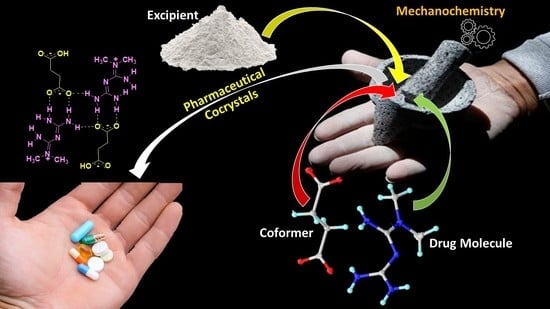
Mechanochemistry: A Green Approach in the Preparation of Pharmaceutical Cocrystals
Abstract
1. Introduction
2. Mechanochemistry
2.1. Definitions, Relevant Historical Aspects, and Applications of Mechanochemistry
2.2. Different Mechanochemical Apparatuses
2.3. Advantages of Reaction Performance among Grinding/Milling and Other Sustainable Methods
2.4. Preparative Conditions of Mechanochemical Methods of Pharmaceutical Cocrystals
- Intersolid reactions, which pertain to the reactivity between solids (mechanochemical reactions), Figure 4.
- Reactions between solids (solid–state reactions).
- Reactions between solids with intermediate local melting.
- Reactions with at least one liquid reagent.
2.5. Mechanistic Aspects in Cocrystal Formation Applying Mechanochemistry
- Molecule migration. The first stage is the reconstruction of the solid phase, suggesting directional long-range migrations of molecules, where component A invades the planes or channels of component B (or vice-versa). The incipient formation of C distorts the original crystal structures of A and B, producing a mixed A-B-C phase.
- Product-phase formation. The concomitant appearance of component C in the mixed phase A-B-C favors spatial discontinuity in particles A and B due to strain and crystal defects.
- Crystal disintegration. In this step, is suggested a chemical and geometrical mismatch between components A and B, produced by the appearance of C causing a disintegration of the particles. The grinding/milling process produces fresh surfaces available for further reaction to completion.
3. Cocrystal
3.1. Cocrystal Definition
- “Only compounds constructed from discrete neutral molecular species will be considered cocrystals. Consequently, all solids containing ions, including complex transition-metal ions, are excluded”.
- “Only cocrystals made from reactants that are solids at ambient conditions will be included. Therefore, all hydrates and other solvates are excluded which, in principle, eliminates compounds that are typically classified as clathrates or inclusion compounds (where the guest is a solvent or a gas molecule)”.
- “A cocrystal is a structurally homogeneous crystalline material that contains two or more neutral building blocks that are present in definite stoichiometric amounts”.
3.2. Design of Pharmaceutical Cocrystals: Selection of Appropriate API/Conformer
3.3. Characterization of Pharmaceutical Cocrystals
3.4. Diverse Preparation Methods of Pharmaceutical Cocrystals
3.5. Benefits of Pharmaceutical Cocrystals
3.6. Chronological Survey of Papers Mentioning Mechanochemical Synthesis of Pharmaceutical Cocrystals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| adi | Adipic acid |
| ana | Anthranilic Acid |
| API | Active Pharmaceutical Ingredient |
| α-alanine | α-ala |
| BA | Barbituric acid |
| BSC | Biopharmaceutics classification system |
| 4,4′-bipy | 4,4′-Bipyridine |
| ca | Citric Acid |
| caf | Caffeine |
| cbz | Carbamazepine |
| CSD | Cambridge structural database |
| CTA | Cyclohexane-1,3-cis-5-cis-tricarboxylic Acid |
| δ | δ parameter |
| dabco | 1,4-diazabicyclo [2.2.2]octane |
| DL-ta | DL-tartaric acid |
| DSC | Differential Scanning Calorimetry |
| DTA | Differential thermal analysis |
| FDA | Food and drug administration |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| GRAS | Generally recognized as safe |
| Glu | Glutaric acid |
| HIMO | 4,5-dimethyl-imidazole-3-oxide |
| IL | Imidazolium based ionic liquids |
| ILAG | Ion liquid-assisted grinding |
| IUPAC | International Union of Pure and Applied Chemistry |
| LAG | Liquid-Assisted Grinding |
| LASI | Liquid-assisted sonochemical irradiation |
| ma | Mesaconic Acid |
| MeNO3 | Methyl nitrate |
| MOF | Metal-organic framework |
| m | weights of cocrystal components |
| NP | Natural product |
| NG | Neat Grinding |
| Nicotinamide | nic |
| 5-nip | 5-Nitroisophtalic acid |
| η | η parameter |
| ox | Oxalic Acid |
| PEG | Polyethylene Glycol |
| phe | Phenazide |
| 4,7-phen | 4,7-phenanthroline |
| POLAG | Polymer-Assisted Grinding |
| PMMA | Poly(methyl methacrylate) |
| PXRD | Powder X-ray Diffraction |
| RAM | Resonant acoustic mixing |
| RS-ibp | RS-ibuprofen |
| sa | Salicylic Acid |
| SCXRD | Single Crystal X-ray Diffraction |
| SDG | Solvent Drop Grinding |
| S-ibp | S-ibuprofen |
| ssNMR | solid-state Nuclear Magnetic Resonance |
| ta | Terephthalate |
| thp | Theophillyne |
| TBA | Thiobarbituric acid |
| TGA | Thermogravimetric Analysis |
| TSE | Twin-screw extrusion |
| tp | Theophylline |
| V | volume |
| VALAG | Variable amount liquid-assisted grinding |
| VATEG | Variable temperature grinding |
| XPS | X-ray photoelectron spectroscopy |
References
- Datta, S.; Grant, D.J.W. Crystal Structures of Drugs: Advances in Determination, Prediction and Engineering. Nat. Rev. Drug Discov. 2004, 3, 42–57. [Google Scholar] [CrossRef]
- Shekunov, B.Y.; York, P. Crystallization Processes in Pharmaceutical Technology and Drug Delivery Design. J. Cryst. Growth 2000, 211, 122–136. [Google Scholar] [CrossRef]
- Grunenberg, A.; Henck, J.O.; Siesler, H.W. Theoretical Derivation and Practical Application of Energy/Temperature Diagrams as an Instrument in Preformulation Studies of Polymorphic Drug Substances. Int. J. Pharm. 1996, 129, 147–158. [Google Scholar] [CrossRef]
- Morissette, S.L.; Soukasene, S.; Levinson, D.; Cima, M.J.; Almarsson, O. Elucidation of Crystal Form Diversity of the HIV Protease Inhibitor Ritonavir by High-throughput Crystallization. Proc. Natl. Acad. Sci. USA 2003, 100, 2180–2184. [Google Scholar] [CrossRef]
- Morissette, S.L.; Almarsson, Ö.; Peterson, M.L.; Remenar, J.F.; Read, M.J.; Lemmo, A.V.; Ellis, S.; Cima, M.J.; Gardner, C.R. High-throughput Crystallization: Polymorphs, Salts, Co-crystals and Solvates of Pharmaceutical Solids. Adv. Drug Deliv. Rev. 2004, 56, 275–300. [Google Scholar] [CrossRef]
- Tiekink, E.R.T.; Vittal, J.; Zaworotko, M. Organic Crystal Engineering: Frontiers in Crystal Engineering; Tiekink, E.R.T., Vittal, J.J., Zaworotko, M.J., Eds.; John Wiley & Sons: New York, NY, USA, 2010; ISBN 9780470319901. [Google Scholar]
- Lee, E.H. A Practical Guide to Pharmaceutical Polymorph Screening & Selection. Asian J. Pharm. Sci. 2014, 9, 163–175. [Google Scholar] [CrossRef]
- Aaltonen, J.; Allesø, M.; Mirza, S.; Koradia, V.; Gordon, K.C.; Rantanen, J. Solid Form Screening—A Review. Eur. J. Pharm. Biopharm. 2009, 71, 23–37. [Google Scholar] [CrossRef]
- Tung, H.H. Industrial Perspectives of Pharmaceutical Crystallization. Org. Process Res. Dev. 2013, 17, 445–454. [Google Scholar] [CrossRef]
- Croker, D.M.; Kelly, D.M.; Horgan, D.E.; Hodnett, B.K.; Lawrence, S.E.; Moynihan, H.A.; Rasmuson, Å.C. Demonstrating the Influence of Solvent Choice and Crystallization Conditions on Phenacetin Crystal Habit and Particle Size Distribution. Org. Process Res. Dev. 2015, 19, 1826–1836. [Google Scholar] [CrossRef]
- Keraliya, R.A.; Soni, T.G.; Thakkar, V.T.; Gandhi, T.R. Effect of Solvent on Crystal Habit and Dissolution Behavior of Tolbutamide by Initial Solvent Screening. Dissolution Technol. 2010, 17, 16–21. [Google Scholar] [CrossRef]
- Schultheiss, N.; Newman, A. Pharmaceutical Cocrystals and Their Physicochemical Properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef]
- Berry, D.J.; Steed, J.W. Pharmaceutical Cocrystals, Salts and Multicomponent Systems; Intermolecular Interactions and Property Based Design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef]
- Karagianni, A.; Malamatari, M.; Kachrimanis, K. Pharmaceutical Cocrystals: New Solid Phase Modification Approaches for the Formulation of APIs. Pharmaceutics 2018, 10, 18. [Google Scholar] [CrossRef]
- Healy, A.M.; Worku, Z.A.; Kumar, D.; Madi, A.M. Pharmaceutical Solvates, Hydrates and Amorphous Forms: A Special Emphasis on Cocrystals. Adv. Drug Deliv. Rev. 2017, 117, 25–46. [Google Scholar] [CrossRef]
- Myrdal, P.B.; Jozwiakowski, M.J. Alteration of the Solid State of the Drug Substances: Polymorphs, Solvates, and Amorphous Forms. In Water-Insoluble Drug Formulation; CRC Press: Boca Raton, FL, USA, 2008; pp. 531–566. [Google Scholar]
- Babu, N.J.; Nangia, A. Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble Drug Delivery Strategies: Review of Recent Advances and Business Prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef]
- Kavanagh, O.N.; Croker, D.M.; Walker, G.M.; Zaworotko, M.J. Pharmaceutical Cocrystals: From Serendipity to Design to Application. Drug Discov. Today 2019, 24, 796–804. [Google Scholar] [CrossRef]
- Bučar, D.-K.; Lancaster, R.W.; Bernstein, J. Disappearing Polymorphs Revisited. Angew. Chem. 2015, 54, 6972–6993. [Google Scholar] [CrossRef]
- Kaushal, A.M.; Gupta, P.; Bansal, A.K. Amorphous Drug Delivery Systems: Molecular Aspects, Design, and Performance. Crit. Rev. Ther. Drug Carrier Syst. 2004, 21, 133–193. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Rinaki, E.; Valsami, G.; Macheras, P. Quantitative Biopharmaceutics Classification System: The Central Role of Dose/Solubility Ratio. Pharm. Res. 2003, 20, 1917–1925. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in vivo Bioavailability. Pharm. Res. An Off. J. Am. Assoc. Pharm. Sci. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernas, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of In Vitro Drug Product Dissolution and In Vivo Bioavailability, Pharm. Res. 12, 413–420, 1995—Backstory of BCS. AAPS J. 2014, 16, 894–898. [Google Scholar] [CrossRef]
- Fujii, S.; Yokoyama, T.; Ikegaya, K.; Sato, F.; Yokoo, N. Promoting Effect of the New Chymotrypsin Inhibitor FK-448 on the Intestinal Absorption of Insulin in Rats and Dogs. J. Pharm. Pharmacol. 1985, 37, 545–549. [Google Scholar] [CrossRef]
- Doroshyenko, O.; Jetter, A.; Odenthal, K.P.; Fuhr, U. Clinical Pharmacokinetics of Trospium Chloride. Clin. Pharmacokinet. 2005, 44, 701–720. [Google Scholar] [CrossRef]
- Aungst, B.J. Intestinal Permeation Enhancers. J. Pharm. Sci. 2000, 89, 429–442. [Google Scholar] [CrossRef]
- Aungst, B.J. Novel Formulation Strategies for Improving Oral Bioavailability of Drugs with Poor Membrane Permeation or Presystemic Metabolism. J. Pharm. Sci. 1993, 82, 979–987. [Google Scholar] [CrossRef]
- Fleisher, D.; Bong, R.; Stewart, B.H. Improved Oral Drug Delivery: Solubility Limitations Overcome by the Use of Prodrugs. Adv. Drug Deliv. Rev. 1996, 19, 115–130. [Google Scholar] [CrossRef]
- Morris, K.R.; Fakes, M.G.; Thakur, A.B.; Newman, A.W.; Singh, A.K.; Venit, J.J.; Spagnuolo, C.J.; Serajuddin, A.T.M. An Integrated Approach to the Selection of Optimal Salt Form for a New Drug Candidate. Int. J. Pharm. 1994, 105, 209–217. [Google Scholar] [CrossRef]
- Huang, Y.; Dai, W.-G. Fundamental Aspects of Solid Dispersion Technology for Poorly Soluble Drugs. Acta Pharm. Sin. B 2014, 4, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Solid Dispersion- Strategy To Enhance Solubility and Dissolution of Poorly Water Soluble Drugs. Univers. J. Pharm. Res. 2017, 2, 54–59. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B. Cyclodextrin Complexes: Perspective from Drug Delivery and Formulation. Drug Dev. Res. 2018, 79, 201–217. [Google Scholar] [CrossRef]
- Shah, S.; Date, A.; Holm, R. Strategies for the Formulation Development of Poorly Soluble Drugs via Oral Route. In Innovative Dosage Forms: Design and Development at Early Stage; Bachhav, Y., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2019; Chapter 2; pp. 49–89. [Google Scholar]
- Tran, P.H.L.; Tran, T.T.D. Dosage Form Designs for the Controlled Drug Release of Solid Dispersions. Int. J. Pharm. 2020, 581, 119274. [Google Scholar] [CrossRef]
- Jornada, D.H.; dos Santos Fernandes, G.F.; Chiba, D.E.; de Melo, T.R.; dos Santos, J.L.; Chung, M.C. The Prodrug Approach: A Successful Tool for Improving Drug Solubility. Molecules 2016, 21, 42. [Google Scholar] [CrossRef]
- Berge, S.M.; Bighley, L.D.; Monkhouse, D.C. Pharmaceutical Salts. J. Pharm. Sci. 1977, 66, 1–19. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and Their Pharmaceutical Applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Swarnakar, N.K.; Venkatesan, N.; Betageri, G. Critical In Vitro Characterization Methods of Lipid-Based Formulations for Oral Delivery: A Comprehensive Review. AAPS PharmSciTech 2019, 20, 16. [Google Scholar] [CrossRef]
- Vo, C.L.-N.; Park, C.; Lee, B.-J. Current Trends and Future Perspectives of Solid Dispersions Containing Poorly Water-soluble Drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar] [CrossRef]
- Miroshnyk, I.; Mirza, S.; Sandler, N. Pharmaceutical Co-crystals-An Opportunity for Drug Product Enhancement. Expert Opin. Drug Deliv. 2009, 6, 333–341. [Google Scholar] [CrossRef]
- Yousef, M.A.E.; Vangala, V.R. Pharmaceutical Cocrystals: Molecules, Crystals, Formulations, Medicines. Cryst. Growth Des. 2019, 19, 7420–7438. [Google Scholar] [CrossRef]
- Shaikh, R.; Singh, R.; Walker, G.M.; Croker, D.M. Pharmaceutical Cocrystal Drug Products: An Outlook on Product Development. Trends Pharmacol. Sci. 2018, 39, 1033–1048. [Google Scholar] [CrossRef]
- Thakuria, R.; Delori, A.; Jones, W.; Lipert, M.P.; Roy, L.; Rodríguez-Hornedo, N. Pharmaceutical Cocrystals and Poorly Soluble Drugs. Int. J. Pharm. 2013, 453, 101–125. [Google Scholar] [CrossRef]
- Sathisaran, I.; Dalvi, S.V. Engineering Cocrystals of Poorly Water-Soluble Drugs to Enhance Dissolution in Aqueous Medium. Pharmaceutics 2018, 10, 108. [Google Scholar] [CrossRef]
- Perlovich, G.L.; Manin, A.N. Design of Pharmaceutical Cocrystals for Drug Solubility Improvement. Russ. J. Gen. Chem. 2014, 84, 407–414. [Google Scholar] [CrossRef]
- Cherukuvada, S.; Nangia, A. Eutectics as Improved Pharmaceutical Materials: Design, Properties and Characterization. Chem. Commun. 2014, 50, 906–923. [Google Scholar] [CrossRef]
- Stoler, E.; Warner, J.C. Non-Covalent Derivatives: Cocrystals and Eutectics. Molecules 2015, 20, 14833–14848. [Google Scholar] [CrossRef]
- Chavan, R.B.; Thipparaboina, R.; Kumar, D.; Shastri, N.R. Co Amorphous Systems: A Product Development Perspective. Int. J. Pharm. 2016, 515, 403–415. [Google Scholar] [CrossRef]
- Shi, Q.; Moinuddin, S.M.; Cai, T. Advances in Coamorphous Drug Delivery Systems. Acta Pharm. Sin. B 2019, 9, 19–35. [Google Scholar] [CrossRef]
- Sarma, B.; Chen, J.; Hsi, H.Y.; Myerson, A.S. Solid Forms of Pharmaceuticals: Polymorphs, Salts and Cocrystals. Korean J. Chem. Eng. 2011, 28, 315–322. [Google Scholar] [CrossRef]
- Sun, C.C. Cocrystallization for Successful Drug Delivery. Expert Opin. Drug Deliv. 2012, 10, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Grothe, E.; Meekes, H.; Vlieg, E.; Ter Horst, J.H.; De Gelder, R. Solvates, Salts, and Cocrystals: A Proposal for a Feasible Classification System. Cryst. Growth Des. 2016, 16, 3237–3243. [Google Scholar] [CrossRef]
- Nangia, A.; Bolla, G. Pharmaceutical Cocrystals: Walking the Talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef]
- Duggirala, N.K.; Perry, M.L.; Almarsson, Ö.; Zaworotko, M.J. Pharmaceutical Cocrystals: Along the Path to Improved Medicines. Chem. Commun. 2016, 52, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Tocher, D.A.; Vickers, M.; Karamertzanis, P.G.; Price, S.L. Salt or Cocrystal? A New Series of Crystal Structures Formed from Simple Pyridines and Carboxylic Acids. Cryst. Growth Des. 2009, 9, 2881–2889. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Fasulo, M.E.; Desper, J. Cocrystal or Salt: Does It Really Matter? Mol. Pharm. 2007, 4, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.L.; Stahly, G.P.; Park, A. The Salt−Cocrystal Continuum: The Influence of Crystal Structure on Ionization Stat. Mol. Pharm. 2007, 4, 323–338. [Google Scholar] [CrossRef]
- Stevens, J.S.; Byard, S.J.; Seaton, C.C.; Sadiq, G.; Davey, R.J.; Schroeder, S.L.M. Proton Transfer and Hydrogen Bonding in the Organic Solid State: A Combined XRD/XPS/ssNMR Study of 17 Organic Acid-base Complexes. Phys. Chem. Chem. Phys. 2014, 16, 1150–1160. [Google Scholar] [CrossRef]
- Carolina Bazzo, G.; Ramos Pezzini, B.; Karine Stulzer, H. Eutectic Mixtures as an Approach to Enhance Solubility, Dissolution Rate and Oral Bioavailability of Poorly Water-soluble Drugs. Int. J. Pharm. 2020, 588, 119741. [Google Scholar] [CrossRef]
- Chavan, R.B.; Yadav, B.; Lodagekar, A.; Shastri, N.R. Multicomponent Solid Forms: A New Boost to Pharmaceuticals. In Multifunctional Nanocarriers for Contemporary Healthcare Applications; Barkat, A., Harshita, A.B., Sarwar, B., Farhan, J.A., Eds.; IGI Global: Hershey, PA, USA, 2018; pp. 273–300. ISBN 9781522547822. [Google Scholar]
- Laitinen, R.; Löbmann, K.; Grohganz, H.; Priemel, P.; Strachan, C.J.; Rades, T. Supersaturating Drug Delivery Systems: The Potential of Co-amorphous Drug Formulations. Int. J. Pharm. 2017, 532, 1–12. [Google Scholar] [CrossRef]
- Newman, A.; Zografi, G. An Examination of Water Vapor Sorption by Multicomponent Crystalline and Amorphous Solids and Its Effects on Their Solid-State Properties. J. Pharm. Sci. 2019, 108, 1061–1080. [Google Scholar] [CrossRef]
- Dengale, S.J.; Grohganz, H.; Rades, T.; Löbmann, K. Recent Advances in Co-amorphous Drug Formulations. Adv. Drug Deliv. Rev. 2016, 100, 116–125. [Google Scholar] [CrossRef]
- Fischer, F.; Scholz, G.; Benemann, S.; Rademann, K.; Emmerling, F. Evaluation of the Formation Pathways of Cocrystal Polymorphs in Liquid-assisted Syntheses. CrystEngComm 2014, 16, 8272–8278. [Google Scholar] [CrossRef]
- Madusanka, N.; Eddleston, M.D.; Arhangelskis, M.; Jones, W. Polymorphs, Hydrates and Solvates of a Co-crystal of Caffeine with Anthranilic Acid. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2014, 70, 72–80. [Google Scholar] [CrossRef]
- Prohens, R.; Barbas, R.; Portell, A.; Font-Bardia, M.; Alcobé, X.; Puigjaner, C. Polymorphism of Cocrystals: The Promiscuous Behavior of Agomelatine. Cryst. Growth Des. 2016, 16, 1063–1070. [Google Scholar] [CrossRef]
- Bevill, M.J.; Vlahova, P.I.; Smit, J.P. Polymorphic Cocrystals of Nutraceutical Compound p-Coumaric Acid with Nicotinamide: Characterization, Relative Solid-State Stability, and Conversion to Alternate Stoichiometries. Cryst. Growth Des. 2014, 14, 1438–1448. [Google Scholar] [CrossRef]
- Eddleston, M.D.; Patel, B.; Day, G.M.; Jones, W. Cocrystallization by Freeze-drying: Preparation of Novel Multicomponent Crystal Forms. Cryst. Growth Des. 2013, 13, 4599–4606. [Google Scholar] [CrossRef]
- Schultheiss, N.; Roe, M.; Boerrigter, S.X.M. Cocrystals of Nutraceutical P-coumaric Acid with Caffeine and Theophylline: Polymorphism and Solid-state Stability Explored in Detail Using Their Crystal Graphs. CrystEngComm 2011, 13, 611–619. [Google Scholar] [CrossRef]
- Mnguni, M.J.; Michael, J.P.; Lemmerer, A. Binary Polymorphic Cocrystals: An Update on the Available Literature in the Cambridge Structural Database, Including a New Polymorph of the Pharmaceutical 1:1 Cocrystal Theophylline–3,4-dihydroxybenzoic Acid. Acta Crystallogr. Sect. C Struct. Chem. 2018, 74, 715–720. [Google Scholar] [CrossRef]
- Aitipamula, S.; Chow, P.S.; Tan, R.B.H. Polymorphism in cocrystals: A review and assessment of its significance. CrystEngComm 2014, 16, 3451–3465. [Google Scholar] [CrossRef]
- Douroumis, D.; Ross, S.A.; Nokhodchi, A. Advanced Methodologies for Cocrystal Synthesis. Adv. Drug Deliv. Rev. 2017, 117, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Steed, J.W. The Role of Co-crystals in Pharmaceutical Design. Trends Pharmacol. Sci. 2013, 34, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Hoang Pham, U.G. Pharmaceutical Applications of Eutectic Mixtures. J. Dev. Drugs 2014, 2, 2–3. [Google Scholar] [CrossRef]
- Kaupp, G. Mechanochemistry: The Varied Applications of Mechanical Bond-breaking. CrystEngComm 2009, 11, 388–403. [Google Scholar] [CrossRef]
- Suslick, K.S. Mechanochemistry and Sonochemistry: Concluding Remarks. Faraday Discuss. 2014, 170, 411–422. [Google Scholar] [CrossRef]
- Friščić, T. New Opportunities for Materials Synthesis Using Mechanochemistry. J. Mater. Chem. 2010, 20, 7599–7605. [Google Scholar] [CrossRef]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for Synthesis. Angew. Chem. 2020, 59, 1018–1029. [Google Scholar] [CrossRef]
- Achar, T.K.; Bose, A.; Mal, P. Mechanochemical Synthesis of Small Organic Molecules. Beilstein J. Org. Chem. 2017, 13, 1907–1931. [Google Scholar] [CrossRef]
- Do, J.-L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef]
- Bruckmann, A.; Krebs, A.; Bolm, C. Organocatalytic Reactions: Effects of Ball Milling, Microwave and Ultrasound Irradiation. Green Chem. 2008, 10, 1131. [Google Scholar] [CrossRef]
- Pickhardt, W.; Grätz, S.; Borchardt, L. Direct Mechanocatalysis: Using Milling Balls as Catalysts. Chem. Eur. J. 2020, 26, 12903–12911. [Google Scholar] [CrossRef]
- Hernández, J.G.; Macdonald, N.A.J.; Mottillo, C.; Butler, I.S.; Friščić, T. A Mechanochemical Strategy for Oxidative Addition: Remarkable Yields and Stereoselectivity in the Halogenation of Organometallic Re(I) Complexes. Green Chem. 2014, 16, 1087–1092. [Google Scholar] [CrossRef]
- Chikunov, I.E.; Ranny, G.S.; Astakhov, A.V.; Tafeenko, V.A.; Chernyshev, V.M. Mechanochemical Synthesis of Platinum(IV) Complexes with N-heterocyclic Carbenes. Russ. Chem. Bull. 2018, 67, 2003–2009. [Google Scholar] [CrossRef]
- Adams, C.J.; Lusi, M.; Mutambi, E.M.; Orpen, A.G. Two-Step Mechanochemical Synthesis of Carbene Complexes of Palladium(II) and Platinum(II). Cryst. Growth Des. 2017, 17, 3151–3155. [Google Scholar] [CrossRef]
- Allenbaugh, R.J.; Zachary, J.R.; Underwood, A.N.; Bryson, J.D.; Williams, J.R.; Shaw, A. Kinetic Analysis of the Complete Mechanochemical Synthesis of a Palladium(II) Carbene Complex. Inorg. Chem. Commun. 2020, 111, 107622. [Google Scholar] [CrossRef]
- Rightmire, N.R.; Hanusa, T.P. Advances in Organometallic Synthesis with Mechanochemical Methods. Dalt. Trans. 2016, 45, 2352–2362. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Varma, R.S. Alternative Energy Input: Mechanochemical, Microwave and Ultrasound-assisted Organic Synthesis. Chem. Soc. Rev. 2012, 41, 1559–1584. [Google Scholar] [CrossRef]
- Andersen, J.; Mack, J. Mechanochemistry and Organic Synthesis: From Mystical to Practical. Green Chem. 2018, 20, 1435–1443. [Google Scholar] [CrossRef]
- Leonardi, M.; Villacampa, M.; Menéndez, J.C. Multicomponent Mechanochemical Synthesis. Chem. Sci. 2018, 9, 2042–2064. [Google Scholar] [CrossRef]
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.S.; Ondruschka, B. Ball Milling in Organic Synthesis: Solutions and Challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. [Google Scholar] [CrossRef]
- Margetić, D.; Štrukil, V. Practical Considerations. In Mechanochemical Organic Synthesis; Margetić, D., Štrukil, V.B.T.-M.O.S., Eds.; Elsevier: Boston, MA, USA, 2016; Chapter 1; pp. 1–54. ISBN 978-0-12-802184-2. [Google Scholar]
- Wang, G.-W. Mechanochemical Organic Synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef]
- Beillard, A.; Métro, T.X.; Bantreil, X.; Martinez, J.; Lamaty, F. Cu(0), O2 and Mechanical Forces: A Saving Combination for Efficient Production of Cu-NHC Complexes. Chem. Sci. 2017, 8, 1086–1089. [Google Scholar] [CrossRef]
- Friščić, T.; Halasz, I.; Štrukil, V.; Eckert-Maksić, M.; Dinnebier, R.E. Clean and Efficient Synthesis Using Mechanochemistry: Coordination Polymers, Metal-organic Frameworks and Metallodrugs. Croat. Chem. Acta 2012, 85, 367–378. [Google Scholar] [CrossRef]
- Garay, A.L.; Pichon, A.; James, S.L. Solvent-free Synthesis of Metal Complexes. Chem. Soc. Rev. 2007, 36, 846. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; García, F. Main Group Mechanochemistry: From Curiosity to Established Protocols. Chem. Soc. Rev. 2019, 48, 2274–2292. [Google Scholar] [CrossRef]
- Gečiauskaitė, A.A.; García, F. Main Group Mechanochemistry. Beilstein J. Org. Chem. 2017, 13, 2068–2077. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, J.; Zhang, P.; Dai, S. Mechanochemical Synthesis of Metal–organic Frameworks. Polyhedron 2019, 162, 59–64. [Google Scholar] [CrossRef]
- Klimakow, M.; Klobes, P.; Thünemann, A.F.; Rademann, K.; Emmerling, F. Mechanochemical Synthesis of Metal-organic Frameworks: A Fast and Facile Approach toward Quantitative Yields and High Specific Surface Areas. Chem. Mater. 2010, 22, 5216–5221. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for New and Cleaner Synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef]
- Stolar, T.; Užarević, K. Mechanochemistry: An Efficient and Versatile Toolbox for Synthesis, Transformation, and Functionalization of Porous Metal–organic Frameworks. CrystEngComm 2020, 22, 4511–4525. [Google Scholar] [CrossRef]
- Mottillo, C.; Friščić, T. Advances in Solid-state Transformations of Coordination Bonds: From the Ball Mill to the Aging Chamber. Molecules 2017, 22, 144. [Google Scholar] [CrossRef]
- Willis-Fox, N.; Rognin, E.; Aljohani, T.A.; Daly, R. Polymer Mechanochemistry: Manufacturing Is Now a Force to Be Reckoned With. Chem 2018, 4, 2499–2537. [Google Scholar] [CrossRef]
- Akbulatov, S.; Boulatov, R. Experimental Polymer Mechanochemistry and Its Interpretational Frameworks. ChemPhysChem 2017, 18, 1422–1450. [Google Scholar] [CrossRef]
- May, P.A.; Moore, J.S. Polymer Mechanochemistry: Techniques to Generate Molecular Force via Elongational Flows. Chem. Soc. Rev. 2013, 42, 7497–7506. [Google Scholar] [CrossRef]
- Zhu, S.-E.; Li, F.; Wang, G.-W. Mechanochemistry of Fullerenes and Related Materials. Chem. Soc. Rev. 2013, 42, 7535–7570. [Google Scholar] [CrossRef]
- Braga, D.; Maini, L.; Grepioni, F. Mechanochemical Preparation of Co-crystals. Chem. Soc. Rev. 2013, 42, 7638–7648. [Google Scholar] [CrossRef]
- Delori, A.; Friscic, T.; Jones, W.; Friščić, T.; Jones, W. The Role of Mechanochemistry and Supramolecular Design in the Development of Pharmaceutical Materials. CrystEngComm 2012, 14, 2350–2362. [Google Scholar] [CrossRef]
- Tan, D.; Loots, L.; Friščić, T. Towards Medicinal Mechanochemistry: Evolution of Milling from Pharmaceutical Solid Form Screening to the Synthesis of Active Pharmaceutical Ingredients (APIs). Chem. Commun. 2016, 52, 7760–7781. [Google Scholar] [CrossRef]
- Friščić, T. Supramolecular Concepts and New Techniques in Mechanochemistry: Cocrystals, Cages, Rotaxanes, Open Metal–organic Frameworks. Chem. Soc. Rev. 2012, 41, 3493. [Google Scholar] [CrossRef]
- Trask, A.V.; Jones, W. Crystal Engineering of Organic Cocrystals by the Solid-state Grinding Approach. Top. Curr. Chem. 2005, 254, 41–70. [Google Scholar] [CrossRef]
- Friščič, T.; Jones, W. Recent Advances in Understanding the Mechanism of Cocrystal Formation via Grinding. Cryst. Growth Des. 2009, 9, 1621–1637. [Google Scholar] [CrossRef]
- Ahluwalia, V.K.; Kidwai, M. Organic Synthesis in Solid State. In New Trends in Green Chemistry; Springer: Dordrecht, The Netherlands, 2004; pp. 189–231. [Google Scholar]
- Varma, R.S. Greener and Sustainable Trends in Synthesis of Organics and Nanomaterials. ACS Sustain. Chem. Eng. 2016, 4, 5866–5878. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; ISBN 9780198502340. [Google Scholar]
- Tang, S.L.Y.; Smith, R.L.; Poliakoff, M. Principles of Green Chemistry: PRODUCTIVELY. Green Chem. 2005, 7, 761–762. [Google Scholar] [CrossRef]
- Loupy, A. Microwaves in Organic Synthesis; Loupy, A., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; ISBN 3-527-31452-0. [Google Scholar]
- Leonelli, C.; Mason, T.J. Microwave and Ultrasonic Processing: Now a Realistic Option for Industry. Chem. Eng. Process. Process Intensif. 2010, 49, 885–900. [Google Scholar] [CrossRef]
- Roy, D.; James, S.L.; Crawford, D.E. Solvent-free Sonochemistry as a Route to Pharmaceutical Co-crystals. Chem. Commun. 2019, 55, 5463–5466. [Google Scholar] [CrossRef]
- Crawford, D.E. Solvent-free Sonochemistry: Sonochemical Organic Synthesis in the Absence of a Liquid Medium. Beilstein J. Org. Chem. 2017, 13, 1850–1856. [Google Scholar] [CrossRef]
- Cole, J.M.; Irie, M. Solid-state Photochemistry. CrystEngComm 2016, 18, 7175–7179. [Google Scholar] [CrossRef]
- Cohen, M.D. Solid-state Photochemical Reactions. Tetrahedron 1987, 43, 1211–1224. [Google Scholar] [CrossRef]
- Guillet, J.E. Photochemistry in the Solid Phase. Polym. Eng. Sci. 1974, 14, 482–486. [Google Scholar] [CrossRef]
- Kaupp, G. Solid-state Photochemistry: New Approaches Based on New Mechanistic Insights. Int. J. Photoenergy 2001, 3, 392659. [Google Scholar] [CrossRef]
- Howard, J.L.; Cao, Q.; Browne, D.L. Mechanochemistry as an Emerging Tool for Molecular Synthesis: What Can It Offer? Chem. Sci. 2018, 9, 3080–3094. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. Mechano-chemical Reaction. IUPAC Compend. Chem. Terminol. 2008, 889, 7141. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of Mechanochemistry: From Nanoparticles to Technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef]
- Ostwald, W. Handbuch der Allgemeinen Chemie, Band I; Akademische Verlagsgesellschaft: Leipzig, Germany, 1919. [Google Scholar]
- Takacs, L. Mechanochemistry and the Other Branches of Chemistry: Similarities and Differences. Acta Phys. Pol. A 2012, 121, 711–714. [Google Scholar] [CrossRef]
- Fernández-Bertran, J.F. Mechanochemistry: An Overview. Pure Appl. Chem. 1999, 71, 581–586. [Google Scholar] [CrossRef]
- Kajdas, C. General Approach to Mechanochemistry and Its Relation to Tribochemistry. In Tribology in Engineering; InTechOpen: London, UK, 2013. [Google Scholar]
- Martini, A.; Eder, S.J.; Dörr, N. Tribochemistry: A Review of Reactive Molecular Dynamics Simulations. Lubricants 2020, 8, 44. [Google Scholar] [CrossRef]
- Boldyreva, E. Mechanochemistry of Inorganic and Organic Systems: What is Similar, What is Different? Chem. Soc. Rev. 2013, 42, 7719–7738. [Google Scholar] [CrossRef]
- Takacs, L. The First Documented Mechanochemical Reaction? J. Met. 2000, 52, 12–13. [Google Scholar] [CrossRef]
- Beyer, M.K.; Clausen-Schaumann, H. Mechanochemistry: The Mechanical Activation of Covalent Bonds. Chem. Rev. 2005, 105, 2921–2948. [Google Scholar] [CrossRef]
- Takacs, L.M. Carey Lea, the First Mechanochemist. J. Mater. Sci. 2004, 39, 4987–4993. [Google Scholar] [CrossRef]
- Takacs, L. The Mechanochemical Reduction of AgCl with Metals: Revisiting an Experiment of M. Faraday. J. Therm. Anal. Calorim. 2007, 90, 81–84. [Google Scholar] [CrossRef]
- Takacs, L. The Historical Development of Mechanochemistry. Chem. Soc. Rev. 2013, 42, 7649–7659. [Google Scholar] [CrossRef]
- Takacs, L. What is Unique about Mechanochemical Reactions? Acta Phys. Pol. A 2014, 126, 1040–1043. [Google Scholar] [CrossRef]
- Takacs, L.M. Carey Lea, the Father of Mechanochemistry. Bull. Hist. Chem. 2003, 28, 26–34. [Google Scholar]
- Etter, M.C. Hydrogen Bonds as Design Elements in Organic Chemistry. J. Phys. Chem. 1991, 95, 4601–4610. [Google Scholar] [CrossRef]
- Pedireddi, V.R.; Jones, W.; Chorlton, A.P.; Docherty, R. Creation of Crystalline Supramolecular Arrays: A Comparison of Co-crystal Formation from Solution and by Solid-state Grinding. Chem. Commun. 1996, 8, 987–988. [Google Scholar] [CrossRef]
- Braga, D.; Giaffreda, S.L.; Curzi, M.; Maini, L.; Polito, M.; Grepioni, F. Mechanical Mixing of Molecular Crystals: A Green Route to Co-crystals and Coordination Networks. J. Therm. Anal. Calorim. 2007, 90, 115–123. [Google Scholar] [CrossRef]
- Weyna, D.R.; Shattock, T.; Vishweshwar, P.; Zaworotko, M.J. Synthesis and Structural Characterization of Cocrystals and Pharmaceutical Cocrystals: Mechanochemistry vs. Slow Evaporation from Solution. Cryst. Growth Des. 2009, 9, 1106–1123. [Google Scholar] [CrossRef]
- Juribašić, M.; Užarević, K.; Gracin, D.; Ćurić, M. Mechanochemical C-H Bond Activation: Rapid and Regioselective Double Cyclopalladation Monitored by in situ Raman Spectroscopy. Chem. Commun. 2014, 50, 10287–10290. [Google Scholar] [CrossRef]
- Tan, D.; Mottillo, C.; Katsenis, A.D.; Štrukil, V.; Friščic, T. Development of C-N Coupling Using Mechanochemistry: Catalytic Coupling of Arylsulfonamides and Carbodiimides. Angew. Chem. 2014, 53, 9321–9324. [Google Scholar] [CrossRef]
- Su, Y.T.; Wang, G.W. FeCl3-mediated Cyclization of [60]fullerene with N-benzhydryl Sulfonamides under High-speed Vibration Milling Conditions. Org. Lett. 2013, 15, 3408–3411. [Google Scholar] [CrossRef]
- Haley, R.A.; Zellner, A.R.; Krause, J.A.; Guan, H.; Mack, J. Nickel Catalysis in a High Speed Ball Mill: A Recyclable Mechanochemical Method for Producing Substituted Cyclooctatetraene Compounds. ACS Sustain. Chem. Eng. 2016, 4, 2464–2469. [Google Scholar] [CrossRef]
- Bučar, D.K.; Friščić, T. Professor William Jones and His Materials Chemistry Group: Innovations and Advances in the Chemistry of Solids. Cryst. Growth Des. 2019, 19, 1479–1487. [Google Scholar] [CrossRef]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Solvent-Drop Grinding: Green Polymorph Control of Cocrystallisation. Chem. Commun. 2004, 890–891. [Google Scholar] [CrossRef]
- Trask, A.V.; Shan, N.; Motherwell, W.D.S.; Jones, W.; Feng, S.; Tan, R.B.H.; Carpenter, K.J. Selective Polymorph Transformation via Solvent-drop Grinding. Chem. Commun. 2005, 880–882. [Google Scholar] [CrossRef]
- Trask, A.V.; Samuel Motherwell, W.D.; Jones, W. Pharmaceutical Cocrystallization: Engineering a Remedy for Caffeine Hydration. Cryst. Growth Des. 2005, 5, 1013–1021. [Google Scholar] [CrossRef]
- Adams, C.J.; Colquhoun, H.M.; Crawford, P.C.; Lusi, M.; Orpen, A.G. Solid-state Interconversions of Coordination Networks and Hydrogen-bonded Salts. Angew. Chem. 2007, 46, 1124–1128. [Google Scholar] [CrossRef]
- Skovsgaard, S.; Bond, A.D. Co-crystallisation of Benzoic Acid Derivatives with N-containing Bases in Solution and by Mechanical Grinding: Stoichiometric Variants, Polymorphism and Twinning. CrystEngComm 2009, 11, 444–453. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, F.; Hughes, C.E.; Browne, D.L.; Peskett, T.R.; Harris, K.D.M. Discovery of New Metastable Polymorphs in a Family of Urea Co-crystals by Solid-state Mechanochemistry. Cryst. Growth Des. 2015, 15, 2901–2907. [Google Scholar] [CrossRef]
- Zbačnik, M.; Nogalo, I.; Cinčić, D.; Kaitner, B. Polymorphism Control in the Mechanochemical and Solution-based Synthesis of a Thermochromic Schiff Base. CrystEngComm 2015, 17, 7870–7877. [Google Scholar] [CrossRef]
- Hasa, D.; Jones, W. Screening for New Pharmaceutical Solid Forms Using Mechanochemistry: A Practical Guide. Adv. Drug Deliv. Rev. 2017, 117, 147–161. [Google Scholar] [CrossRef]
- Toda, F.; Yagi, M.; Kiyoshige, K. Baeyer-Villiger Reaction in the Solid State. J. Chem. Soc. Chem. Commun. 1988, 958–959. [Google Scholar] [CrossRef]
- Stolle, A.; Schmidt, R.; Jacob, K. Scale-up of Organic Reactions in Ball Mills: Process Intensification with Regard to Energy Efficiency and Economy of Scale. Faraday Discuss. 2014, 170, 267–286. [Google Scholar] [CrossRef]
- Hernández, J.G. C–H Bond Functionalization by Mechanochemistry. Chem. Eur. J. 2017, 23, 17157–17165. [Google Scholar] [CrossRef]
- Hermann, G.N.; Becker, P.; Bolm, C. Mechanochemical Iridium(III)-Catalyzed C-H Bond Amidation of Benzamides with Sulfonyl Azides under Solvent-Free Conditions in a Ball Mill. Angew. Chem. 2016, 55, 3781–3784. [Google Scholar] [CrossRef]
- Jörres, M.; Aceña, J.L.; Soloshonok, V.A.; Bolm, C. Asymmetric Carbon-carbon Bond Formation under Solventless Conditions in Ball Mills. ChemCatChem 2015, 7, 1265–1269. [Google Scholar] [CrossRef]
- Hernández, J.G.; Bolm, C. Altering Product Selectivity by Mechanochemistry. J. Org. Chem. 2017, 82, 4007–4019. [Google Scholar] [CrossRef] [PubMed]
- Swinburne, A.N.; Steed, J.W. The Mechanochemical Synthesis of Podand Anion Receptors. CrystEngComm 2009, 11, 433–438. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Chopade, P.D. Oxime Decorated Cavitands Functionalized through Solvent-assisted Grinding. Org. Lett. 2011, 13, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; Szuppa, T.; Stolle, A.; Ondruschka, B.; Hopf, H. Energetic Assessment of the Suzuki–Miyaura Reaction: A Curtate Life Cycle Assessment as an Easily Understandable and Applicable Tool for Reaction Optimization. Green Chem. 2009, 11, 1894–1899. [Google Scholar] [CrossRef]
- Ling, A.R.; Baker, J.L. XCVI.—Halogen Derivatives of Quinone. Part III. Derivatives of Quinhydrone. J. Chem. Soc. Trans. 1893, 63, 1314–1327. [Google Scholar] [CrossRef]
- Patil, A.O.; Curtin, D.Y.; Paul, I.C. Solid-State Formation of Quinhydrones from Their Components. Use of Solid-Solid Reactions to Prepare Compounds Not Accessible from Solution. J. Am. Chem. Soc. 1984, 106, 348–353. [Google Scholar] [CrossRef]
- Etter, M.C.; Urbañczyk-Lipkowska, Z.; Zia-Ebrahimi, M.; Panunto, T.W. Hydrogen Bond Directed Cocrystallization and Molecular Recognition Properties of Diarylureas. J. Am. Chem. Soc 1990, 112, 8415–8426. [Google Scholar] [CrossRef]
- Etter, M.C.; Adsmond, D.A. The Use of Cocrystallization as a Method of Studying Hydrogen Bond Preferences of 2-aminopyrimidine. J. Chem. Soc. Chem. Commun. 1990, 589–591. [Google Scholar] [CrossRef]
- Ribas-Arino, J.; Marx, D. Covalent Mechanochemistry: Theoretical Concepts and Computational Tools with Applications to Molecular Nanomechanics. Chem. Rev. 2012, 112, 5412–5487. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Mechanochemistry of Inorganic Solids. Proc. Indian Natl. Sci. Acad. Part A 1986, 52, 400–417. [Google Scholar]
- Suryanarayana, C. Mechanical Alloying and Milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Takacs, L.; McHenry, J.S. Temperature of the Milling Balls in Shaker and Planetary Mills. J. Mater. Sci. 2006, 41, 5246–5249. [Google Scholar] [CrossRef]
- Burmeister, C.F.; Kwade, A. Process Engineering with Planetary Ball Mills. Chem. Soc. Rev. 2013, 42, 7660–7667. [Google Scholar] [CrossRef]
- Medina, C.; Daurio, D.; Nagapudi, K.; Alvarez-Nunez, F. Manufacture of Pharmaceutical Co-crystals Using Twin Screw Extrusion: A Solvent-less and Scalable Process. J. Pharm. Sci. 2010, 99, 1693–1696. [Google Scholar] [CrossRef]
- Daurio, D.; Medina, C.; Saw, R.; Nagapudi, K.; Alvarez-Núñez, F. Application of Twin Screw Extrusion in the Manufacture of Cocrystals, Part I: Four Case Studies. Pharmaceutics 2011, 3, 582–600. [Google Scholar] [CrossRef]
- Moradiya, H.G.; Islam, M.T.; Scoutaris, N.; Halsey, S.A.; Chowdhry, B.Z.; Douroumis, D. Continuous Manufacturing of High Quality Pharmaceutical Cocrystals Integrated with Process Analytical Tools for In-Line Process Control. Cryst. Growth Des. 2016, 16, 3425–3434. [Google Scholar] [CrossRef]
- Crawford, D.E.; Miskimmin, C.K.G.; Albadarin, A.B.; Walker, G.; James, S.L. Organic Synthesis by Twin Screw Extrusion (TSE): Continuous, Scalable and Solvent-free. Green Chem. 2017, 19, 1507–1518. [Google Scholar] [CrossRef]
- Cao, Q.; Howard, J.L.; Crawford, D.E.; James, S.L.; Browne, D.L. Translating Solid State Organic Synthesis from a Mixer Mill to a Continuous Twin Screw Extruder. Green Chem. 2018, 20, 4443–4447. [Google Scholar] [CrossRef]
- Crawford, D.; Casaban, J.; Haydon, R.; Giri, N.; McNally, T.; James, S.L. Synthesis by Extrusion: Continuous, Large-scale Preparation of MOFs Using Little or no Solvent. Chem. Sci. 2015, 6, 1645–1649. [Google Scholar] [CrossRef]
- Crawford, D.E.; Wright, L.A.; James, S.L.; Abbott, A.P. Efficient Continuous Synthesis of High Purity Deep Eutectic Solvents by Twin Screw Extrusion. Chem. Commun. 2016, 52, 4215–4218. [Google Scholar] [CrossRef] [PubMed]
- Thorwirth, R.; Bernhardt, F.; Stolle, A.; Ondruschka, B.; Asghari, J. Switchable Selectivity during Oxidation of Anilines in a Ball Mill. Chem. Eur. J. 2010, 16, 13236–13242. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; Grepioni, F. Reactions between or within Molecular Crystals. Angew. Chem. 2004, 43, 4002–4011. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; D’Addario, D.; Giaffreda, S.L.; Maini, L.; Polito, M.; Grepioni, F. Intra-solid and Inter-solid Reactions of Molecular Crystals: A Green Route to Crystal Engineering. Top. Curr. Chem. 2005, 254, 71–94. [Google Scholar] [CrossRef]
- Schmidt, G.M.J. Photodimerization in the Solid State. Pure Appl. Chem. 1971, 27, 647–678. [Google Scholar] [CrossRef]
- Naumov, P.; Bharadwaj, P.K. Single-crystal-to-single-crystal Transformations. CrystEngComm 2015, 17, 8775. [Google Scholar] [CrossRef]
- Martí-Rujas, J. Thermal Reactivity in Metal Organic Materials (MOMs): From Single-crystal-to-single-crystal Reactions and Beyond. Materials 2019, 12, 4088. [Google Scholar] [CrossRef]
- Rodríguez, B.; Bruckmann, A.; Rantanen, T.; Bolm, C. Solvent-free Carbon-carbon Bond Formations in Ball Mills. Adv. Synth. Catal. 2007, 349, 2213–2233. [Google Scholar] [CrossRef]
- Chen, R.; Gokus, M.K.; Pagola, S. Tetrathiafulvalene: A Gate to the Mechanochemical Mechanisms of Electron Transfer Reactions. Crystals 2020, 10, 482. [Google Scholar] [CrossRef]
- Trukil, V.; Fábián, L.; Reid, D.G.; Duer, M.J.; Jackson, G.J.; Eckert-Maksić, M.; Friić, T. Towards an Environmentally-friendly Laboratory: Dimensionality and Reactivity in the Mechanosynthesis of Metal-organic Compounds. Chem. Commun. 2010, 46, 9191–9193. [Google Scholar] [CrossRef]
- Halasz, I.; Friščić, T.; Kimber, S.A.J.; Užarević, K.; Puškarić, A.; Mottillo, C.; Julien, P.; Štrukil, V.; Honkimäki, V.; Dinnebier, R.E. Quantitative in situ and Real-time Monitoring of Mechanochemical Reactions. Faraday Discuss. 2014, 170, 203–221. [Google Scholar] [CrossRef]
- Friščić, T.; Halasz, I.; Beldon, P.J.; Belenguer, A.M.; Adams, F.; Kimber, S.A.J.; Honkimäki, V.; Dinnebier, R.E. Real-time and in situ Monitoring of Mechanochemical Milling Reactions. Nat. Chem. 2013, 5, 66–73. [Google Scholar] [CrossRef]
- Halasz, I.; Kimber, S.A.J.; Beldon, P.J.; Belenguer, A.M.; Adams, F.; Honkimäki, V.; Nightingale, R.C.; Dinnebier, R.E.; Friščić, T. In situ and Real-time Monitoring of Mechanochemical Milling Reactions Using Synchrotron X-ray Diffraction. Nat. Protoc. 2013, 8, 1718–1729. [Google Scholar] [CrossRef]
- Halasz, I.; Puškaric, A.; Kimber, S.A.J.; Beldon, P.J.; Belenguer, A.M.; Adams, F.; Honkimäki, V.; Dinnebier, R.E.; Patel, B.; Jones, W.; et al. Real-time in situ Powder X-ray Diffraction Monitoring of Mechanochemical Synthesis of Pharmaceutical Cocrystals. Angew. Chem. 2013, 52, 11538–11541. [Google Scholar] [CrossRef]
- Užarević, K.; Halasz, I.; Friščić, T. Real-Time and in Situ Monitoring of Mechanochemical Reactions: A New Playground for All Chemists. J. Phys. Chem. Lett. 2015, 6, 4129–4140. [Google Scholar] [CrossRef] [PubMed]
- Gracin, D.; Štrukil, V.; Friščic, T.; Halasz, I.; Užarevic, K. Laboratory Real-time and in situ Monitoring of Mechanochemical Milling Reactions by Raman Spectroscopy. Angew. Chem. 2014, 53, 6193–6197. [Google Scholar] [CrossRef] [PubMed]
- Julien, P.A.; Malvestiti, I.; Friščić, T. The Effect of Milling Frequency on a Mechanochemical Organic Reaction Monitored by in situ Raman Spectroscopy. Beilstein J. Org. Chem. 2017, 13, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Bowmaker, G.A. Solvent-assisted Mechanochemistry. Chem. Commun. 2013, 49, 334–348. [Google Scholar] [CrossRef]
- Shan, N.; Toda, F.; Jones, W. Mechanochemistry and Co-crystal Formation: Effect of Solvent on Reaction Kinetics. Chem. Commun. 2002, 2372–2373. [Google Scholar] [CrossRef]
- Friščić, T.; Childs, S.L.; Rizvi, S.A.A.; Jones, W. The Role of Solvent in Mechanochemical and Sonochemical Cocrystal Formation: A Solubility-based Approach for Predicting Cocrystallisation Outcome. CrystEngComm 2009, 11, 418–426. [Google Scholar] [CrossRef]
- Hasa, D.; Miniussi, E.; Jones, W. Mechanochemical Synthesis of Multicomponent Crystals: One Liquid for One Polymorph? A Myth to Dispel. Cryst. Growth Des. 2016, 16, 4582–4588. [Google Scholar] [CrossRef]
- Pfund, L.Y.; Matzger, A.J. Towards Exhaustive and Automated High-throughput Screening for Crystalline Polymorphs. ACS Comb. Sci. 2014, 16, 309–313. [Google Scholar] [CrossRef]
- Lopez-Mejías, V.; Knight, J.L.; Brooks, C.L.; Matzger, A.J. On the Mechanism of Crystalline Polymorph Selection by Polymer Heteronuclei. Langmuir 2011, 27, 7575–7579. [Google Scholar] [CrossRef][Green Version]
- McClelland, A.A.; López-Mejías, V.; Matzger, A.J.; Chen, Z. Peering at a Buried Polymer-crystal Interface: Probing Heterogeneous Nucleation by Sum Frequency Generation Vibrational Spectroscopy. Langmuir 2011, 27, 2162–2165. [Google Scholar] [CrossRef]
- Hasa, D.; Schneider Rauber, G.; Voinovich, D.; Jones, W. Cocrystal Formation through Mechanochemistry: From Neat and Liquid-Assisted Grinding to Polymer-Assisted Grinding. Angew. Chem. 2015, 54, 7371–7375. [Google Scholar] [CrossRef]
- Karki, S.; Friščić, T.; Jones, W.; Motherwell, W.D.S. Screening for Pharmaceutical Cocrystal Hydrates via Neat and Liquid-assisted Grinding. Mol. Pharm. 2007, 4, 347–354. [Google Scholar] [CrossRef]
- Batchelor, E.; Klinowski, J.; Jones, W. Crystal Engineering Using Co-crystallisation of Phenazine with Dicarboxylic Acids. J. Mater. Chem. 2000, 10, 839–848. [Google Scholar] [CrossRef]
- Germann, L.S.; Emmerling, S.T.; Wilke, M.; Dinnebier, R.E.; Moneghini, M.; Hasa, D. Monitoring Polymer-assisted Mechanochemical Cocrystallisation through in situ X-ray Powder Diffraction. Chem. Commun. 2020, 56, 8743–8746. [Google Scholar] [CrossRef]
- Thakuria, R.; Eddleston, M.D.; Chow, E.H.H.; Lloyd, G.O.; Aldous, B.J.; Krzyzaniak, J.F.; Bond, A.D.; Jones, W. Use of in situ Atomic Force Microscopy to Follow Phase Changes at Crystal Surfaces in Real Time. Angew. Chem. 2013, 52, 10541–10544. [Google Scholar] [CrossRef]
- Friščić, T.; Reid, D.G.; Halasz, I.; Stein, R.S.; Dinnebier, R.E.; Duer, M.J. Ion- and Liquid-assisted Grinding: Improved Mechanochemical Synthesis of Metal-organic Frameworks Reveals Salt Inclusion and Anion Templating. Angew. Chem. 2010, 49, 712–715. [Google Scholar] [CrossRef]
- Mukherjee, A.; Rogers, R.D.; Myerson, A.S. Cocrystal Formation by Ionic Liquid-assisted Grinding: Case Study with Cocrystals of Caffeine. CrystEngComm 2018, 20, 3817–3821. [Google Scholar] [CrossRef]
- Smit, J.P.; Hagen, E.J. Polymorphism in Caffeine Citric Acid Cocrystals. J. Chem. Crystallogr. 2015, 45, 128–133. [Google Scholar] [CrossRef]
- Hasa, D.; Carlino, E.; Jones, W. Polymer-Assisted Grinding, a Versatile Method for Polymorph Control of Cocrystallization. Cryst. Growth Des. 2016, 16, 1772–1779. [Google Scholar] [CrossRef]
- Von Colbe, J.M.B.; Felderhoff, M.; Bogdanović, B.; Schüth, F.; Weidenthaler, C. One-step Direct Synthesis of a Ti-doped Sodium Alanate Hydrogen Storage Material. Chem. Commun. 2005, 4732–4734. [Google Scholar] [CrossRef]
- Doppiu, S.; Schultz, L.; Gutfleisch, O. In situ Pressure and Temperature Monitoring during the Conversion of Mg into MgH2 by High-pressure Reactive Ball Milling. J. Alloys Compd. 2007, 427, 204–208. [Google Scholar] [CrossRef]
- Užarević, K.; Štrukil, V.; Mottillo, C.; Julien, P.A.; Puškarić, A.; Friščić, T.; Halasz, I. Exploring the Effect of Temperature on a Mechanochemical Reaction by in Situ Synchrotron Powder X-ray Diffraction. Cryst. Growth Des. 2016, 16, 2342–2347. [Google Scholar] [CrossRef]
- Chadwick, K.; Davey, R.; Cross, W. How Does Grinding Produce Co-crystals? Insights from the Case of Benzophenone and Diphenylamine. CrystEngComm 2007, 9, 732–734. [Google Scholar] [CrossRef]
- Lien Nguyen, K.; Friščić, T.; Day, G.M.; Gladden, L.F.; Jones, W. Terahertz Time-domain Spectroscopy and the Quantitative Monitoring of Mechanochemical Cocrystal Formation. Nat. Mater. 2007, 6, 206–209. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Bassi, P.S.; Chadha, S.L. Mechanism of the Reaction between Hydrocarbons and Picric Acid in the Solid State. J. Phys. Chem. 1963, 67, 2569–2573. [Google Scholar] [CrossRef]
- Kaupp, G. Solid-state Molecular Syntheses: Complete Reactions without Auxiliaries Based on the New Solid-state Mechanism. CrystEngComm 2003, 5, 117–133. [Google Scholar] [CrossRef]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating Cocrystals: A Review of Pharmaceutical Cocrystal Preparation Routes and Applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Rothenberg, G.; Downie, A.P.; Raston, C.L.; Scott, J.L. Understanding Solid/Solid Organic Reactions. J. Am. Chem. Soc. 2001, 123, 8701–8708. [Google Scholar] [CrossRef]
- Germann, L.S.; Arhangelskis, M.; Etter, M.; Dinnebier, R.E.; Friščić, T. Challenging the Ostwald Rule of Stages in Mechanochemical Cocrystallisation. Chem. Sci. 2020, 11, 10092–10100. [Google Scholar] [CrossRef]
- Ma, X.; Yuan, W.; Bell, S.E.J.; James, S.L. Better Understanding of Mechanochemical Reactions: Raman Monitoring Reveals Surprisingly Simple ‘Pseudo-fluid’ Model for a Ball Milling Reaction. Chem. Commun. 2014, 50, 1585–1587. [Google Scholar] [CrossRef]
- Kulla, H.; Becker, C.; Michalchuk, A.A.L.; Linberg, K.; Paulus, B.; Emmerling, F. Tuning the Apparent Stability of Polymorphic Cocrystals through Mechanochemistry. Cryst. Growth Des. 2019, 19, 7271–7279. [Google Scholar] [CrossRef]
- Schmidt, R.; Martin Scholze, H.; Stolle, A. Temperature Progression in a Mixer Ball Mill. Int. J. Ind. Chem. 2016, 7, 181–186. [Google Scholar] [CrossRef]
- Kulla, H.; Fischer, F.; Benemann, S.; Rademann, K.; Emmerling, F. The Effect of the Ball to Reactant Ratio on Mechanochemical Reaction Times Studied by in situ PXRD. CrystEngComm 2017, 19, 3902. [Google Scholar] [CrossRef]
- Schmidt, R.; Burmeister, C.F.; Baláž, M.; Kwade, A.; Stolle, A. Effect of Reaction Parameters on the Synthesis of 5-arylidene Barbituric Acid Derivatives in Ball Mills. Org. Process Res. Dev. 2015, 19, 427–436. [Google Scholar] [CrossRef]
- Michalchuk, A.A.L.; Tumanov, I.A.; Boldyreva, E.V. Ball Size or Ball Mass-What Matters in Organic Mechanochemical Synthesis? CrystEngComm 2019, 21, 2174–2179. [Google Scholar] [CrossRef]
- McKissic, K.S.; Caruso, J.T.; Blair, R.G.; Mack, J. Comparison of Shaking vs. Baking: Further Understanding the Energetics of a Mechanochemical Reaction. Green Chem. 2014, 16, 1628–1632. [Google Scholar] [CrossRef]
- Tumanov, I.A.; Achkasov, A.F.; Boldyreva, E.V.; Boldyrev, V.V. Following the Products of Mechanochemical Synthesis Step by Step. CrystEngComm 2011, 13, 2213–2216. [Google Scholar] [CrossRef]
- Urakaev, F.K.; Boldyrev, V.V. Mechanism and Kinetics of Mechanochemical Processes in Comminuting Devices: 1. Theory. Powder Technol. 2000, 107, 93–107. [Google Scholar] [CrossRef]
- Urakaev, F.K.; Boldyrev, V.V. Mechanism and Kinetics of Mechanochemical Processes in Comminuting Devices: 2. Applications of the Theory. Experiment. Powder Technol. 2000, 107, 197–206. [Google Scholar] [CrossRef]
- Fox, P.G. Mechanically Initiated Chemical Reactions in Solids. J. Mater. Sci. 1975, 10, 340–360. [Google Scholar] [CrossRef]
- Karki, S.; Friščić, T.; Jones, W. Control and Interconversion of Cocrystal Stoichiometry in Grinding: Stepwise Mechanism for the Formation of a Hydrogen-bonded Cocrystal. CrystEngComm 2009, 11, 470–481. [Google Scholar] [CrossRef]
- Burley, J.C.; Duer, M.J.; Stein, R.S.; Vrcelj, R.M. Enforcing Ostwald’s Rule of Stages: Isolation of Paracetamol Forms III and II. Eur. J. Pharm. Sci. 2007, 31, 271–276. [Google Scholar] [CrossRef]
- Guerain, M.; Guinet, Y.; Correia, N.T.; Paccou, L.; Danède, F.; Hédoux, A. Polymorphism and Stability of Ibuprofen/Nicotinamide Cocrystal: The Effect of the Crystalline Synthesis Method. Int. J. Pharm. 2020, 584, 119454. [Google Scholar] [CrossRef]
- Berry, D.J.; Seaton, C.C.; Clegg, W.; Harrington, R.W.; Coles, S.J.; Horton, P.N.; Hursthouse, M.B.; Storey, R.; Jones, W.; Friščić, T.; et al. Applying Hot-stage Microscopy to Co-crystal Screening: A Study of Nicotinamide with Seven Active Pharmaceutical Ingredients. Cryst. Growth Des. 2008, 8, 1697–1712. [Google Scholar] [CrossRef]
- Dudek, M.K.; Śniechowska, J.; Wróblewska, A.; Kaźmierski, S.; Potrzebowski, M.J. Cocrystals “Divorce and Marriage”: When a Binary System Meets an Active Multifunctional Synthon in a Ball Mill. Chem. Eur. J. 2020, 26, 13264–13273. [Google Scholar] [CrossRef]
- Shemchuk, O.; Braga, D.; Grepioni, F. Alloying Barbituric and Thiobarbituric Acids: From Solid Solutions to a Highly Stable Keto Co-crystal Form. Chem. Commun. 2016, 52, 11815–11818. [Google Scholar] [CrossRef]
- Almarsson, O.; Zaworotko, M.J. Crystal Engineering of the Composition of Pharmaceutical Phases. Do Pharmaceutical Co-Crystals Represent a New Path to Improved Medicines? Chem. Commun. 2004, 1889–1896. [Google Scholar] [CrossRef]
- Reutzel-Edens, S. Chapter 10—Analytical Techniques and Strategies for Salt/Co-crystal Characterization. In Pharmaceutical Salts and Co-Crystals; The Royal Society of Chemistry: London, UK, 2012; pp. 212–246. ISBN 978-1-84973-158-4. [Google Scholar]
- Aakeröy, C.B.; Salmon, D.J. Building Co-crystals with Molecular Sense and Supramolecular Sensibility. CrystEngComm 2005, 7, 439–448. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J. Acid–base Crystalline Complexes and the pKa Rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Ramon, G.; Davies, K.; Nassimbeni, L.R. Structures of Benzoic Acids with Substituted Pyridines and Quinolines: Salt vs. Co-crystal Formation. CrystEngComm 2014, 16, 5802–5810. [Google Scholar] [CrossRef]
- Shattock, T.R.; Arora, K.K.; Vishweshwar, P.; Zaworotko, M.J. Hierarchy of Supramolecular Synthons: Persistent Carboxylic Acid·Pyridine Hydrogen Bonds in Cocrystals That also Contain a Hydroxyl Moiety. Cryst. Growth Des. 2008, 8, 4533–4545. [Google Scholar] [CrossRef]
- Tothadi, S.; Shaikh, T.R.; Gupta, S.; Dandela, R.; Vinod, C.P.; Nangia, A.K. Can We Identify the Salt–Cocrystal Continuum State Using XPS? Cryst. Growth Des. 2021, 21, 735–747. [Google Scholar] [CrossRef]
- Swapna, B.; Nangia, A. Epalrestat–Cytosine Cocrystal and Salt Structures: Attempt To Control E,Z → Z,Z Isomerization. Cryst. Growth Des. 2017, 17, 3350–3360. [Google Scholar] [CrossRef]
- Steiner, T.; Majerz, I.; Wilson, C.C. First O-H-N Hydrogen Bond with a Centered Proton Obtained by Thermally Induced Proton Migration. Angew. Chem. Int. Ed. 2001, 4, 2651–2654. [Google Scholar] [CrossRef]
- Losev, E.; Boldyreva, E. The Effect of Amino Acid Backbone Length on Molecular Packing: Crystalline Tartrates of Glycine, β-alanine, γ-aminobutyric Acid (GABA) and dl-α-aminobutyric Acid (AABA). Acta Crystallogr. Sect. C Struct. Chem. 2018, 74, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, J.; Wang, L.; Wu, B.; Xu, X.; Deng, Z.; Zhang, H. Pharmaceutical Crystalline Complexes of Sulfamethazine with Saccharin: Same Interaction Site but Different Ionization States. RSC Adv. 2016, 6, 26474–26478. [Google Scholar] [CrossRef]
- Losev, E.A.; Boldyreva, E.V. A Salt or a Co-crystal-when Crystallization Protocol Matters. CrystEngComm 2018, 20, 2299–2305. [Google Scholar] [CrossRef]
- Zhang, C.; Xiong, Y.; Jiao, F.; Wang, M.; Li, H. Redefining the Term of “Cocrystal” and Broadening Its Intention. Cryst. Growth Des. 2019, 19, 1471–1478. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Shemchuk, O. Organic-inorganic Ionic Co-crystals: A New Class of Multipurpose Compounds. CrystEngComm 2018, 20, 2212–2220. [Google Scholar] [CrossRef]
- Generally Recognized as Safe (GRAS). Available online: http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/ (accessed on 12 November 2020).
- Singh Sekhon, B. Drug-drug Co-crystals. DARU J. Pharm. Sci 2012, 20, 45. [Google Scholar] [CrossRef]
- Thipparaboina, R.; Kumar, D.; Chavan, R.B.; Shastri, N.R. Multidrug Co-crystals: Towards the Development of Effective Therapeutic Hybrids. Drug Discov. Today 2016, 21, 481–490. [Google Scholar] [CrossRef]
- Thakuria, R.; Sarma, B. Drug-Drug and Drug-Nutraceutical Cocrystal/Salt as Alternative Medicine for Combination Therapy: A Crystal Engineering Approach. Crystals 2018, 8, 101. [Google Scholar] [CrossRef]
- Kale, D.P.; Zode, S.S.; Bansal, A.K. Challenges in Translational Development of Pharmaceutical Cocrystals. J. Pharm. Sci. 2017, 106, 457–470. [Google Scholar] [CrossRef]
- Almarsson, Ö.; Peterson, M.L.; Zaworotko, M. The A to Z of Pharmaceutical Cocrystals: A Decade of Fast-moving New Science and Patents. Pharm. Pat. Anal. 2012, 1, 313–327. [Google Scholar] [CrossRef]
- Trask, A.V. An Overview of Pharmaceutical Cocrystals as Intellectual Property. Mol. Pharm. 2007, 4, 301–309. [Google Scholar] [CrossRef]
- Desiraju, G.R. The Supramolecular Synthon in Crystal Engineering—A New Organic Synthesis. Angew. Chem. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Abramov, Y.A.; Loschen, C.; Klamt, A. Rational Coformer or Solvent Selection for Pharmaceutical Cocrystallization or Desolvation. J. Pharm. Sci. 2012, 101, 3687–3697. [Google Scholar] [CrossRef]
- Kumar, S.; Nanda, A. Approaches to Design of Pharmaceutical Cocrystals: A Review. Mol. Cryst. Liq. Cryst. 2018, 667, 54–77. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Mehta, B.K.; Singh, S.S.; Chaturvedi, S.; Wahajuddin, M.; Thakur, T.S. Rational Coformer Selection and the Development of New Crystalline Multicomponent Forms of Resveratrol with Enhanced Water Solubility. Cryst. Growth Des. 2018, 18, 1581–1592. [Google Scholar] [CrossRef]
- Buddhadev, S.S.; Garala, K.C. Pharmaceutical Cocrystals—A Review. Proceedings 2020, 62, 14. [Google Scholar] [CrossRef]
- Sarkar, N.; Mitra, J.; Vittengl, M.; Berndt, L.; Aakeröy, C.B. A User-friendly Application for Predicting the Outcome of Co-crystallizations. CrystEngComm 2020, 22, 6776–6779. [Google Scholar] [CrossRef]
- Sarkar, N.; Sinha, A.S.; Aakeröy, C.B. Systematic Investigation of Hydrogen-bond Propensities for Informing Co-crystal Design and Assembly. CrystEngComm 2019, 21, 6048–6055. [Google Scholar] [CrossRef]
- Pindelska, E.; Sokal, A.; Kolodziejski, W. Pharmaceutical Cocrystals, Salts and Polymorphs: Advanced Characterization Techniques. Adv. Drug Deliv. Rev. 2017, 117, 111–146. [Google Scholar] [CrossRef]
- Qiao, Y.; Qiao, R.; He, Y.; Shi, C.; Liu, Y.; Hao, H.; Su, J.; Zhong, J. Instrumental Analytical Techniques for the Characterization of Crystals in Pharmaceutics and Foods. Cryst. Growth Des. 2017, 17, 6138–6148. [Google Scholar] [CrossRef]
- Thipparaboina, R.; Kumar, D.; Mittapalli, S.; Balasubramanian, S.; Nangia, A.; Shastri, N.R. Ionic, Neutral, and Hybrid Acid-Base Crystalline Adducts of Lamotrigine with Improved Pharmaceutical Performance. Cryst. Growth Des. 2015, 15, 5816–5826. [Google Scholar] [CrossRef]
- Barbas, R.; Font-Bardia, M.; Paradkar, A.; Hunter, C.A.; Prohens, R. Combined Virtual/Experimental Multicomponent Solid Forms Screening of Sildenafil: New Salts, Cocrystals, and Hybrid Salt-Cocrystals. Cryst. Growth Des. 2018, 18, 7618–7627. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An Optimization Program Integrating Computer Algebra and Crystallographic Objects Written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- David, W.I.F.; Shankland, K.; Van De Streek, J.; Pidcock, E.; Motherwell, W.D.S.; Cole, J.C. DASH: A Program for Crystal Structure Determination from Powder Diffraction Data. J. Appl. Crystallogr. 2006, 39, 910–915. [Google Scholar] [CrossRef]
- Rycerz, L. Practical Remarks Concerning Phase Diagrams Determination on the Basis of Differential Scanning Calorimetry Measurements. J. Therm. Anal. Calorim. 2013, 113, 231–238. [Google Scholar] [CrossRef]
- Patrick Stahly, G. A Survey of Cocrystals Reported Prior to 2000. Cryst. Growth Des. 2009, 9, 4212–4229. [Google Scholar] [CrossRef]
- Lu, E.; Rodríguez-Hornedo, N.; Suryanarayanan, R. A Rapid Thermal Method for Cocrystal Screening. CrystEngComm 2008, 10, 665–668. [Google Scholar] [CrossRef]
- Malamatari, M.; Ross, S.A.; Douroumis, D.; Velaga, S.P. Experimental Cocrystal Screening and Solution Based Scale-up Cocrystallization Methods. Adv. Drug Deliv. Rev. 2017, 117, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Bezerra Melo Figueirêdo, C.; Nadvorny, D.; Quintas de Medeiros Vieira, A.C.; Lamartine Soares Sobrinho, J.; Rolim Neto, P.J.; Lee, P.I.; Felts de La Roca Soares, M. Enhancement of Dissolution Rate through Eutectic Mixture and Solid Solution of Posaconazole and Benznidazole. Int. J. Pharm. 2017, 525, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.P.A.; Bansal, A.K.; Jain, S. Molecular Understanding and Implication of Structural Integrity in the Deformation Behavior of Binary Drug-Drug Eutectic Systems. Mol. Pharm. 2018, 15, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.L.; Riegelman, S. Pharmaceutical Applications of Solid Dispersion Systems. J. Pharm. Sci. 1971, 60, 1281–1302. [Google Scholar] [CrossRef] [PubMed]
- Skieneh, J.M.; Sathisaran, I.; Dalvi, S.V.; Rohani, S. Co-amorphous Form of Curcumin-folic Acid Dihydrate with Increased Dissolution Rate. Cryst. Growth Des. 2017, 17, 6273–6280. [Google Scholar] [CrossRef]
- Stevens, J.S.; Byard, S.J.; Schroeder, S.L.M. Salt or Co-Crystal? Determination of Protonation State by X-ray Photoelectron Spectroscopy (XPS). J. Pharm. Sci. 2010, 99, 4453–4457. [Google Scholar] [CrossRef]
- Stevens, J.S.; Byard, S.J.; Schroeder, S.L.M. Characterization of Proton Transfer in Co-Crystals by X-ray Photoelectron Spectroscopy (XPS). Cryst. Growth Des. 2010, 10, 1435–1442. [Google Scholar] [CrossRef]
- Bryce, D.L. NMR Crystallography: Structure and Properties of Materials from Solid-state Nuclear Magnetic Resonance Observables. IUCrJ 2017, 4, 350–359. [Google Scholar] [CrossRef]
- Rodrigues, M.; Baptista, B.; Lopes, J.A.; Sarraguça, M.C. Pharmaceutical Cocrystallization Techniques. Advances and Challenges. Int. J. Pharm. 2018, 547, 404–420. [Google Scholar] [CrossRef]
- Daurio, D.; Nagapudi, K.; Li, L.; Quan, P.; Nunez, F.A. Application of Twin Screw Extrusion to the Manufacture of Cocrystals: Scale-up of AMG 517-sorbic Acid Cocrystal Production. Faraday Discuss. 2014, 170, 235–249. [Google Scholar] [CrossRef]
- Kelly, A.L.; Gough, T.; Dhumal, R.S.; Halsey, S.A.; Paradkar, A. Monitoring Ibuprofen-nicotinamide Cocrystal Formation during Solvent Free Continuous Cocrystallization (SFCC) Using near Infrared Spectroscopy as a PAT Tool. Int. J. Pharm. 2012, 426, 15–20. [Google Scholar] [CrossRef]
- Michalchuk, A.A.L.; Hope, K.S.; Kennedy, S.R.; Blanco, M.V.; Boldyreva, E.V.; Pulham, C.R. Ball-free Mechanochemistry: In situ Real-time Monitoring of Pharmaceutical Co-crystal Formation by Resonant Acoustic Mixing. Chem. Commun. 2018, 54, 4033–4036. [Google Scholar] [CrossRef]
- Tanaka, R.; Takahashi, N.; Nakamura, Y.; Hattori, Y.; Ashizawa, K.; Otsuka, M. In-line and Real-time Monitoring of Resonant Acoustic Mixing by Near-infrared Spectroscopy Combined with Chemometric Technology for Process Analytical Technology Applications in Pharmaceutical Powder Blending Systems. Anal. Sci. 2017, 33, 41–46. [Google Scholar] [CrossRef]
- Am Ende, D.J.; Anderson, S.R.; Salan, J.S. Development and Scale-up of Cocrystals Using Resonant Acoustic Mixing. Org. Process Res. Dev. 2014, 18, 331–341. [Google Scholar] [CrossRef]
- Nagapudi, K.; Umanzor, E.Y.; Masui, C. High-throughput Screening and Scale-up of Cocrystals Using Resonant Acoustic Mixing. Int. J. Pharm. 2017, 521, 337–345. [Google Scholar] [CrossRef]
- Fleischman, S.G.; Kuduva, S.S.; McMahon, J.A.; Moulton, B.; Bailey Walsh, R.D.; Rodríguez-Hornedo, N.; Zaworotko, M.J. Crystal Engineering of the Composition of Pharmaceutical Phases: Multiple-Component Crystalline Solids Involving Carbamazepine. Cryst. Growth Des. 2003, 3, 909–919. [Google Scholar] [CrossRef]
- Morrison, H.; Mrozek-Morrison, M.; Toschi, J.; Luu, V.; Tan, H.; Daurio, D. High throughput Bench-top Co-crystal Screening via a Floating Foam Rack/Sonic Bath Method. Org. Process Res. Dev. 2013, 17, 533–539. [Google Scholar] [CrossRef]
- Cheney, M.L.; Weyna, D.R.; Shan, N.; Hanna, M.; Wojtas, L.; Zaworotko, M.J. Coformer Selection in Pharmaceutical Cocrystal Development: A Case Study of a Meloxicam Aspirin Cocrystal that Exhibits Enhanced Solubility and Pharmacokinetics. J. Pharm. Sci. 2011, 100, 2172–2181. [Google Scholar] [CrossRef]
- Friščić, T.; Jones, W. Benefits of Cocrystallisation in Pharmaceutical Materials Science: An Update. J. Pharm. Pharmacol. 2010, 62, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Dalpiaz, A.; Pavan, B.; Ferretti, V. Can Pharmaceutical Co-crystals Provide an Opportunity to Modify the Biological Properties of Drugs? Drug Discov. Today 2017, 22, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Siahi-Shadbad, M.; Adibkia, K.; Barzegar-Jalali, M. Recent Advances in Improving Oral Drug Bioavailability by Cocrystals. BioImpacts 2018, 8, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Friščić, T.; Fabián, L.; Laity, P.R.; Day, G.M.; Jones, W. Improving Mechanical Properties of Crystalline Solids by Cocrystal Formation: New Compressible Forms of Paracetamol. Adv. Mater. 2009, 21, 3905–3909. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, W.; Sun, W.J.; Lu, T.; Tong, H.H.Y.; Sun, C.C.; Zheng, Y. Resveratrol Cocrystals with Enhanced Solubility and Tabletability. Int. J. Pharm. 2016, 509, 391–399. [Google Scholar] [CrossRef]
- Grepioni, F.; Wouters, J.; Braga, D.; Nanna, S.; Fours, B.; Coquerel, G.; Longfils, G.; Rome, S.; Aerts, L.; Quere, L. Ionic Co-crystals of Racetams: Solid-state Properties Enhancement of Neutral Active Pharmaceutical Ingredients via Addition of Mg2+ and Ca2+ Chlorides. CrystEngComm 2014, 16, 5887–5896. [Google Scholar] [CrossRef]
- Etter, M.C.; Choo, C.G.; Reutzel, S.M. Self-Organization of Adenine and Thymine in the Solid State. J. Am. Chem. Soc. 1993, 115, 4411–4412. [Google Scholar] [CrossRef]
- Caira, M.R.; Nassimbeni, L.R.; Wildervanck, A.F. Selective Formation of Hydrogen Bonded Cocrystals between a Sulfonamide and Aromatic Carboxylic Acids in the Solid State. J. Chem. Soc. Perkin Trans. 1995, 2, 2213–2216. [Google Scholar] [CrossRef]
- Friščić, T.; Fábián, L.; Burley, J.C.; Jones, W.; Motherwell, W.D.S. Exploring Cocrystal–cocrystal Reactivity via Liquid-assisted Grinding: The Assembling of Racemic and Dismantling of Enantiomeric Cocrystals. Chem. Commun. 2006, 5009–5011. [Google Scholar] [CrossRef]
- Friščić, T.; Trask, A.V.; Jones, W.; Motherwell, W.D.S. Screening for Inclusion Compounds and Systematic Construction of Three-Component Solids by Liquid-Assisted Grinding. Angew. Chem. 2006, 45, 7546–7550. [Google Scholar] [CrossRef]
- Friščić, T.; Jones, W. Cocrystal Architecture and Properties: Design and Building of Chiral and Racemic Structures by Solid-solid Reactions. Faraday Discuss. 2007, 136, 167–178. [Google Scholar] [CrossRef]
- Myz, S.A.; Shakhtshneider, T.P.; Fucke, K.; Fedotov, A.P.; Boldyreva, E.V.; Boldyrev, V.V.; Kuleshova, N.I. Synthesis of Co-crystals of Meloxicam with Carboxylic Acids by Grinding. Mendeleev Commun. 2009, 19, 272–274. [Google Scholar] [CrossRef]
- Lemmerer, A.; Esterhuysen, C.; Bernstein, J. Synthesis, Characterization, and Molecular Modeling of a Pharmaceutical Co-crystal: (2-chloro-4-nitrobenzoic Acid):(Nicotinamide). J. Pharm. Sci. 2010, 99, 4054–4071. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Alhalaweh, A.; Velaga, S.P. Hansen Solubility Parameter as a Tool to Predict Cocrystal Formation. Int. J. Pharm. 2011, 407, 63–71. [Google Scholar] [CrossRef]
- Rehder, S.; Klukkert, M.; Löbmann, K.A.M.; Strachan, C.J.; Sakmann, A.; Gordon, K.; Rades, T.; Leopold, C.S. Investigation of the Formation Process of Two Piracetam Cocrystals during Grinding. Pharmaceutics 2011, 3, 706–722. [Google Scholar] [CrossRef]
- Fábián, L.; Hamill, N.; Eccles, K.S.; Moynihan, H.A.; Maguire, A.R.; McCausland, L.; Lawrence, S.E. Cocrystals of Fenamic Acids with Nicotinamide. Cryst. Growth Des. 2011, 11, 3522–3528. [Google Scholar] [CrossRef]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.B.R.; Nangia, A. Fast Dissolving Curcumin Cocrystals. Cryst. Growth Des. 2011, 11, 4135–4145. [Google Scholar] [CrossRef]
- Nanjwade, V.K.; Manvi, F.; Shamrez, A.; Nanjwade, B. Characterization of Prulifloxacin-Salicylic Acid Complex by IR, DSC and PXRD. J. Pharm. Biomed. Sci. 2011, 5, 1–6. [Google Scholar]
- Goud, N.R.; Gangavaram, S.; Suresh, K.; Pal, S.; Manjunatha, S.G.; Nambiar, S.; Nangia, A. Novel Furosemide Cocrystals and Selection of High Solubility Drug Forms. J. Pharm. Sci. 2012, 101, 664–680. [Google Scholar] [CrossRef]
- Arenas-García, J.I.; Herrera-Ruiz, D.; Mondragón-Vásquez, K.; Morales-Rojas, H.; Höpfl, H. Modification of the Supramolecular Hydrogen-bonding Patterns of Acetazolamide in the Presence of Different Cocrystal Formers: 3:1, 2:1, 1:1, and 1:2 Cocrystals from Screening with the Structural Isomers of Hydroxybenzoic Acids, Aminobenzoic Acids, Hydroxy. Cryst. Growth Des. 2012, 12, 811–824. [Google Scholar] [CrossRef]
- Padrela, L.; De Azevedo, E.G.; Velaga, S.P. Powder X-ray Diffraction Method for the Quantification of Cocrystals in the Crystallization Mixture. Drug Dev. Ind. Pharm. 2012, 38, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Alhalaweh, A.; George, S.; Basavoju, S.; Childs, S.L.; Rizvi, S.A.A.; Velaga, S.P. Pharmaceutical Cocrystals of Nitrofurantoin: Screening, Characterization and Crystal Structure Analysis. CrystEngComm 2012, 14, 5078–5088. [Google Scholar] [CrossRef]
- Fucke, K.; Myz, S.A.; Shakhtshneider, T.P.; Boldyreva, E.V.; Griesser, U.J. How Good are the Crystallisation Methods for Co-crystals? A Comparative Study of Piroxicam. New J. Chem. 2012, 36, 1969–1977. [Google Scholar] [CrossRef]
- Tumanov, N.A.; Myz, S.A.; Shakhtshneider, T.P.; Boldyreva, E.V. Are Meloxicam Dimers Really the Structure-forming Units in the “Meloxicam-carboxylic Acid” Co-crystals Family? Relation between Crystal Structures and Dissolution Behaviour. CrystEngComm 2012, 14, 305–313. [Google Scholar] [CrossRef]
- Eddleston, M.D.; Arhangelskis, M.; Frišcic, T.; Jones, W. Solid State Grinding as a Tool to Aid Enantiomeric Resolution by Cocrystallisation. Chem. Commun. 2012, 48, 11340–11342. [Google Scholar] [CrossRef]
- Suresh, K.; Goud, N.R.; Nangia, A. Andrographolide: Solving Chemical Instability an Poor Solubility by Means of Cocrystals. Chem. Asian J. 2013, 8, 3032–3041. [Google Scholar] [CrossRef]
- Losev, E.A.; Mikhailenko, M.A.; Achkasov, A.F.; Boldyreva, E.V. The Effect of Carboxylic Acids on Glycine Polymorphism, Salt and Co-crystal Formation. A Comparison of Different Crystallisation Techniques. New J. Chem. 2013, 37, 1973–1981. [Google Scholar] [CrossRef]
- Espinosa-Lara, J.C.; Guzman-Villanueva, D.; Arenas-García, J.I.; Herrera-Ruiz, D.; Rivera-Islas, J.; Román-Bravo, P.; Morales-Rojas, H.; Höpfl, H. Cocrystals of Active Pharmaceutical Ingredients–Praziquantel in Combination with Oxalic, Malonic, Succinic, Maleic, Fumaric, Glutaric, Adipic, and Pimelic Acids. Cryst. Growth Des. 2013, 13, 169–185. [Google Scholar] [CrossRef]
- Losev, E.A.; Boldyreva, E.V. The Role of a Liquid in “Dry” Co-grinding: A Case Study of the Effect of Water on Mechanochemical Synthesis in a “l-serine–oxalic Acid” System. CrystEngComm 2014, 16, 3857. [Google Scholar] [CrossRef]
- Sládková, V.; Cibulková, J.; Eigner, V.; Šturc, A.; Kratochvíl, B.; Rohlíček, J. Application and Comparison of Cocrystallization Techniques on Trospium Chloride Cocrystals. Cryst. Growth Des. 2014, 14, 2931–2936. [Google Scholar] [CrossRef]
- Song, J.X.; Yan, Y.; Yao, J.; Chen, J.M.; Lu, T.B. Improving the Solubility of Lenalidomide via Cocrystals. Cryst. Growth Des. 2014, 14, 3069–3077. [Google Scholar] [CrossRef]
- Sugandha, K.; Kaity, S.; Mukherjee, S.; Isaac, J.; Ghosh, A. Solubility Enhancement of Ezetimibe by a Cocrystal Engineering Technique. Cryst. Growth Des. 2014, 14, 4475–4486. [Google Scholar] [CrossRef]
- Michalchuk, A.A.L.; Tumanov, I.A.; Drebushchak, V.A.; Boldyreva, E.V. Advances in Elucidating Mechanochemical Complexities via Implementation of a Simple Organic System. Faraday Discuss. 2014, 170, 311–335. [Google Scholar] [CrossRef]
- Tilborg, A.; Springuel, G.; Norberg, B.; Wouters, J.; Leyssens, T. How Cocrystallization Affects Solid-state Tautomerism: Stanozolol Case Study. Cryst. Growth Des. 2014, 14, 3408–3422. [Google Scholar] [CrossRef]
- Abourahma, H.; Shah, D.D.; Melendez, J.; Johnson, E.J.; Holman, K.T. A Tale of Two Stoichiometrically Diverse Cocrystals. Cryst. Growth Des. 2015, 15, 3101–3104. [Google Scholar] [CrossRef]
- Fischer, F.; Scholz, G.; Batzdorf, L.; Wilke, M.; Emmerling, F. Synthesis, Structure Determination, and Formation of a Theobromine:oxalic Acid 2:1 Cocrystal. CrystEngComm 2015, 17, 824–829. [Google Scholar] [CrossRef]
- Fernandes, J.A.; Sardo, M.; Mafra, L.; Choquesillo-Lazarte, D.; Masciocchi, N. X-ray and NMR Crystallography Studies of Novel Theophylline Cocrystals Prepared by Liquid Assisted Grinding. Cryst. Growth Des. 2015, 15, 3674–3683. [Google Scholar] [CrossRef]
- Odiase, I.; Nicholson, C.E.; Ahmad, R.; Cooper, J.; Yufit, D.S.; Cooper, S.J. Three Cocrystals and a Cocrystal Salt of Pyrimidin-2-amine and Glutaric Acid. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 276–283. [Google Scholar] [CrossRef]
- Batzdorf, L.; Fischer, F.; Wilke, M.; Wenzel, K.J.; Emmerling, F. Direct in situ Investigation of Milling Reactions Using Combined X-ray Diffraction and Raman Spectroscopy. Angew. Chem. 2015, 54, 1799–1802. [Google Scholar] [CrossRef]
- Fischer, F.; Joester, M.; Rademann, K.; Emmerling, F. Survival of the Fittest: Competitive Co-crystal Reactions in the Ball Mill. Chem. Eur. J. 2015, 21, 14969–14974. [Google Scholar] [CrossRef]
- Stepanovs, D.; Jure, M.; Kuleshova, L.N.; Hofmann, D.W.M.; Mishnev, A. Cocrystals of Pentoxifylline: In Silico and Experimental Screening. Cryst. Growth Des. 2015, 15, 3652–3660. [Google Scholar] [CrossRef]
- Li, A.Y.; Xu, L.L.; Chen, J.M.; Lu, T.B. Solubility and Dissolution Rate Enhancement of Triamterene by a Cocrystallization Method. Cryst. Growth Des. 2015, 15, 3785–3791. [Google Scholar] [CrossRef]
- Saikia, B.; Bora, P.; Khatioda, R.; Sarma, B. Hydrogen Bond Synthons in the Interplay of Solubility and Membrane Permeability/Diffusion in Variable Stoichiometry Drug Cocrystals. Cryst. Growth Des. 2015, 15, 5593–5603. [Google Scholar] [CrossRef]
- Jung, S.; Choi, I.; Kim, I.W. Liquid-assisted Grinding to Prepare a Cocrystal of Adefovir Dipivoxil Thermodynamically Less Stable Than Its Neat Phase. Crystals 2015, 5, 583–591. [Google Scholar] [CrossRef]
- Mannava, M.K.C.; Suresh, K.; Nangia, A. Enhanced Bioavailability in the Oxalate Salt of the Anti-Tuberculosis Drug Ethionamide. Cryst. Growth Des. 2016, 16, 1591–1598. [Google Scholar] [CrossRef]
- Lin, H.L.; Huang, Y.T.; Lin, S.Y. Spectroscopic and Thermal Approaches to Investigate the Formation Mechanism of Piroxicam-saccharin Co-crystal Induced by Liquid-assisted Grinding or Thermal Stress. J. Therm. Anal. Calorim. 2016, 123, 2345–2356. [Google Scholar] [CrossRef]
- Fischer, F.; Heidrich, A.; Greiser, S.; Benemann, S.; Rademann, K.; Emmerling, F. Polymorphism of Mechanochemically Synthesized Cocrystals: A Case Study. Cryst. Growth Des. 2016, 16, 1701–1707. [Google Scholar] [CrossRef]
- Chadha, R.; Rani, D.; Goyal, P. Novel Cocrystals of Gliclazide: Characterization and Evaluation. CrystEngComm 2016, 18, 2275–2283. [Google Scholar] [CrossRef]
- Fischer, F.; Wenzel, K.J.; Rademann, K.; Emmerling, F. Quantitative Determination of Activation Energies in Mechanochemical Reactions. Phys. Chem. Chem. Phys. 2016, 18, 23320–23325. [Google Scholar] [CrossRef]
- Sopyan, I.; Fudholi, A.; Muchtaridi, M.; Puspitasari, I. A Novel of Cocrystalization to Improve Solubility and Dissolution Rate of Simvastatin. Int. J. PharmTech Res. 2016, 9, 483–491. [Google Scholar]
- Fischer, F.; Schmidt, M.U.; Greiser, S.; Emmerling, F. The Challenging Case of the Theophylline-benzamide Cocrystal. Acta Crystallogr. Sect. C Struct. Chem. 2016, 72, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Tantardini, C.; Arkhipov, S.G.; Cherkashina, K.A.; Kil’Met’Ev, A.S.; Boldyreva, E.V. Crystal Structure of a 2:1 Co-crystal of Meloxicam with Acetylendicarboxylic Acid. Acta Crystallogr. Sect. E Crystallogr. Commun. 2016, 72, 1856–1859. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.; Lubjuhn, D.; Greiser, S.; Rademann, K.; Emmerling, F. Supply and Demand in the Ball Mill: Competitive Cocrystal Reactions. Cryst. Growth Des. 2016, 16, 5843–5851. [Google Scholar] [CrossRef]
- Kulla, H.; Greiser, S.; Benemann, S.; Rademann, K.; Emmerling, F. In situ Investigation of a Self-accelerated Cocrystal Formation by Grinding Pyrazinamide with Oxalic Acid. Molecules 2016, 21, 917. [Google Scholar] [CrossRef]
- Dai, X.L.; Li, S.; Chen, J.M.; Lu, T.B. Improving the Membrane Permeability of 5-Fluorouracil via Cocrystallization. Cryst. Growth Des. 2016, 16, 4430–4438. [Google Scholar] [CrossRef]
- Du, S.; Wang, Y.; Wu, S.; Yu, B.; Shi, P.; Bian, L.; Zhang, D.; Hou, J.; Wang, J.; Gong, J. Two Novel Cocrystals of Lamotrigine with Isomeric Bipyridines and in situ Monitoring of the Cocrystallization. Eur. J. Pharm. Sci. 2017, 110, 19–25. [Google Scholar] [CrossRef]
- Cho, M.Y.; Kim, P.; Kim, G.Y.; Lee, J.Y.; Song, K.H.; Lee, M.J.; Yoon, W.; Yun, H.; Choi, G.J. Preparation and Characterization of Aripiprazole Cocrystals with Coformers of Multihydroxybenzene Compounds. Cryst. Growth Des. 2017, 17, 6641–6652. [Google Scholar] [CrossRef]
- Gopi, S.P.; Banik, M.; Desiraju, G.R. New Cocrystals of Hydrochlorothiazide: Optimizing Solubility and Membrane Diffusivity. Cryst. Growth Des. 2017, 17, 308–316. [Google Scholar] [CrossRef]
- Chadha, R.; Rani, D.; Goyal, P. Supramolecular Cocrystals of Gliclazide: Synthesis, Characterization and Evaluation. Pharm. Res. 2017, 34, 552–563. [Google Scholar] [CrossRef]
- Cugovčan, M.; Jablan, J.; Lovrić, J.; Cinčić, D.; Galić, N.; Jug, M. Biopharmaceutical Characterization of Praziquantel Cocrystals and Cyclodextrin Complexes Prepared by Grinding. J. Pharm. Biomed. Anal. 2017, 137, 42–53. [Google Scholar] [CrossRef]
- Michalchuk, A.A.L.; Tumanov, I.A.; Konar, S.; Kimber, S.A.J.; Pulham, C.R.; Boldyreva, E.V. Challenges of Mechanochemistry: Is in situ Real-Time Quantitative Phase Analysis Always Reliable? A Case Study of Organic Salt Formation. Adv. Sci. 2017, 4, 1700132. [Google Scholar] [CrossRef]
- Zeng, Q.Z.; Ouyang, J.; Zhang, S.; Zhang, L. Structural Characterization and Dissolution Profile of Mycophenolic Acid Cocrystals. Eur. J. Pharm. Sci. 2017, 102, 140–146. [Google Scholar] [CrossRef]
- Samie, A.; Desiraju, G.R.; Banik, M. Salts and Cocrystals of the Antidiabetic Drugs Gliclazide Tolbutamide and Glipizide: Solubility Enhancements through Drug-coformer Interactions. Cryst. Growth Des. 2017, 17, 2406–2417. [Google Scholar] [CrossRef]
- Kulla, H.; Greiser, S.; Benemann, S.; Rademann, K.; Emmerling, F. Knowing When to Stop–Trapping Metastable Polymorphs in Mechanochemical Reactions. Cryst. Growth Des. 2017, 17, 1190–1196. [Google Scholar] [CrossRef]
- Drozd, K.V.; Manin, A.N.; Churakov, A.V.; Perlovich, G.L. Novel Drug-drug Cocrystals of Carbamazepine with: Paraminosalicylic Acid: Screening, Crystal Structures and Comparative Study of Carbamazepine Cocrystal Formation Thermodynamics. CrystEngComm 2017, 19, 4273–4286. [Google Scholar] [CrossRef]
- Kulla, H.; Wilke, M.; Fischer, F.; Röllig, M.; Maierhofer, C.; Emmerling, F. Warming up for Mechanosynthesis–Temperature Development in Ball Mills during Synthesis. Chem. Commun. 2017, 53, 1664–1667. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.; Fendel, N.; Greiser, S.; Rademann, K.; Emmerling, F. Impact Is Important–Systematic Investigation of the Influence of Milling Balls in Mechanochemical Reactions. Org. Process Res. Dev. 2017, 21, 655–659. [Google Scholar] [CrossRef]
- Plata-Vargas, E.; De la Cruz-Hernández, C.; Dorazco-González, A.; Fuentes-Noriega, I.; Morales-Morales, D.; Germán-Acacio, J.M. Synthesis of Metforminium Succinate by Melting. Crystal Structure, Thermal, Spectroscopic and Dissolution Properties. J. Mex. Chem. Soc. 2017, 61, 197–204. [Google Scholar] [CrossRef]
- Aljohani, M.; Pallipurath, A.R.; McArdle, P.; Erxleben, A. A Comprehensive Cocrystal Screening Study of Chlorothiazide. Cryst. Growth Des. 2017, 17, 5223–5232. [Google Scholar] [CrossRef]
- Nisar, M.; Sung, H.H.Y.; Puschmann, H.; Lakerveld, R.; Haynes, R.K.; Williams, I.D. 11-Azaartemisinin Cocrystals with Preserved Lactam: Acid Heterosynthons. CrystEngComm 2018, 20, 1205–1219. [Google Scholar] [CrossRef]
- Peach, A.A.; Hirsh, D.A.; Holmes, S.T.; Schurko, R.W. Mechanochemical Syntheses and 35Cl Solid-state NMR Characterization of Fluoxetine HCl Cocrystals. CrystEngComm 2018, 20, 2780–2792. [Google Scholar] [CrossRef]
- Nisar, M.; Wong, L.W.Y.; Sung, H.H.Y.; Haynes, R.K.; Williams, I.D. Cocrystals of the Antimalarial Drug 11-azaartemisinin with Three Alkenoic Acids of 1:1 or 2:1 Stoichiometry. Acta Crystallogr. Sect. C Struct. Chem. 2018, 74, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Hussain, E.; Kumar, R.; Choudhary, M.I.; Yousuf, S. Crystal Engineering of Naturally Occurring Seselin to Obtain Cocrystal with Enhanced Anti-Leishmanial Activity, Hirshfeld Surface Analysis, and Computational Insight. Cryst. Growth Des. 2018, 18, 4628–4636. [Google Scholar] [CrossRef]
- Tumanova, N.; Tumanov, N.; Fischer, F.; Morelle, F.; Ban, V.; Robeyns, K.; Filinchuk, Y.; Wouters, J.; Emmerling, F.; Leyssens, T. Exploring Polymorphism and Stoichiometric Diversity in Naproxen/Proline Cocrystals. CrystEngComm 2018, 20, 7308–7321. [Google Scholar] [CrossRef]
- Do Amaral, L.H.; do Carmo, F.A.; Amaro, M.I.; de Sousa, V.P.; da Silva, L.C.R.P.; de Almeida, G.S.; Rodrigues, C.R.; Healy, A.M.; Cabral, L.M. Development and Characterization of Dapsone Cocrystal Prepared by Scalable Production Methods. AAPS PharmSciTech 2018, 19, 2687–2699. [Google Scholar] [CrossRef]
- Darwish, S.; Zeglinski, J.; Krishna, G.R.; Shaikh, R.; Khraisheh, M.; Walker, G.M.; Croker, D.M. A New 1:1 Drug-Drug Cocrystal of Theophylline and Aspirin: Discovery, Characterization, and Construction of Ternary Phase Diagrams. Cryst. Growth Des. 2018, 18, 7526–7532. [Google Scholar] [CrossRef]
- Batool, F.; Ahmad, M.; Minhas, M.U.; Khalid, Q.; Idrees, H.A. Fabrication of Glipizide-glycolic Acid Cocrystals for Solubility Enhancement and Their in vitro Evaluation. Lat. Am. J. Pharm. 2018, 37, 1828–1836. [Google Scholar]
- Belenguer, A.M.; Lampronti, G.I.; De Mitri, N.; Driver, M.; Hunter, C.A.; Sanders, J.K.M. Understanding the Influence of Surface Solvation and Structure on Polymorph Stability: A Combined Mechanochemical and Theoretical Approach. J. Am. Chem. Soc. 2018, 140, 17051–17059. [Google Scholar] [CrossRef]
- Nugrahani, I.; Utami, D.; Permana, B.; Ibrahim, S. Development of the NSAID-L-proline Amino Acid Zwitterionic Cocrystals. J. Appl. Pharm. Sci. 2018, 8, 57–63. [Google Scholar] [CrossRef]
- Gunnam, A.; Suresh, K.; Nangia, A. Salts and Salt Cocrystals of the Antibacterial Drug Pefloxacin. Cryst. Growth Des. 2018, 18, 2824. [Google Scholar] [CrossRef]
- Takata, N.; Tanida, S.; Nakae, S.; Shiraki, K.; Tozuka, Y.; Ishigai, M. Tofogliflozin Salt Cocrystals with Sodium Acetate and Potassium Acetate. Chem. Pharm. Bull. 2018, 66, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Tumanova, N.; Tumanov, N.; Robeyns, K.; Fischer, F.; Fusaro, L.; Morelle, F.; Ban, V.; Hautier, G.; Filinchuk, Y.; Wouters, J.; et al. Opening Pandora’s Box: Chirality, Polymorphism, and Stoichiometric Diversity in Flurbiprofen/Proline Cocrystals. Cryst. Growth Des. 2018, 18, 954–961. [Google Scholar] [CrossRef]
- Emami, S.; Adibkia, K.; Barzegar-Jalali, M.; Siahi-Shadbad, M. Piroxicam Cocrystals with Phenolic Coformers: Preparation, Characterization, and Dissolution Properties. Pharm. Dev. Technol. 2019, 24, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Kuang, W.J.; Ji, S.C.; Xu, S.M.; Lan, P.; Liao, A.P.; Zhou, J.Y.; Zhang, J.Y. Thermodynamic and Crystallization of Lamotrigne Cocrystal. Cryst. Growth Des. 2019, 19, 6603–6610. [Google Scholar] [CrossRef]
- Panzade, P.; Rathi, P.; Somani, P. Nevirapine Pharmaceutical Cocrystal: Design, Development and Formulation. Drug Deliv. Lett. 2019, 9, 240–247. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, S.; Zhou, J.; Chen, J.; Tian, L.; Gao, W.; Zhang, Y.; Ma, A.; Li, L.; Zhou, Z. Luteolin Cocrystals: Characterization, Evaluation of Solubility, Oral Bioavailability and Theoretical Calculation. J. Drug Deliv. Sci. Technol. 2019, 50, 248–254. [Google Scholar] [CrossRef]
- Fernandes, R.P.; do Nascimento, A.L.C.S.; Carvalho, A.C.S.; Teixeira, J.A.; Ionashiro, M.; Caires, F.J. Mechanochemical Synthesis, Characterization, and Thermal Behavior of Meloxicam Cocrystals with Salicylic Acid, Fumaric Acid, and Malic Acid. J. Therm. Anal. Calorim. 2019, 138, 765–777. [Google Scholar] [CrossRef]
- Li, X.; Yu, G.; Chen, X.; He, L.; Zhou, Z.; Ren, Z. Investigating the Solubilization Effect of Oxcarbazepine by Forming Cocrystals. CrystEngComm 2019, 21, 4718–4729. [Google Scholar] [CrossRef]
- Linberg, K.; Ali, N.Z.; Etter, M.; Michalchuk, A.A.L.; Rademann, K.; Emmerling, F. A Comparative Study of the Ionic Cocrystals NaX (α-d-Glucose)2 (X = Cl, Br, I). Cryst. Growth Des. 2019, 19, 4293–4299. [Google Scholar] [CrossRef]
- Roca-Paixão, L.; Correia, N.T.; Affouard, F. Affinity Prediction Computations and Mechanosynthesis of Carbamazepine Based Cocrystals. CrystEngComm 2019, 21, 6991–7001. [Google Scholar] [CrossRef]
- Mazur, L.; Materek, I.; Bond, A.D.; Jones, W. Multicomponent Crystal Forms of a Biologically Active Hydrazone with Some Dicarboxylic Acids: Salts or Cocrystals? Cryst. Growth Des. 2019, 19, 2663–2678. [Google Scholar] [CrossRef]
- Myz, S.A.; Mikhailovskaya, A.V.; Mikhailenko, M.A.; Bulina, N.V.; Kuznetsova, S.A.; Shakhtshneider, T.P. New Crystalline Betulin-based Materials: Improving Betulin Solubility via Cocrystal Formation. Mater. Today Proc. 2019, 12, 82–85. [Google Scholar] [CrossRef]
- Surov, A.O.; Vasilev, N.A.; Churakov, A.V.; Stroh, J.; Emmerling, F.; Perlovich, G.L. Solid Forms of Ciprofloxacin Salicylate: Polymorphism, Formation Pathways, and Thermodynamic Stability. Cryst. Growth Des. 2019, 19, 2979–2990. [Google Scholar] [CrossRef]
- Kulla, H.; Michalchuk, A.A.L.; Emmerling, F. Manipulating the Dynamics of Mechanochemical Ternary Cocrystal Formation. Chem. Commun. 2019, 55, 9793–9796. [Google Scholar] [CrossRef]
- Kumari, N.; Bhattacharya, B.; Roy, P.; Michalchuk, A.A.L.; Emmerling, F.; Ghosh, A. Enhancing the Pharmaceutical Properties of Pirfenidone by Mechanochemical Cocrystallization. Cryst. Growth Des. 2019, 19, 6482–6492. [Google Scholar] [CrossRef]
- Batool, F.; Ahmad, M.; Usman Minhas, M.; Khan, F.M.; Idrees, H.A.; Khalid, Q. Use of Glutaric Acid to Improve the Solubility and Dissolution Profile of Glipizide through Pharmaceutical Cocrystallization. Acta Pol. Pharm. Drug Res. 2019, 76, 103–114. [Google Scholar] [CrossRef]
- Holanda, B.B.C.; Alarcon, R.T.; Gaglieri, C.; de Souza, A.R.; Castro, R.A.E.; Rosa, P.C.P.; Tangerino, D.J.A.; Bannach, G. Thermal Studies, Degradation Kinetic, Equilibrium Solubility, DFT, MIR, and XRPD Analyses of a New Cocrystal of Gemfibrozil and Isonicotinamide. J. Therm. Anal. Calorim. 2019, 136, 2049–2062. [Google Scholar] [CrossRef]
- Martins, I.C.B.; Al-Sabbagh, D.; Meyer, K.; Maiwald, M.; Scholz, G.; Emmerling, F. Insight into the Structure and Properties of Novel Imidazole-based Salts of Salicylic Acid. Molecules 2019, 24, 4144. [Google Scholar] [CrossRef]
- Ouiyangkul, P.; Tantishaiyakul, V.; Hirun, N. Exploring Potential Coformers for Oxyresveratrol Using Principal Component Analysis. Int. J. Pharm. 2020, 587, 119630. [Google Scholar] [CrossRef]
- Cruz, R.M.; Boleslavská, T.; Beránek, J.; Tieger, E.; Twamley, B.; Santos-Martinez, M.J.; Dammer, O.; Tajber, L. Identification and Pharmaceutical Characterization of a New Itraconazole Terephthalic Acid Cocrystal. Pharmaceutics 2020, 12, 741. [Google Scholar] [CrossRef]
- De Almeida, A.C.; Torquetti, C.; Ferreira, P.O.; Fernandes, R.P.; dos Santos, E.C.; Kogawa, A.C.; Caires, F.J. Cocrystals of Ciprofloxacin with Nicotinic and Isonicotinic Acids: Mechanochemical Synthesis, Characterization, Thermal and Solubility Study. Thermochim. Acta 2020, 685, 178346. [Google Scholar] [CrossRef]
- Mikhailovskaya, A.V.; Myz, S.A.; Bulina, N.V.; Gerasimov, K.B.; Kuznetsova, S.A.; Shakhtshneider, T.P. Screening and Characterization of Cocrystal Formation between Betulin and Terephthalic Acid. Mater. Today Proc. 2020, 25, 381–383. [Google Scholar] [CrossRef]
- Shemchuk, O.; D’Agostino, S.; Fiore, C.; Sambri, V.; Zannoli, S.; Grepioni, F.; Braga, D. Natural Antimicrobials Meet a Synthetic Antibiotic: Carvacrol/Thymol and Ciprofloxacin Cocrystals as a Promising Solid-State Route to Activity Enhancement. Cryst. Growth Des. 2020, 20, 6796–6803. [Google Scholar] [CrossRef]
- Lv, W.-T.; Liu, X.-X.; Dai, X.-L.; Long, X.; Chen, J.-M. A 5-fluorouracil-kaempferol drug-drug Cocrystal: Ternary Phase Diagram, Characterization and Property Evaluation. CrystEngComm 2020, 22, 8127–8135. [Google Scholar] [CrossRef]
- Abidi, S.S.A.; Garg, U.; Azim, Y.; Alam, M.; Gupta, A.K.; Pradeep, C.P.; Azum, N.; Asiri, A.M. Spectroscopic, Structural, DFT and Molecular Docking Studies on Novel Cocrystal Salt Hydrate of Chromotropic Acid and Its Antibiofilm Activity. Arab. J. Sci. Eng. 2020, 46, 353–364. [Google Scholar] [CrossRef]
- Wróblewska, A.; Śniechowska, J.; Kaźmierski, S.; Wielgus, E.; Bujacz, G.D.; Mlostoń, G.; Chworos, A.; Suwara, J.; Potrzebowski, M.J. Application of 1-hydroxy-4,5-dimethyl-imidazole 3-oxide as Coformer in Formation of Pharmaceutical Cocrystals. Pharmaceutics 2020, 12, 359. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Shete, S.; Hol, V.; Bachhav, R. Novel Pharmaceutical Cocrystal of Telmisartan and Hydrochlorothiazide. Asian J. Pharm. Clin. Res. 2020, 13, 104–112. [Google Scholar] [CrossRef]
- Aljohani, M.; McArdle, P.; Erxleben, A. Influence of Excipients on Cocrystal Stability and Formation. Cryst. Growth Des. 2020, 20, 4523–4532. [Google Scholar] [CrossRef]
- Nikam, V.J.; Patil, S.B. Pharmceutical Cocrystals of Nebivolol Hydrochloride with Enhanced Solubility. J. Cryst. Growth 2020, 534, 125488. [Google Scholar] [CrossRef]
- Roselló, Y.; Benito, M.; Bagués, N.; Martínez, N.; Moradell, A.; Mata, I.; Galcerà, J.; Barceló-Oliver, M.; Frontera, A.; Molins, E. 9-Ethyladenine: Mechanochemical Synthesis, Characterization, and DFT Calculations of Novel Cocrystals and Salts. Cryst. Growth Des. 2020, 20, 2985–2997. [Google Scholar] [CrossRef]
- Guerain, M.; Derollez, P.; Roca-Paixão, L.; Dejoie, C.; Correia, N.T.; Affouard, F. Structure Determination of a New Cocrystal of Carbamazepine and DL-tartaric Acid by Synchrotron Powder X-ray Diffraction. Acta Crystallogr. Sect. C Struct. Chem. 2020, 76, 225–230. [Google Scholar] [CrossRef]
- Campillo-Alvarado, G.; Keene, E.A.; Swenson, D.C.; MacGillivray, L.R. Repurposing of the Anti-HIV Drug Emtricitabine as a Hydrogen-bonded Cleft for Bipyridines via Cocrystallization. CrystEngComm 2020, 22, 3563–3566. [Google Scholar] [CrossRef]
- Fandiño, O.E.; Reviglio, L.; Linck, Y.G.; Monti, G.A.; Marcos Valdez, M.M.; Faudone, S.N.; Caira, M.R.; Sperandeo, N.R. Novel Cocrystals and Eutectics of the Antiprotozoal Tinidazole: Mechanochemical Synthesis, Cocrystallization, and Characterization. Cryst. Growth Des. 2020, 20, 2930–2942. [Google Scholar] [CrossRef]
- De Almeida, A.C.; Ferreira, P.O.; Torquetti, C.; Ekawa, B.; Carvalho, A.C.S.; dos Santos, E.C.; Caires, F.J. Mechanochemical Synthesis, Characterization and Thermal Study of New Cocrystals of Ciprofloxacin with Pyrazinoic Acid and p-aminobenzoic Acid. J. Therm. Anal. Calorim. 2020, 140, 2293–2303. [Google Scholar] [CrossRef]
- Panzade, P.; Shendarkar, G. Design and Preparation of Zaltoprofen-nicotinamide Pharmaceutical Cocrystals via Liquid Assisted Grinding Method. Indian J. Pharm. Educ. Res. 2019, 53, S563–S570. [Google Scholar] [CrossRef]
- Seera, R.; Guru Row, T.N. Evaluation of Cocrystallization Outcomes of Multicomponent Adducts: Rapid Fabrication to Achieve Uniform Particle Size Distribution Using Thermal Inkjet Printing. Cryst. Growth Des. 2020, 20, 4667–4677. [Google Scholar] [CrossRef]
- Topić, F.; Friščić, T. No Regioselectivity for the Steroid α-face in Cocrystallization of Exemestane with Aromatic Cocrystal Formers Based on Phenanthrene and Pyrene. Can. J. Chem. 2020, 98, 386–393. [Google Scholar] [CrossRef]
- Yuan, Z.J.; Dai, X.L.; Huang, Y.L.; Lu, T.B.; Chen, J.M. Cocrystals of Penciclovir with Hydroxybenzoic Acids: Synthesis, Crystal Structures, and Physicochemical Evaluation. Cryst. Growth Des. 2020, 20, 4108–4119. [Google Scholar] [CrossRef]
- Singh, M.; Bhowal, R.; Vishwakarma, R.; Chopra, D. Assessing the Impact on Aqueous Solubility of Berberine Chloride via Co-crystallization with Different Stoichiometric Ratios of Pyromellitic Dianhydride. J. Mol. Struct. 2020, 1200, 127086. [Google Scholar] [CrossRef]
- Dai, X.L.; Yao, J.; Wu, C.; Deng, J.H.; Mo, Y.H.; Lu, T.B.; Chen, J.M. Solubility and Permeability Improvement of Allopurinol by Cocrystallization. Cryst. Growth Des. 2020, 20, 5160–5168. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Das, S.; Lal, G.; Soni, S.R.; Ghosh, A.; Reddy, C.M.; Ghosh, S. Screening, Crystal Structures and Solubility Studies of a Series of Multidrug Salt Hydrates and Cocrystals of Fenamic Acids with Trimethoprim and Sulfamethazine. J. Mol. Struct. 2020, 1199, 127028. [Google Scholar] [CrossRef]
- Surov, A.O.; Vasilev, N.A.; Voronin, A.P.; Churakov, A.V.; Emmerling, F.; Perlovich, G.L. Ciprofloxacin Salts with Benzoic Acid Derivatives: Structural Aspects, Solid-state Properties and Solubility Performance. CrystEngComm 2020, 22, 4238–4249. [Google Scholar] [CrossRef]
- Jia, J.-L.; Dai, X.-L.; Che, H.-J.; Li, M.-T.; Zhuang, X.-M.; Lu, T.-B.; Chen, J.-M. Cocrystals of Regorafenib with Dicarboxylic Acids: Synthesis, Characterization and Property Evaluation. CrystEngComm 2021, 23, 653–662. [Google Scholar] [CrossRef]
- Gołdyn, M.R.; Larowska, D.; Bartoszak-Adamska, E. Novel Purine Alkaloid Cocrystals with Trimesic and Hemimellitic Acids as Coformers: Synthetic Approach and Supramolecular Analysis. Cryst. Growth Des. 2021, 21, 396–413. [Google Scholar] [CrossRef]
- Wang, J.; Dai, X.-L.; Lu, T.-B.; Chen, J.-M. Temozolomide–Hesperetin Drug–Drug Cocrystal with Optimized Performance in Stability, Dissolution, and Tabletability. Cryst. Growth Des. 2021, 21, 838–846. [Google Scholar] [CrossRef]

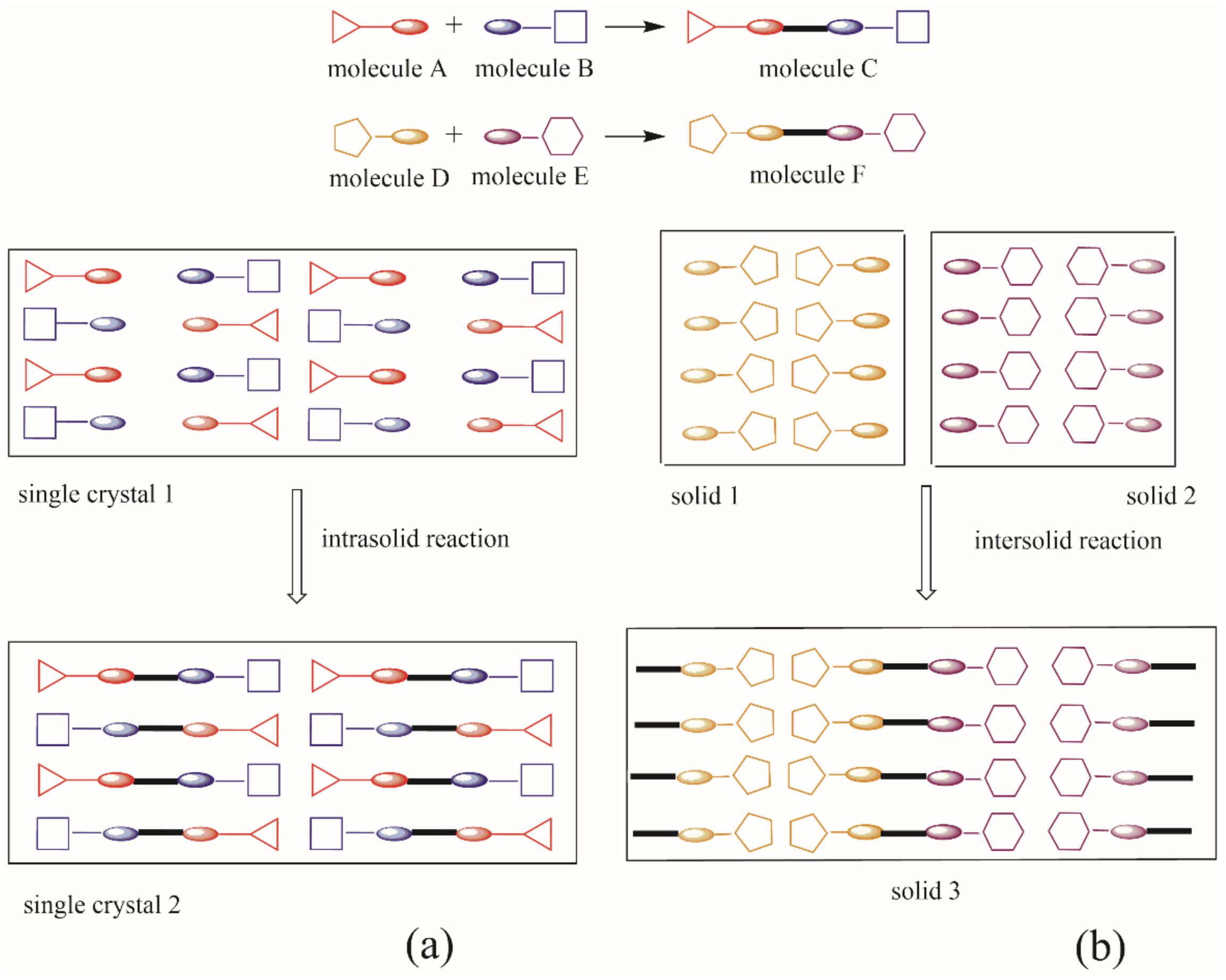

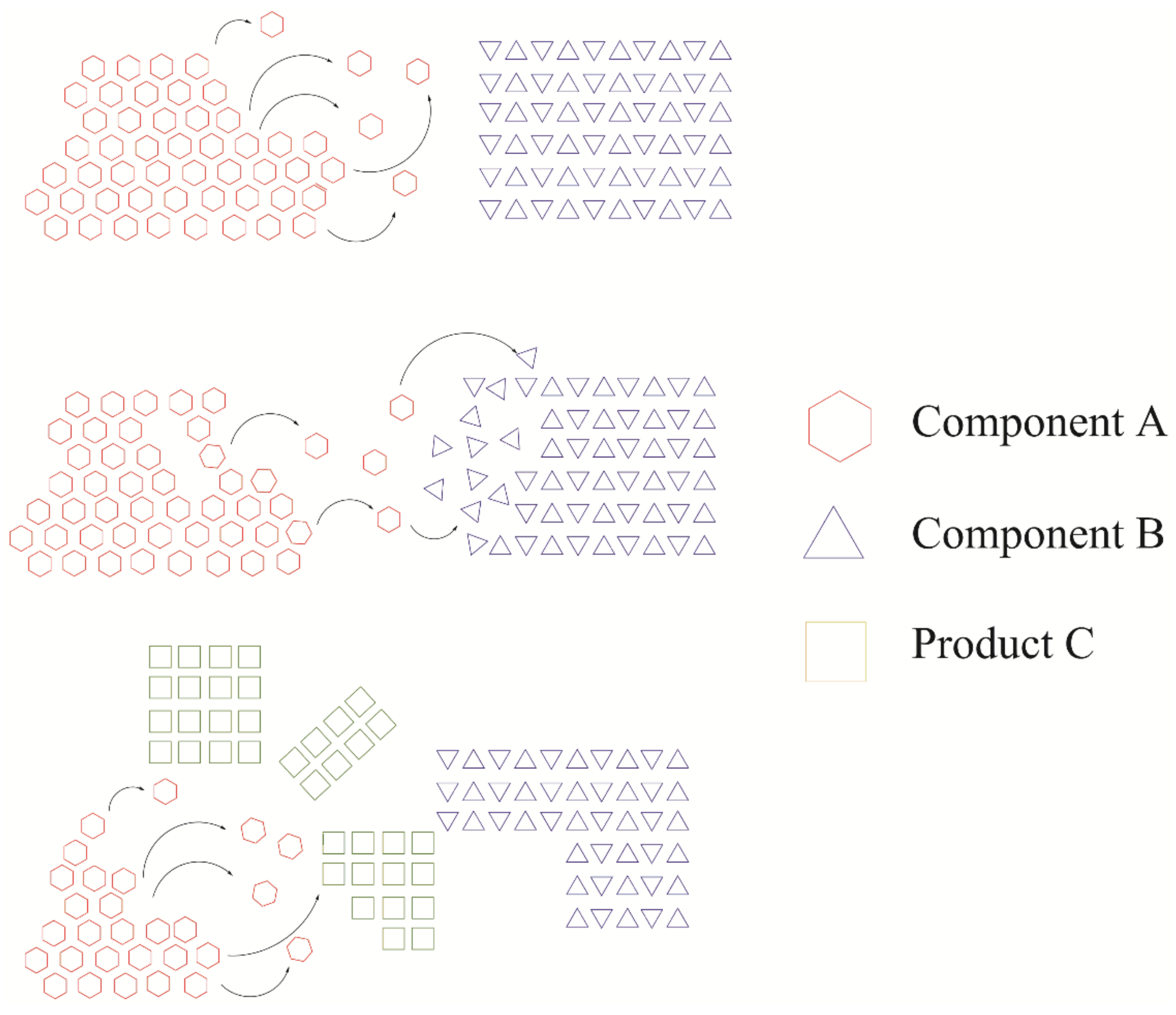
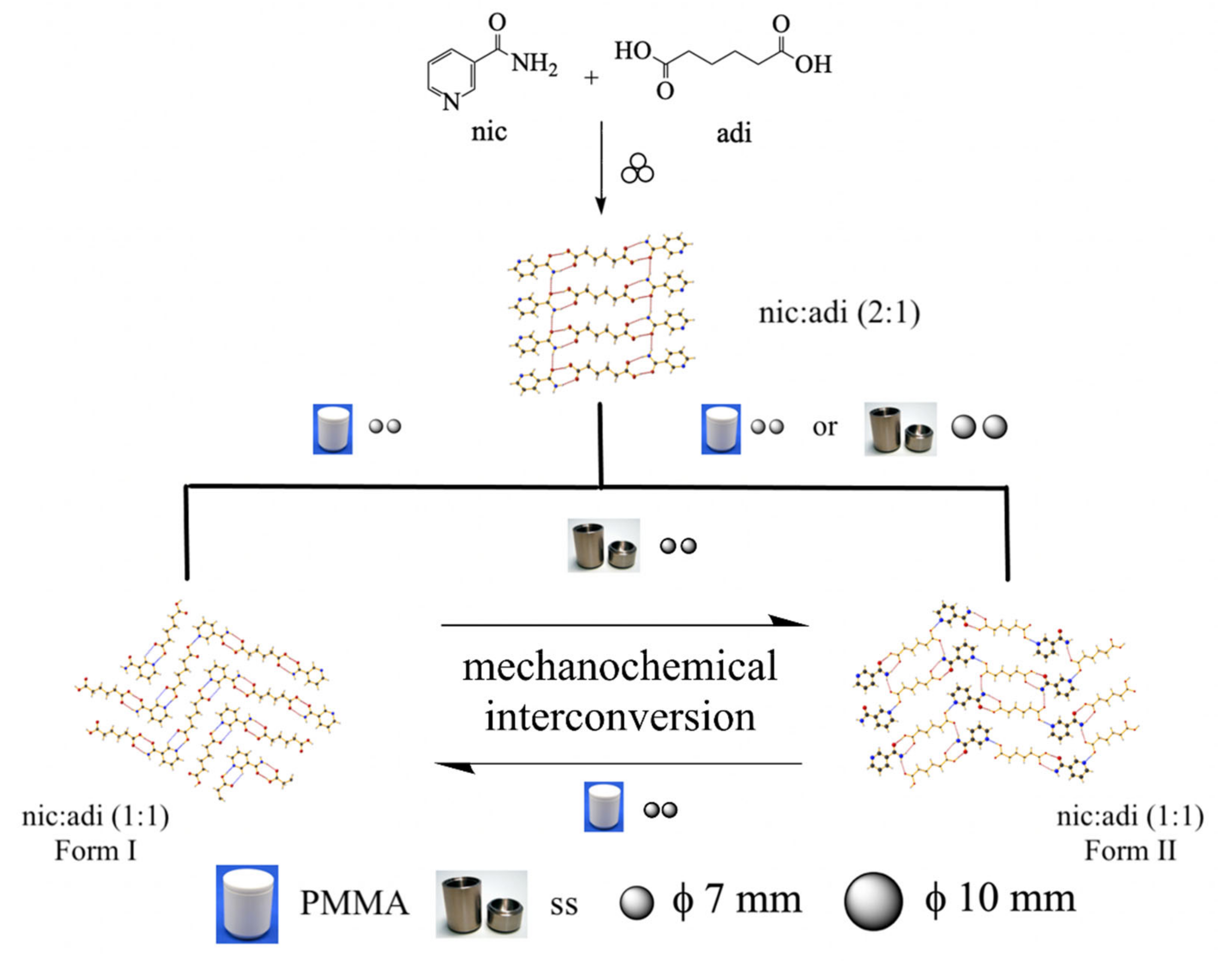
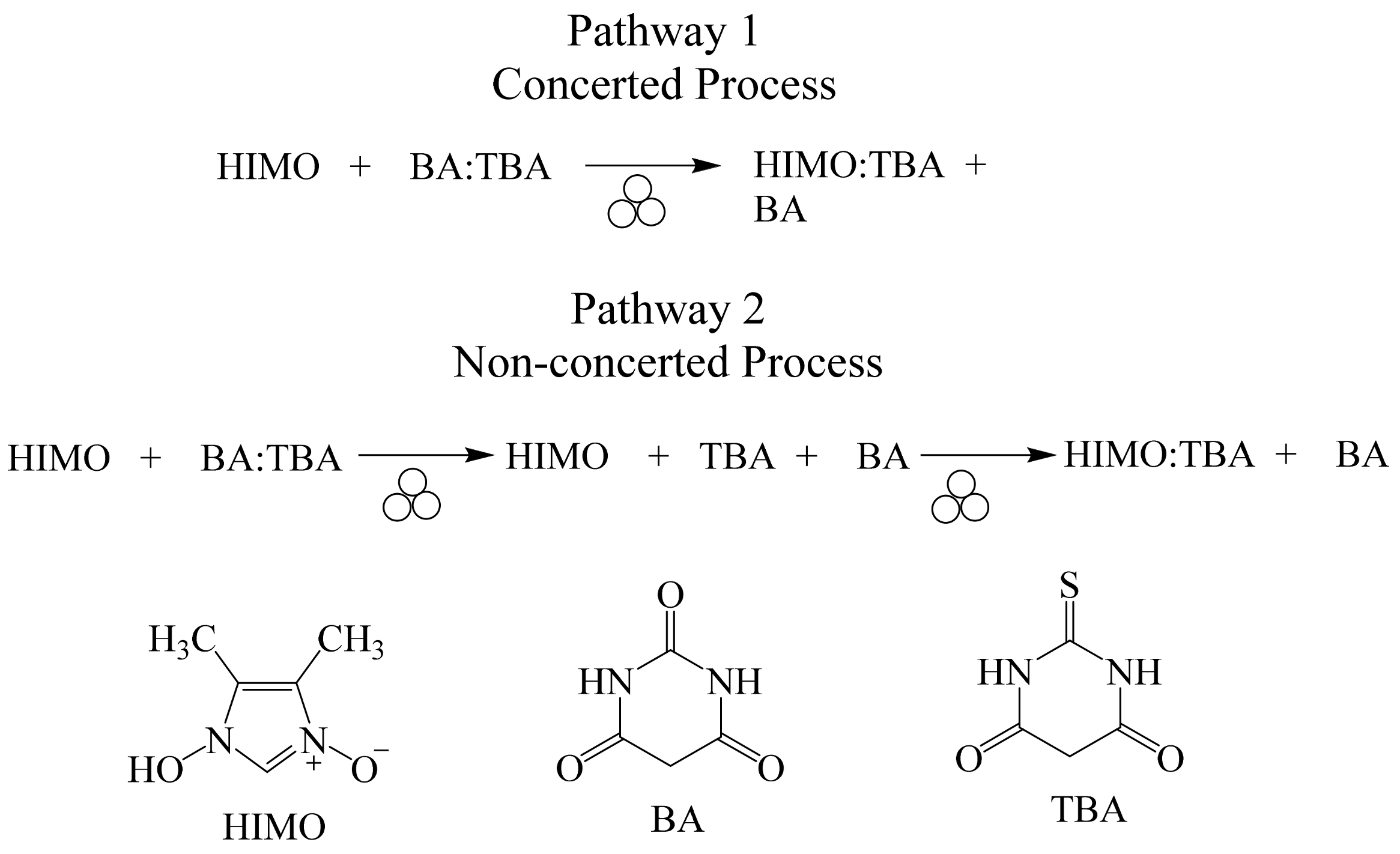
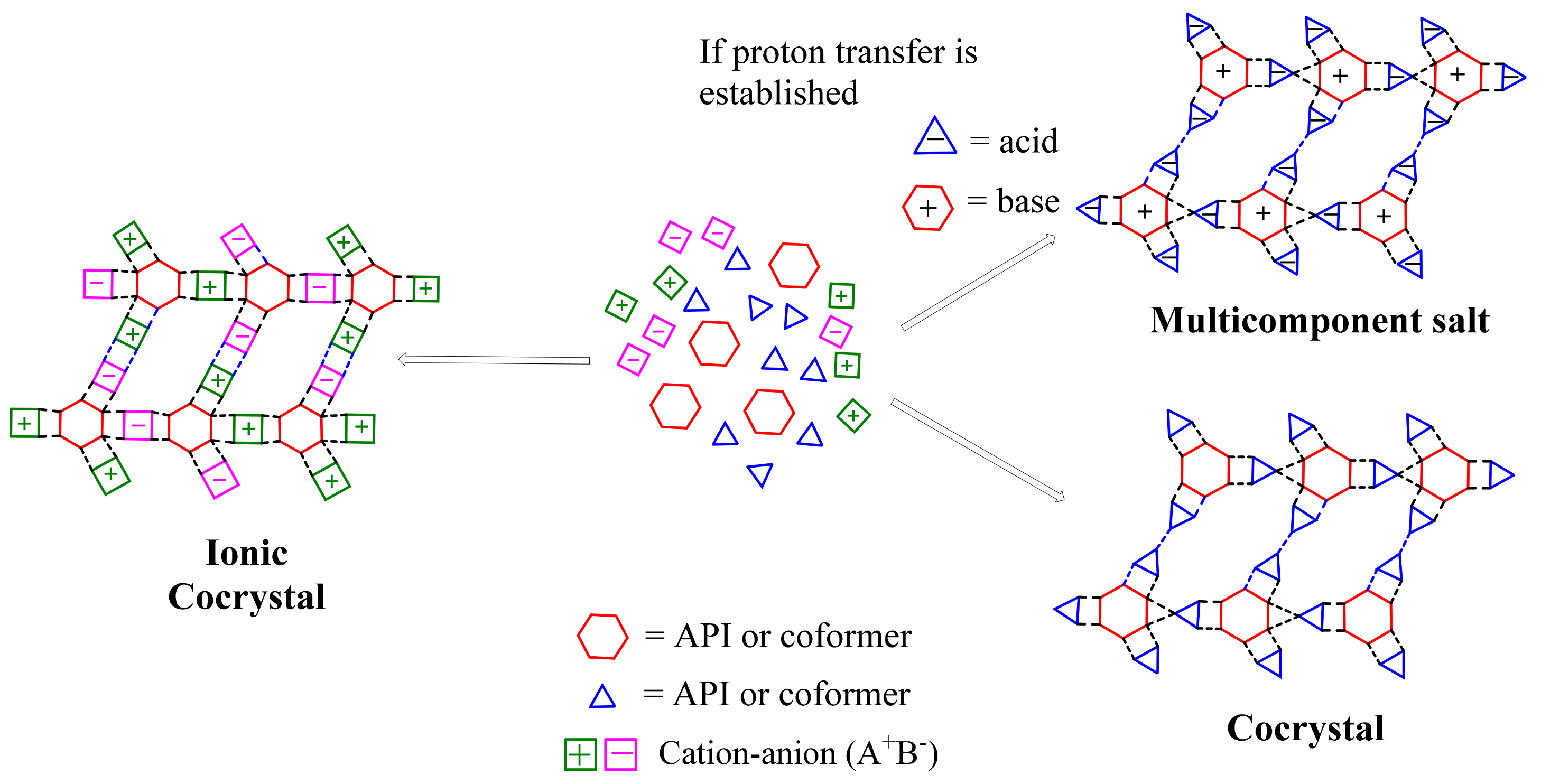
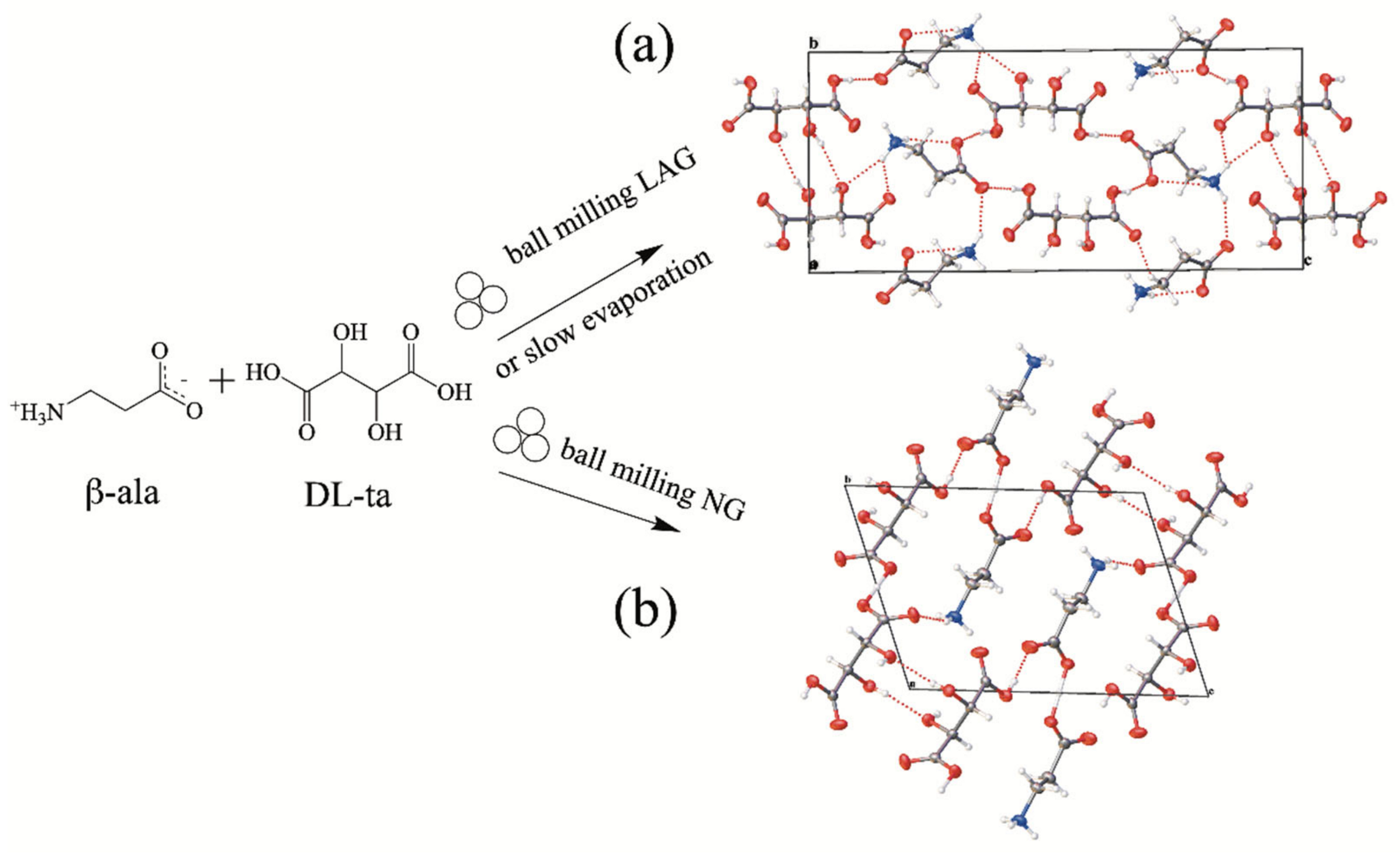
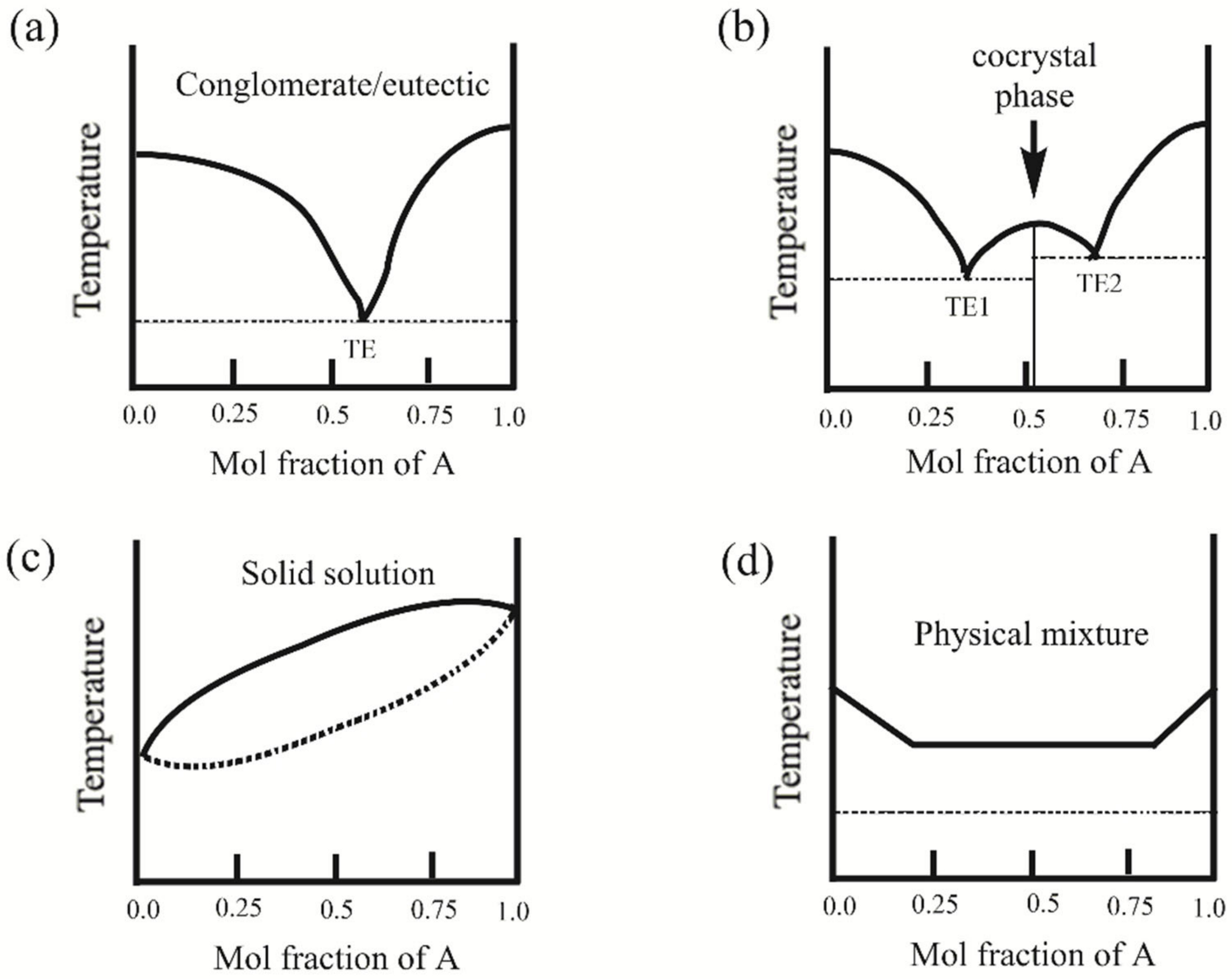

| API or NP + Coformer or API + API | Year of the Paper | Type of Grinding or Method of Synthesis and Characterization | Type of Multicomponent Form | Reference |
|---|---|---|---|---|
| Adenine + Thymine | 1993 | NG/slow evaporation | Cocrystal | [309] |
| Sulfadimidine + 8 diverse Carboxylic acids coformers | 1995 | NG | Cocrystal | [310] |
| Caffeine + Glutaric acid | 2004 | SDG | Cocrystal | [155] |
| Caffeine or Theophylline + chiral or racemic forms D,L-tartaric acid | 2006 | LAG | Cocrystal | [311] |
| Ternary system Caffeine + Succinic acid + diverse solvent guest | 2006 | NG/LAG/slow evaporation | Cocrystal solvent | [312] |
| Caffeine or Theophylline + citric acid | 2007 | NG/LAG | Cocrystal | [212] |
| Theophylline + 1,7-Heptanediamine | 2007 | NG | Salt-Cocrystal Continuum | [61] |
| Nicotinamide + Mandelic acid or Ibuprofen | 2007 | NG/LAG | Cocrystal | [313] |
| Carbamazepine + diverse coformers | 2009 | SDG/slow evaporation | Cocrystal | [149] |
| Theophylline or Caffeine + L-Malic or L-Tartaric acid | 2009 | LAG/sonochemical reactions | Cocrystal | [206] |
| Meloxicam + Succinic acid or Maleic acid | 2009 | SDG | Cocrystal | [314] |
| Nicotinamide + 10 diverse dicarboxylic acids coformers | 2009 | Melt/NG/LAG/slow evaporation | Cocrystal | [241] |
| Paracetamol + 13 diverse coformers | 2009 | NG/LAG | Cocrystal | [306] |
| 2-Chloro-4-nitrobenzoic acid + Nicotinamide | 2010 | LAG/slow evaporation | Cocrystal | [315] |
| Indomethacin + 30 diverse coformers | 2011 | LAG/Prediction of cocrystal formation employing Hansen solubility parameter | Cocrystal | [316] |
| Piracetam + Citric acid or Tartaric acid | 2011 | NG/LAG | Cocrystal | [317] |
| Nicotinamide + five Fenamic acid derivatives | 2011 | LAG/liquid assisted sonication/slow evaporation | Cocrystal | [318] |
| Curcumin + Resorcinol or Pyrogallol | 2011 | LAG | Cocrystal | [319] |
| Prulifloxacin + Salicylic acid | 2011 | Kneading | Cocrystal | [320] |
| Furosemide + 8 diverse coformers | 2012 | LAG | Cocrystal | [321] |
| Acetazolamide + diverse carboxilic acids or amide derivatives coformers | 2012 | LAG | Cocrystal | [322] |
| Indomethacin + Saccharin | 2012 | LAG | Cocrystal | [323] |
| Nitrofurantoin + 4-Hydroxybenzoic acid or Nicotinamide or L-Proline or Vanillic acid | 2012 | LAG | Cocrystal | [324] |
| Piroxicam + 20 different carboxylic acids | 2012 | LAG/fast cooling and slow cooling of a hot saturated solution/precipitation with an antisolvent/slow evaporation/melting | Cocrystal/cocrystal hydrate/coamorphous | [325] |
| Meloxicam + diverse carboxylic acids | 2012 | SDG | Cocrystal | [326] |
| DL-Malic acid + L-Tartaric acid and L-Malic acid + DL-Tartaric acid | 2012 | LAG | Cocrystal | [327] |
| Andrographolide (NP) + Vanilin or Vanillic acid or Salicylic acid or Guaiacol | 2013 | LAG | Cocrystal | [328] |
| α- or γ-Glycine + 7 carboxylic acids coformers | 2013 | NG/spray drying/fast and slow anti-solvent techniques | Cocrystal/multicomponent salt | [329] |
| Racemic Praziquantel + diverse aliphatic dicarboxylic acids coformers | 2013 | LAG/slurry method | Cocrystal | [330] |
| Carbamazepine + Saccharin and Nicotinamide + Suberic acid | 2013 | LAG | Cocrystal | [200] |
| L-Serine (anhydrous or monohydrate) + Oxalic acid (anhydrous or dihydrate) | 2014 | NG/LAG/slow evaporation/precipitation on antisolvent crystallization | Multicomponent salt/multicomponent salt hydrate/multicomponent salt polymorph | [331] |
| Trospium chloride + diverse carboxylic acid coformers | 2014 | NG/LAG/slurry method/slow evaporation | Cocrystal | [332] |
| AMG 517 + Sorbic acid | 2014 | Ball-milling/TSE | Cocrystal | [294] |
| Caffeine + Anthranilic acid | 2014 | LAG | Cocrystal polymorph | [68] |
| Lenalidomide + urea or 3,5-Dihydroxybenzoic acid (1:1 and 1:2:1 monohydrate) | 2014 | LAG | Cocrystal | [333] |
| Ezetimibe + Methyl paraben | 2014 | LAG/slow evaporation | Cocrystal | [334] |
| α-Glycine + β-Malonic acid | 2014 | NG/LAG/Impact treatment/shear treatment/vibratory treatment | Multicomponent salt | [335] |
| Stanozolol + Malonic acid or D-Phenyllactic acid or 6-Hydroxy-2-Naphthoic Acid | 2014 | LAG | Cocrystal | [336] |
| Caffeine + Citric acid or Anthranilic acid and Phenazine + Mesaconic acid | 2015 | LAG/POLAG | Cocrystal | [211] |
| Pyrazinamide + p-Nitrobenzoic acid | 2015 | LAG | Stoichiometric cocrystals | [337] |
| Theobromine + Oxalic acid | 2015 | NG/structure solved based on the powder X-ray data/in situ using synchrotron powder X-ray diffraction | Cocrystal | [338] |
| Theophylline + 4-Aminosalicylic acid or 4-Aminobenzoic acid | 2015 | LAG/slow evaporation | Cocrystal | [339] |
| Pyrimidin-2-amine + Glutaric acid | 2015 | NG/slow evaporation | Cocrystal/salt-cocrystal continuum | [340] |
| Theophylline + Benzoic acid | 2015 | NG/In Situ investigations of milling reactions using combined powder X-ray Diffraction and Raman spectroscopy | Cocrystal | [341] |
| Anthranilic acid + Carbamazepine or Salicylic acid or Theophylline and Salicylic acid + theobromine | 2015 | NG/LAG/slurry methods/competitive milling reactions | Cocrystal | [342] |
| Pentoxifylline + diverse carboxylic acid derivatives or Furosemide or L-Ascorbic acid | 2015 | NG/LAG/In Silico screening | Cocrystal | [343] |
| Triamterene + DL-Mandelic acid or Saccharin | 2015 | LAG/slurry method | Cocrystal | [344] |
| Theophylline + o-Aminobenzoic acid or m-Aminobenzoic acid or p-Aminobenzoic acid | 2015 | LAG/slow evaporation | Cocrystal | [345] |
| Adefovir Dipivoxil + Glutaric acid | 2015 | LAG | Cocrystal | [346] |
| Resveratrol + 4-Aminobenzamide or Isoniazid | 2016 | LAG/rapid solvent removal | Cocrystal | [307] |
| Ethionamide + Oxalic acid or Glutaric acid or Adipic acid or Sebacic acid or Fumaric acid | 2016 | LAG | Cocrystal/multicomponent salt | [347] |
| Piroxicam + Saccharin | 2016 | NG/LAG/slow evaporation | Cocrystal | [348] |
| Theophylline + Benzamide | 2016 | LAG/solvent screening/in situ synchrotron powder X-ray diffraction | Cocrystal/Cocrystal polymorph | [349] |
| Glicazide + Malic acid or Succinic acid | 2016 | LAG | Cocrystal | [350] |
| Ibuprofen + Nicotinamide | 2016 | NG/in situ Raman spectroscopy | Cocrystal | [351] |
| Simvastatin + Malic acid | 2016 | LAG | Cocrystal | [352] |
| Theophylline + Benzamide | 2016 | NG/synchrotron X-ray powder diffraction data | Cocrystal | [353] |
| Meloxicam + Acetylendicarboxylic acid | 2016 | LAG/slow evaporation | Cocrystal | [354] |
| Theophylline + Benzamide or Benzoic acid or Isonicotinamide | 2016 | NG/Competitive Cocrystal Reactions/in situ powder X-ray diffraction | Cocrystal | [355] |
| Pyrazinamide + Oxalic acid | 2016 | NG/LAG/in situ using combined synchrotron Powder X-ray Diffraction and Raman | Cocrystal | [356] |
| 5-Fluorouracil + 3-Hydroxybenzoic acid or 4-Aminobenzoic acid or Cinnamic acid | 2016 | LAG/slurry method | Cocrystal | [357] |
| Lamotrigine + 4,4′-Bipyridine or 2,2′-Bipyridine | 2017 | LAG | Cocrystal | [358] |
| Aripiprazole + Orcinol | 2017 | LAG | Cocrystal | [359] |
| Hydrochlorothiazide + Piperazine or Tetramethylpyrazine or Picolinamide or Isoniazid or Malonamide or Isonicotinic acid | 2017 | NG/LAG | Cocrystal | [360] |
| Glicazide + Sebacic acid or α-Hydroxyacetic acid | 2017 | LAG | Cocrystal | [361] |
| Praziquantel + Citric acid or Malic acid or Salicylic acid or Tartaric acid | 2017 | NG/LAG | Cocrystal | [362] |
| γ-Glycine + Oxalic acid dihydrate | 2017 | NG/Real-time in situ X-ray powder diffraction | Multicomponent salt | [363] |
| Mycophenolic acid + Isonicotinamide or Minoxidil or 2,2′-Dipyridylamine | 2017 | LAG/slow evaporation | Cocrystal | [364] |
| Gliclazide + Catechol or Resorcinol or p-Toluene sulfonic acid or Piperazine | 2017 | LAG/slow evaporation | Cocrystal/multicomponent salt | [365] |
| Pyrazinamide + Malonic acid | 2017 | NG/LAG/slurry methods/In situ Powder X-ray Diffraction | Cocrystal polymorph | [366] |
| Carbamazepine + p-Aminosalicylic acid | 2017 | LAG/slurry methods/slow evaporation | Cocrystal | [367] |
| Theobromine + Oxalic acid and Pyrazinamide + Oxalic acid | 2017 | NG/LAG/in situ Raman experiments | Cocrystal | [368] |
| Felodipine + Imidazole | 2017 | LAG/in situ Raman experiments | Cocrystal | [369] |
| Metformin hydrochloride + Dehydrated disodium succinate | 2017 | NG/melting/slow evaporation | Multicomponent salt | [370] |
| Chlorothiazide + 13 diverse coformers | 2017 | LAG/slow evaporation | Cocrystal/multicomponent salt | [371] |
| 11-Azaartemisinin + 13 diverse carboxylic acids coformers | 2018 | LAG | Cocrystal | [372] |
| Fluoxetine·HCl + Fumaric acid or Benzoic acid or Succinic acid | 2018 | NG/LAG/solvothermal synthesis/slow evaporation | Cocrystal | [373] |
| 11-Azaartemisinin + trans-Cinnamic or Maleic acid (1:1 and 2:1) or Fumaric acid | 2018 | LAG | Cocrystal | [374] |
| Seselin (NP) + Thiourea | 2018 | LAG/slow evaporation | Cocrystal | [375] |
| β-Alanine + DL-Tartaric acid | 2018 | NG/LAG | Cocrystal/multicomponent salt | [258] |
| Naproxen + Proline | 2018 | LAG | Cocrystal polymorph/cocrystal hydrate/cocrystal solvate | [376] |
| Caffeine + Dapsone | 2018 | Slow evaporation/LAG/spray drying | Cocrystal | [377] |
| Theophylline + Aspirin | 2018 | NG/LAG/slurry method/ternary phase diagram | Multidrug cocrystal | [378] |
| Caffeine + Citric acid or Glutaric acid | 2018 | ILAG | Cocrystal polymorph | [217] |
| Glipizide + Glycolic acid | 2018 | NG/LAG/slurry method/slow evaporation | Cocrystal | [379] |
| Theophylline + Benzamide | 2018 | LAG | Cocrystal polymorph | [380] |
| Diclofenac acid + L-Proline | 2018 | NG/LAG | Cocrystal | [381] |
| Pefloxacin + 10 diverse dicarboxylic acids | 2018 | LAG/solvent evaporation | Multicomponent salt/multicomponent salt hydrate/salt cocrystal | [382] |
| Tofogliflozin + Sodium acetate or Potassium acetate | 2018 | LAG | Salt cocrystal | [383] |
| Flurbiprofen + Proline | 2018 | LAG/in situ Variable Temperature Synchrotron X-ray Diffraction | Chiral cocrystal/cocrystal solvate/stoichiometric cocrystal/cocrystal polymorph | [384] |
| Piroxicam + Succinic acid or Methylparaben or Resorcinol | 2019 | LAG | Cocrystal | [385] |
| Lamotrigine + Phthalimide or Succinimide | 2019 | LAG/slow evaporation/Ternary phase diagram | Cocrystal/Cocrystal hydrate | [386] |
| Pyrazinamide + Pimelic acid | 2019 | NG/LAG/Time Resolved In situ Powder X-ray Diffraction | Cocrystal polymorph | [231] |
| Nevirapine + p-Aminobenzoic acid | 2019 | NG/LAG | Cocrystal | [387] |
| Luteolin (NP) + Isoniazid or Caffeine | 2019 | LAG/Rapid solvent removal | Cocrystal | [388] |
| Meloxicam + Salicylic acid or Fumaric acid or Malic acid | 2019 | LAG | Cocrystal | [389] |
| Oxcarbazepine + Oxalic acid or 2,5-Dihydroxybenzoic acid or Salicylic acid | 2019 | LAG/slow evaporation | Cocrystal | [390] |
| α-D-Glucose + NaCl or NaBr or NaI | 2019 | NG/LAG | Ionic cocrystal | [391] |
| Carbamazepine + DL-Mandelic acid or DL-Tartaric acid | 2019 | LAG/computational prediction | Cocrystal/cocrystal polymorph | [392] |
| 2-Pyridine-carboxaldehyde benzoylhydrazone (hydrazone) + Malonic acid + Succinic acid + Glutaric acid + Mesaconic acid | 2019 | NG/LAG/slow evaporation | Cocrystal/cocrystal solvate/multicomponent salt | [393] |
| Betulin + Adipic acid or Succinic acid or Suberic acid | 2019 | LAG | Cocrystal | [394] |
| Ciprofloxacin + Salicylic acid | 2019 | LAG/in situ Raman spectroscopy experiments | Multicomponent salt/multicomponent salt hydrate and solvate/salt-cocrystal | [395] |
| Pyrazinamide + Glutaric acid + Isonicotinamide and Pyrazin-2-carboxylic acid + Glutaric acid + Isonicotinamide | 2019 | LAG/In situ Powder X-ray Diffraction | Ternary cocristal | [396] |
| Pirfenidone + Fumaric acid or Trimesic acid | 2019 | LAG/slow evaporation | Cocrystal | [397] |
| Glipizide + Glutaric acid | 2019 | NG/LAG/slow evaporation/slurry method | Cocrystal | [398] |
| Gemfibrozil + Isonicotinamide | 2019 | Milling | Cocrystal | [399] |
| Salicylic acid + diverse Imidazole coformers | 2019 | NG/structures were solved by powder X-ray diffraction | Multicomponent salt | [400] |
| Caffeine + Glutaric acid | 2020 | NG/LAG/POLAG | Cocrystal | [214] |
| Oxyresveratrol + Nicotinamide or Proline | 2020 | LAG/employing principal component analysis | Cocrystal | [401] |
| Itraconazole + Terephtalic acid | 2020 | LAG | Cocrystal | [402] |
| Ciprofloxacin + Nicotinic acid or Isonicotinic acid | 2020 | LAG | Cocrystal | [403] |
| Nicotinamide + Adipic acid | 2020 | Ball-milling LAG/Demonstration of reversible mechanochemical cocrystal polymorph interconversion (stable → metastable phase transformation). This process can be controlled by the choice of milling assembly/Real-time X-ray powder diffraction | Cocrystal polymorph | [229] |
| Betulin + Terephthalic acid | 2020 | LAG | Cocrystal | [404] |
| Ciprofloxacin + Carvacrol or Thymol | 2020 | NG/ball-milling LAG/slow evaporation/slurry method | Cocrystal | [405] |
| 5-Fluorouracil + Kaempferol | 2020 | LAG/slurry method/slow evaporation/ternary phase diagram | Multidrug cocrystal | [406] |
| Chromotropic acid + 1,10-Phenanthroline | 2020 | LAG/solvent evaporation | Cocrystal salt hydrate | [407] |
| Ibuprofen + Nicotinamide | 2020 | Ball-milling NG/melting/slow evaporation. Detection of cocrystal polymorphism (2 forms). Formation of one or another polymorph depend on the synthetic method used. | Cocrystal polymorph | [243] |
| Thiobarbituric acid or Barbituric acid + 1-Hydroxy-4,5-Dimethyl-Imidazole 3-Oxide | 2020 | LAG/solvent evaporation | Cocrystal | [408] |
| Telmisartan + Hydroclorothiazide | 2020 | LAG/slow evaporation | Multidrug cocrystal | [409] |
| Chlorothiazide + 13 diverse coformers | 2020 | Ball-milling LAG/Study of the effect of grinding on 11 cocrystals an one salt in the presence of the excipients polyvinylpyrrolidone and microcrystalline cellulose | Cocrystal/multicomponent drug | [410] |
| Nebivolol hydrochloride + 4-Hydroxybenzoic acid or Nicotinamide | 2020 | LAG | Cocrystal | [411] |
| 9-Ethyladenine + Malonic acid or Succinic acid or Fumaric acid or Glutaric acid or Adipic acid | 2020 | LAG/slow evaporation | Cocrystal/multicomponent salt | [412] |
| Carbamazepine + DL-Tartaric acid | 2020 | LAG | Cocrystal | [413] |
| Emtricitabine + 1,2-Bis(4-pyridyl)ethane or 1,2-Bis(4-pyridyl)ethylene or 4,4′-Azopyridine or 4,4′-Bipyridine | 2020 | LAG | Cocrystal | [414] |
| Caffeine + Glutaric acid | 2020 | NG/LAG/POLAG/in situ X-ray powder diffraction | Cocrystal | [214] |
| Cocrystal (Barbituric acid/Thiobarbituric acid) BA0.5TBA0.5 + 1-Hydroxy-4,5-dimethyl-imidazole 3-oxide | 2020 | Ball-milling LAG/Preparation of ternary cocrystal system beginning from binary cocrystal system. Evaluation of the possible pathways involved, the evidence suggests a non-concerted process. | Binary and ternary cocrystal | [245] |
| Tinidazol + p-Aminobenzoic acid or Citric acid or Salicylic acid | 2020 | NG/LAG | Cocrystal | [415] |
| Ciprofloxacin + Pyrazinoic acid or p-Aminobenzoic acid | 2020 | NG/LAG | Cocrystal | [416] |
| Zaltoprofen + Nicotinamide (1:1 or 1:2) | 2020 | LAG | Cocrystal | [417] |
| Metronidazole + 3,5-Dihydroxybenzoic or 3,4,5-Trihydroxybenzoic acid | 2020 | LAG/melt/slow evaporation | Cocrystal | [418] |
| Exemestane + 9-Hydroxyphenanthrene and 1-Hydroxypyrene | 2020 | LAG | Cocrystal | [419] |
| Penciclovir + 3,5-Dihydroxybenzoic acid or Gallic acid (1:1 or 1:1:1 hydrate) or 4-Hydroxycinnamic acid (1:1 or 1:1:1 hydrate) | 2020 | LAG | Cocrystal/cocrystal hydrate | [420] |
| Berberine chloride + Pyromellitic dianhydride | 2020 | LAG | Diverse multicomponent stoichiomorphs: multicomponent salt/multicomponent salt polymorph/ionic cocrystal hydrate | [421] |
| Allopurinol + Isonicotinamide or Piperazine or 2,4-Dihydroxybenzoic acid | 2020 | LAG/slurry methods/slow evaporation | Cocrystal | [422] |
| Trimethoprim + Flufenamic acid or Tolfenamic acid or Mefenamic acid and Sulfamethazine + Flufenamic acid or Niflumic acid | 2020 | LAG/slow evaporation | Multicomponent salt hydrate/multidrug cocrystal | [423] |
| Ciprofloxacin + 4-Hydroxybenzoic acid or 4-Aminobenzoic acid or Gallic acid | 2020 | LAG | Multicomponent salt hydrate | [424] |
| Regorafenib + Malonic acid or Glutaric acid or Pimelic acid | 2021 | LAG/slurry methods | Cocrystal | [425] |
| Theobromine + Trimesic acid or Hemimellitic acid and Caffeine + Trimesic acid or Hemimellitic acid | 2021 | NG/LAG/slow evaporation | Cocrystal/multicomponent salt/cocrystal hydrate | [426] |
| Temozolomide + Hesperetin | 2021 | LAG/slurry methods/slow evaporation | Multidrug cocrystal | [427] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solares-Briones, M.; Coyote-Dotor, G.; Páez-Franco, J.C.; Zermeño-Ortega, M.R.; de la O Contreras, C.M.; Canseco-González, D.; Avila-Sorrosa, A.; Morales-Morales, D.; Germán-Acacio, J.M. Mechanochemistry: A Green Approach in the Preparation of Pharmaceutical Cocrystals. Pharmaceutics 2021, 13, 790. https://doi.org/10.3390/pharmaceutics13060790
Solares-Briones M, Coyote-Dotor G, Páez-Franco JC, Zermeño-Ortega MR, de la O Contreras CM, Canseco-González D, Avila-Sorrosa A, Morales-Morales D, Germán-Acacio JM. Mechanochemistry: A Green Approach in the Preparation of Pharmaceutical Cocrystals. Pharmaceutics. 2021; 13(6):790. https://doi.org/10.3390/pharmaceutics13060790
Chicago/Turabian StyleSolares-Briones, Mizraín, Guadalupe Coyote-Dotor, José C. Páez-Franco, Miriam R. Zermeño-Ortega, Carmen Myriam de la O Contreras, Daniel Canseco-González, Alcives Avila-Sorrosa, David Morales-Morales, and Juan M. Germán-Acacio. 2021. "Mechanochemistry: A Green Approach in the Preparation of Pharmaceutical Cocrystals" Pharmaceutics 13, no. 6: 790. https://doi.org/10.3390/pharmaceutics13060790
APA StyleSolares-Briones, M., Coyote-Dotor, G., Páez-Franco, J. C., Zermeño-Ortega, M. R., de la O Contreras, C. M., Canseco-González, D., Avila-Sorrosa, A., Morales-Morales, D., & Germán-Acacio, J. M. (2021). Mechanochemistry: A Green Approach in the Preparation of Pharmaceutical Cocrystals. Pharmaceutics, 13(6), 790. https://doi.org/10.3390/pharmaceutics13060790