A Safety and Tolerability Study of Thin Film Freeze-Dried Tacrolimus for Local Pulmonary Drug Delivery in Human Subjects
Abstract
1. Introduction
2. Materials and Methods
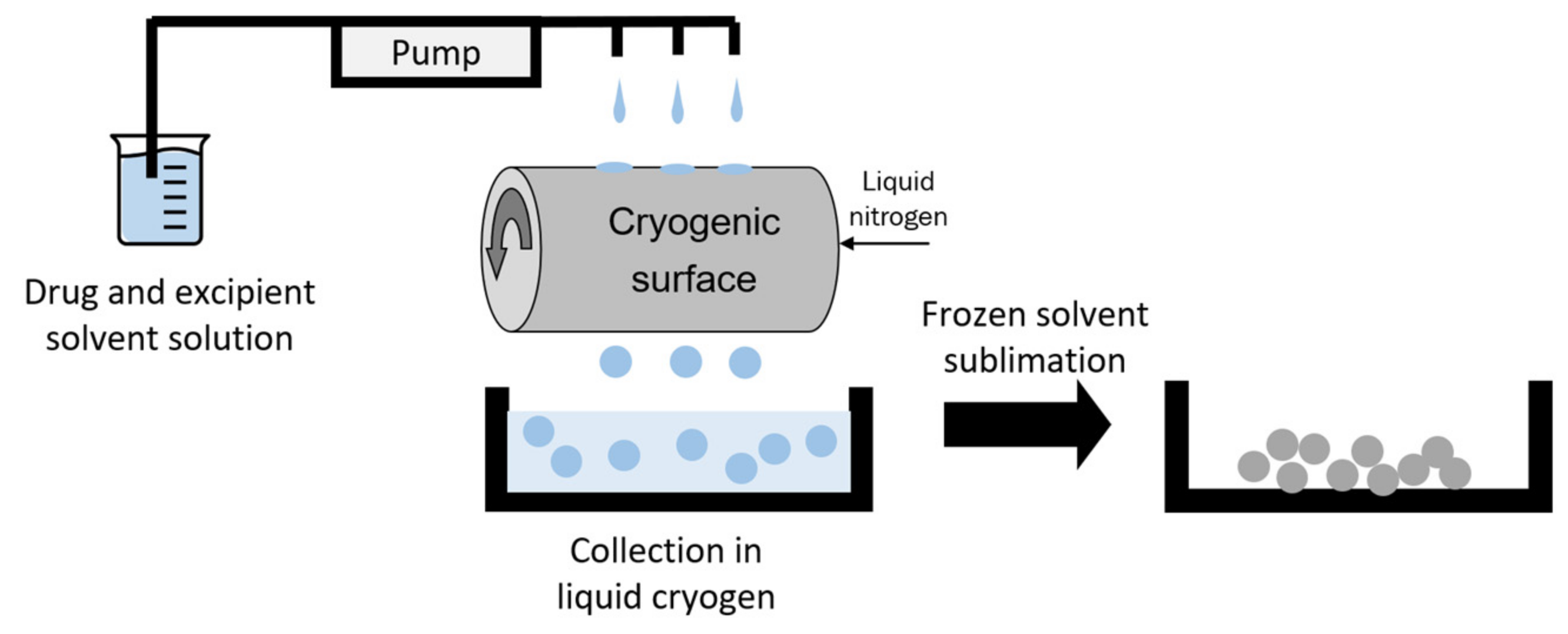
2.1. Materials and Sample Preparation
2.2. Study Design and Population
2.2.1. Stage I—Pulmonary Administration of TFF TAC-LAC Colloidal Dispersion via a Vibrating Mesh Nebulizer
2.2.2. Stage II—Pulmonary Administration of TFF TAC-LAC Inhalable Powder via a DPI
2.3. Exposures, Covariates and Outcomes
2.3.1. Outcomes
2.3.2. Ascertainment of Other Covariates
2.4. Statistical Analysis
3. Results
3.1. Pulmonary Administration of TFF LAC-LAC Colloidal Dispersion via a Nebulizer
3.2. Pulmonary Admistration of TFF LAC-LAC Inhalable Powder via A DPI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yusen, R.D.; Edwards, L.B.; Dipchand, A.I.; Goldfarb, S.B.; Kucheryavaya, A.Y.; Levvey, B.J.; Lund, L.H.; Meiser, B.; Rossano, J.W.; Stehlik, J.; et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J. Heart Lung Transplant. 2016, 35, 1170–1184. [Google Scholar] [CrossRef]
- Chambers, D.C.; Zuckermann, A.; Cherikh, W.S.; Harhay, M.O.; Hayes, D., Jr.; Hsich, E.; Khush, K.K.; Potena, L.; Sadavarte, A.; Singh, T.P.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: 37th adult lung transplantation report—2020; focus on deceased donor characteristics. J. Heart Lung Transplant. 2020, 39, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Bos, S.; Vos, R.; Van Raemdonck, D.E.; Verleden, G.M. Survival in adult lung transplantation: Where are we in 2020? Curr. Opin. Organ Transplant. 2020, 25, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.A.; Dilling, D.F. Immunosuppressive strategies in lung transplantation. Ann. Transl. Med. 2020, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Farmer, J.D., Jr.; Lane, W.S.; Friedman, J.; Weissman, I.; Schreiber, S.L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 1991, 66, 807–815. [Google Scholar] [CrossRef]
- Hachem, R.R.; Yusen, R.D.; Chakinala, M.M.; Meyers, B.F.; Lynch, J.P.; Aloush, A.A.; Patterson, G.A.; Trulock, E.P. A randomized controlled trial of tacrolimus versus cyclosporine after lung transplantation. J. Heart Lung Transplant. 2007, 26, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Keenan, R.J.; Konishi, H.; Kawai, A.; Paradis, I.L.; Nunley, D.R.; Iacono, A.T.; Hardesty, R.L.; Weyant, R.J.; Griffith, B.P. Clinical trial of tacrolimus versus cyclosporine in lung transplantation. Ann. Thorac. Surg. 1995, 60, 580–584; discussion 584–585. [Google Scholar] [CrossRef]
- Zuckermann, A.; Reichenspurner, H.; Birsan, T.; Treede, H.; Deviatko, E.; Reichart, B.; Klepetko, W. Cyclosporine A versus tacrolimus in combination with mycophenolate mofetil and steroids as primary immunosuppression after lung transplantation: One-year results of a 2-center prospective randomized trial. J. Thorac. Cardiovasc. Surg. 2003, 125, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Treede, H.; Klepetko, W.; Reichenspurner, H.; Zuckermann, A.; Meiser, B.; Birsan, T.; Wisser, W.; Reichert, B.; Munich and Vienna Lung Transplant Group. Tacrolimus versus cyclosporine after lung transplantation: A prospective, open, randomized two-center trial comparing two different immunosuppressive protocols. J. Heart Lung Transplant. 2001, 20, 511–517. [Google Scholar] [CrossRef]
- Fan, Y.; Xiao, Y.B.; Weng, Y.G. Tacrolimus versus cyclosporine for adult lung transplant recipients: A meta-analysis. Transplant. Proc. 2009, 41, 1821–1824. [Google Scholar] [CrossRef]
- Gordana Pavliša, A.V.D.; Peter, J.; Miroslav, S. Immunosupressive Therapy In The Lung Transplant Recipient. Med. Sci. 2015, 41, 47–54. [Google Scholar]
- Venkataramanan, R.; Shaw, L.M.; Sarkozi, L.; Mullins, R.; Pirsch, J.; MacFarlane, G.; Scheller, D.; Ersfeld, D.; Frick, M.; Fitzsimmons, W.E.; et al. Clinical Utility of Monitoring Tacrolimus Blood Concentrations in Liver Transplant Patients. J. Clin. Pharmacol. 2001, 41, 542–551. [Google Scholar] [CrossRef]
- Scheffert, J.L.; Raza, K. Immunosuppression in lung transplantation. J. Thorac. Dis. 2014, 6, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Overhoff, K.A.; Johnston, K.P.; Tam, J.; Engstrom, J.; Williams, R.O. Use of thin film freezing to enable drug delivery: A review. J. Drug Deliv. Sci. Technol. 2009, 19, 89–98. [Google Scholar] [CrossRef]
- Sinswat, P.; Overhoff, K.A.; McConville, J.T.; Johnston, K.P.; Williams, R.O., 3rd. Nebulization of nanoparticulate amorphous or crystalline tacrolimus--single-dose pharmacokinetics study in mice. Eur. J. Pharm. Biopharm. 2008, 69, 1057–1066. [Google Scholar] [CrossRef]
- Watts, A.B.; Wang, Y.B.; Johnston, K.P.; Williams, R.O., 3rd. Respirable low-density microparticles formed in situ from aerosolized brittle matrices. Pharm. Res. 2013, 30, 813–825. [Google Scholar] [CrossRef]
- Watts, A.B.; Cline, A.M.; Saad, A.R.; Johnson, S.B.; Peters, J.I.; Williams, R.O., 3rd. Characterization and pharmacokinetic analysis of tacrolimus dispersion for nebulization in a lung transplanted rodent model. Int. J. Pharm. 2010, 384, 46–52. [Google Scholar] [CrossRef]
- Sahakijpijarn, S.; Moon, C.; Ma, X.; Su, Y.; Koleng, J.J.; Dolocan, A.; Williams, R.O., 3rd. Using thin film freezing to minimize excipients in inhalable tacrolimus dry powder formulations. Int. J. Pharm. 2020, 586, 119490. [Google Scholar] [CrossRef]
- Watts, A.B.; Peters, J.I.; Talbert, R.L.; O’Donnell, K.P.; Coalson, J.J.; Williams, R.O., 3rd. Preclinical evaluation of tacrolimus colloidal dispersion for inhalation. Eur. J. Pharm. Biopharm. 2011, 77, 207–215. [Google Scholar] [CrossRef]
- Ide, N.; Nagayasu, T.; Matsumoto, K.; Tagawa, T.; Tanaka, K.; Taguchi, T.; Sumida, Y.; Nakashima, M. Efficacy and safety of inhaled tacrolimus in rat lung transplantation. J. Thorac. Cardiovasc. Surg. 2007, 133, 548–553. [Google Scholar] [CrossRef][Green Version]
- Das, N.A.; Peters, J.I.; Simmons, J.D.; Wang, Y.; O’Donnell, K.P.; DeArmond, D.T.; Coalson, J.J.; Brooks, E.G.; Johnson, S.B. The efficacy of inhaled nanoparticle tacrolimus in preventing rejection in an orthotopic rat lung transplant model. J. Thorac. Cardiovasc. Surg. 2017, 154, 2144–2151.e1. [Google Scholar] [CrossRef] [PubMed]
- Sahakijpijarn, S.; Moon, C.; Koleng, J.J.; Christensen, D.J.; Williams Iii, R.O. Development of Remdesivir as a Dry Powder for Inhalation by Thin Film Freezing. Pharmaceutics 2020, 12, 1002. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.C.; Raghu, G.; Verleden, G.M.; Corris, P.A.; Aurora, P.; Wilson, K.C.; Brozek, J.; Glanville, A.R.; ISHLT/ATS/ERS BOS Task Force Committee. An international ISHLT/ATS/ERS clinical practice guideline: Diagnosis and management of bronchiolitis obliterans syndrome. Eur. Respir. J. 2014, 44, 1479–1503. [Google Scholar] [CrossRef] [PubMed]
- Kroshus, T.J.; Kshettry, V.R.; Savik, K.; John, R.; Hertz, M.I.; Bolman, R.M., 3rd. Risk factors for the development of bronchiolitis obliterans syndrome after lung transplantation. J. Thorac. Cardiovasc. Surg. 1997, 114, 195–202. [Google Scholar] [CrossRef]
- Venkataramanan, R.; Swaminathan, A.; Prasad, T.; Jain, A.; Zuckerman, S.; Warty, V.; McMichael, J.; Lever, J.; Burckart, G.; Starzl, T. Clinical Pharmacokinetics of Tacrolimus. Clin. Pharmacokinet. 1995, 29, 404–430. [Google Scholar] [CrossRef]
- Chambers, D.C.; Cherikh, W.S.; Goldfarb, S.B.; Hayes, D., Jr.; Kucheryavaya, A.Y.; Toll, A.E.; Khush, K.K.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report-2018; Focus theme: Multiorgan Transplantation. J. Heart Lung Transplant. 2018, 37, 1169–1183. [Google Scholar] [CrossRef]
- Ivulich, S.; Dooley, M.; Kirkpatrick, C.; Snell, G. Clinical Challenges of Tacrolimus for Maintenance Immunosuppression Post–Lung Transplantation. Transplant. Proc. 2017, 49, 2153–2160. [Google Scholar] [CrossRef]
- Yusen, R.D.; Christie, J.D.; Edwards, L.B.; Kucheryavaya, A.Y.; Benden, C.; Dipchand, A.I.; Dobbels, F.; Kirk, R.; Lund, L.H.; Rahmel, A.O.; et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report--2013; focus theme: Age. J. Heart Lung Transplant. 2013, 32, 965–978. [Google Scholar] [CrossRef]
- Paradela de la Morena, M.; De La Torre Bravos, M.; Prado, R.F.; Roel, M.D.; Salcedo, J.A.; Costa, E.F.; Rivas, D.G.; Mate, J.M. Chronic kidney disease after lung transplantation: Incidence, risk factors, and treatment. Transplant. Proc. 2010, 42, 3217–3219. [Google Scholar] [CrossRef]
- Bloom, R.D.; Reese, P.P. Chronic kidney disease after nonrenal solid-organ transplantation. J. Am. Soc. Nephrol. 2007, 18, 3031–3041. [Google Scholar] [CrossRef]
- Abu-Elmagd, K.; Fung, J.J.; Alessiani, M.; Jain, A.; Venkataramanan, R.; Warty, V.S.; Takaya, S.; Todo, S.; Shannon, W.D.; Starzl, T.E. The effect of graft function on FK506 plasma levels, dosages, and renal function, with particular reference to the liver. Transplantation 1991, 52, 71–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abu-Elmagd, K.M.; Fung, J.J.; Alessiani, M.; Jain, A.; Takaya, S.; Venkataramanan, R.; Warty, V.S.; Shannon, W.; Todo, S.; Tzakis, A.; et al. Strategy of FK 506 therapy in liver transplant patients: Effect of graft function. Transplant. Proc. 1991, 23, 2771–2774. [Google Scholar]
- Ragette, R.; Kamler, M.; Weinreich, G.; Teschler, H.; Jakob, H. Tacrolimus Pharmacokinetics in Lung Transplantation: New Strategies for Monitoring. J. Heart Lung Transplant. 2005, 24, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Schrepfer, S.; Deuse, T.; Reichenspurner, H.; Hoffmann, J.; Haddad, M.; Fink, J.; Fischbein, M.P.; Robbins, R.C.; Pelletier, M.P. Effect of inhaled tacrolimus on cellular and humoral rejection to prevent posttransplant obliterative airway disease. Am. J. Transplant. 2007, 7, 1733–1742. [Google Scholar] [CrossRef]
- Konstan, M.W.; Flume, P.A.; Kappler, M.; Chiron, R.; Higgins, M.; Brockhaus, F.; Zhang, J.; Angyalosi, G.; He, E.; Geller, D.E. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: The EAGER trial. J. Cyst. Fibros. 2011, 10, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Sommerwerck, U.; Virella-Lowell, I.; Angyalosi, G.; Viegas, A.; Cao, W.; Debonnett, L. Long-term safety of tobramycin inhalation powder in patients with cystic fibrosis: Phase IV (ETOILES) study. Curr. Med. Res. Opin. 2016, 32, 1789–1795. [Google Scholar] [CrossRef]
- Sahakijpijarn, S.; Smyth, H.D.C.; Miller, D.P.; Weers, J.G. Post-inhalation cough with therapeutic aerosols: Formulation considerations. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Sarmento, A.; de Andrade, A.F.D.; Lima, Í.N.D.; Aliverti, A.; de Freitas Fregonezi, G.A.; Resqueti, V.R. Air Stacking: A Detailed Look Into Physiological Acute Effects on Cough Peak Flow and Chest Wall Volumes of Healthy Subjects. Respir. Care 2017, 62, 432–443. [Google Scholar] [CrossRef]
- Jaques, A.; Daviskas, E.; Turton, J.A.; McKay, K.; Cooper, P.; Stirling, R.G.; Robertson, C.F.; Bye, P.T.P.; LeSouëf, P.N.; Shadbolt, B.; et al. Inhaled Mannitol Improves Lung Function in Cystic Fibrosis. Chest 2008, 133, 1388–1396. [Google Scholar] [CrossRef]
- Deuse, T.; Blankenberg, F.; Haddad, M.; Reichenspurner, H.; Phillips, N.; Robbins, R.C.; Schrepfer, S. Mechanisms behind local immunosuppression using inhaled tacrolimus in preclinical models of lung transplantation. Am J. Respir. Cell Mol. Biol. 2010, 43, 403–412. [Google Scholar] [CrossRef]
- Purvis, T.P. Nanoparticle Formulations of Poorly Water Soluble Drugs and Their Action In Vivo and In Vitro; University of Texas Libraries: Austin, TX, USA, 2007. [Google Scholar]
| Lab Parameter | Pre-Inhalation (N = 20) | 24 h Post-Inhalation (N = 20) |
|---|---|---|
| Hemoglobin (Hgb) g/dL | 13.5 ± 1.5 | 13.4 ± 1.5 |
| White Blood Cell (WBC) × 109/L | 6.0 ± 1.9 | 6.2 ± 1.4 |
| Potassium mEq/L | 3.8 ± 0.3 | 3.9 ± 0.2 |
| Blood Urea Nitrogen (BUN) mg/dL | 11.7 ± 3.1 | 11.0 ± 3.1 |
| Creatinine mg/dL | 0.76 ± 0.18 | 0.78 ± 0.22 |
| Aspartate Aminotransferase (AST) IU | 25.2 ± 19.5 | 31.1 ± 8.3 |
| Variable | Pre-Inhalation (N = 20) | 1 h Post-Inhalation (N = 20) | 24 h Post-Inhalation (N = 10) |
|---|---|---|---|
| FEV1 (L) | 3.34 ± 0.69 | 3.31 ± 0.71 | 3.31 ± 0.80 |
| FVC (L) | 4.16 ± 0.83 | 4.08 ± 0.82 | 4.09 ± 0.92 |
| Ratio | 0.80 ± 0.06 | 0.81 ± 0.06 | 0.81 ± 0.06 |
| FEF 25–75% (L/sec) | 3.23 ± 1.13 | 3.33 ± 1.08 | 3.29 ± 1.21 |
| Symptoms | % Number of Patients (N = 20) |
|---|---|
| 1 h following inhalation | |
| • Cough | 5 (1) |
| • Shortness of breath | 5 (1) |
| • Abnormal throat sensation | 15 (3) |
| 24 h following inhalation | |
| • Abnormal taste | 20 (16) |
| 48 h following inhalation | |
| • Abnormal taste and throat sensation | 0 (0) |
| Lab Parameter | Pre-Inhalation (N = 10) | 24-h Post-Inhalation (N = 10) |
|---|---|---|
| Hemoglobin (Hgb) g/dL | 13.4 ± 1.6 | 13.5 ± 1.5 |
| White Blood Cell (WBC) × 109/L | 6.2 ± 1.9 | 6.5 ± 1.4 |
| Potassium mEq/L | 4.0 ± 0.3 | 3.9 ± 0.2 |
| Blood Urea Nitrogen (BUN) mg/dL | 10.7 ± 3.2 | 11.0 ± 3.1 |
| Creatinine mg/dL | 0.71 ± 0.17 | 0.74 ± 0.21 |
| Aspartate Aminotransferase (AST) IU | 25.6 ± 10.5 | 29.1 ± 8.3 |
| Alanine Aminotransferase (ALT) IU | 24.5 ± 10.2 | 25.4 ± 10.6 |
| Alkaline Phosphatase IU | 65.4 ± 18.4 | 64.5 ± 16.3 |
| Variable | Pre-Inhalation (N = 10) | 1 h Post-Inhalation (N = 10) | 24 h Post-Inhalation (N = 10) |
|---|---|---|---|
| FEV1 (L) | 3.36 ± 0.70 | 3.35 ± 0.79 | 3.37 ± 0.81 |
| FVC (L) | 4.16 ± 0.83 | 4.18 ± 0.80 | 4.08 ± 0.90 |
| Ratio | 0.8 1± 0.06 | 0.80 ± 0.05 | 0.82 ± 0.06 |
| FEF 25–75% (L/sec) | 3.30 ± 1.16 | 3.31 ± 1.09 | 3.30 ± 1.19 |
| Symptoms | % Number of Patients (N = 10) |
|---|---|
| During inhalation | |
| • Cough | 80 (8) |
| • Throat irritation | 30 (3) |
| • Distate | 80 (8) |
| 1 h following inhalation | |
| • Cough (mild) | 90 (9) |
| • Distaste (mild) | 100 (10) |
| 24 and 48 h following inhalation | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahakijpijarn, S.; Beg, M.; Levine, S.M.; Peters, J.I.; Williams, R.O., III. A Safety and Tolerability Study of Thin Film Freeze-Dried Tacrolimus for Local Pulmonary Drug Delivery in Human Subjects. Pharmaceutics 2021, 13, 717. https://doi.org/10.3390/pharmaceutics13050717
Sahakijpijarn S, Beg M, Levine SM, Peters JI, Williams RO III. A Safety and Tolerability Study of Thin Film Freeze-Dried Tacrolimus for Local Pulmonary Drug Delivery in Human Subjects. Pharmaceutics. 2021; 13(5):717. https://doi.org/10.3390/pharmaceutics13050717
Chicago/Turabian StyleSahakijpijarn, Sawittree, Moeezullah Beg, Stephanie M. Levine, Jay I. Peters, and Robert O. Williams, III. 2021. "A Safety and Tolerability Study of Thin Film Freeze-Dried Tacrolimus for Local Pulmonary Drug Delivery in Human Subjects" Pharmaceutics 13, no. 5: 717. https://doi.org/10.3390/pharmaceutics13050717
APA StyleSahakijpijarn, S., Beg, M., Levine, S. M., Peters, J. I., & Williams, R. O., III. (2021). A Safety and Tolerability Study of Thin Film Freeze-Dried Tacrolimus for Local Pulmonary Drug Delivery in Human Subjects. Pharmaceutics, 13(5), 717. https://doi.org/10.3390/pharmaceutics13050717







