1. Introduction
Effective treatment of cancers with peritoneal dissemination by cytoreductive surgery is challenging because of the presence of residual tumor cells and micrometastases. The eradication of small intraperitoneal (i.p.) tumors and single cells can be achieved through the use of highly energetic and short-range alpha radiation in combination with a carrier compound that facilitates i.p. containment of the radioactive payload. Radium-224-labeled calcium carbonate microparticles (
224Ra-CaCO
3 MPs) in suspension were developed according to this concept [
1], and their therapeutic potential was evaluated in mice with i.p. ovarian cancer [
2,
3,
4]. As a medically promising alpha-emitter,
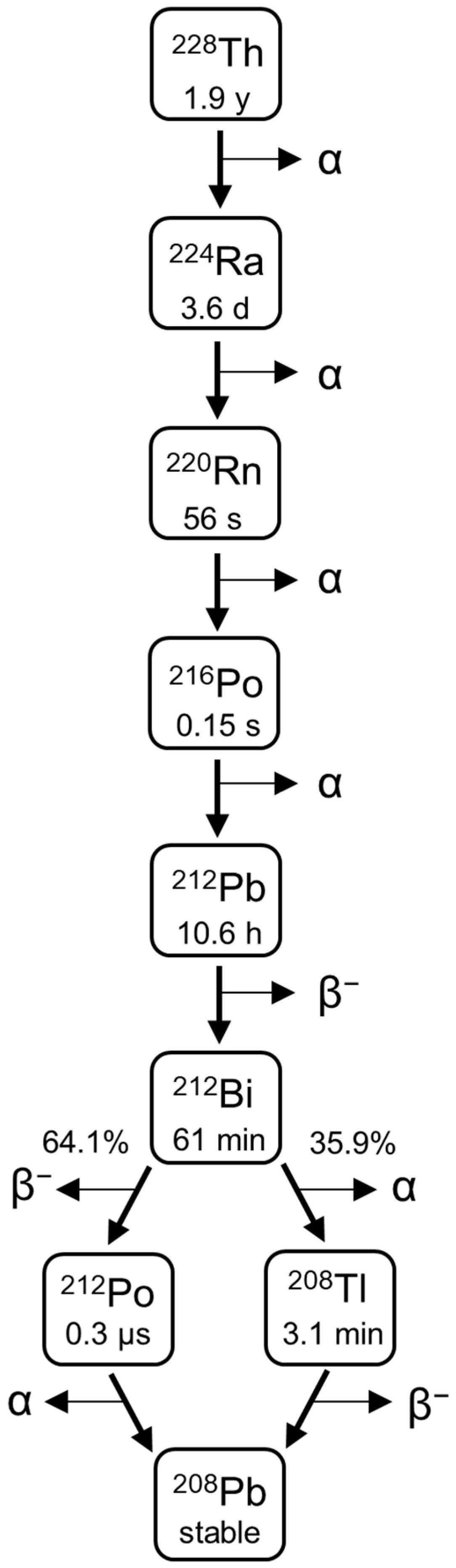
224Ra is a radionuclide with a convenient half-life of 3.6 days. It has more than 90% of its decay energy associated with alpha emissions;
224Ra and its progenies emit four alpha particles in total (
Figure 1). Further,
224Ra can be adsorbed by CaCO
3 MPs, which are suitable carriers because CaCO
3 is nontoxic and biodegradable. We previously demonstrated the importance of the CaCO
3 MPs as a carrier compound by comparing
224Ra-CaCO
3 MPs to free radium-224 dichloride (
224RaCl
2) when both were administered i.p. The
224Ra-CaCO
3 MPs resulted in increased i.p. retention of
224Ra [
1] and extended the survival of mice with tumors [
3]. This radiopharmaceutical (Radspherin) is currently being tested in two ongoing clinical phase I trials, as a postoperative treatment to combat the remaining micrometastatic tumors following the complete cytoreductive surgery of peritoneal carcinomatosis originating from ovarian and colorectal cancer [
5,
6].
One important factor in terms of product stability during the 7-day shelf-life of the radiopharmaceutical is preserving the particle size. The particle size itself influences the ability of MPs to remain suspended, as larger particles settle faster. Calcium carbonate can exist in three different crystalline polymorphs: vaterite, aragonite, and calcite. These different forms have characteristic morphologies: vaterite is typically present as spherical particles, aragonite as needle-like structures, and calcite as rhombohedral particles [
7,
8]. The thermodynamic stability of the polymorphs also varies; calcite is the only stable form. The dissolution and subsequent reprecipitation of CaCO
3 in aqueous solution, a process known as recrystallization, causes a transformation from metastable vaterite or aragonite to stable calcite [
9]. Moreover, recrystallization causes the growth of individual CaCO
3 particles. Larger particles grow at the expense of dissolving smaller ones, in a process known as Ostwald ripening [
10,
11], or the smaller particles may aggregate into larger particles [
11]. This leads to increasing particle diameter with time, especially at elevated temperature [
11]. Therefore, additives that inhibit recrystallization are necessary to control the size of
224Ra-CaCO
3 MPs, which ensures a stable and dispersed suspension of MPs over time.
Various compounds ranging from small molecules to (bio)polymers have been reported to influence the morphology of CaCO
3 crystals, e.g., by inhibiting recrystallization [
7,
10,
12,
13], and therefore, have a stabilizing effect on crystal structure and/or size. Among these, phosphonic acid derivatives, or phosphonates, are interesting candidates. In relation to CaCO
3, these compounds have been used in industrial water management to prevent scale formation attributed to CaCO
3 precipitating on surfaces [
14]. It has been proposed that the phosphonates inhibit CaCO
3 nucleation, adsorb to and block crystal growth sites, distort the crystal lattice, and change the surface charge of crystals [
14,
15]. The phosphonate ethylenediamine tetra(methylene phosphonic acid) (EDTMP) can retard the transformation from vaterite to calcite [
16]. Furthermore, the calcium binding property of phosphonates can be exploited therapeutically due to their consequential skeletal accumulation in diseases such as osteoporosis, where bone resorption by osteoclasts is inhibited by bisphosphonates [
17].
Phosphonates have strong chelation properties toward many divalent metal ions, including radiometals [
18]. Therefore, in combination with their skeletal targeting, phosphonate-based radiopharmaceuticals have been developed to both diagnose and relieve pain from skeletal metastases [
18]. Samarium lexidronam (
153Sm-EDTMP) is approved worldwide for the pain relief of osteoblastic metastatic bone lesions. While radium itself is inherently a bone-seeker, EDTMP has been used to increase the proportion of daughter nuclides
212Pb and
212Bi delivered to the bone in mouse models [
19,
20]. However, if the goal is to ensure radionuclide accumulation at other target sites, the complexation and bone-seeking properties may be problematic. In our application, the CaCO
3 MPs retain
224Ra and progeny, reducing the extraperitoneal release of
224Ra and therefore, the level of
224Ra in the skeleton [
1]. For any stabilizing additive, its influence on the ability of CaCO
3 MPs to retain radionuclides, i.e., radiochemical purity (RCP), must be clarified.
The aim of this work was to evaluate the crystal growth inhibitor and chelator EDTMP as an additive in suspensions of 224Ra-CaCO3 MPs in order to determine both its ability to stabilize the particle size during the shelf-life of the product and any potential negative effect on RCP, the in vivo biodistribution of radionuclides, or therapeutic efficacy.
2. Materials and Methods
2.1. Producing 224Ra-CaCO3 MPs
Calcium carbonate MPs were produced by spontaneous precipitation according to a previously reported procedure [
1,
3,
21], with the exception of the drying process; the collected precipitate was dried under vacuum at 100 °C for 1 h. Radiolabeling was also performed as described earlier [
3], with a few modifications. Radium-224 was extracted from a generator consisting of
228Th (Oak Ridge National Laboratory, Oak Ridge, TN, USA) that was either immobilized on a TrisKem Actinide Resin (TrisKem International, Bruze, France) and eluted in 1 M HCl (Suprapur, Merck Group, Darmstadt, Germany) or temporarily immobilized on a Dowex anion exchange resin (Sigma-Aldrich, St. Louis, MO, USA) and then eluted in 0.5 M HNO
3 (PlasmaPURE Plus, SCP Science, Baie-d’Urfé, QC, Canada) and 80% methanol (Merck Group, Darmstadt, Germany) before evaporation to dryness, which was followed by dissolution in 1 M HCl. In the latter case, the dissolved residue was run through a TrisKem Actinide Resin. In both cases, 5 M NH
4OAc (Sigma-Aldrich, St. Louis, MO, USA) and 1 M NaOH (VWR International, Radnor, PA, USA) were added to obtain a pH of 7.5–9 in the final
224RaCl
2 solution to be used for radiolabeling. The
224RaCl
2 solution was sterile filtered prior to labeling. The CaCO
3 MPs were surface-labeled with
224Ra by incubation in the
224RaCl
2 solution (0.1–2.7 kBq per mg of CaCO
3) in the presence of Ba
2+ (0.004% (
w/
w) relative to CaCO
3) and SO
42− (0.6% (
w/
w) relative to CaCO
3) for the coprecipitation of
224Ra. The labeled MPs were then washed once with 0.9% NaCl (Fresenius Kabi AG, Bad Homburg, Germany) before any addition of EDTMP (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) and final suspension in 0.9% NaCl.
A modified layer-encapsulated 224Ra-CaCO3 MP was prepared by adding an outer CaCO3 layer after labeling in an effort to encapsulate its radioactivity. Calcium carbonate microparticles were first surface-labeled as described earlier. After the removal of the incubation solution, equimolar amounts of Na2CO3 and CaCl2 (Merck Group, Darmstadt, Germany) were added to the labeled MPs under vigorous stirring. This led to an additional precipitation process on the surface of the MPs that would increase the total precipitated CaCO3 mass by a factor of 2.2–2.4, corresponding to a 30–33% increase in the MP diameter, assuming precipitation exclusively took place on the surface of existing MPs. In some cases, additional Ba2+ and SO42− were added during the precipitation process to account for the increase in total CaCO3 mass to final relative concentrations of 0.004% (w/w) and 0.6% (w/w), respectively. The encapsulated and labeled MPs were washed once with 0.9% NaCl before EDTMP was added, and the MPs were suspended in 0.9% NaCl.
In some cases, where radioactivity was deemed unimportant for the study outcome, CaCO3 MPs were either suspended directly in saline (“unlabeled”) or mock-labeled. As for the unlabeled MPs, mock-labeling resulted in a nonradioactive suspension of CaCO3 MPs but involved the same preparation steps and reagents as for the radiolabeled and layer-encapsulated MPs, except for the use of 0.9% NaCl in place of a solution of 224RaCl2.
In all cases, the MPs were suspended in 0.9% NaCl, sealed in a crimp neck glass headspace vial, and sterilized in an autoclave at 121 °C for 20 min. The suspension cooled to room temperature before further handling.
The remaining sections will distinguish surface-labeled MPs from layer-encapsulated MPs for clarity, despite the fact that both were surface-labeled initially.
2.2. Particle Size Measurements
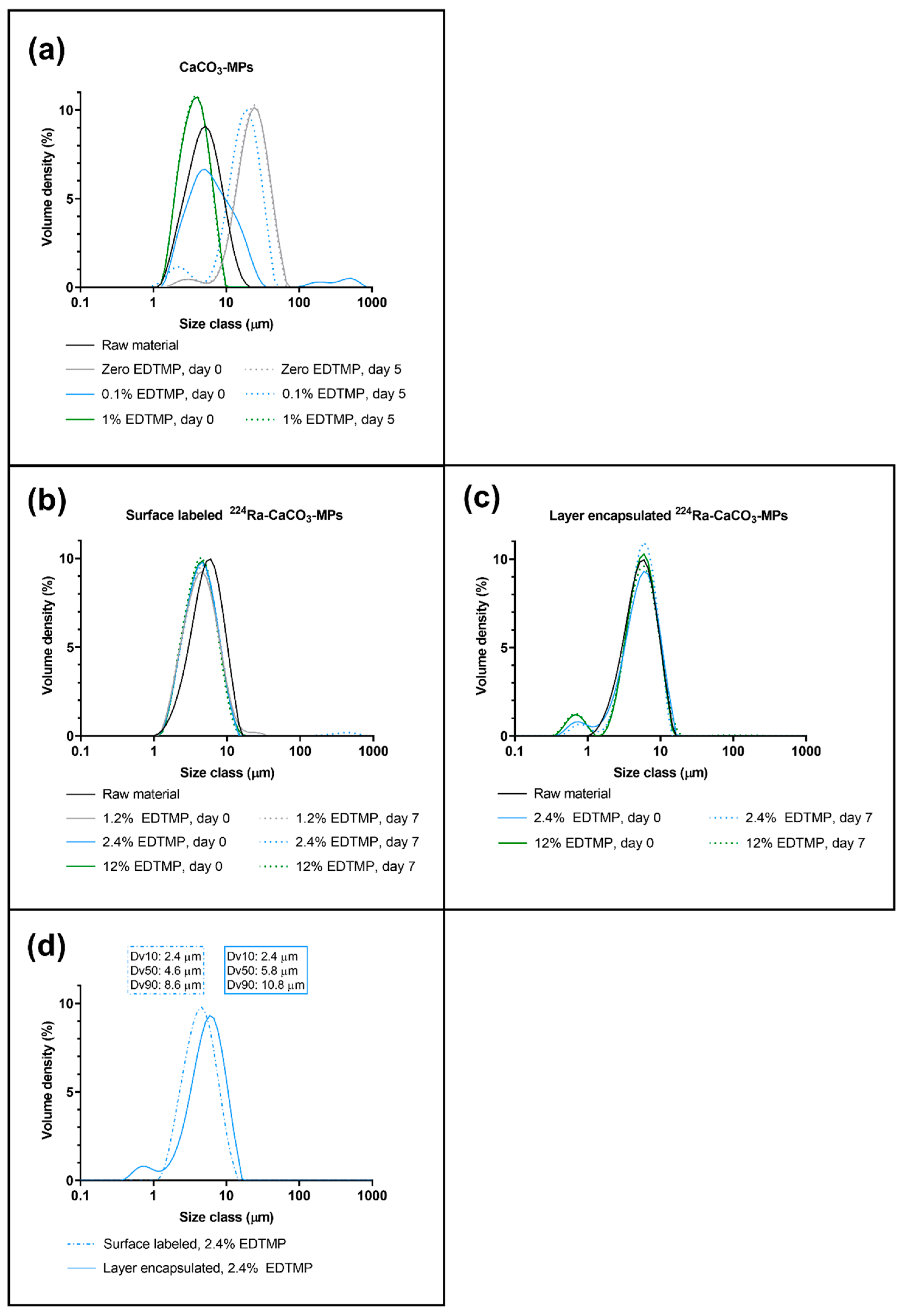
The size of unlabeled, mock-labeled, and radiolabeled CaCO3 MPs in suspension with varying concentrations of EDTMP was measured with laser diffraction (Mastersizer 3000, Malvern Instruments Ltd., Worcestershire, UK). The unautoclaved CaCO3 MPs used as raw material for radio- and mock-labeling were used as a reference by dispersing a small amount of dried CaCO3 MPs in water and ultrasonicating to disperse. Size stability over time was evaluated in radiolabeled CaCO3 MPs by measuring after seven days of storage at room temperature; surface-labeled MPs were compared with layer-encapsulated MPs.
2.3. Influence of EDTMP on the Sedimentation Rate of MPs
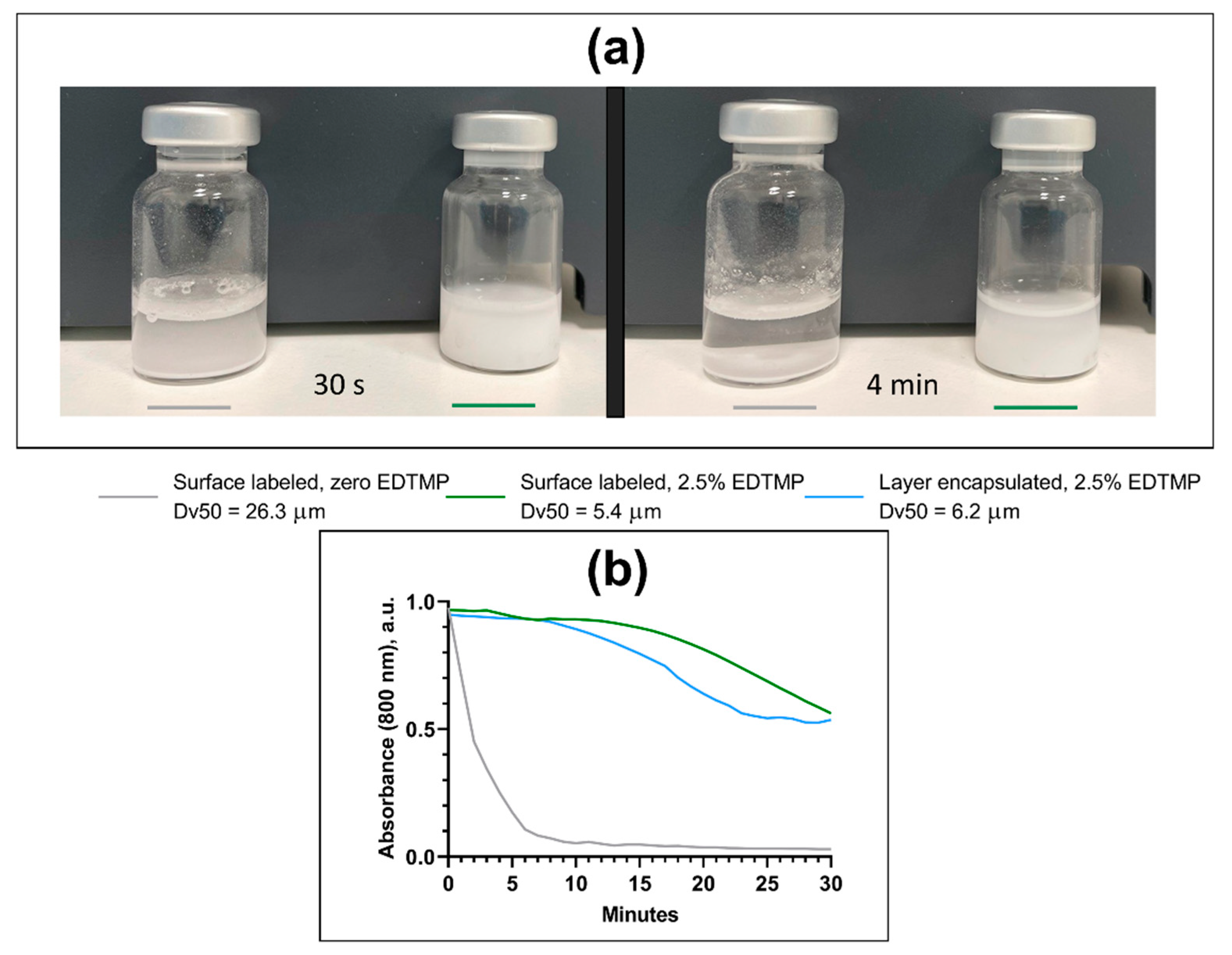
The ability of MPs to remain suspended in solution was evaluated by the sedimentation rate, which was investigated through the visual inspection of samples and by evaluating the turbidity of different suspensions of nonradioactive mock-labeled CaCO3 MPs, with and without EDTMP. Turbidity was assessed by diluting the CaCO3 MP suspension with water (water for injection) and then measuring the change in optical density at a wavelength of 800 nm over 30 min using a spectrophotometer (Hitachi U-1900, Hitachi High-Tech, Tokyo, Japan). The 800 nm wavelength was chosen to reduce potential light absorbance by CaCO3 and improve light scattering by particles. A decrease in optical density with time is, therefore, directly related to decreased light scattering by MPs and thereby, decreased turbidity of the sample due to sedimentation.
2.4. Influence of EDTMP on Radiochemical Properties
The intrinsic product stability of
224Ra-CaCO
3 MPs during shelf-life was assessed by measuring the radiochemical purity and comparing surface-labeled MPs to layer-encapsulated MPs. Radiochemical purity was defined as the percentage of radionuclides retained on the MPs after a certain period. A small aliquot of suspension was separated into MP fraction P and supernatant fraction S by centrifugation. The percentage radiochemical purity, % RCP, was defined as the proportion of radioactivity in the P fraction: CPM(P)/CPM(P+S), with CPM denoting counts per minute. The radioactivity of the two fractions was measured separately using a Hidex Automatic Gamma Counter (Hidex Oy, Turku, Finland). Sample tubes with air-tight lids were used to avoid potential
220Rn escape from the samples [
4]. Radioactivity of
212Pb was quantified by counts in the 60–110 keV window [
22]. For
224Ra, radioactivity was determined indirectly by assuming a transient equilibrium between
224Ra and progeny
212Pb after allowing the two fractions to decay for at least two days and then measuring
212Pb activity in the 65–345 keV window, in which gamma energy and X-rays mainly originated from this daughter. Sampling and measurement were repeated after up to seven days of storage at room temperature to evaluate the stability of
212Pb and
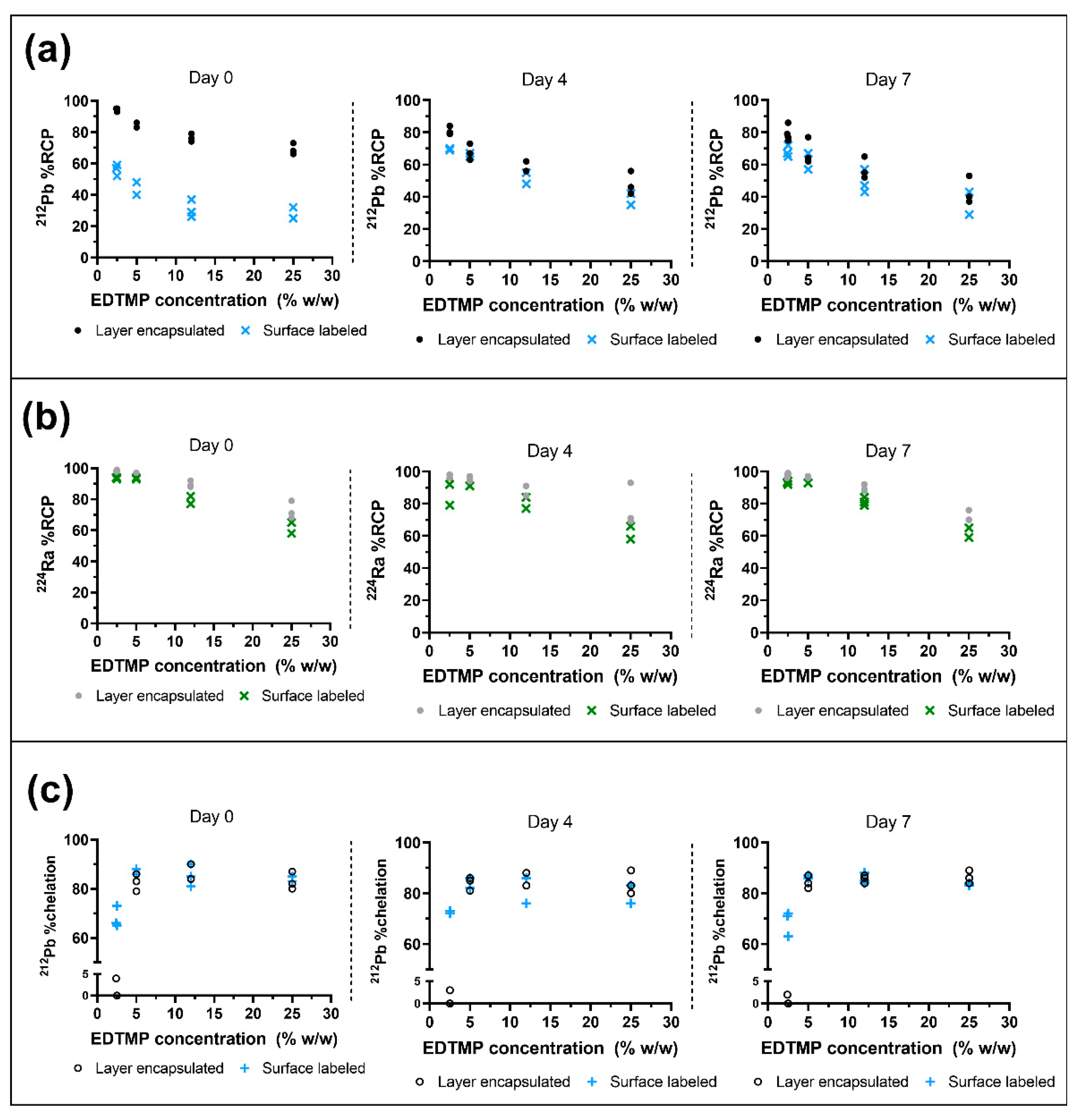
224Ra % RCP over time.
The complexation between the released
212Pb from MPs and EDTMP in the solution was evaluated in the liquid phase of different variants of
224Ra-CaCO
3 MPs. The liquid fraction was first separated from the MPs by centrifugation. The degree of
212Pb-EDTMP complexation in the obtained supernatant was then measured using instant thin-layer chromatography (ITLC) strips (Tec-Control Chromatography Systems #150-772, Biodex Medical Systems, Inc., Shirley, NY, USA). Chelated
212Pb will migrate with the mobile phase in this system, while most (>90%) unbound
212Pb
2+ will remain at the origin line, allowing for the evaluation of
212Pb-EDTMP complexation. Water (pharmaceutical grade) or 0.9% NaCl was used as the mobile phase, and the strips were cut in half after the solvent front had reached the top line. The radioactivity of
212Pb in the two parts was measured with a Hidex Automatic Gamma Counter as described earlier. The degree of chelation was defined by the proportion of migrated
212Pb in the liquid fraction of
224Ra-CaCO
3 MPs and was quantified by subtracting the unspecific migration of free
212Pb
2+ in a 0.9% NaCl solution without EDTMP. Equation (1) describes the percentage chelation; A
212Pb-EDTMP denotes the measured activity in the supernatant of
224Ra-CaCO
3 MPs; A
212Pb denotes the measured activity of free
212Pb
2+ in the 0.9% NaCl solution, and
m and
o denote the two parts of the ITLC strip;
m denotes migration with the mobile phase and
o denotes the origin line.
2.5. Biodistribution
The biodistribution of layer-encapsulated
224Ra-CaCO
3 MPs with added EDTMP was evaluated in institutionally bred nontumor-bearing female athymic nude mice (Hsd: Athymic Nude-
Foxn1nu, Department of Comparative Medicine, The Norwegian Radium Hospital, Oslo University Hospital, Oslo, Norway). Calcium carbonate microparticles were labeled and autoclaved as described earlier. The impact of mass dose (mg dose) was considered by testing doses ranging from 1–12 mg CaCO
3 and 6–18 kBq by creating dilutions with an isotonic infusion solution (Plasmalyte, Baxter International Inc., Deerfield, IL, USA). One day after a single i.p. administration, the mice were euthanized by cervical dislocation, and tissue samples were obtained to measure radioactivity. Three standard samples corresponding to 25–50% of the administered dose of each treatment were used to determine the injected radioactivity dose. The radioactivity of
212Pb and
224Ra of tissue and standard samples was measured using a gamma counter as described above, from which the percentage injected dose per gram tissue (% ID/g) was calculated. Correction for decay and/or ingrowth of
224Ra and
212Pb was not performed in the calculation of the % ID/g for two reasons: firstly, standard samples and tissue samples were counted with less than 2–3 h time interval (i.e., 3% of the half-life of
224Ra), and secondly, error propagation as a result of uncertainty in the measurement of
224Ra, when the measured activity was close to or below the limit of quantification of the instrument, could be avoided. As a reference for the skeletal accumulation of free
224Ra
2+ one day after i.p. injection, one group of mice received ~30 kBq
224RaCl
2 prepared as described previously [
3]. An overview of the experimental groups is provided in
Supplementary Table S1.
2.6. Therapeutic Efficacy
The therapeutic effect of radiolabeled MPs of different sizes with
224Ra adsorbed either on the surface or beneath an outer protective layer was evaluated. Layer-encapsulated
224Ra-CaCO
3 MPs suspended in EDTMP (1% (
w/
w) relative to CaCO
3) and 0.9% NaCl solution were compared to that of the surface-labeled variant suspended in 0.9% NaCl only, by evaluating the survival rate of mice with tumors. To establish i.p. ovarian cancer xenografts, nude mice aged 4–5 weeks were inoculated with 1 million ES-2 cells (ATCC, Wesel, Germany) [
2,
3,
4]. For a syngeneic colorectal cancer model, BALB/c mice (BALB/cAnNRj, Janvier Labs, Le Genest-Saint-Isle, France) aged six weeks were inoculated i.p. with 50,000 CT26.WT cells (LGC Standards, ATCC, Wesel, Germany) in a study managed by Minerva Imaging (Ølstykke, Denmark). In both cases, mice were randomized and received a single i.p. injection of autoclaved
224Ra-CaCO
3 MPs (14–26 kBq, 4–14 mg), or 0.9% NaCl as vehicle control, one day after tumor inoculation. The dosing was selected based on previously tested efficacious doses with former generations of unautoclaved
224Ra-CaCO
3 MPs in the ES-2 model [
2,
3,
4]. An overview of the experimental groups can be found in
Supplementary Table S2. Animals were supplied with food and water ad libitum and euthanized by cervical dislocation when reaching predetermined study endpoints, which included rapid body weight loss or severe build-up of ascites. Animals were censored if they lived beyond the timepoint corresponding to three times the median survival time of the longest surviving group.
2.7. Statistical Analysis
A statistical analysis of the differences between the experimental groups in the animal studies was performed with GraphPad Prism (Version 8.1.2, GraphPad Software, San Diego, CA, USA). A t-test was performed on each pair of experimental groups in the biodistribution study to detect differences in the % ID/g while adjusting the obtained p-value to account for multiple comparisons using the Holm–Sidak method. In the studies of therapeutic efficacy, differences in the survival curves were analyzed using the Gehan–Breslow–Wilcoxon method while adjusting the obtained p-values using the Holm–Sidak method. In both cases, an adjusted p < 0.05 was considered statistically significant.
4. Discussion
This work has shown that the size of 224Ra-CaCO3 MPs in suspension can be stabilized for at least one week by adding the recrystallization inhibitor EDTMP to the suspension and that the percentage of the daughter nuclide 212Pb retained on the MPs can be increased by an outer encapsulating CaCO3 surface layer.
Additives that inhibit recrystallization are necessary to prevent the growth of CaCO
3 MPs in suspension, while the particle size itself is important for the MP to remain suspended. The present work considered only autoclaved suspensions of MPs, contrasting with our previously published work on the
224Ra-CaCO
3 MPs [
1,
2,
3,
4], because a sterilization procedure is compulsory for a radiopharmaceutical intended for clinical use. The growth of CaCO
3 MPs with no EDTMP added was detected immediately after the suspension was autoclaved. Even though the increased particle diameter remained stable thereafter, the particles sedimented fast and the suspension was difficult to disperse. The size distribution of CaCO
3 MPs remained constant from unlabeled raw material to radiolabeled MPs in suspension when EDTMP was added. It is important to note that the sedimentation rate was substantially reduced when the particle size was decreased and that the smaller MPs were easier to disperse and handle, resulting in a significant advantage in terms of clinical administration of the product.
After obtaining size control via EDTMP, layer encapsulation was introduced to optimize the radiochemical properties of the
224Ra-CaCO
3 MPs, as EDTMP is known to chelate
212Pb and other divalent metals [
18,
19,
20]. The presence of an outer CaCO
3 layer on CaCO
3 MPs that had already been surface-labeled was supported by comparing their size distribution to that of CaCO
3 MPs that were only surface-labeled. The slight increase in MP diameters from the surface-labeled analog did not influence their ability to disperse. However, the additional precipitation process resulted in the formation of a small volume of submicrometer CaCO
3 particles that could only be detected after layer-encapsulation.
It was suspected that complexation of
212Pb with EDTMP would result in a decrease in MP-bound
212Pb, which could potentially lead to the undesired release of
212Pb to systemic circulation in vivo and localization of
212Pb-EDTMP to the skeleton. The largest difference between surface-labeled and layer-encapsulated
224Ra-CaCO
3 MPs with EDTMP was detected in their ability to retain
212Pb. At 2.4–2.5% (
w/
w) EDTMP relative to CaCO
3, the
212Pb % RCP of layer-encapsulated
224Ra-CaCO
3 MPs was highest on day zero (94%) and remained above 79% on average over the course of seven days. The cumulative amount of the chemically equivalent stable daughter nuclide
208Pb (
Figure 1) adsorbed on the MPs increases with time (
Supplementary Figure S2), although this seems to have little effect on the adsorption of
212Pb. For the surface-labeled variant at this EDTMP concentration, % RCP of
212Pb was only 56% on day zero, with an increase to 70% on day four. The increased adsorption of
212Pb from day zero to day four was a general observation for surface-labeled MPs. For both MP variants, the persistence of adsorbed
212Pb over time is a result of either retention of
224Ra daughters after decay, reassociation of released
212Pb, or a combination of the two. We have previously shown that a certain emanation of the gaseous
220Rn, the parent of
212Pb, occurs from
224Ra-CaCO
3 MPs, with
212Pb being substantially readsorbed by the MPs [
4]. For the optimized formulation herein, readsorption may also be mediated by EDTMP, as released
212Pb
2+ is sequestered by EDTMP at sufficient EDTMP concentration. The known complexation property of EDTMP with both
212Pb and calcium indicates that it is also possible for the
212Pb-EDTMP complex to associate with the MPs. To test this hypothesis, adsorption of
212Pb on nonradioactive mock-labeled CaCO
3 MPs, both with and without layer-encapsulation, was evaluated after the addition of a solution of
212Pb-EDTMP. It was found that 17–20% of the
212Pb-EDTMP adsorbed on the MPs; the adsorption increased to 96% when unbound
212Pb
2+ (
212PbCl
2) was added instead (
Supplementary Figure S3). The reduced adsorption of
212Pb-EDTMP is in line with the general observation that the % RCP of
212Pb of both surface-labeled MPs and layer-encapsulated MPs decreased at higher EDTMP concentrations in the MP suspension. The exact distribution of EDTMP in solution versus on the MPs themselves is not known, although it can be argued that the majority is adsorbed on the MPs due to the low presence of chelated
212Pb in solution for the layer encapsulated
224Ra-CaCO
3 MPs with 2.4–2.5% (
w/
w) EDTMP (
Figure 4c).
The stable particle size in combination with the promising radioactivity-retention properties of the layer-encapsulated
224Ra-CaCO
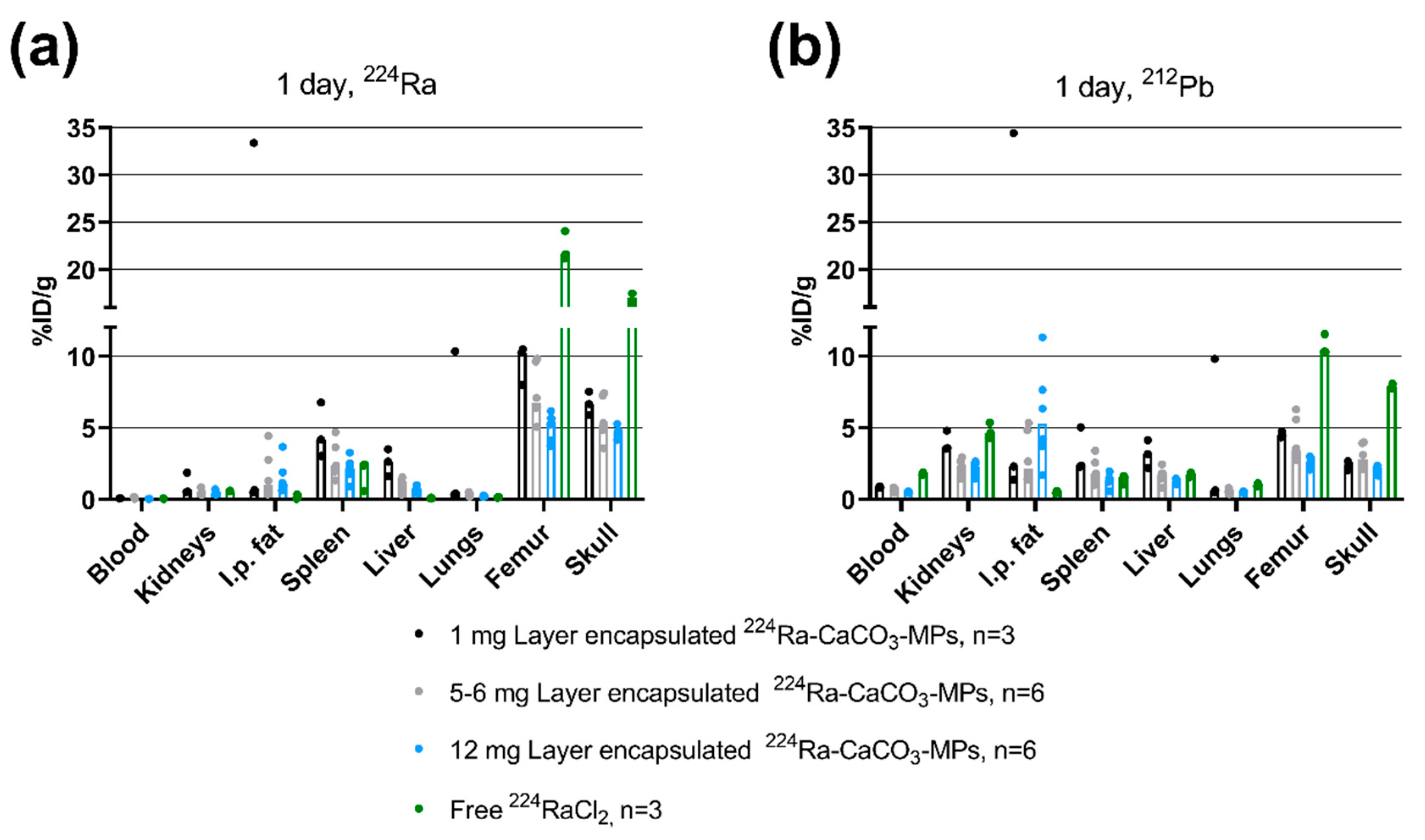
3 MPs with low EDTMP concentration warranted further investigation in animal models. A suitable biodistribution pattern of both
212Pb and
224Ra was achieved after i.p. administration at 1.2–2.5% (
w/
w) EDTMP. The substantially decreased % ID/g of both
212Pb and
224Ra detected in the skeleton when compared with i.p. injection of
224RaCl translates to low levels of radionuclides leaking from the peritoneal cavity. The slightly higher skeletal activity for the 1 mg dose as compared to 5–12 mg is attributed to the difference in specific activity, i.e., activity per CaCO
3 mass (1–1.8 kBq/mg vs. ~6 kBq/mg), which is in line with previous work [
1,
3]. Low levels of
212Pb were detected in the skeleton as compared to the previously published biodistribution results of
212Pb-EDTMP after i.v. administration to mice [
19], indicating a limited release of any potential
212Pb-EDTMP to systemic circulation. The localization of EDTMP to bone is attributed to its affinity to hydroxyapatite and is dependent on calcium concentration rather than the number of osteoblasts [
23]. Hence, the presence of Ca
2+ from CaCO
3 may possibly contribute to retaining EDTMP on MPs within the peritoneal cavity, at least within the relatively short half-lives of
224Ra and its daughters.
The loading of radionuclides into carrier MPs for tumor radiotherapy is an approach that has historically been used for beta emitters. [
24]. For alpha emitters, the incorporation of the nuclide into the bulk of the carrier can be advantageous as a means of retaining recoiling daughter nuclides to prevent unintentional escape and redistribution in vivo. Incorporation of alpha emitters such as
225Ra/
225Ac, and/or
223Ra into nanoparticles has been reported in liposomes [
25,
26], LaPO
4 [
27], LaVO
4 [
28] and hydroxyapatite [
29], among others. Further, a study of
225Ac-labeled CaCO
3 MPs was recently published, with the
225Ac incorporated into MPs and submicron particles that were coated with a protein and polyphenol on the particle surface as stabilizing agents [
30]. Ensuring that the encapsulation does not compromise the already short range of the alpha particles (50–100 µm in tissue) is a prerequisite for alpha emitters to be carried inside MPs. The MP size and the thickness of the encapsulating layer of
224Ra-CaCO
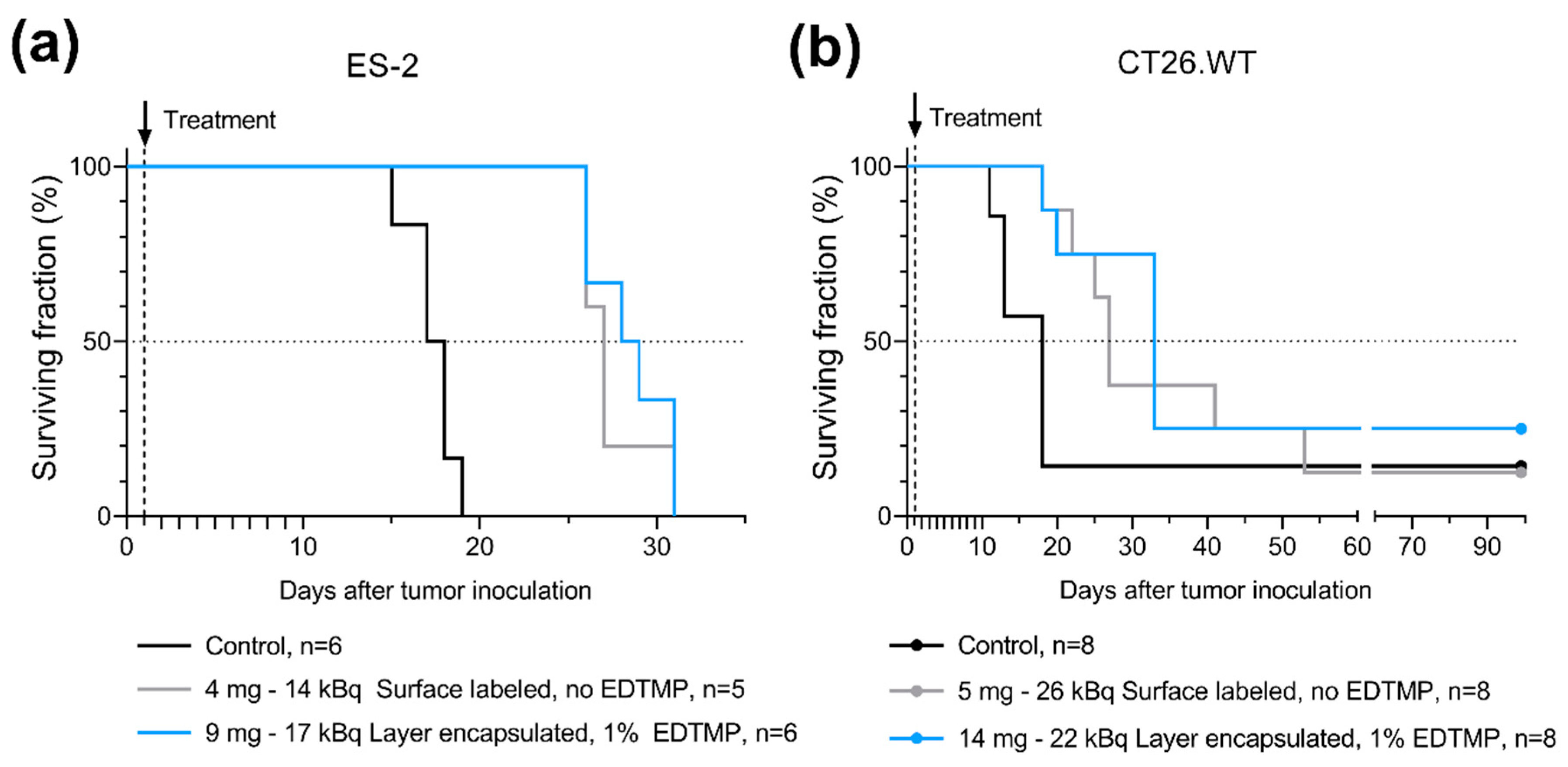
3 MPs are both very small compared to the range of alpha particles and are therefore not expected to significantly limit the range. However, size-dependent sedimentation differences may potentially affect the distribution of the infused microparticles. In this work, the data from studies of mice with tumors was inconclusive in terms of the potential influence of particle size and EDTMP on survival. A survival benefit in mice was observed in two different tumor models after a single i.p. injection, but no statistical difference was detected when the smaller layer-encapsulated
224Ra-CaCO
3 MPs with EDTMP were compared to the larger surface-labeled
224Ra-CaCO
3 MPs without EDTMP.
Ongoing clinical studies (NCT03732768 [
5], NCT03732781 [
6]) with
224Ra-CaCO
3 MPs (Radspherin) have been initiated on the basis of the promising preclinical data on layer-encapsulated
224Ra-CaCO
3 MPs with 1.2–2.5% (
w/
w) EDTMP added.