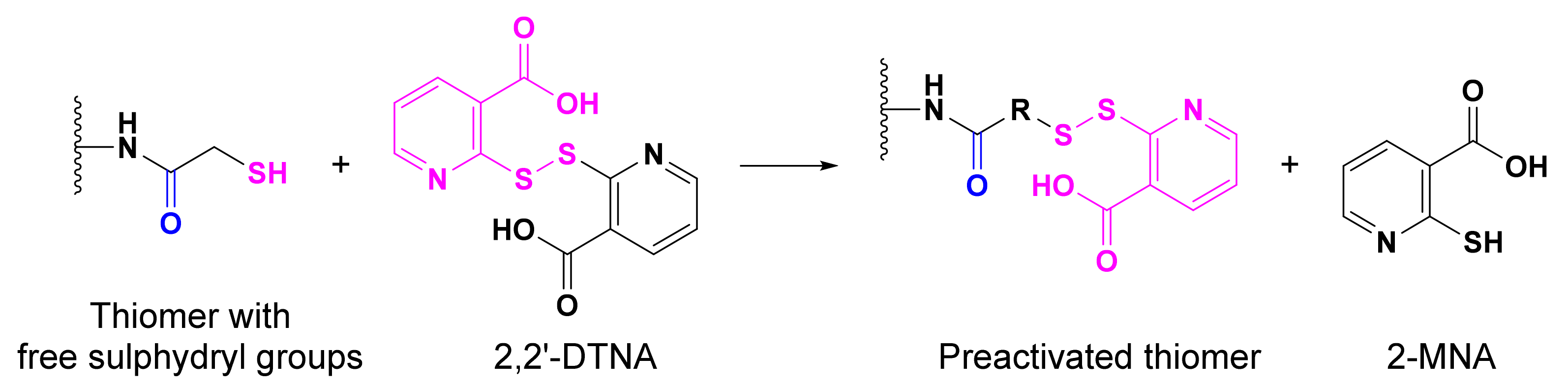
- Menzel, C.; Silbernagl, J.; Laffleur, F.; Leichner, C.; Jelkmann, M.; Huck, C.W.; Hussain, S.; Bernkop-Schnürch, A. 2,2′Dithiodinicotinyl ligands: Key to more reactive thiomers. Int. J. Pharm. 2016, 503, 199–206. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol-ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Hlil, A.R.; Lepiller, C.; Gaudet, J.; Guay, D.; Shi, Z.; Holdcroft, S.; Hay, A.S. Stability and Utility of Pyridyl Disulfide Functionality in RAFT and Conventional Radical Polymerizations. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7207–7224. [Google Scholar] [CrossRef]
- Kade, M.J.; Burke, D.J.; Hawker, C.J. The power of thiol-ene chemistry. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 743–750. [Google Scholar] [CrossRef]
- Stichler, S.; Jungst, T.; Schamel, M.; Zilkowski, I.; Kuhlmann, M.; Böck, T.; Blunk, T.; Teßmar, J.; Groll, J. Thiol-ene Clickable Poly(glycidol) Hydrogels for Biofabrication. Ann. Biomed. Eng. 2017, 45, 273–285. [Google Scholar] [CrossRef]
- Cao, W.; Sui, J.; Ma, M.; Xu, Y.; Lin, W.; Chen, Y.; Man, Y.; Sun, Y.; Fan, Y.; Zhang, X. The preparation and biocompatible evaluation of injectable dual crosslinking hyaluronic acid hydrogels as cytoprotective agents. J. Mater. Chem. B 2019, 7, 4413–4423. [Google Scholar] [CrossRef]
- Dubbini, A.; Censi, R.; Butini, M.E.; Sabbieti, M.G.; Agas, D.; Vermonden, T.; Di Martino, P. Injectable hyaluronic acid/PEG-p(HPMAm-lac)-based hydrogels dually cross-linked by thermal gelling and Michael addition. Eur. Polym. J. 2015, 72, 423–437. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Granja, S.; Boni, F.I.; Prezotti, F.G.; Ferreira, L.M.B.; Cury, B.S.F.; Reis, R.M.; Baltazar, F.; Gremião, M.P.D. Modulating chitosan-PLGA nanoparticle properties to design a co-delivery platform for glioblastoma therapy intended for nose-to-brain route. Drug Deliv. Transl. Res. 2020, 10, 1729–1747. [Google Scholar] [CrossRef]
- Khire, V.S.; Lee, T.Y.; Bowman, C.N. Synthesis, Characterization and Cleavage of Surface-Bound Linear Polymers Formed Using Thiol−Ene Photopolymerizations. Macromolecules 2008, 41, 7440–7447. [Google Scholar] [CrossRef]
- Shin, J.; Matsushima, H.; Chan, J.W.; Hoyle, C.E. Segmented Polythiourethane Elastomers through Sequential Thiol−Ene and Thiol−Isocyanate Reactions. Macromolecules 2009, 42, 3294–3301. [Google Scholar] [CrossRef]
- Müller, C.; Ma, B.N.; Gust, R.; Bernkop-Schnürch, A. Thiopyrazole preactivated chitosan: Combining mucoadhesion and drug delivery. Acta Biomater. 2013, 9, 6585–6593. [Google Scholar] [CrossRef]
- Palmberger, T.F.; Albrecht, K.; Loretz, B.; Bernkop-Schnürch, A. Thiolated polymers: Evaluation of the influence of the amount of covalently attached l-cysteine to poly(acrylic acid). Eur. J. Pharm. Biopharm. 2007, 66, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Baus, R.A.; Innerhofer, J.; Rohrer, J.; Lupo, N.; Bernkop-Schnürch, A. Anhydrous thiomers: Strategy for enhanced mucoadhesion. Eur. J. Pharm. Biopharm. 2018, 129, 273–281. [Google Scholar] [CrossRef]
- Nitave, S.A.; Patil, V.A.; Kagalkar, A.A. Review on gastro retentive drug delivery system (GRDDS). Int. J. Pharm. Sci. Rev. Res. 2014, 27, 90–95. [Google Scholar]
- Pereira de Sousa, I.; Suchaoin, W.; Zupančič, O.; Leichner, C.; Bernkop-Schnürch, A. Totally S-protected hyaluronic acid: Evaluation of stability and mucoadhesive properties as liquid dosage form. Carbohydr. Polym. 2016, 152, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Mun, E.A.; Williams, A.C.; Khutoryanskiy, V.V. Adhesion of thiolated silica nanoparticles to urinary bladder mucosa: Effects of PEGylation, thiol content and particle size. Int. J. Pharm. 2016, 512, 32–38. [Google Scholar] [CrossRef]
- Cevher, E.; Taha, M.A.M.; Orlu, M.; Araman, A. Evaluation of Mechanical and Mucoadhesive Properties of Clomiphene Citrate Gel Formulations Containing Carbomers and Their Thiolated Derivatives. Drug Deliv. 2008, 15, 57–67. [Google Scholar] [CrossRef]
- Kafedjiiski, K.; Föger, F.; Werle, M.; Bernkop-Schnürch, A. Synthesis and in Vitro Evaluation of a Novel Chitosan–Glutathione Conjugate. Pharm. Res. 2005, 22, 1480–1488. [Google Scholar] [CrossRef]
- Langoth, N.; Kalbe, J.; Bernkop-Schnürch, A. Development of buccal drug delivery systems based on a thiolated polymer. Int. J. Pharm. 2003, 252, 141–148. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, S.; Deng, F.; Chen, X.; Wang, X.; Wang, Y.; Zhang, H.; Dai, W.; He, B.; Zhang, Q.; et al. The function and mechanism of preactivated thiomers in triggering epithelial tight junctions opening. Eur. J. Pharm. Biopharm. 2018, 133, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Zambito, Y.; Fogli, S.; Zaino, C.; Stefanelli, F.; Breschi, M.C.; Di Colo, G. Synthesis, characterization and evaluation of thiolated quaternary ammonium-chitosan conjugates for enhanced intestinal drug permeation. Eur. J. Pharm. Sci. 2009, 38, 112–120. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Kast, C.E.; Guggi, D. Permeation enhancing polymers in oral delivery of hydrophilic macromolecules: Thiomer/GSH systems. J. Control. Release 2003, 93, 95–103. [Google Scholar] [CrossRef]
- Kafedjiiski, K.; Hoffer, M.; Werle, M.; Bernkop-Schnürch, A. Improved synthesis and in vitro characterization of chitosan- thioethylamidine conjugate. Biomaterials 2006, 27, 127–135. [Google Scholar] [CrossRef]
- Iqbal, J.; Shahnaz, G.; Dünnhaupt, S.; Müller, C.; Hintzen, F.; Bernkop-Schnürch, A. Preactivated thiomers as mucoadhesive polymers for drug delivery. Biomaterials 2012, 33, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L.; Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta (BBA) Biomembr. 1976, 455, 152–162. [Google Scholar] [CrossRef]
- Khan, S.; Faisal, S.; Shams, D.F.; Zia, M.; Nadhman, A. Photo-inactivation of bacteria in hospital effluent via thiolated iron-doped nanoceria. IET Nanobiotechnol. 2019, 13, 875–879. [Google Scholar] [CrossRef]
- Thanki, K.; Gangwal, R.P.; Sangamwar, A.T.; Jain, S. Oral delivery of anticancer drugs: Challenges and opportunities. J. Control. Release 2013, 170, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Mok, H.; Lee, S.H.; Park, J.W.; Park, T.G. Multimeric small interfering ribonucleic acid for highly efficient sequence-specific gene silencing. Nat. Mater. 2010, 9, 272–278. [Google Scholar] [CrossRef]
- Ferris, C.; de Paz, M.V.; Aguilar-de-Leyva, A.; Caraballo, I.; Galbis, J.A. Reduction-sensitive functionalized copolyurethanes for biomedical applications. Polym. Chem. 2014, 5, 2370–2381. [Google Scholar] [CrossRef]
- Ma, G. Microencapsulation of protein drugs for drug delivery: Strategy, preparation, and applications. J. Control. Release 2014, 193, 324–340. [Google Scholar] [CrossRef]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Enescu, D.; Olteanu, C.E. Functionalized Chitosan and its use in pharmaceutical, biomedical, and biotechnological research. Chem. Eng. Commun. 2008, 195, 1269–1291. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.D.; Leroux, J.C.; Atkinson, B.L.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Feng, Z.; Simeone, A.; Odelius, K.; Hakkarainen, M. Biobased Nanographene Oxide Creates Stronger Chitosan Hydrogels with Improved Adsorption Capacity for Trace Pharmaceuticals. ACS Sustain. Chem. Eng. 2017, 5, 11525–11535. [Google Scholar] [CrossRef]
- Bravo-Osuna, I.; Teutonico, D.; Arpicco, S.; Vauthier, C.; Ponchel, G. Characterization of chitosan thiolation and application to thiol quantification onto nanoparticle surface. Int. J. Pharm. 2007, 340, 173–181. [Google Scholar] [CrossRef]
- Denora, N.; Lopedota, A.; Perrone, M.; Laquintana, V.; Iacobazzi, R.M.; Milella, A.; Fanizza, E.; Depalo, N.; Cutrignelli, A.; Lopalco, A.; et al. Spray-dried mucoadhesives for intravesical drug delivery using N-acetylcysteine- and glutathione-glycol chitosan conjugates. Acta Biomater. 2016, 43, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Hauptstein, S.; Bonengel, S.; Griessinger, J.; Bernkop-Schnürch, A. Synthesis and Characterization of pH Tolerant and Mucoadhesive (Thiol–Polyethylene Glycol) Chitosan Graft Polymer for Drug Delivery. J. Pharm. Sci. 2014, 103, 594–601. [Google Scholar] [CrossRef]
- Santos, J.C.C.; Moreno, P.M.D.; Mansur, A.A.P.; Leiro, V.; Mansur, H.S.; Pêgo, A.P. Functionalized chitosan derivatives as nonviral vectors: Physicochemical properties of acylated N,N,N-trimethyl chitosan/oligonucleotide nanopolyplexes. Soft Matter 2015, 11, 8113–8125. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.S.; Oh, H.M.; Cho, M.O.; Jang, B.S.; Cho, J.K.; Park, K.H.; Kang, S.W.; Huh, K.M. Synthesis and characterization of thiolated hexanoyl glycol chitosan as a mucoadhesive thermogelling polymer. Biomater. Res. 2018, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Talaei, F.; Azizi, E.; Dinarvand, R.; Atyabi, F. Thiolated chitosan nanoparticles as a delivery system for antisense therapy: Evaluation against EGFR in T47D breast cancer cells. Int. J. Nanomed. 2011, 6, 1963–1975. [Google Scholar] [CrossRef]
- Millotti, G.; Laffleur, F.; Perera, G.; Vigl, C.; Pickl, K.; Sinner, F.; Bernkop-Schnürch, A. In Vivo Evaluation of Thiolated Chitosan Tablets for Oral Insulin Delivery. J. Pharm. Sci. 2014, 103, 3165–3170. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Piras, A.M.; Uccello-Barretta, G.; Balzano, F.; Cesari, A.; Testai, L.; Citi, V.; Zambito, Y. Impact of mucoadhesive polymeric nanoparticulate systems on oral bioavailability of a macromolecular model drug. Eur. J. Pharm. Biopharm. 2018, 130, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Dünnhaupt, S.; Barthelmes, J.; Thurner, C.C.; Waldner, C.; Sakloetsakun, D.; Bernkop-Schnürch, A. S-protected thiolated chitosan: Synthesis and in vitro characterization. Carbohydr. Polym. 2012, 90, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wu, D.; Cheng, G.; Zhao, H. Reductant-triggered rapid self-gelation and biological functionalization of hydrogels. Polym. Chem. 2015, 6, 8275–8283. [Google Scholar] [CrossRef]
- Zambito, Y.; Felice, F.; Fabiano, A.; Di Stefano, R.; Di Colo, G. Mucoadhesive nanoparticles made of thiolated quaternary chitosan crosslinked with hyaluronan. Carbohydr. Polym. 2013, 92, 33–39. [Google Scholar] [CrossRef]
- Lupo, N.; Jalil, A.; Nazir, I.; Gust, R.; Bernkop-Schnürch, A. In vitro evaluation of intravesical mucoadhesive self-emulsifying drug delivery systems. Int. J. Pharm. 2019, 564, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kafedjiiski, K.; Krauland, A.H.; Hoffer, M.H.; Bernkop-Schnürch, A. Synthesis and in vitro evaluation of a novel thiolated chitosan. Biomaterials 2005, 26, 819–826. [Google Scholar] [CrossRef]
- Lee, S.J.; Huh, M.S.; Lee, S.Y.; Min, S.; Lee, S.; Koo, H.; Chu, J.U.; Lee, K.E.; Jeon, H.; Choi, Y.; et al. Tumor-homing poly-siRNA/glycol chitosan self-cross-linked nanoparticles for systemic siRNA delivery in cancer treatment. Angew. Chem. Int. Ed. 2012, 51, 7203–7207. [Google Scholar] [CrossRef]
- Verheyen, E.; Van Der Wal, S.; Deschout, H.; Braeckmans, K.; De Smedt, S.; Barendregt, A.; Hennink, W.E.; Van Nostrum, C.F. Protein macromonomers containing reduction-sensitive linkers for covalent immobilization and glutathione triggered release from dextran hydrogels. J. Control. Release 2011, 156, 329–336. [Google Scholar] [CrossRef]
- Yhee, J.Y.; Song, S.; Lee, S.J.; Park, S.G.; Kim, K.S.; Kim, M.G.; Son, S.; Koo, H.; Kwon, I.C.; Jeong, J.H.; et al. Cancer-targeted MDR-1 siRNA delivery using self-cross-linked glycol chitosan nanoparticles to overcome drug resistance. J. Control. Release 2015, 198, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, J.; Pan, H.; He, F.; Liu, Z.; Wu, Q.; Bai, C.; Yu, S.; Yang, X. Potential advantages of a novel chitosan-N-acetylcysteine surface modified nanostructured lipid carrier on the performance of ophthalmic delivery of curcumin. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Singh, R.; Kats, L.; Blättler, W.A.; Lambert, J.M. Formation of N-substituted 2-iminothiolanes when amino groups in proteins and peptides are modified by 2-iminothiolane. Anal. Biochem. 1996, 236, 114–125. [Google Scholar] [CrossRef]
- Nagireddi, S.; Golder, A.K.; Uppaluri, R. Role of EDTA on the Pd(II) adsorption characteristics of chitosan cross-linked 3-amino-1,2,4-triazole-5-thiol derivative from synthetic electroless plating solutions. Int. J. Biol. Macromol. 2019, 127, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Guggi, D.; Pinter, Y. Thiolated chitosans: Development and in vitro evaluation of a mucoadhesive, permeation enhancing oral drug delivery system. J. Control. Release 2004, 94, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ferris, C.; Casas, M.; Lucero, M.J.; De Paz, M.V.; Jiménez-Castellanos, M.R. Synthesis and characterization of a novel chitosan-N-acetyl-homocysteine thiolactone polymer using MES buffer. Carbohydr. Polym. 2014, 111, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Juntapram, K.; Praphairaksit, N.; Siraleartmukul, K.; Muangsin, N. Synthesis and characterization of chitosan-homocysteine thiolactone as a mucoadhesive polymer. Carbohydr. Polym. 2012, 87, 2399–2408. [Google Scholar] [CrossRef]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef]
- Goodarzi, N.; Varshochian, R.; Kamalinia, G.; Atyabi, F.; Dinarvand, R. A review of polysaccharide cytotoxic drug conjugates for cancer therapy. Carbohydr. Polym. 2013, 92, 1280–1293. [Google Scholar] [CrossRef]
- Brannigan, R.P.; Khutoryanskiy, V.V. Progress and Current Trends in the Synthesis of Novel Polymers with Enhanced Mucoadhesive Properties. Macromol. Biosci. 2019, 19, 1–11. [Google Scholar] [CrossRef]
- Zarembinski, T.I.; Doty, N.J.; Erickson, I.E.; Srinivas, R.; Wirostko, B.M.; Tew, W.P. Thiolated hyaluronan-based hydrogels crosslinked using oxidized glutathione: An injectable matrix designed for ophthalmic applications. Acta Biomater. 2014, 10, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Fernandes-Cunha, G.M.; Myung, D. In situ-forming hyaluronic acid hydrogel through visible light-induced thiol-ene reaction. React. Funct. Polym. 2018, 131, 29–35. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, L.; Zhou, L.; Yu, S.; Lan, Y.; Liang, Q.; Liu, J.; Cao, A.; Liu, Y. Tumor Microenvironment-Triggered Charge Reversal Polymetformin-Based Nanosystem Co-Delivered Doxorubicin and IL-12 Cytokine Gene for Chemo-Gene Combination Therapy on Metastatic Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12, 45873–45890. [Google Scholar] [CrossRef]
- Shu, X.Z.; Liu, Y.; Palumbo, F.; Prestwich, G.D. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: A covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials 2003, 24, 3825–3834. [Google Scholar] [CrossRef]
- Choh, S.Y.; Cross, D.; Wang, C. Facile synthesis and characterization of disulfide-cross-linked hyaluronic acid hydrogels for protein delivery and cell encapsulation. Biomacromolecules 2011, 12, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Yin Win, K.; Feng, S.-S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Luangtana-anan, M.; Opanasopit, P.; Ngawhirunpat, T.; Nunthanid, J.; Sriamornsak, P.; Limmatvapirat, S.; Lim, L.Y. Effect of Chitosan Salts and Molecular Weight on a Nanoparticulate Carrier for Therapeutic Protein. Pharm. Dev. Technol. 2005, 10, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, E.; Kingston, D.G.I. A new semisynthesis of paclitaxel from Baccatin III. J. Nat. Prod. 1999, 62, 1068–1071. [Google Scholar] [CrossRef]
- Shriner, D.L.; Mccoy, D.K.; Goldberg, D.J.; Wagner, R.F. Docetaxel: A taxoid for the treatment of metastatic breast cancer. Am. J. Health Syst. Pharm. 1998, 55, 79–97. [Google Scholar]
- Santra, M.; Santra, S.; Roberts, C.; Zhang, R.L.; Chopp, M. Doublecortin induces mitotic microtubule catastrophe and inhibits glioma cell invasion. J. Neurochem. 2009, 108, 231–245. [Google Scholar] [CrossRef]
- Rowinsky, E.; Donehower, R. Paclitaxel (taxol). N. Engl. J. Med. 1995, 332, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Kelland, L.R.; Abel, G. Comparative in vitro cytotoxicity of taxol and Taxotere against cisplatin-sensitive and -resistant human ovarian carcinoma cell lines. Cancer Chemother. Pharmacol. 1992, 30, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Danhier, F.; Lecouturier, N.; Vroman, B.; Jérôme, C.; Marchand-Brynaert, J.; Feron, O.; Préat, V. Paclitaxel-loaded PEGylated PLGA-based nanoparticles: In vitro and in vivo evaluation. J. Control. Release 2009, 133, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef]
- Roldo, M.; Hornof, M.; Caliceti, P.; Bernkop-Schnürch, A. Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: Synthesis and in vitro evaluation. Eur. J. Pharm. Biopharm. 2004, 57, 115–121. [Google Scholar] [CrossRef]
- Jiang, L.; Li, X.; Liu, L.; Zhang, Q. Thiolated chitosan-modified PLA-PCL-TPGS nanoparticles for oral chemotherapy of lung cancer. Nanoscale Res. Lett. 2013, 8, 1–11. [Google Scholar] [CrossRef]
- Leitner, V.M.; Guggi, D.; Krauland, A.H.; Bernkop-Schnärch, A. Nasal delivery of human growth hormone: In vitro and in vivo evaluation of a thiomer/glutathione microparticulate delivery system. J. Control. Release 2004, 100, 87–95. [Google Scholar] [CrossRef]
- Fischer, J.R.; Harkin, K.R.; Freeman, L.C. Concurrent administration of water-soluble vitamin E can increase the oral bioavailability of cyclosporine a in healthy dogs. Vet 2002, 3, 465–473. [Google Scholar]
- Ma, Y.; Zheng, Y.; Liu, K.; Tian, G.; Tian, Y.; Xu, L.; Yan, F.; Huang, L.; Mei, L. Nanoparticles of poly(Lactide-Co-Glycolide)-D-α-Tocopheryl polyethylene glycol 1000 succinate random copolymer for cancer treatment. Nanoscale Res. Lett. 2010, 5, 1161–1169. [Google Scholar] [CrossRef]
- Zhang, M.; Asghar, S.; Jin, X.; Hu, Z.; Ping, Q.; Chen, Z.; Shao, F.; Xiao, Y. The enhancing effect of N-acetylcysteine modified hyaluronic acid-octadecylamine micelles on the oral absorption of paclitaxel. Int. J. Biol. Macromol. 2019, 138, 636–647. [Google Scholar] [CrossRef]
- Föger, F.; Malaivijitnond, S.; Wannaprasert, T.; Huck, C.; Bernkop-Schnürch, A.; Werle, M. Effect of a thiolated polymer on oral paclitaxel absorption and tumor growth in rats. J. Drug Target. 2008, 16, 149–155. [Google Scholar] [CrossRef]
- Iglesias, N.; Galbis, E.; Romero-Azogil, L.; Benito, E.; Lucas, R.; García-Martín, M.G.; De-Paz, M.V. In-depth study into polymeric materials in low-density gastroretentive formulations. Pharmaceutics 2020, 12, 636. [Google Scholar] [CrossRef]
- Zhou, L.; Lv, F.; Liu, L.; Shen, G.; Yan, X.; Bazan, G.C.; Wang, S. Cross-Linking of Thiolated Paclitaxel–Oligo(p-phenylene vinylene) Conjugates Aggregates inside Tumor Cells Leads to “Chemical Locks” That Increase Drug Efficacy. Adv. Mater. 2018, 30, 1–8. [Google Scholar] [CrossRef]
- Levit, M.; Zashikhina, N.; Vdovchenko, A.; Dobrodumov, A.; Zakharova, N.; Kashina, A.; Rühl, E.; Lavrentieva, A.; Scheper, T.; Tennikova, T.; et al. Bio-Inspired Amphiphilic Block-Copolymers Based on Synthetic Glycopolymer and Poly(Amino Acid) as Potential Drug Delivery Systems. Polymers 2020, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.I.; Ayub, A.D.; Mat Yusuf, S.N.A.; Yahaya, N.; Kadir, E.A.; Lim, V. Docetaxel-loaded disulfide cross-linked nanoparticles derived from thiolated sodium alginate for colon cancer drug delivery. Pharmaceutics 2020, 12, 38. [Google Scholar] [CrossRef]
- Gao, Y.; Kieltyka, R.E.; Jesse, W.; Norder, B.; Korobko, A.V.; Kros, A. Thiolated human serum albumin cross-linked dextran hydrogels as a macroscale delivery system. Soft Matter 2014, 10, 4869–4874. [Google Scholar] [CrossRef] [PubMed]
- Schmidl, D.; Werkmeister, R.; Kaya, S.; Unterhuber, A.; Witkowska, K.J.; Baumgartner, R.; Höller, S.; O’Rourke, M.; Peterson, W.; Wolter, A.; et al. A Controlled, Randomized Double-Blind Study to Evaluate the Safety and Efficacy of Chitosan-N-Acetylcysteine for the Treatment of Dry Eye Syndrome. J. Ocul. Pharmacol. Ther. 2017, 33, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Bayat, N.; McOrist, N.; Ariotti, N.; Lai, M.; Sia, K.C.S.; Li, Y.; Grace, J.L.; Quinn, J.F.; Whittaker, M.R.; Kavallaris, M.; et al. Thiol-reactive star polymers functionalized with short ethoxy-containing moieties exhibit enhanced uptake in acute lymphoblastic leukemia cells. Int. J. Nanomed. 2019, 14, 9795–9808. [Google Scholar] [CrossRef]
- Cella, D.; Peterman, A.; Hudgens, S.; Webster, K.; Socinski, M.A. Measuring the side effects of taxane therapy in oncology: The Functional Assessment of Cancer Therapy-Taxane (FACT-Taxane). Cancer 2003, 98, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Krauland, A.H.; Guggi, D.; Bernkop-Schnürch, A. Oral insulin delivery: The potential of thiolated chitosan-insulin tablets on non-diabetic rats. J. Control. Release 2004, 95, 547–555. [Google Scholar] [CrossRef]
- Sudhakar, S.; Chandran, S.V.; Selvamurugan, N.; Nazeer, R.A. Biodistribution and pharmacokinetics of thiolated chitosan nanoparticles for oral delivery of insulin in vivo. Int. J. Biol. Macromol. 2020, 150, 281–288. [Google Scholar] [CrossRef]
- Uthaman, S.; Zheng, S.; Han, J.; Choi, Y.J.; Cho, S.; Nguyen, V.D.; Park, J.O.; Park, S.H.; Min, J.J.; Park, S.; et al. Preparation of Engineered Salmonella Typhimurium-Driven Hyaluronic-Acid-Based Microbeads with Both Chemotactic and Biological Targeting Towards Breast Cancer Cells for Enhanced Anticancer Therapy. Adv. Healthc. Mater. 2016, 5, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Leake, D.; Boese, Q.; Scaringe, S.; Marshall, W.S.; Khvorova, A. Rational siRNA design for RNA interference. Nat. Biotechnol. 2004, 22, 326–330. [Google Scholar] [CrossRef]
- Sharp, P.A. RNA interference. Genes Dev. 2000, 485–490. [Google Scholar] [CrossRef]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef]
- Soutschek, J.; Akinc, A.; Bramlage, B.; Charisse, K.; Constien, R.; Donoghue, M.; Elbashir, S.; Geick, A.; Hadwiger, P.; Harborth, J.; et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 2004, 432, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Judge, A.D.; Sood, V.; Shaw, J.R.; Fang, D.; McClintock, K.; MacLachlan, I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 2005, 23, 457–462. [Google Scholar] [CrossRef]
- Judge, A.D.; Bola, G.; Lee, A.C.H.; MacLachlan, I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol. Ther. 2006, 13, 494–505. [Google Scholar] [CrossRef]
- Lee, S.Y.; Huh, M.S.; Lee, S.; Lee, S.J.; Chung, H.; Park, J.H.; Oh, Y.K.; Choi, K.; Kim, K.; Kwon, I.C. Stability and cellular uptake of polymerized siRNA (poly-siRNA)/polyethylenimine (PEI) complexes for efficient gene silencing. J. Control. Release 2010, 141, 339–346. [Google Scholar] [CrossRef]
- Deng, Z.J.; Morton, S.W.; Ben-Akiva, E.; Dreaden, E.C.; Shopsowitz, K.E.; Hammond, P.T. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano 2013, 7, 9571–9584. [Google Scholar] [CrossRef]
- York, A.W.; Huang, F.; McCormick, C.L. Rational Design of Targeted Cancer Therapeutics through the Multi-Conjugation of Folate and Cleavable siRNA to RAFT- synthesized (HPMA-s-APMA) Copolymers. Biomacromolecules 2010. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.K.; Sarkar, A.; Feldmann, D.P.; Hoffmann, P.; Merkel, O. Revisiting the Value of Competition Assays in Folate Receptor-Mediated Drug Delivery. Biomaterials 2017, 138, 35–45. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Lammers, T.; Schiffelers, R.M.; Van Steenbergen, M.J.; Hennink, W.E.; Storm, G. Gene silencing activity of siRNA polyplexes based on biodegradable polymers. Eur. J. Pharm. Biopharm. 2011, 77, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Van De Wetering, P.; Schuurmans-Nieuwenbroek, N.M.E.; Hennink, W.E.; Storm, G. Comparative Transfection Studies of Human Ovarian Carcinoma Cells in vitro, ex vivo and in vivo with Poly(2-(dimethylamino)ethyl methacrylate)-based Polyplexes. J. Gene Med. 1999, 1, 156–165. [Google Scholar] [CrossRef]
- Yin, T.; Liu, J.; Zhao, Z.; Dong, L.; Cai, H.; Yin, L.; Zhou, J.; Huo, M. Smart nanoparticles with a detachable outer shell for maximized synergistic antitumor efficacy of therapeutics with varying physicochemical properties. J. Control. Release 2016, 243, 54–68. [Google Scholar] [CrossRef]
- Bastaki, S.; Aravindhan, S.; Ahmadpour Saheb, N.; Afsari Kashani, M.; Dorofeev, A.E.; Karoon Kiani, F.; Jahandideh, H.; Beigi Dargani, F.; Aksoun, M.; Nikkhoo, A.; et al. Codelivery of STAT3 and PD-L1 siRNA by hyaluronate-TAT trimethyl/thiolated chitosan nanoparticles suppresses cancer progression in tumor-bearing mice. Life Sci. 2021, 266, 118847. [Google Scholar] [CrossRef] [PubMed]
- Muthiah, M.; Che, H.L.; Kalash, S.; Jo, J.; Choi, S.Y.; Kim, W.J.; Cho, C.S.; Lee, J.Y.; Park, I.K. Formulation of glutathione responsive anti-proliferative nanoparticles from thiolated Akt1 siRNA and disulfide-crosslinked PEI for efficient anti-cancer gene therapy. Colloids Surf. B Biointerfaces 2015, 126, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yhee, J.Y.; Kim, S.H.; Kwon, I.C.; Kim, K. Biocompatible gelatin nanoparticles for tumor-targeted delivery of polymerized siRNA in tumor-bearing mice. J. Control. Release 2013, 172, 358–366. [Google Scholar] [CrossRef] [PubMed]




| Reaction Code | Compound | Ref. | Reaction Code | Compound | Ref. | ||
|---|---|---|---|---|---|---|---|
| Formula | Abbreviations | Formula | Abbreviations | ||||
| 2 |  2,2’-Dithiodinicotinic acid | 2,2′-DTNA | [72] | 2 |  N-acetyl-S-((5-carbamoylpyridin-2-yl)thio)-l-cysteine | NAC-6-MNAm | [120] |
| Reaction Code | Compound | Ref. | Reaction Code | Compound | Ref. | ||
|---|---|---|---|---|---|---|---|
| Formula | Abbrev. | Formula | Abbrev. | ||||
| 3 | 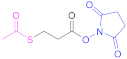 N-Succinimidyl-S-acetylthiopropionate | SATP | [60] | 3 | 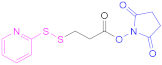 N-Succinimidyl 3-(2-pyridyldithio) propionate | SPDP | [55,93] |
| 3 | 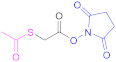 N-Succinimidyl S-(acetyl) thioglycolate | SATA | [80,123] | 3 | 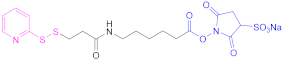 N-Sulfosuccinimidyl 6-(3-(pyridin-2-yldisulfaneyl) propionamido) hexanoate | Sulfo-LC-SPDP | [122,124] |
| 3 |  NAC activated | -- | [125] | 3 |  GSH activated | -- | [60] |
| 4 | 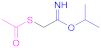 Isopropyl (acetylthio)acetimidate | i-PATAI | [95,121] | ||||
| Reaction Code | Compound | Ref. | Reaction Code | Compound | Ref. | ||
|---|---|---|---|---|---|---|---|
| Formula | Abbreviations | Formula | Abbreviations | ||||
| 5 6 | 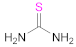 Thiourea | -- | Reacc. 5: [51,52,54] Reacc. 6: [49,52] | 6 | 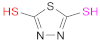 2,5-Dimercapto-1,3,4-thiadiazole | DMTD | [50] |
| 6 | 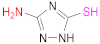 3-Amino-1,2,4-triazole-5-thiol | AZ | [127] | ||||
| System | Particle Formation Method | Crosslinker(s) | APIs | Thiomer | NP Size (nm)/PdI | Ref. |
|---|---|---|---|---|---|---|
| Microparticles | Spray-drying technique | --- | FDA | CTS–NAC CTS–GSH | 800–2270/--- | [110] |
| NP | Self-assembly of the amphiphilic material | --- | Docetaxel | CTS–GSH–graft–PMMA | <200/<0.15 | [27] |
| NP | Self-assembly of the amphiphilic material | Air core XrL (ultrasonication) | Methotrexate | CM–CTS–Ph–SH a | 160/0.055 | [25] |
| NP | Ionic gelation | Na2SO4 | DOX or ASOND | CTS–NAC CTS–NAP | 150–300/--- | [114] |
| NP | Ionic gelation | TPP Ca2+ | FD4 | CTS–TGA with TPP PAA–Cys with Ca2+ | 220–280/--- | [29] |
| NLC | Polyelectrolyte complex formation | Negatively charged CUR-loaded NLC | Curcumin | CTS–NAC | 89/--- | [125] |
| Hybrid nanoplex | Polyelectrolyte complex formation | HA of reduced Mw | FD4 | QA–CTS–TGA–SH or QA–CTS–TGA–SS–6-MNAm | 371–376/0.39–0.41 | [116] |
| Micelleplexes | Double polyelectrolyte complex formation: 1. pIL-12 + DOX-Loaded polymetformin micelles 2. Micelleplexes + HA-SH | For step 1: pIL-12 For step 2: Polymetformin-based micelleplexes | DOX and pIL-12 | HA–SH | 98–192/--- | [136] |
| Hybrid nanoplex | Polyelectrolyte complex formation and ionic gelation | Negatively charged insulin TPP | Insulin | Thiomalyl–CTS | 365/--- | [24] |
| Nanosuspension | Polyelectrolyte complex formation and ionic gelation | HA TPP | Isoniazid | CTS–TGA | 300–500/--- | [23] |
| NP | Polyelectrolyte complex method and disulfide bond formation | bFGF Thiol oxidation (air, pH 7.4) | bFGF | Carboxymethyl(sulfate)–CTS–thiobutylamidine | 218–290/--- | [30] |
| NP | Polyelectrolyte complex method and disulfide bond formation | Poly-siRNA Thiol oxidation (air, pH 8.0, 37 °C) | siRNA | Glycol–CTS–SH | ~300/--- | [122,124] |
| NP | Polyelectrolyte complex method, Ionic gelation and disulfide bond formation | Poly-siRNA Calcium phosphate | siRNA | HA–SH | 169/0.108 | [31] |
| Thiomer | Formulation | Loaded Compound | Cancer Cells Studied In Vitro | Ref. |
|---|---|---|---|---|
| Thiolated CTS | Nanoparticles (PMMA core + CTS–GSH conjugate coating) | Paclitaxel | NIH 3T3 and T47D (breast carcinoma) cells | [37] |
| Thiolated CTS | Nanoparticles (PMMA core + thiolated chitosan coating) | Docetaxel | Caco-2 (colorectal adenocarcinoma) and MCF-7 (breast carcinoma) cell lines | [27] |
| Thiolated CTS | Nanoparticles (PLA–PCL–TPGS core + thiolated chitosan coating) | Paclitaxel | A549 (adenocarcinomic human alveolar basal epithelia) cells | [151] |
| Thiolated HA–OA | Nanoparticles (HA–OA conjugate functionalized with NAC) | Paclitaxel | Caco-2 cells and Caco-2/HT29 cell monolayer (human colon cancer cell lines) | [155] |
| Thiolated polycarbophil | Nanoparticles | Paclitaxel | Only in vivo studies (wild-type rats vs. mammary-cancer-induced rats) | [156] |
| Thiolated OPV | Nanoparticles | Paclitaxel | A549 (adenocarcinomic human alveolar basal epithelia) cells | [158] |
| Thiolated sodium alginate | Nanoparticles (fluorescein-labelled wheat germ agglutinin-conjugated disulfide-crosslinked sodium alginate) | Docetaxel | HT-29 (human colon cancer) cells and L929 (mouse fibroblast) cells | [160] |
| Thiolated PEG + maleimide-functionalized HA | Microbeads | Docetaxel | NIH/3T3 (mouse embryonic fibroblast) cells and 4T1 (mouse breast cancer) cells | [167] |
| Dextran a | Hydrogel (dextran + thiolated human serum albumin) | Paclitaxel | - | [161] |
| PMAG + poly(amino acid) | Nanoparticles | Paclitaxel | A549 (human lung carcinoma) and MCF-7 (human breast adenocarcinoma) cells | [159] |
| Thiolated CTS c | Nanostructured lipid carrier (lipid core + thiolated chitosan coating) | Curcumin | Isolated rabbit cornea cells | [125] |
| Thiolated CTS c | Nanoparticles (based on CTS–NAC or CTS–NAP) | Doxorubicin and antisense oligonucleotides | T47D (human breast epithelial tumor) cells | [114] |
| Thiolated hexanoyl glycol CTS c | Mucoadhesive thermogelling polymer | - | HeLa (cervical cancer) cells, human fibroblasts, epithelial cells, and epithelial cell aggregate | [113] |
| Thiolated HA c | Micelleplexes (polymetformin + hyaluronic acid) | Doxorubicin and plasmid-encoding IL-12 gene | 4T1 (mouse breast cancer) cell line | [136] |
| Thiolated amidized glycol CTS c | Dual secured nano-sting | Melittin and other amphipathic peptides | MCF-7 (breast carcinoma), HCT-116 (human colon cancer), SKOV-3 (human ovarian cancer), and NCI/ADR–RES (ovarian tumor) cell lines | [55] |
| PLGA and OCTS b,c | Nanoparticles | CHC and CTX | U251 and SW1088 (glioma cell lines) | [80] |
| Thiolated carboxymethyl chitosan c | Nanoparticles | Methotrexate | HeLa (cervical cancer) cells | [25] |
| POEGA + PDEGA (star polymers) | Nanoparticles | - | Jurkat (T-ALL) and Nalm-6 (B-ALL) cancer cell lines | [163] |
| Thiomer | Formulation | Loaded Compound | Cancer Cells Studied In Vitro | Ref. |
|---|---|---|---|---|
| Thiolated glycol CTS | Nanoparticles | MDR-1 siRNA | MCF-7/ADR (Adriamycin-resistant breast cancer cells) | [124] |
| Thiolated glycol CTS | Nanoparticles | VEGF siRNA | RFP-expressing B16F10 (murine melanoma) cells. In vivo studies in SCC-7 and RFP-B16F10 tumor-bearing mic | [122] |
| HPMAm-s-AMPAm a | Multiconjugates | siRNA and folate | - | [176] |
| Thiolated TMC (both alone and with HA) | Polyplexes | siRNA | H1299 (human lung cancer) cells | [178] |
| Thiolated HA + PSR | Nanoparticles (PSR core + thiolated hyaluronic acid coating) | Hydrophobic chemotherapeutics + siRNA | A549 (adenocarcinomic human alveolar basal epithelia) cells | [180] |
| Thiolated HA | Nanoparticles (siRNA with calcium phosphate core + thiolated hyaluronic acid coating) | Bcl-2 siRNA | B16-F10 (murine melanoma) cells | [31] |
| Thiolated chitosan + TMC | Nanoparticles (thiolated chitosan + TMC completed with HA and HIV-derived TAT peptide) | PD-L1 and STAT-3 siRNA | B16-F10 (murine melanoma) and 4T1 (mouse breast cancer) cell lines | [181] |
| Thiolated PEI | Nanoparticles | Akt1 siRNA | CT-26 (mouse colon cancer) cells. In vivo studies in mouse tumor models | [182] |
| Thiolated gelatin | Nanoparticles | siRNA | RFP-expressing B16F10 (murine melanoma) cells. In vivo studies in tumor-bearing mice | [183] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosso, R.; de-Paz, M.-V. Thiolated-Polymer-Based Nanoparticles as an Avant-Garde Approach for Anticancer Therapies—Reviewing Thiomers from Chitosan and Hyaluronic Acid. Pharmaceutics 2021, 13, 854. https://doi.org/10.3390/pharmaceutics13060854
Grosso R, de-Paz M-V. Thiolated-Polymer-Based Nanoparticles as an Avant-Garde Approach for Anticancer Therapies—Reviewing Thiomers from Chitosan and Hyaluronic Acid. Pharmaceutics. 2021; 13(6):854. https://doi.org/10.3390/pharmaceutics13060854
Chicago/Turabian StyleGrosso, Roberto, and M.-Violante de-Paz. 2021. "Thiolated-Polymer-Based Nanoparticles as an Avant-Garde Approach for Anticancer Therapies—Reviewing Thiomers from Chitosan and Hyaluronic Acid" Pharmaceutics 13, no. 6: 854. https://doi.org/10.3390/pharmaceutics13060854
APA StyleGrosso, R., & de-Paz, M.-V. (2021). Thiolated-Polymer-Based Nanoparticles as an Avant-Garde Approach for Anticancer Therapies—Reviewing Thiomers from Chitosan and Hyaluronic Acid. Pharmaceutics, 13(6), 854. https://doi.org/10.3390/pharmaceutics13060854







