Fabrication of Capsaicin Loaded Nanocrystals: Physical Characterizations and In Vivo Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Capsaicin Nano-Crystals
2.3. Lyophilization of Nano-Crystals
2.4. Physicochemical Characterizations of Capsaicin Nano-Crystals
2.4.1. Stability Studies
2.4.2. Zeta Potential, Particle Size and Polydispersity Index of Nano-Crystals
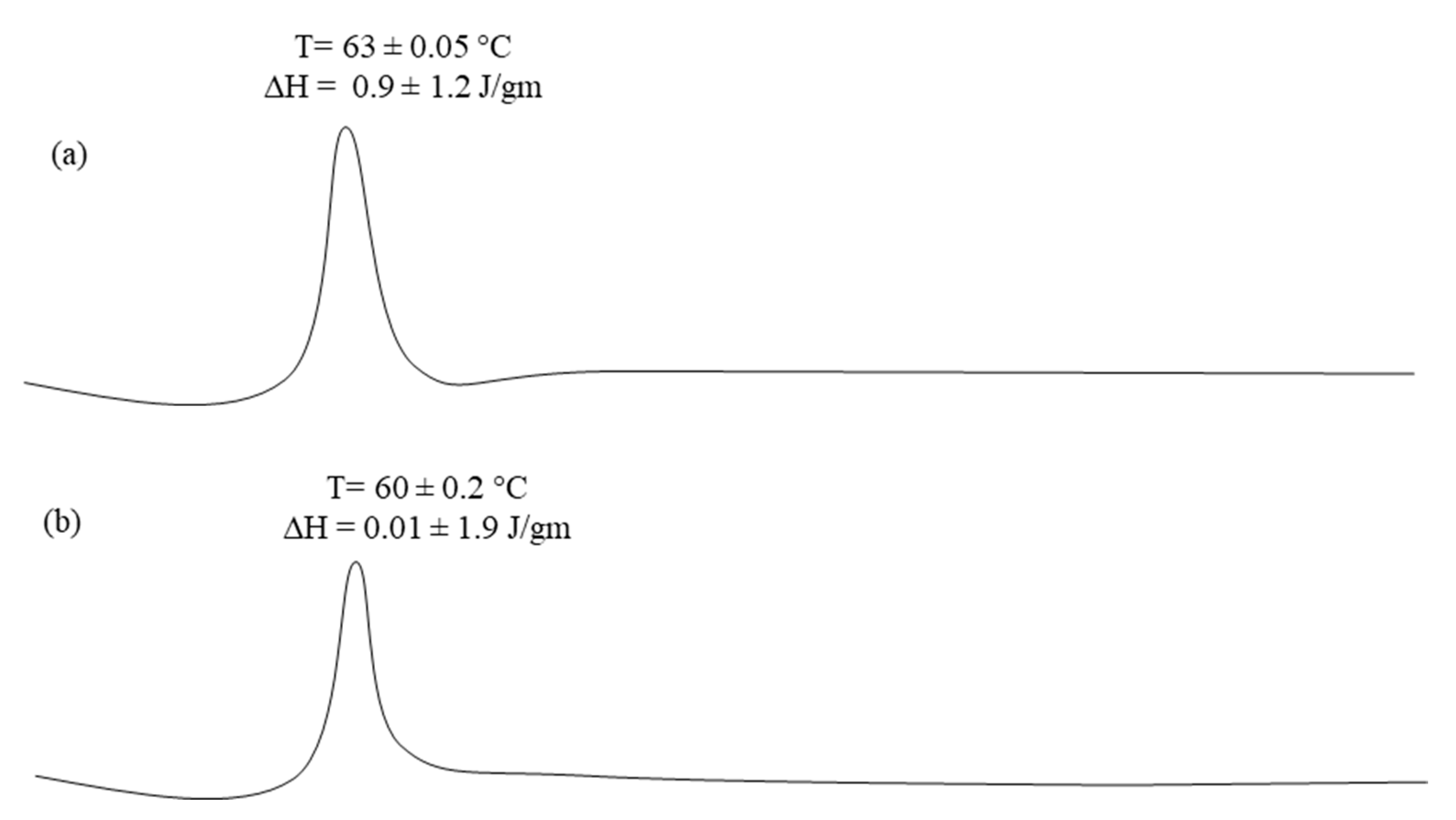
2.4.3. Differential Scanning Calorimetric Studies
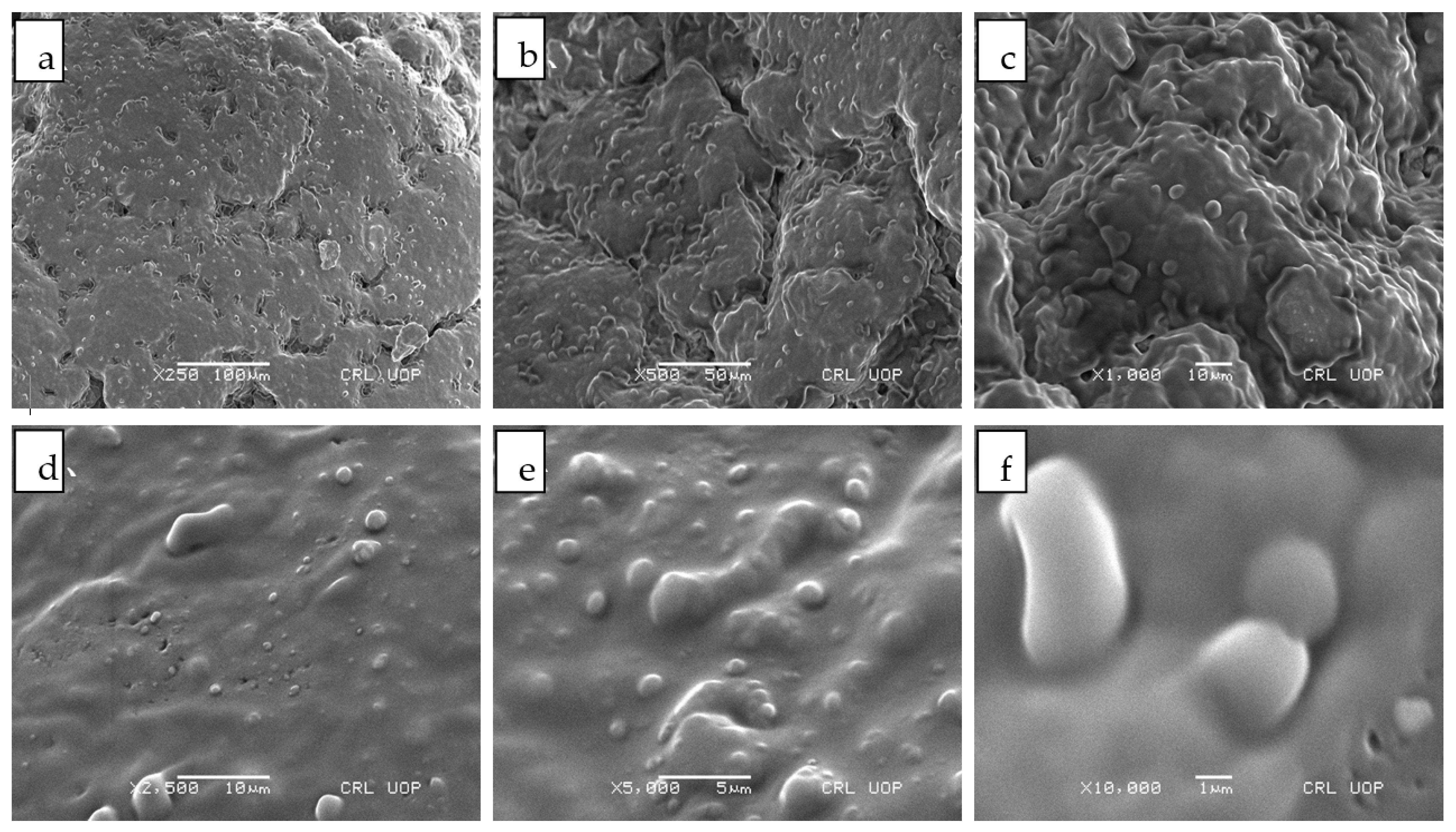
2.4.4. Scanning Electron Microscopy
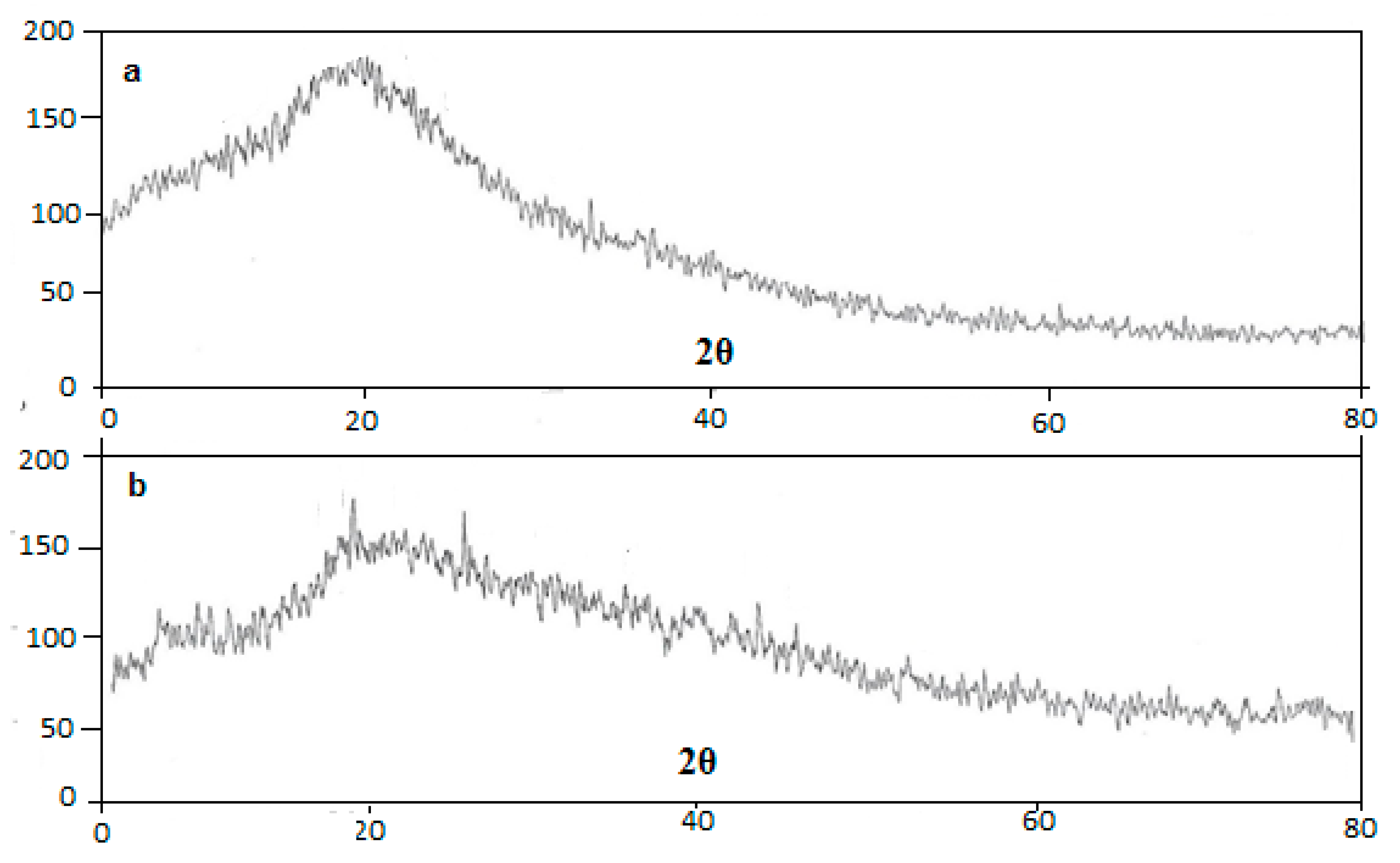
2.4.5. XRD Studies
2.4.6. Drug Content of the Nano-Crystals
2.4.7. Solubility Studies
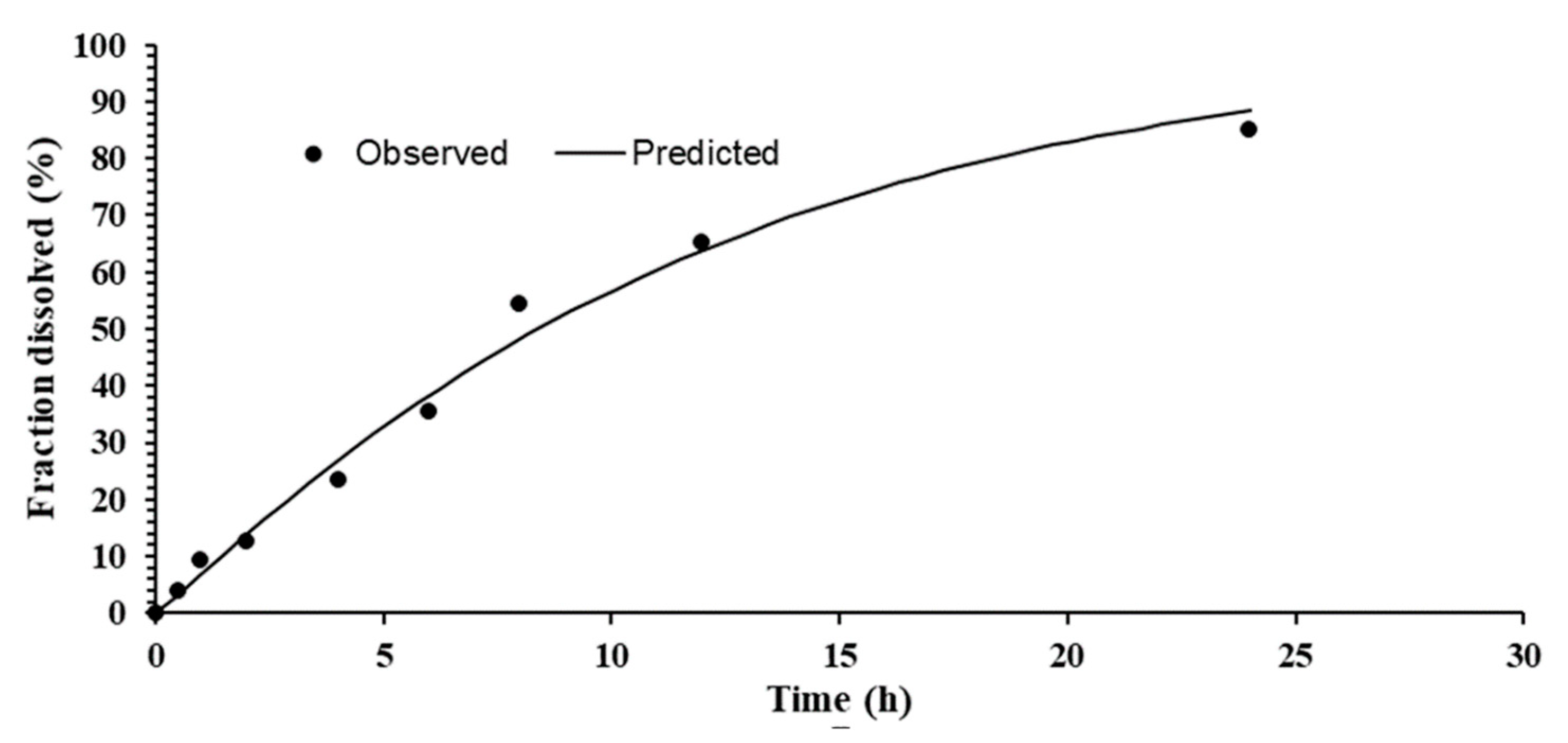
2.4.8. In Vitro Release
2.4.9. Kinetic Models
2.4.10. Drug Retention/Permeation Study
2.4.11. Vibrational Analysis
2.5. In Vivo Studies
2.5.1. Animals
2.5.2. In Vivo Anti-Inflammatory Activity
2.5.3. Treatment Protocols
2.5.4. Statistical Analysis
3. Results and Discussion
3.1. Surface Charge, Size and Polydispersity Index of Capsaicin Nano-Crystals
3.2. Drug Content and Entrapment Efficiency
3.3. Differential Scanning Calorimeter
3.4. Scanning Electron Microscopy
3.5. XRD Studies
3.6. Solubility Studies
3.7. In Vitro Drug Release
3.8. Kinetic Model
3.9. Ex Vivo Permeation/Drug Retention Studies
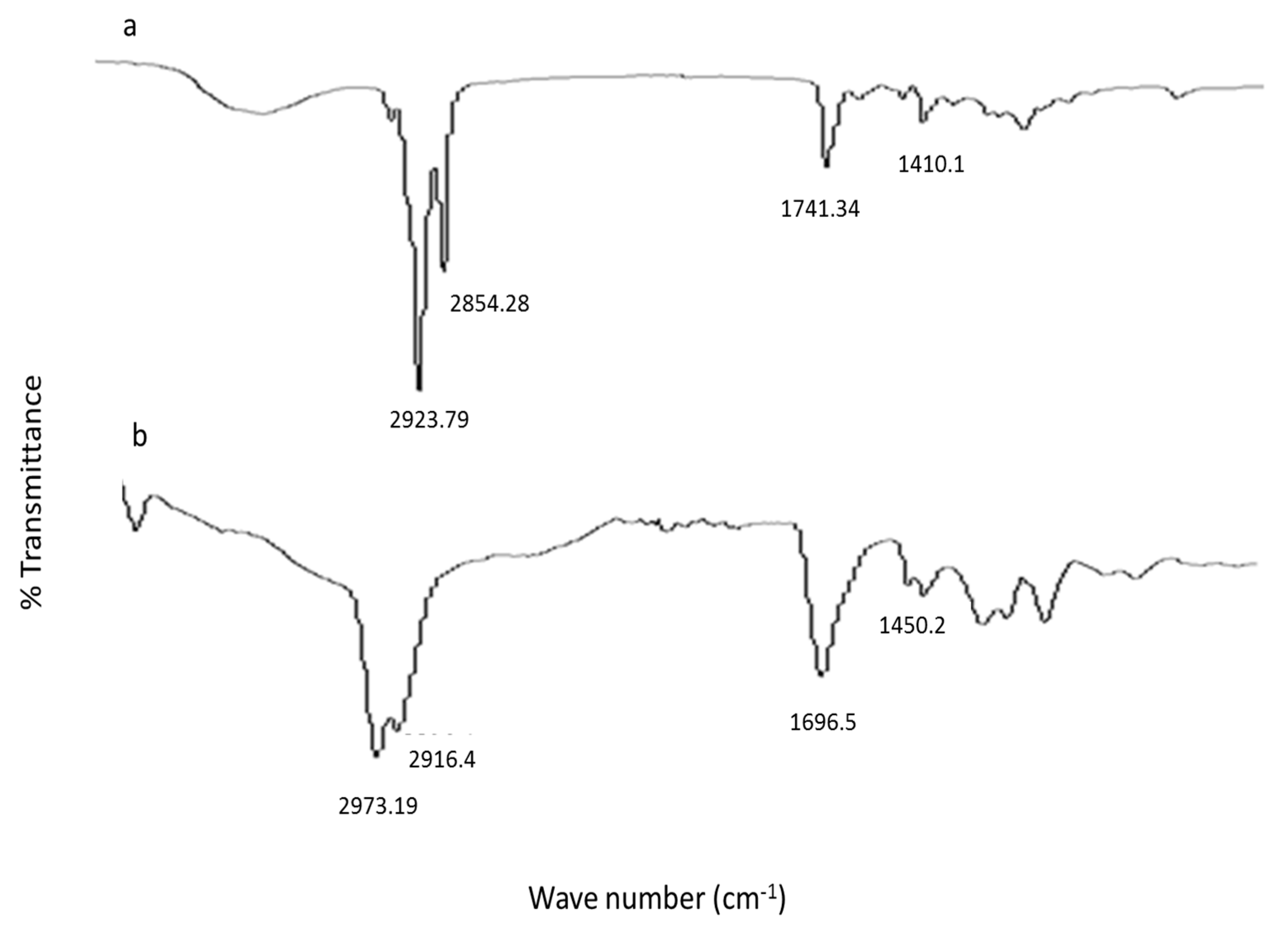
3.10. ATR-FTIR
3.11. Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, X.; Guy, R.H. Applications of nanoparticles in topical drug delivery and in cosmetics. J. Drug Deliv. Sci. Technol. 2009, 19, 371–384. [Google Scholar] [CrossRef]
- Gao, L.; Liu, G.; Ma, J.; Wang, X.; Zhou, L.; Li, X. Drug nanocrystals: In vivo performances. J. Control. Release 2012, 160, 418–430. [Google Scholar] [CrossRef]
- Sinha, B.; Müller, R.H.; Möschwitzer, J.P. Bottom-up approaches for preparing drug nanocrystals: Formulations and factors affecting particle size. Int. J. Pharm. 2013, 453, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Shete, G.; Jain, H.; Punj, D.; Prajapat, H.; Akotiya, P.; Bansal, A.K. Stabilizers used in nanocrystal based drug delivery systems. J. Excip. Food Chem. 2014, 5, 184–209. [Google Scholar]
- Müller, R.H.; Zhai, X.; Romero, G.B.; Keck, C.M. Nanocrystals for passive dermal penetration enhancement. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Nanocarriers, 1st ed.; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 283–295. [Google Scholar]
- Zhai, X.; Lademann, J.; Keck, C.; Müller, R.H. Nanocrystals of medium soluble actives—Novel concept for improved dermal delivery and production strategy. Int. J. Pharm. 2014, 470, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Al Shaal, L.; Shegokar, R.; Müller, R.H. Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. Int. J. Pharm. 2011, 420, 133–140. [Google Scholar] [CrossRef]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lutein nanocrystals as antioxidant formulation for oral and dermal delivery. Int. J. Pharm. 2011, 420, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Pireddu, R.; Corrias, F.; Fadda, A.M.; Valenti, D.; Pini, E.; Sinico, C. Nanosuspension improves tretinoin photostability and delivery to the skin. Int. J. Pharm. 2013, 458, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Pireddu, R.; Sinico, C.; Ennas, G.; Marongiu, F.; Muzzalupo, R.; Lai, F.; Fadda, A.M. Novel nanosized formulations of two diclofenac acid polymorphs to improve topical bioavailability. Eur. J. Pharm. Sci. 2015, 77, 208–215. [Google Scholar] [CrossRef]
- Vidlářová, L.; Romero, G.B.; Hanuš, J.; Štěpánek, F.; Müller, R.H. Nanocrystals for dermal penetration enhancement—Effect of concentration and underlying mechanisms using curcumin as model. Eur. J. Pharm. Biopharm. 2016, 104, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Hatahet, T.; Morille, M.; Hommoss, A.; Dorandeu, C.; Müller, R.; Bégu, S. Dermal quercetin smartCrystals®: Formulation development, antioxidant activity and cellular safety. Eur. J. Pharm. Biopharm. 2016, 102, 51–63. [Google Scholar] [CrossRef]
- Sinico, C.; Pireddu, R.; Pini, E.; Valenti, D.; Caddeo, C.; Fadda, A.M.; Lai, F. Enhancing topical delivery of resveratrol through a nanosizing approach. Planta Med. 2016, 83, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.M.; Meinke, M.C.; Keck, C.M.; Müller, R.H. Rutin—Increased antioxidant activity and skin penetration by nanocrystal technology (smartCrystals). Cosmetics 2016, 3, 9. [Google Scholar] [CrossRef]
- Shen, C.; Shen, B.; Liu, X.; Yuan, H. Nanosuspensions based gel as delivery system of nitrofurazone for enhanced dermal bioavailability. J. Drug Deliv. Sci. Technol. 2018, 43, 1–11. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z. The two faces of capsaicin. Cancer Res. 2011, 71, 2809–2813. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Hohmann, M.S.N.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef]
- Gregory, S.; Devassy, R.K. Integrating TRPV1 receptor function with capsaicin psychophysics. Adv. Pharmacol. Sci. 2016, 2016, 16. [Google Scholar]
- Khan, S.; Matas, M.D.; Zhang, J.; Anwar, J. Nanocrystal preparation: Low-energy precipitation method revisit-ed. Cryst. Growth Des. 2013, 13, 2766–2777. [Google Scholar] [CrossRef]
- Choi, J.-S. Design of cilostazol nanocrystals for improved solubility. J. Pharm. Innov. 2020, 15, 416–423. [Google Scholar] [CrossRef]
- Basit, H.M.; Cairul, M.; Mohd, I.; Ng, S.; Katas, H.; Shah, S.U.; Khan, N.R. Formulation and Evaluation of Microwave-Modified Chitosan-Curcumin Nanoparticles—A Promising Applications Following Burn Wounds. Polymers 2020, 12, 2608. [Google Scholar] [CrossRef]
- Karri, V.V.S.N.R.; Raman, S.; Kuppusamy, G.; Mulukutla, S.; Ramaswamy, S.; Malayandi, R. Terbinafine hydrochloride loaded nanoemulsion based gel for topical application. J. Pharm. Investig. 2014, 45, 79–89. [Google Scholar] [CrossRef]
- Ali, M.; Khan, N.R.; Hussain, Z.; Basit, H.M.; Mahmood, S. Novel Composite pH Controlled Drug Release Hydrogel Containing Dexibuprofen. RADS J. Pharm. Pharm. Sci. 2018, 6, 223–235. [Google Scholar]
- Madeha, S.; Zahida, P.; Muhammad, R.K. Evaluation of antioxidant, antiinflammatory, analgesic and antipyreticactivities of the stem bark of Sapindus mukorossi. Complement. Altern. Med. 2017, 17, 526. [Google Scholar]
- Simunkova, H.; Pessenda-Garcia, P.; Wosik, J.; Angerer, P.; Kronberger, H.; Nauer, G.E. The fundamentals of nano- and submicro-scaled ceramic particles incorporation into electrodeposited nickel layers: Zeta potential measurements. Surf. Coat. Technol. 2009, 203, 1806–1814. [Google Scholar] [CrossRef]
- Verma, S.; Gokhale, R.; Burgess, D.J. A comparative study of top-down and bottom-up approaches for the preparation of micro/nanosuspensions. Int. J. Pharm. 2009, 380, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Khan, S.; Ahmed, S.; Govender, T.; Faidah, H.S.; de Matas, M.; Shahid, M.; Minhas, M.U.; Sohail, M.; Khurram, M. Dexibuprofen nanocrystals with improved therapeu-tic performance: Fabrication, characterization, in silico modeling, and in vivo evaluation. Int. J. Nanomed. 2018, 13, 1677–1692. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Shanthi, N.; Mahato, A.K.; Soni, S.; Rajnikanth, P. Preparation of luliconazole nanocrystals loaded hydrogel for improvement of dissolution and antifungal activity. Heliyon 2019, 5, e01688. [Google Scholar] [CrossRef]



| Formulation Code | Carbopol (w/w) | Tween-80 (w/w) | Capsaicin (w/w) | Water (w/w) |
|---|---|---|---|---|
| T1 | 0.1 g | 0.5 g | -- | 49.4 g |
| T2 | 0.5 g | 0.5 g | 200 mg | 48.8 g |
| T3 | 1 g | 0.5 g | 200 mg | 48.8 g |
| T4 | 1.5 g | 0.5 g | 250 mg | 48.5 g |
| T5 | 2 g | 0.5 g | 250 mg | 47.5 g |
| Formulations | Particle Size (nm) | Surface Charge (mV) | PDI |
|---|---|---|---|
| T1 | 130.5 ± 1.7 | −12.7 ± 2.4 | 0.45 ± 0.2 |
| T2 | 150 ± 2.8 | −15.2 ± 4.1 | 0.36 ± 0.4 |
| T3 | 120 ± 3.0 | −20.7 ± 3.5 | 0.48 ± 1.5 |
| Formulations Codes | Capsaicin Concentration (mg) | Capsaicin Obtained (mg) | Capsaicin Content % | Percent Encapsulation Efficiency ± SD |
|---|---|---|---|---|
| T2 | 200 mg | 165 ± 1.3 mg | 82.5% | 85 ± 3.4 |
| T3 | 200 mg | 170 ± 1.6 mg | 85% | 90 ± 1.9 |
| S.No | Solvents | Solubility of Capsaicin Loaded Nanocrystals μg/mL |
|---|---|---|
| 1 | Water * | 12.0 ± 0.013 |
| 2 | Methanol | 18.40 ± 0.42 |
| 3 | Acetone | 6.30 ± 0.015 |
| 4 | Ethanol | 13.84 ± 0.01 |
| 5 | PBS 7.4 | 10.0 ± 0.033 |
| 6 | Acetate buffer 5.5 | 12.0 ± 0.029 |
| Time Intervals | % Drug Permeated | Retained in Skin (%) | Retained on Skin (%) |
|---|---|---|---|
| 0 | 0 | 16.13 ± 1.11 | 9.12 ± 0.14 |
| 1 | 26.21 ± 0.17 | ||
| 2 | 27.11 ± 0.21 | ||
| 4 | 35.30 ± 1.10 | ||
| 6 | 40.00 ± 1.19 | ||
| 8 | 48.19 ± 1.48 | ||
| 10 | 55.00 ± 1.66 | ||
| 12 | 68.32 ± 1.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, B.A.; Rashid, F.; Khan, M.K.; Alqahtani, S.S.; Sultan, M.H.; Almoshari, Y. Fabrication of Capsaicin Loaded Nanocrystals: Physical Characterizations and In Vivo Evaluation. Pharmaceutics 2021, 13, 841. https://doi.org/10.3390/pharmaceutics13060841
Khan BA, Rashid F, Khan MK, Alqahtani SS, Sultan MH, Almoshari Y. Fabrication of Capsaicin Loaded Nanocrystals: Physical Characterizations and In Vivo Evaluation. Pharmaceutics. 2021; 13(6):841. https://doi.org/10.3390/pharmaceutics13060841
Chicago/Turabian StyleKhan, Barkat Ali, Furqan Rashid, Muhammad Khalid Khan, Saad Saeed Alqahtani, Muhammad Hadi Sultan, and Yosif Almoshari. 2021. "Fabrication of Capsaicin Loaded Nanocrystals: Physical Characterizations and In Vivo Evaluation" Pharmaceutics 13, no. 6: 841. https://doi.org/10.3390/pharmaceutics13060841
APA StyleKhan, B. A., Rashid, F., Khan, M. K., Alqahtani, S. S., Sultan, M. H., & Almoshari, Y. (2021). Fabrication of Capsaicin Loaded Nanocrystals: Physical Characterizations and In Vivo Evaluation. Pharmaceutics, 13(6), 841. https://doi.org/10.3390/pharmaceutics13060841







