In Vitro and Clinical Safety Assessment of the Multiple W/O/W Emulsion Based on the Active Ingredients from Rosmarinus officinalis L., Avena sativa L. and Linum usitatissimum L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Plant Extracts
2.3. Preparation of the W/O/W Emulsion
2.4. Microbiological Challenge Testing
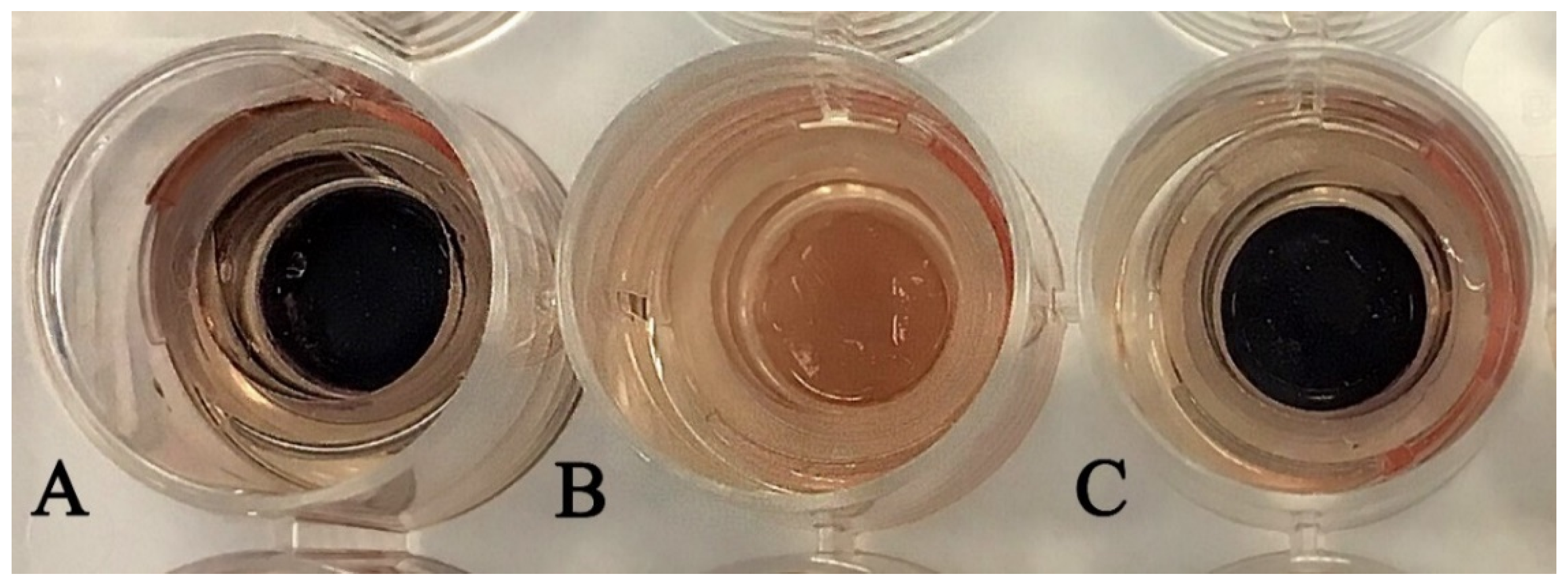
2.5. In Vitro Skin Irritation Test
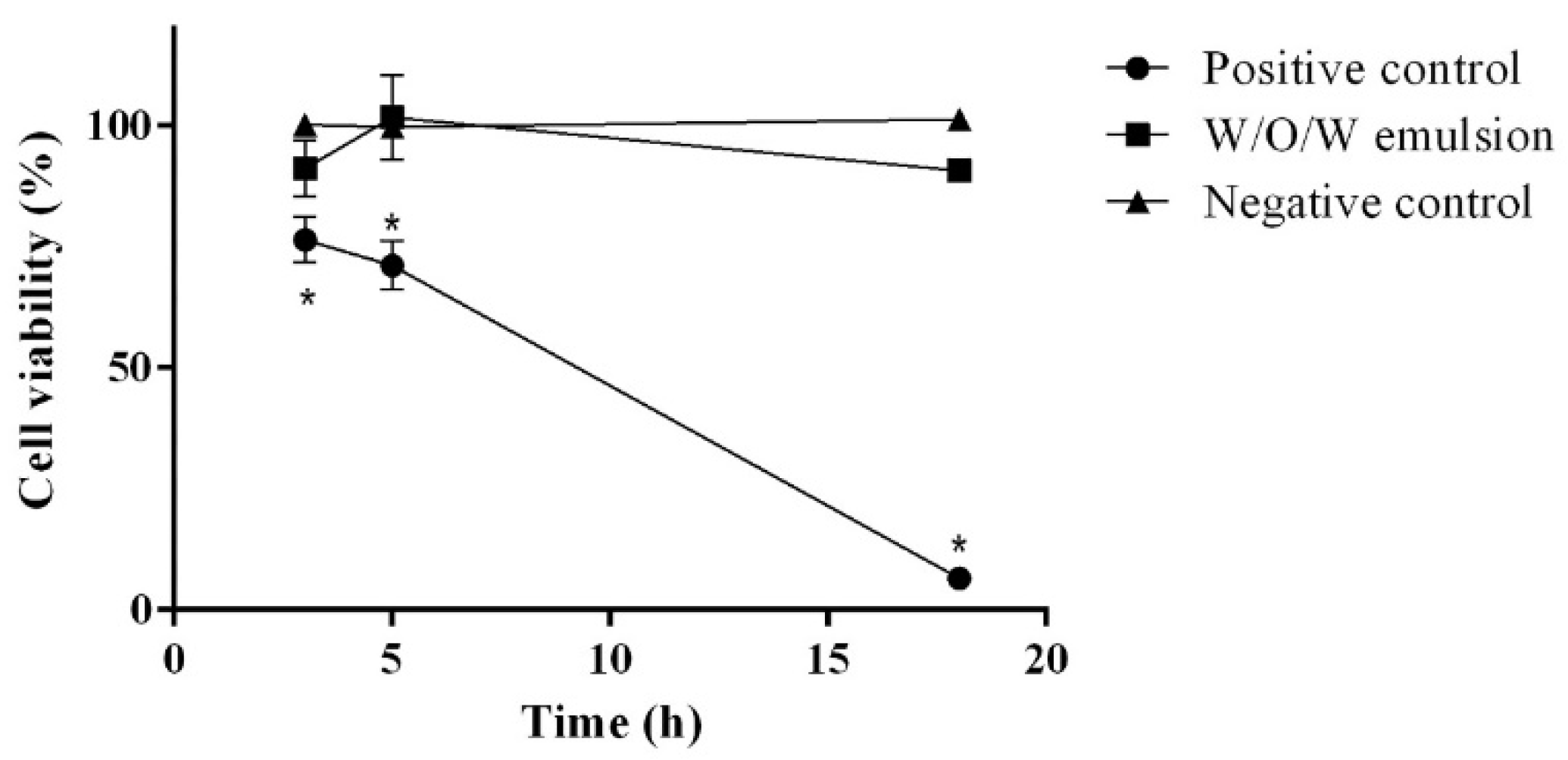
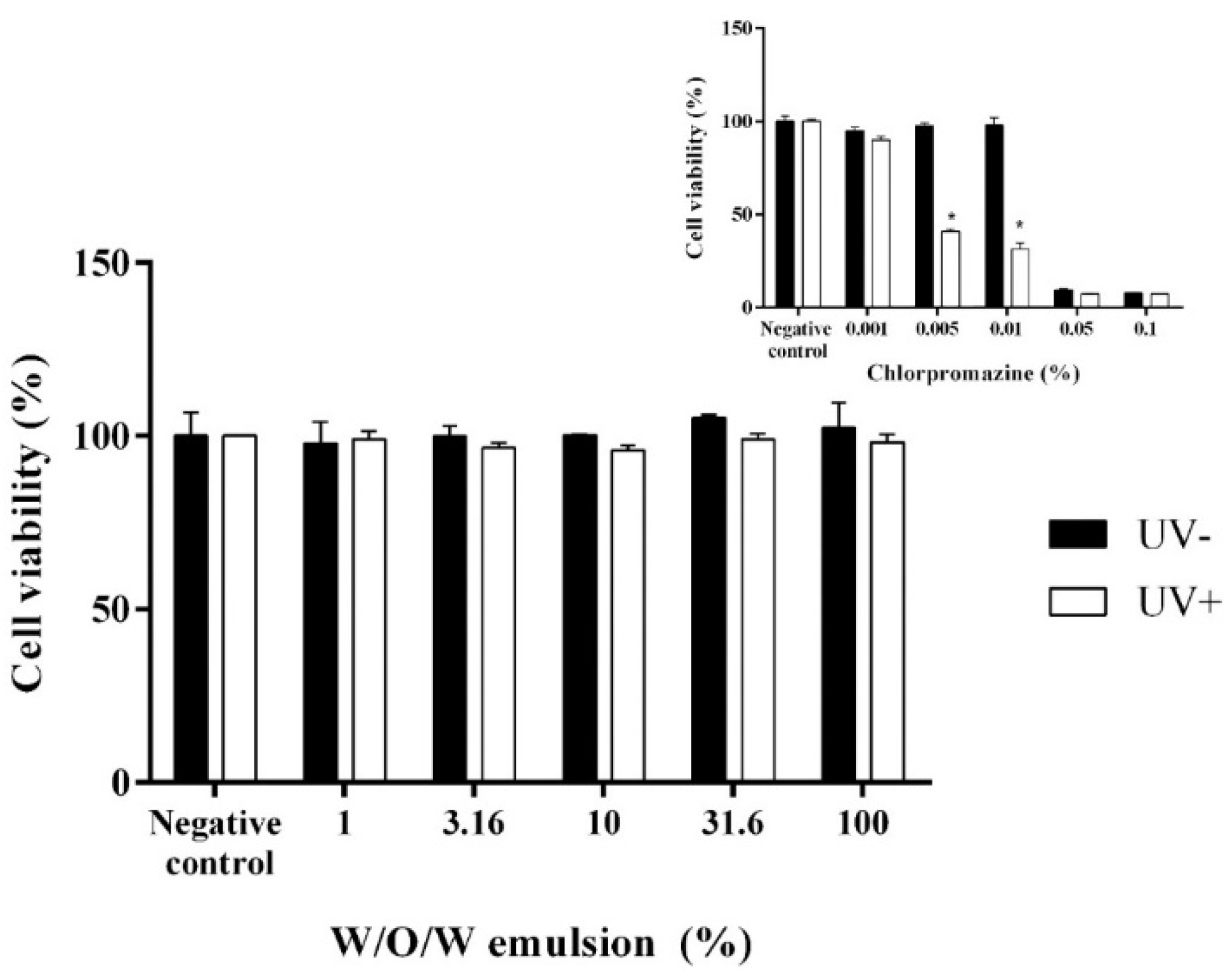
2.6. In Vitro Skin Phototoxicity Test
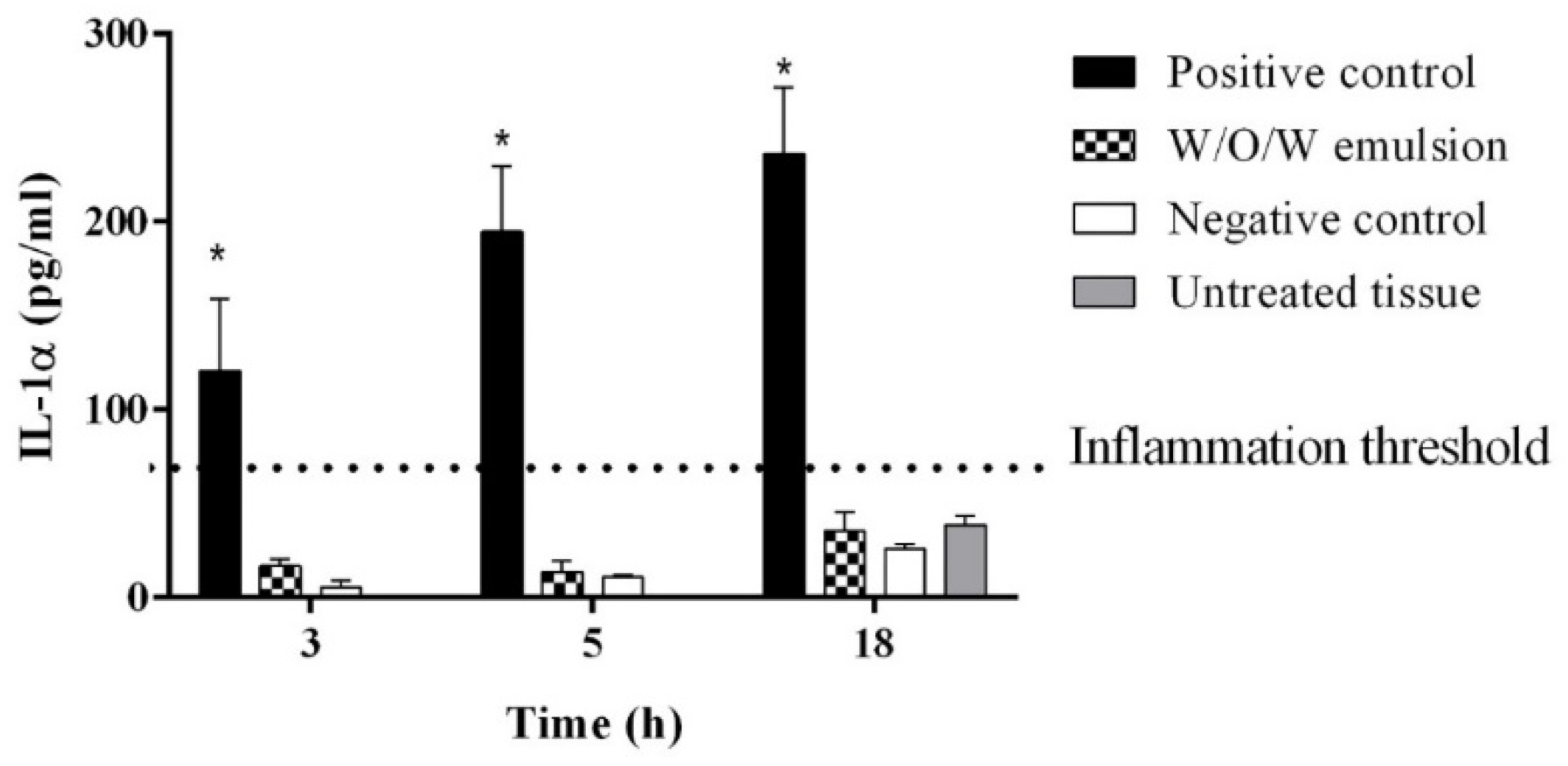
2.7. IL-1α Assay for SKIN inflammation Testing
2.8. 48 Hours Human Skin Patch Test
2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baumann, L.S. Less-known botanical cosmeceuticals. Dermatol. Ther. 2007, 20, 330–342. [Google Scholar] [CrossRef]
- Akhtar, M.; Murray, B.S.; Afeisume, E.I.; Khew, S.H. Encapsulation of flavonoid in multiple emulsion using spinning disc reactor technology. Food Hydrocoll. 2014, 34, 62–67. [Google Scholar] [CrossRef]
- Mahmood, T.; Akhtar, N. Stability of a cosmetic multiple emulsion loaded with green tea extract. Sci. World J. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pal, R. Rheology of simple and multiple emulsions. Curr. Opin. Colloid Interface Sci. 2011, 16, 41–60. [Google Scholar] [CrossRef]
- Cizauskaite, U.; Marksiene, R.; Viliene, V.; Gruzauskas, R.; Bernatoniene, J. New strategy of multiple emulsion formation based on the interactions between polymeric emulsifier and natural ingredients. Colloids Surf. A Physicochem. Eng. Asp. 2017, 515, 22–33. [Google Scholar] [CrossRef]
- Sun, C.; Gunasekaran, S. Effects of protein concentration and oil-phase volume fraction on the stability and rheology of menhaden oil-in-water emulsions stabilized by whey protein isolate with xanthan gum. Food Hydrocoll. 2009, 23, 165–174. [Google Scholar] [CrossRef]
- Pootongkam, S.; Nedorost, S. Oat and wheat as contact allergens in personal care products. Dermatitis 2013, 24, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Astier, C.; Benchad Yel, A.; Moneret-Vautrin, D.A.; Bihain, B.E.; Kanny, G. Anaphylaxis to argan oil. Allergy 2010, 65, 662–663. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, K.J.; Yiannias, J.A. Contact dermatitis to cosmetics, fragrances, and botanicals. Dermatol. Ther. 2004, 17, 264–271. [Google Scholar] [CrossRef]
- Kiken, D.A.; Cohen, D.E. Contact dermatitis to botanical extracts. Am. J. Contact Dermat. Off. J. Am. Contact Dermat. Soc. 2002, 13, 148–152. [Google Scholar]
- Yamakawa, Y.; Ohsuna, H.; Aihara, M.; Tsubaki, K.; Ikezawa, Z. Contact urticaria from rice. Contact Dermat. 2001, 44, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Amaro, C.; Goossens, A. Immunological occupational contact urticaria and contact dermatitis from proteins: A review. Contact Dermat. 2008, 58, 67–75. [Google Scholar] [CrossRef]
- EU, Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32009R1223 (accessed on 2 August 2017).
- Draize, J.H.; Woodard, G.; Calvery, H.O. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar]
- El Ghalbzouri, A.; Siamari, R.; Willemze, R.; Ponec, M. Leiden reconstructed human epidermal model as a tool for the evaluation of the skin corrosion and irritation potential according to the ECVAM guidelines. Toxicol. Vitro 2008, 22, 1311–1320. [Google Scholar] [CrossRef]
- Basketter, D.A.; Chamberlain, M.; Griffiths, H.A.; Rowson, M.; Whittle, E.; York, M. The classification of skin irritants by human patch test. Food Chem. Toxicol. 1997, 35, 845–852. [Google Scholar] [CrossRef]
- Robinson, M.K.; McFadden, J.P.; Basketter, D.A. Validity and ethics of the human 4-h patch test as an alternative method to assess acute skin irritation potential. Contact Dermat. 2001, 45, 1–12. [Google Scholar] [CrossRef] [PubMed]
- McKim, J.M.; Keller, D.J.; Gorski, J.R. An in vitro method for detecting chemical sensitization using human reconstructed skin models and its applicability to cosmetic, pharmaceutical, and medical device safety testing. Cutan. Ocular Toxicol. 2012, 31, 292–305. [Google Scholar] [CrossRef]
- Schlotmann, K.; Kaeten, M.; Black, A.F.; Damour, O.; Waldmann-Laue, M.; Förster, T. Cosmetic efficacy claims in vitro using a three-dimensional human skin model. Int. J. Cosmet. Sci. 2001, 23, 309–318. [Google Scholar] [CrossRef][Green Version]
- Nohynek, G.J.; Antignac, E.; Re, T.; Toutain, H. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol. Appl. Pharmacol. 2010, 243, 239–259. [Google Scholar] [CrossRef]
- Krausz, A.; Gunn, H.; Friedman, A. The basic science of natural ingredients. J. Drugs Dermatol. 2014, 13, 937–945. [Google Scholar]
- Jirova, D.; Basketter, D.; Liebsch, M.; Bendova, H.; Kejlova, K.; Marriott, M.; Kandarova, H. Comparison of human skin irritation patch test data with in vitro skin irritation assays and animal data. Contact Dermat. 2010, 62, 109–116. [Google Scholar] [CrossRef]
- Spielmann, H.; Liebsch, M.; Pape, W.J.; Balls, M.; Dupuis, J.; Klecak, G.; Lovell, W.W.; Maurer, T.; De Silva, O.; Steiling, W. EEC/COLIPA in vitro photoirritancy program: Results of the first stage of validation. Curr. Probl. Dermatol. 1995, 23, 256–264. [Google Scholar]
- Kanto, H.; Washizaki, K.; Ito, M.; Matsunaga, K.; Akamatsu, H.; Kawai, K.; Katoh, N.; Natsuaki, M.; Yoshimura, I.; Kojima, H.; et al. Optimal patch application time in the evaluation of skin irritation. J. Dermatol. 2013, 40, 363–369. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Cizauskaite, U.; Ivanauskas, L.; Jakstas, V.; Kalveniene, Z.; Kopustinskiene, D.M. Novel approaches to optimize extraction processes of ursolic, oleanolic and rosmarinic acids from Rosmarinus officinalis leaves. Ind. Crop. Prod. 2016, 84, 72–79. [Google Scholar] [CrossRef]
- Cizauskaite, U.; Bernatoniene, J. Innovative Natural Ingredients-Based Multiple Emulsions: The Effect on Human Skin Moisture, Sebum Content, Pore Size and Pigmentation. Molecules 2018, 23, 1428. [Google Scholar] [CrossRef]
- Cizauskaite, U.; Ivanauskas, L.; Jakštas, V.; Marksiene, R.; Jonaitiene, L.; Bernatoniene, J. Rosmarinus officinalis L. extract and some of its active ingredients as potential emulsion stabilizers: A new approach to the formation of multiple (W/O/W) emulsion. Pharm. Dev. Technol. 2016, 21, 716–724. [Google Scholar] [PubMed]
- Cizauskaite, U.; Marksa, M.; Bernatoniene, J. The optimization of technological processes, stability and microbiological evaluation of innovative natural ingredients-based multiple emulsion. Pharm. Dev. Technol. 2017, 23, 636–645. [Google Scholar] [CrossRef] [PubMed]
- CIOMS. International Ethical Guidelines for Biomedical Research Involving Human Subjects; Council for International Organizations of Medical Sciences (CIOMS): Geneva, Switzerland, 2002. [Google Scholar]
- Fonacier, L. A Practical Guide to Patch Testing. J. Allergy Clin. Immunol. Pract. 2015, 3, 669–675. [Google Scholar] [CrossRef]
- Fonacier, L.; Bernstein, D.I.; Pacheco, K.; Holness, D.L.; Blessing-Moore, J.; Khan, D.; Lang, D.; Nicklas, R.; Oppenheimer, J.; Portnoy, J.; et al. Contact dermatitis: A practice parameter-update 2015. J. Allergy Clin. Immunol. Pract. 2015, 3, S1–S39. [Google Scholar] [CrossRef]
- Anand, S.; Sati, N. Artificial preservatives and their harmful effects: Looking toward nature for safer alternatives. Int. J. Pharm. Sci. Res. 2013, 4, 2496. [Google Scholar]
- Varvaresou, A.; Papageorgiou, S.; Tsirivas, E.; Protopapa, E.; Kintziou, H.; Kefala, V.; Demetzos, C. Self-preserving cosmetics. Int. J. Cosmet. Sci. 2009, 31, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Choulitoudi, E.; Ganiari, S.; Tsironi, T.; Ntzimani, A.; Tsimogiannis, D.; Taoukis, P.; Oreopoulou, V. Edible coating enriched with rosemary extracts to enhance oxidative and microbial stability of smoked eel fillets. Food Packag. Shelf Life 2017, 12, 107–113. [Google Scholar] [CrossRef]
- Kaczmarczyk, D.; Strub, D.; Polowy, A.; Lochyński, S. Selected essential oils in cosmetic emulsions: Process oriented stability studies and antimicrobial activity. Nat. Volatiles Essent. Oils 2015, 2, 27–39. [Google Scholar]
- Ozogul, Y.; Yuvka, İ.; Ucar, Y.; Durmus, M.; Kösker, A.R.; Öz, M.; Ozogul, F. Evaluation of effects of nanoemulsion based on herb essential oils (rosemary, laurel, thyme and sage) on sensory, chemical and microbiological quality of rainbow trout (Oncorhynchus mykiss) fillets during ice storage. LWT Food Sci. Technol. 2017, 75, 677–684. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Adham, A.N.; Hui-Duan, L. Comparative extraction methods, phytochemical constituents, fluorescence analysis and HPLC validation of rosmarinic acid content in Mentha piperita, Mentha longifolia and Osimum basilicum. J. Pharmacogn. Phytochem. 2015, 3, 130–139. [Google Scholar]
- Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W. Safety Assessment of Rosmarinus officinalis (Rosemary)-Derived Ingredients as Used in Cosmetics. 2013. Available online: http://www.cir-safety.org/sites/default/files/rosmarinus_0.pdf (accessed on 15 May 2021).
- Lachenmeier, D.W. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J. Occup. Med. Toxicol. 2008, 3, 26. [Google Scholar] [CrossRef]
- Cartner, T.; Brand, N.; Tian, K.; Saud, A.; Carr, T.; Stapleton, P.; Lane, M.; Rawlings, A. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A2 together with epidermal keratinocytes and skin irritation. Int. J. Cosmet. Sci. 2017, 39, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.F.; Rato, M.; Martins, C. Drug-induced photosensitivity: Photoallergic and phototoxic reactions. Clin. Dermatol. 2016, 34, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Broeders, J.J.; Blaauboer, B.J.; Hermens, J.L. In vitro biokinetics of chlorpromazine and the influence of different dose metrics on effect concentrations for cytotoxicity in Balb/c 3T3, Caco-2 and HepaRG cell cultures. Toxicol. Vitro 2013, 27, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Gill, L.; Lim, H.W. Drug-induced photosensitivity. In Cutaneous Drug Eruptions; Springer: Berlin/Heidelberg, Germany, 2015; pp. 107–121. [Google Scholar]
- Nobile, V.; Michelotti, A.; Cestone, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Pérez-Sánchez, A.; Micol, V. Skin photoprotective and antiageing effects of a combination of rosemary (Rosmarinus officinalis) and grapefruit (Citrus paradisi) polyphenols. Food Nutr. Res. 2016, 60, 31871. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.; Kimball, A. Topical vitamins, minerals and botanical ingredients as modulators of environmental and chronological skin damage. Br. J. Dermatol. 2003, 149, 681–691. [Google Scholar] [CrossRef]
- Peng, C.-H.; Su, J.-D.; Chyau, C.-C.; Sung, T.-Y.; Ho, S.-S.; Peng, C.-C.; Peng, R.Y. Supercritical fluid extracts of rosemary leaves exhibit potent anti-inflammation and anti-tumor effects. Biosci. Biotechnol. Biochem. 2007, 71, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Rabetafika, H.N.; Van Remoortel, V.; Danthine, S.; Paquot, M.; Blecker, C. Flaxseed proteins: Food uses and health benefits. Int. J. Food Sci. Technol. 2011, 46, 221–228. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Olsen, L.M.; Carbonero, E.R.; Baggio, C.H.; Freitas, C.S.; Marcon, R.; Santos, A.R.; Gorin, P.A.; Iacomini, M. Anti-inflammatory and analgesic properties in a rodent model of a (1→3),(1→6)-linked β-glucan isolated from Pleurotus pulmonarius. Eur. J. Pharmacol. 2008, 597, 86–91. [Google Scholar] [CrossRef]
- Boussault, P.; Léauté-Labrèze, C.; Saubusse, E.; Maurice-Tison, S.; Perromat, M.; Roul, S.; Sarrat, A.; Taïeb, A.; Boralevi, F. Oat sensitization in children with atopic dermatitis: Prevalence, risks and associated factors. Allergy 2007, 62, 1251–1256. [Google Scholar] [CrossRef]
- Rokaitė, R.; Labanauskas, L.; Vaidelienė, L. Role of the skin patch test in diagnosing food allergy in children with atopic dermatitis. Medicina 2004, 40, 1081–1087. [Google Scholar]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W. Safety Assessment of Rosmarinus officinalis (Rosemary)-Derived Ingredients as Used in Cosmetics. Int. J. Toxicol. 2018, 37, 12S–50S. [Google Scholar] [CrossRef]
| Microorganisms | N, cfu/mL | N0, cfu/mL | Nvf, cfu/mL | Nvn, cfu/mL | Nvf ≥ 0.5 Nvn | Nv, cfu/mL |
|---|---|---|---|---|---|---|
| Escherichia coli | 3.2 × 107 | 3.2 × 105 | 2.5 × 102 | 2.6 × 102 | >0.5 | 3.0 × 102 |
| Staphylococcus aureus | 2.9 × 107 | 2.9 × 105 | 2.0 × 102 | 2.1 × 102 | >0.5 | 2.9 × 102 |
| Pseudomonas aeruginosa | 3.7 × 107 | 3.7 × 105 | 1.2 × 102 | 1.4 × 102 | >0.5 | 1.2 × 102 |
| Candida albicans | 1.4 × 106 | 1.4 × 104 | 1.1 × 102 | 9.5 × 101 | >0.5 | 1.0 × 102 |
| Aspergillus brasiliensis | 1.0 × 106 | 1.0 × 104 | 8.0 × 101 | 5.9 × 101 | >0.5 | 7.2 × 101 |
| Microorganisms | Log Reduction Values (Rx = lgN0 − lgNx) | |||||
|---|---|---|---|---|---|---|
| T7 | Criteria | T14 | Criteria | T28 | Criteria | |
| Escherichia coli | 4.5 | ≥3 | 4.5 | ≥3 and NI | 4.5 | ≥3 and NI |
| Staphylococcus aureus | 4.5 | ≥3 | 4.5 | ≥3 and NI | 4.5 | ≥3 and NI |
| Pseudomonas aeruginosa | 4.6 | ≥3 | 4.6 | ≥3 and NI | 4.6 | ≥3 and NI |
| Candida albicans | 3.1 | ≥1 | 3.1 | ≥1 and NI | 3.1 | ≥1 and NI |
| Aspergillus brasiliensis | 3.0 | - | 3.0 | ≥0 | 3.0 | ≥0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zlabiene, U.; Baranauskaite, J.; Kopustinskiene, D.M.; Bernatoniene, J. In Vitro and Clinical Safety Assessment of the Multiple W/O/W Emulsion Based on the Active Ingredients from Rosmarinus officinalis L., Avena sativa L. and Linum usitatissimum L. Pharmaceutics 2021, 13, 732. https://doi.org/10.3390/pharmaceutics13050732
Zlabiene U, Baranauskaite J, Kopustinskiene DM, Bernatoniene J. In Vitro and Clinical Safety Assessment of the Multiple W/O/W Emulsion Based on the Active Ingredients from Rosmarinus officinalis L., Avena sativa L. and Linum usitatissimum L. Pharmaceutics. 2021; 13(5):732. https://doi.org/10.3390/pharmaceutics13050732
Chicago/Turabian StyleZlabiene, Ugne, Juste Baranauskaite, Dalia M. Kopustinskiene, and Jurga Bernatoniene. 2021. "In Vitro and Clinical Safety Assessment of the Multiple W/O/W Emulsion Based on the Active Ingredients from Rosmarinus officinalis L., Avena sativa L. and Linum usitatissimum L." Pharmaceutics 13, no. 5: 732. https://doi.org/10.3390/pharmaceutics13050732
APA StyleZlabiene, U., Baranauskaite, J., Kopustinskiene, D. M., & Bernatoniene, J. (2021). In Vitro and Clinical Safety Assessment of the Multiple W/O/W Emulsion Based on the Active Ingredients from Rosmarinus officinalis L., Avena sativa L. and Linum usitatissimum L. Pharmaceutics, 13(5), 732. https://doi.org/10.3390/pharmaceutics13050732







