1. Introduction
Programmed cell death protein 1 (PD-1) is a cell surface receptor usually expressed on activated T cells [
1]. Programmed cell death ligand 1 (PD-L1) is overexpressed in various cancerous cells, and overexpressed PD-L1 often binds with PD-1 and inhibits the antitumor immune response mediated by T cells, eventually causing the immune evasion of tumors [
2,
3]. PD-1/PD-L1 checkpoint pathway blocking is an effective immunotherapy strategy for cancer treatment [
4]. In the past decade, several specific PD-1/PD-L1 antibodies have been developed and marketed as anti-cancer drugs that have achieved spectacular success in anti-cancer treatments. Moreover, the number of anti-PD-1/PD-L1 antibodies is still rapidly increasing, and they are clinically proven to treat various malignancies [
5]. However, antibody-derived drugs usually have inherent disadvantages, such as the need for intravenous injections, complex production, instability, high cost, low penetration into tissues, and immune-related adverse events [
6,
7]. Thus, it is desirable to develop small-molecule PD-1/PD-L1 inhibitors, which are expected to overcome the problems with antibody-derived drugs but retain the desirable anti-cancer efficacy [
8].
Since the crystal structure of the human PD-1/PD-L1 complex was first published in 2015, several small-molecule PD-1/PD-L1 inhibitors with potent inhibitory activities have been reported [
9]. YPD-29B blocks the binding of PD-1 and PD-L1 with an IC
50 of less than 10
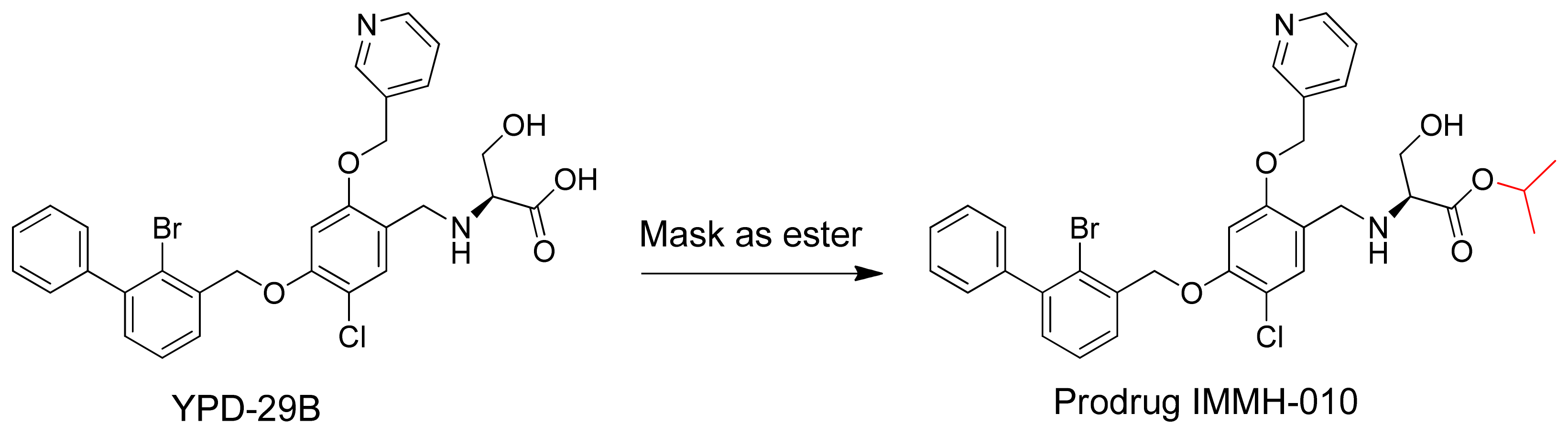
−13 M (patent: CN109153670), measured using a homogeneous time-resolved fluorescence protein-protein interaction assay. However, during drug chemistry, manufacturing, and controls (CMC), YPD-29B encountered intractable problems. Thus, the carboxylic acid of YPD-29B was masked, giving the ester prodrug IMMH-010 (
Figure 1). IMMH-010 is expected to be hydrolyzed efficiently to produce YPD-29B as the active metabolite in vivo. In addition, IMMH-010 exhibited some PD-L1 inhibitory activity with an IC
50 of 10
−8–10
−7 M. Currently, IMMH-010 is in phase I clinical trials [
10].
Although the prodrug strategy can improve the druggability of some compounds, it is necessary to investigate prodrug metabolism to determine whether the parent compound is released in vivo as desired. In addition, mouse models are widely used in various pharmacology studies, particularly in anti-cancer research. However, there are differences in metabolism among species. For example, there are species and tissue differences in esterase activities that cause species differences in hydrolase activity and efficacy [
11]. Thus, prodrug metabolism should also be investigated in different species, particularly the pharmacokinetics (PK)/pharmacodynamics (PD) relationship. These data provide important information for the drug CMC development and for clinical applications.
In this study, we clarify the metabolic pathway of IMMH-010, compare the differences in IMMH-010 metabolism among species in vivo and in vitro, and analyze the in vivo PK/PD relationship of IMMH-010 in tumor-bearing mice.
2. Materials and Methods
2.1. Chemicals and Reagents
IMMH-010, YPD-29B (M1, purity ≥99%), M2, M3, and M4 (purity ≥98%) were synthesized by the Department of Medicinal Chemistry (Institute of Materia Medica, Chinese Academy of Medical Sciences). β-Nicotinamide adenine dinucleotide phosphate (NADP), glucose-6-phosphate, glucose-6-phosphate dehydrogenase, midazolam, telmisartan, and phenacetin were obtained from Sigma Aldrich (St. Louis, MO, USA). Lidocaine and CPT-11 were purchased from J&K Scientific Ltd. (Beijing, China). Male mouse and rat liver microsomes and liver S9 homogenate fractions were prepared by our group. Pooled mixed-gender human liver microsomes (HLM); male beagle dog and monkey liver microsomes; pooled mixed-gender dog, monkey, and human liver S9 homogenate fractions; recombinant human cytochrome P450s (CYPs); and recombinant human flavin-containing monooxygenases (FMOs) were obtained from BD Gentest (Woburn, MA, USA). HPLC-grade acetonitrile and methanol were purchased from Merck (Darmstadt, Germany). Deionized water was obtained from a Milli-Q system (Millipore Co., Billerica, MA, USA). All other reagents and solvents were commercially available. Recombinant human carboxylesterases (CESs; CES1 and CES2) were purchased from Cypex Ltd. (Dundee, United Kingdom), and recombinant human arylacetamide deacetylase (AADAC) was purchased from CUSABIO Biotech Co., Ltd. (Wuhan, China).
2.2. Animals
Eight-week-old male C57BL/6 mice and six-week-old male Sprague-Dawley rats were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Standard pelleted laboratory chow and water were allowed ad libitum. The animal protocol followed was approved by the Experimental Animal Management and Welfare Committee at the Institute of Materia Medica, Peking Union Medical College. Healthy male cynomolgus monkeys (3–4 years old, approximately 3 kg) were purchased from Guangdong Frontier Biotechnology Co., Ltd. (Guangzhou, China). The animal protocol was approved by the Animal Experimental Ethics Committee of Joinn Laboratories (Joinn Laboratories, Inc., Beijing, China) (No.ACU18-324) and the Experimental Animal Management and Welfare Committee at the Institute of Materia Medica, Peking Union Medical College (No. 00000522, No. 00000523, No. 00000538, No. 00000539).
2.3. Identification of IMMH-010 Metabolites
After oral administration of IMMH-010 maleate (5 mg/kg), two rats were housed in metabolic cages to collect urine and feces samples for 24 h. Feces samples were homogenized with methanol at 1:10 (w/v) and urine samples were diluted 10-fold in methanol. Additionally, two rats were bile duct cannulated under isoflurane anesthesia. After oral administration of IMMH-010 maleate (5 mg/kg), bile samples were collected over 0–2 h after dosing.
LC-MS was performed using a Thermo Scientific Q Exactive HF hybrid quadrupole-Orbitrap mass spectrometer. Reverse-phase liquid chromatography was performed on a Zorbax C18 column (100 × 2.1 mm, 3.5 μm). Mobile phase A was 0.1% aqueous formic acid and mobile phase B was 0.1% formic acid in methanol. The mobile phase was delivered with the following gradient elution profile: 0 min, 10% B; 15 min, 40% B; 17–19 min, 100% B; 20–23 min, 10% B. The total runtime for each injection was 23 min. The flow rate was 0.3 mL/min, and the column oven was maintained at 30 °C.
The analysis was performed in full mass spectrometry with data-dependent tandem mass spectrometry (MS/dd-MS2) mode. For a full mass spectrometry scan, the selected scan range was from m/z 100 to 500, the resolution was 70,000, and the automatic gain control (AGC) target was set to 1.0 × 106 with a maximum injection time (IT) of 100 ms. For the dd-MS2 scan, the fragmentation mass spectra were recorded at a mass resolving power of 17,500 fwhm with a quadrupole isolation window of 1.5 m/z for precursor ions. The AGC target was 2.0 × 105 and the maximum IT was 50 ms.
Xcalibur 4.1 software (Thermo Fisher Scientific, San Jose, CA, USA) was used for instrument control and data processing. Compound Discover 2.0 software was used for metabolite identification.
2.4. IMMH-010 Metabolism in Plasma, S9 Fractions and Microsomes from Liver and Intestine
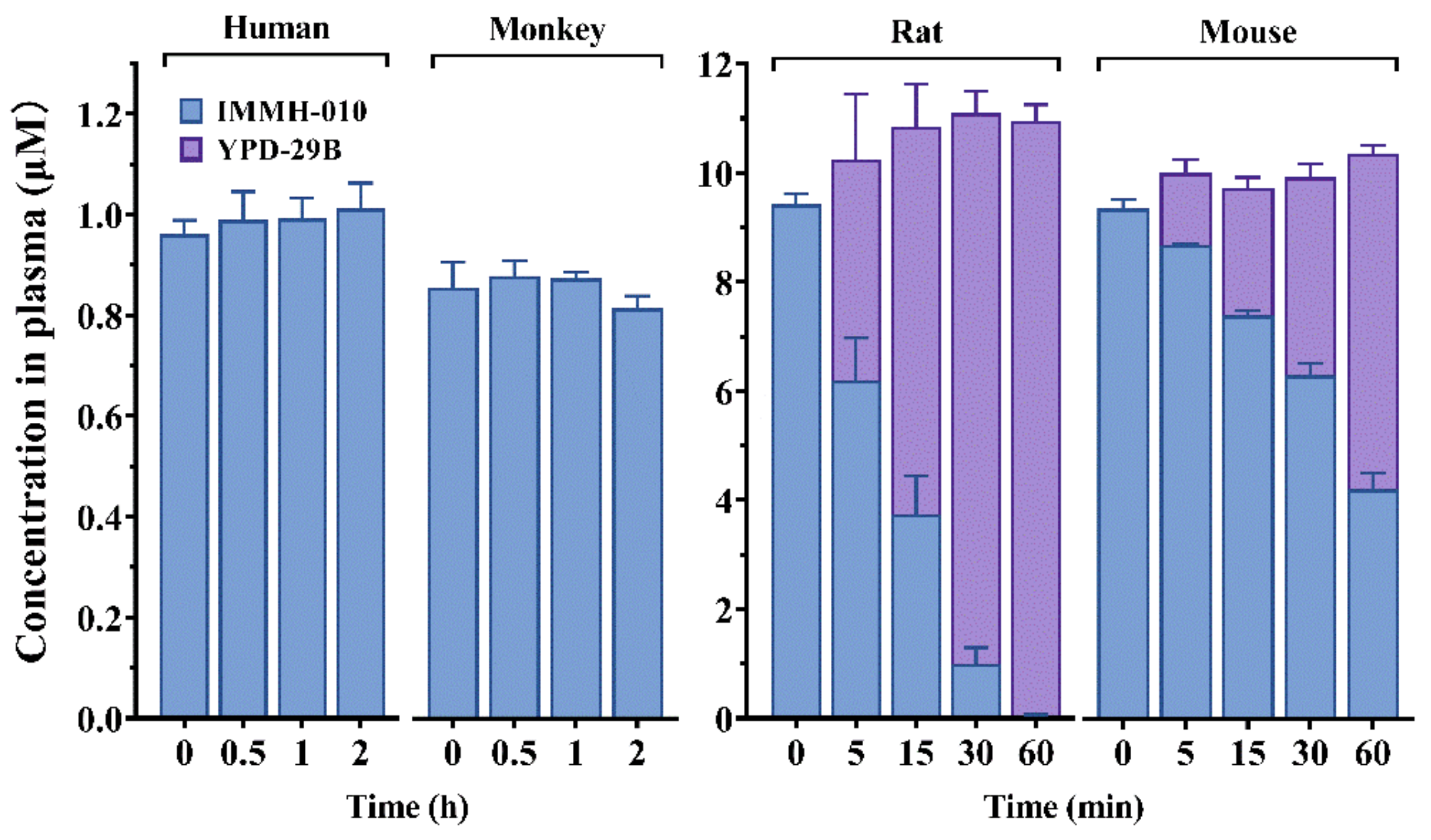
IMMH-010 hydrolysis was investigated in human, monkey, rat, and mouse plasma. IMMH-010 (1 μM) was incubated in human, monkey, or dog plasma (0.2 mL) at 37 °C for 120 min. IMMH-010 (10 μM) was incubated in rat or mouse plasma (0.2 mL) at 4 °C for 60 min. The experiments were performed in triplicate. The reactions were initiated by the addition of IMMH-010 (10 μM). After incubation, the reactions were stopped by adding 2 volumes of ice-cold acetonitrile. After vortexing and centrifuging, the concentrations of IMMH-010 and YPD-29B in the supernatant were determined by liquid chromatography with tandem mass spectrometry (LC-MS/MS). The compound olmesartan (10 μM), a substrate of paraoxonase 1, was set as the positive control.
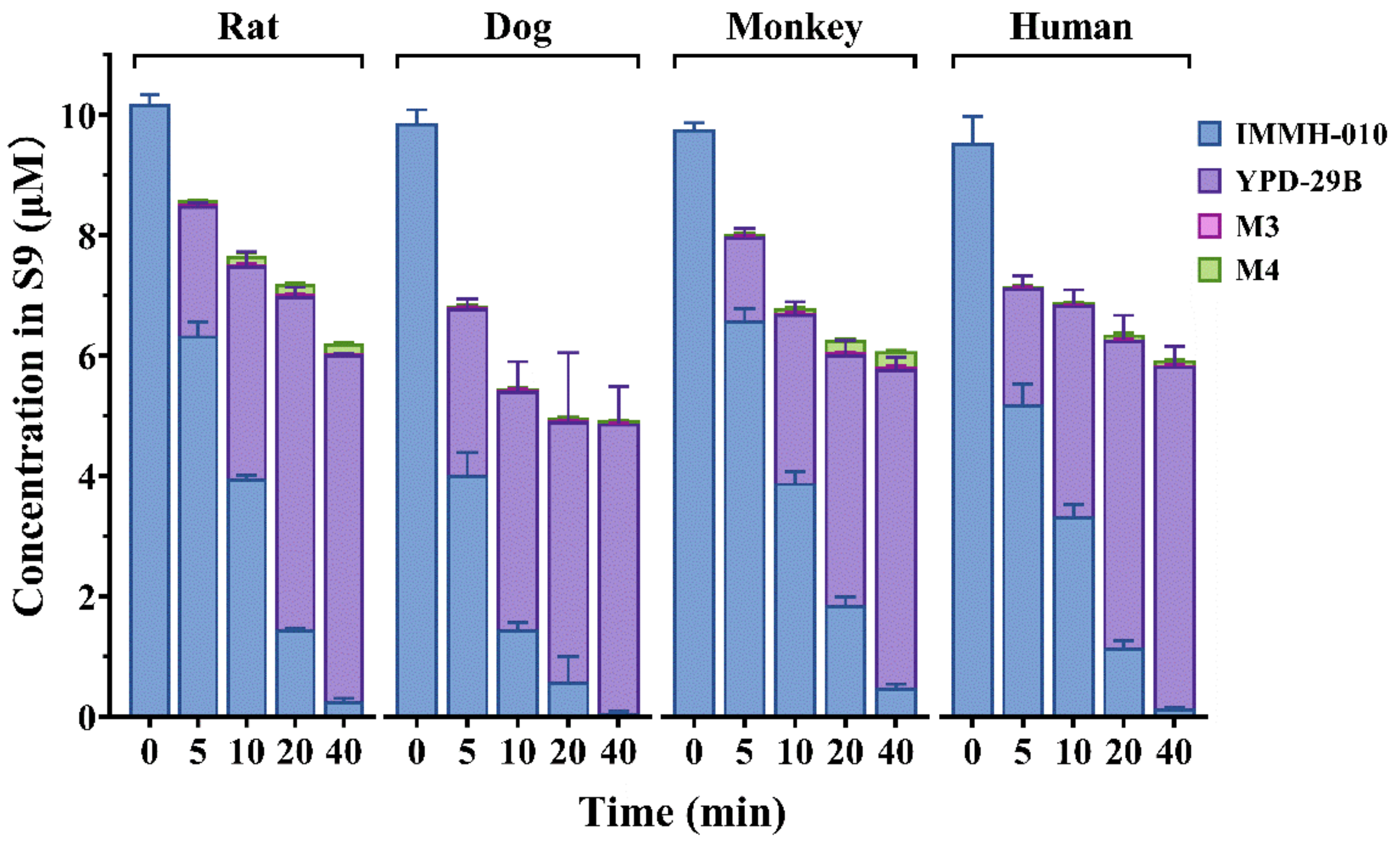
IMMH-010 metabolism was compared in human, monkey, dog, and rat liver S9 homogenates. IMMH-010 (10 μM) was incubated with liver S9 homogenate protein (0.5 mg protein/mL) and a reduced nicotinamide adenine dinucleotide phosphate (NADPH) regenerating system in a final volume of 0.2 mL Tris-HCl buffer (50 mM, pH 7.4) containing 5 mM MgCl2. The reactions were performed in triplicate. After 2 min of preincubation at 37 °C, reactions were initiated by the addition of IMMH-010 (10 μM). The samples were collected 0, 5, 10, and 20 min after incubation, 2 volumes of acetonitrile were added to terminate the reaction. After vortexing and centrifugation, IMMH-010, YPD-29B, M3, and M4 in the supernatant were analyzed by LC-MS/MS.
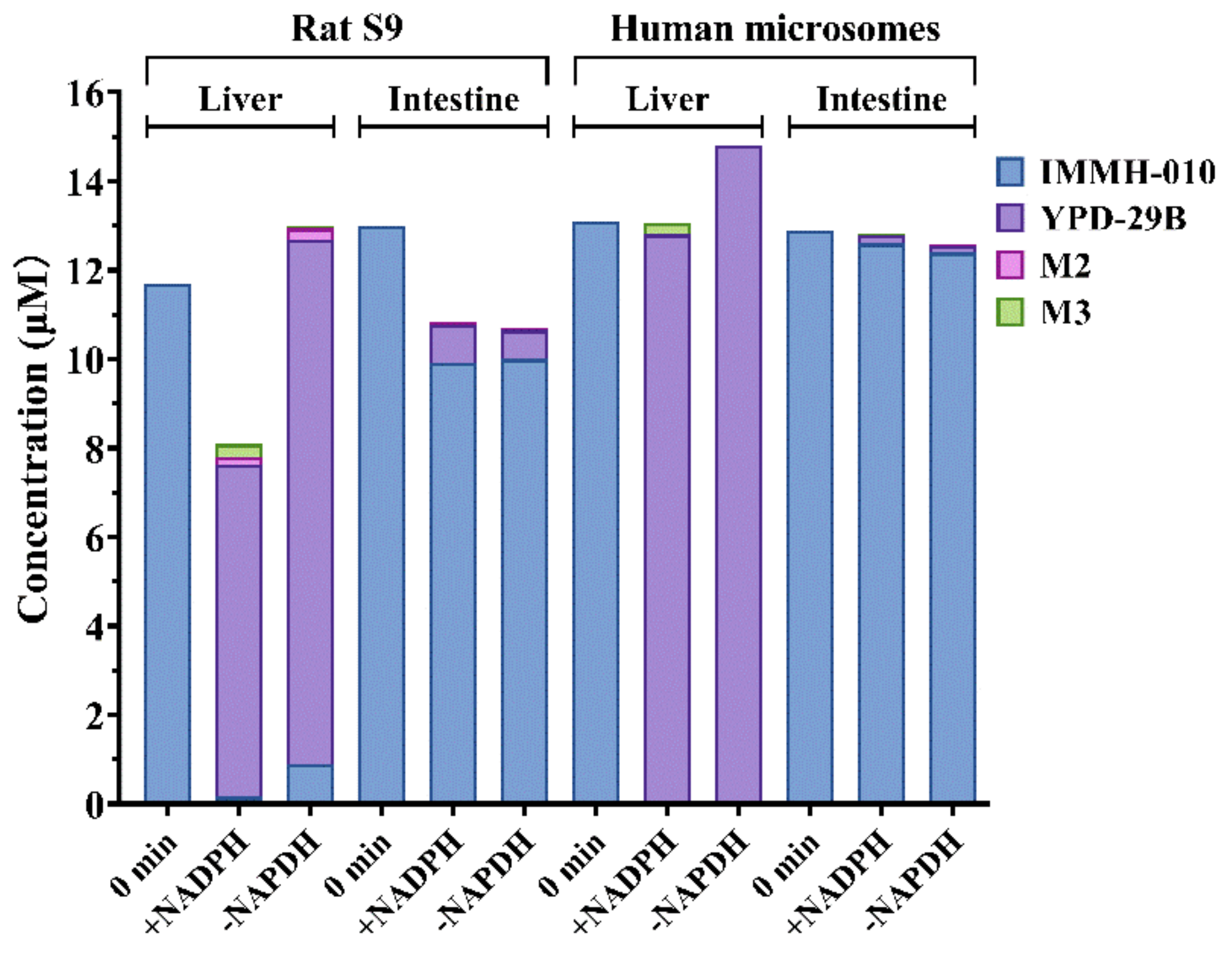
To compare the differences in IMMH-010 metabolism between the liver and intestine, IMMH-010 (10 μM) was incubated with rat liver and intestinal S9 homogenate protein (1 mg protein/mL) and with human liver and intestinal microsomes (0.2 mg protein/mL) in a final volume of 0.2 mL Tris-HCl buffer (50 mM, pH 7.4) containing 5 mM MgCl2. The incubations were performed in duplicate in the presence and absence of an NADPH regenerating system. After 2 min preincubation at 37 °C, reactions were initiated by the addition of IMMH-010 (10 μM). Incubations were terminated by acetonitrile at 0, 10, and 20 min. IMMH-010, YPD-29B, M2, and M3 were quantified by LC-MS/MS assay. Midazolam (10 μM, a substrate of CYP3A4) was set as the positive control.
2.5. Identification of Metabolizing Enzymes
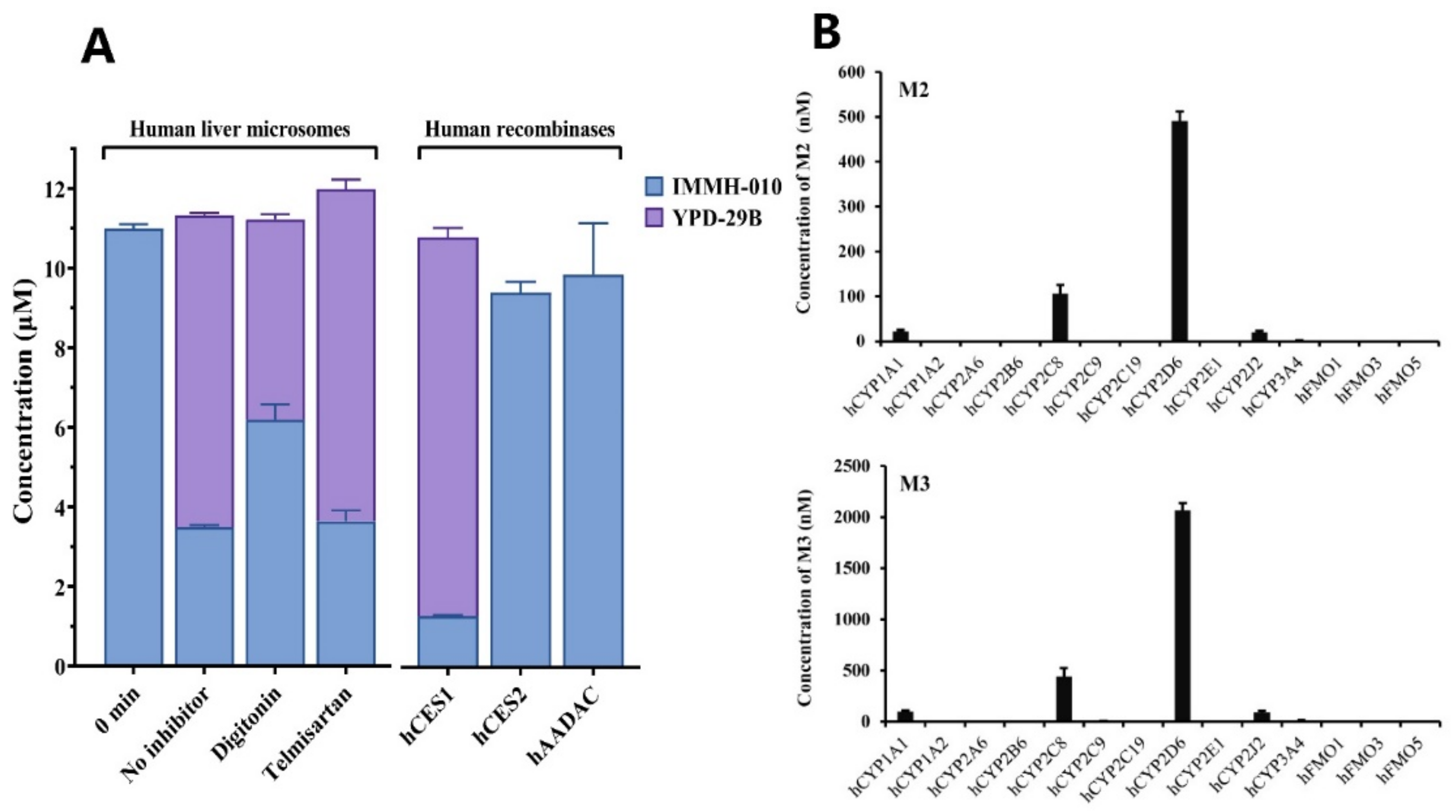
Selective chemical inhibitors and recombinant human enzymes were used to investigate the esterase responsible for IMMH-010 metabolism in HLM. The selective CES1 and CES2 inhibitors were digitonin (100 μM) and telmisartan (50 μM), respectively. IMMH-010 (10 µM) was incubated with HLM (0.2 mg/mL) at 37 °C for 0 and 15 min in the presence of chemical inhibitors. Control samples were incubated without inhibitors.
To verify the role of CES1, CES2, and AADAC in human metabolism of IMMH-010, IMMH-010 (10 µM) was incubated individually with recombinant human CES1, CES2, and AADAC (0.1 mg protein/mL) at 37 °C for 15 min. Lidocaine (500 μM), CPT-11 (2 μM), and phenacetin (1 mM), which are substrates of CES1, CES2, and AADAC, were used as positive controls.
cDNA-expressed human CYPs were also used to investigate the enzymes mediating IMMH-010 metabolism. IMMH-010 (10 µM) was incubated individually with 50 pmol of 10 individual cDNA-expressed human CYPs (CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP2J2, or CYP3A4) and three cDNA-expressed human FMO enzymes (FMO1, FMO3, and FMO5) at 37 °C for 30 min. An NADPH regeneration system was added to initiate the reactions. Probe substrates of the CYPs were used as the positive controls. Incubations without NADPH served as the negative controls. All reactions were carried out in a final volume of 0.2 mL Tris-HCl buffer (50 mM, pH 7.4) containing 5 mM MgCl2 and conducted in triplicate. All incubations were terminated by 2 volumes of acetonitrile. After centrifugation at 14,000 rpm for 5 min, the samples were analyzed by LC-MS/MS.
2.6. PK/PD Study in Mice
The antitumor activity of IMMH-010 was evaluated by B16F10 melanoma and MC38 colon carcinoma xenograft models. The B16F10 and MC38 cells were resuspended in saline (1.5 × 10
6 cells/0.2 mL saline) and injected subcutaneously into the right flanks of each mouse on day 0. The treated mice received once-daily oral administration of IMMH-010 maleate (1.25, 2.5, 5, and 10 mg/kg,
n = 10) by oral gavage (PO) for 19 consecutive days, whereas the control mice received vehicle (0.5% carboxymethyl cellulose,
n = 10). The mice in the positive control group received the anti-mouse PD-L1 antibody (696618M2, Bio X Cell, West Lebanon, NH, USA) at 10 mg/kg intraperitoneally every 3 days (
n = 10). The first-line antineoplastic drug cyclophosphamide (CTX) was administered at doses of 80 and 40 mg/kg weekly in the B16F10 and MC38 models (
n = 10), respectively [
12]. After the last treatment, on day 19, the mice were sacrificed, and the tumor and spleen were stripped and weighed. The tumor growth inhibition (TGI) was calculated as TGI = (1 − treatment group tumor weight/vehicle group tumor weight) × 100.
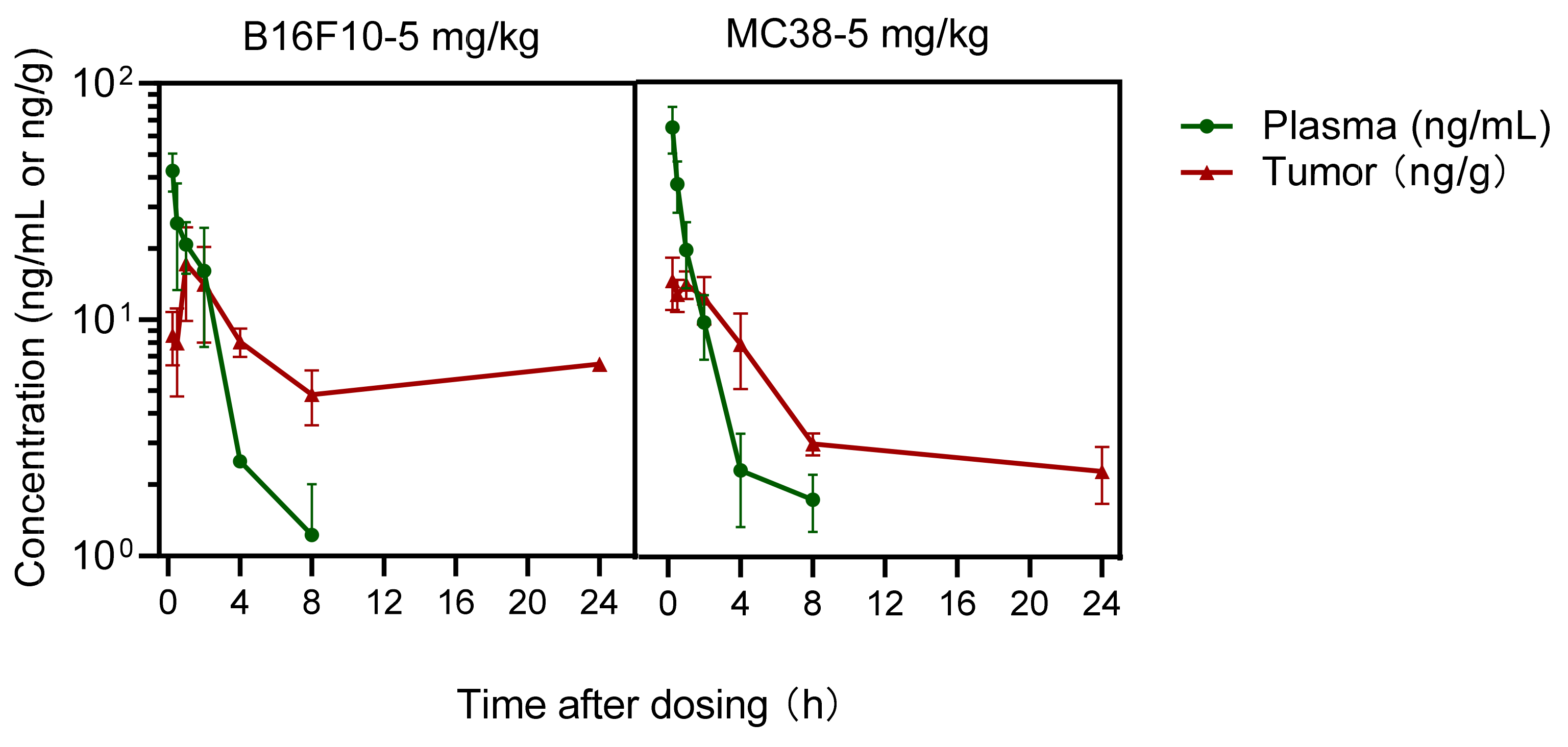
After the final dose, tumor and blood samples treated with IMMH-010 maleate (5 mg/kg) were collected over 72 h. Blood was exposed in an EP tube containing 500 mM NaF and 0.5% heparin sodium. Plasma samples were obtained by centrifugation, and tumor tissues were homogenized with 3 volumes (w/v) of saline on ice. The concentration of IMMH-010 and YPD-29B in plasma and tumor samples was quantitated by an LC-MS/MS method.
2.7. PK Study in Monkeys
IMMH-010 maleate was suspended with 0.5% carboxymethyl cellulose to make a 1 mg/mL suspension for PO gavage. Four male cynomolgus monkeys received a single oral dose of IMMH-010 maleate (5 mg/kg). Serial blood samples were collected upto 48 h. The plasma was separated and the plasma concentrations of IMMH-010 and YPD-29B were determined by LC-MS/MS.
2.8. LC-MS/MS Analysis
LC-MS/MS was performed using a triple quadrupole mass spectrometer (API 4000, AB Sciex, Framingham, MA, USA) with an ultra-performance liquid chromatography system (LC-30A, Shimadzu, Kyoto, Japan). Analyst 1.6.2 software (AB Sciex) was used for data acquisition and analysis. Mobile phase A was 1 mM ammonium acetate and mobile phase B was methanol. The mobile phase was delivered with the following gradient elution profile: 0–2.8 min, 70% B; 2.9 min, 85% B; 3 min, 90% B; 3.1–6.8 min, 100% B; 6.9–12 min, 70% B. The flow rate was 0.3 mL/min, and the column oven was maintained at 35 °C. The specific transitions monitored were 641.3 → 494.2 for IMMH-010, 597 → 155 for YPD-29B, 512 → 247 for M3, and 524 → 246 for M4.
2.9. Data Analysis
The apparent permeability coefficient (Papp) was determined according to the following equation: Papp = dQ/dt × (1/AC0), where dQ/dt is the permeability rate, A is the area of the inserts, and C0 is the initial concentration. The efflux ratio was defined as the ratio of Papp in the basolateral-to-apical direction divided by Papp in the apical-to-basolateral direction.
All statistics were calculated using GraphPad Prism 8.0 software (San Diego, CA, USA) designed for one-way ANOVA. Pharmacokinetic parameters were calculated with a non-compartmental analysis using WinNonlin Version 6.3 (Pharsight, Mountain View, CA, USA). ADMET predictor V10.0 (Simulations Plus, Inc., Lancaster, CA, USA) and Microsoft Excel (Microsoft, Redmond, WA, USA) were used to process the data (i.e., half-life (t1/2) and hepatic clearance (CLhep) determination). Data were expressed as means ± standard deviations (SD). Results were considered statistically significant if the p-value <0.05, <0.01 and <0.001.
4. Discussion
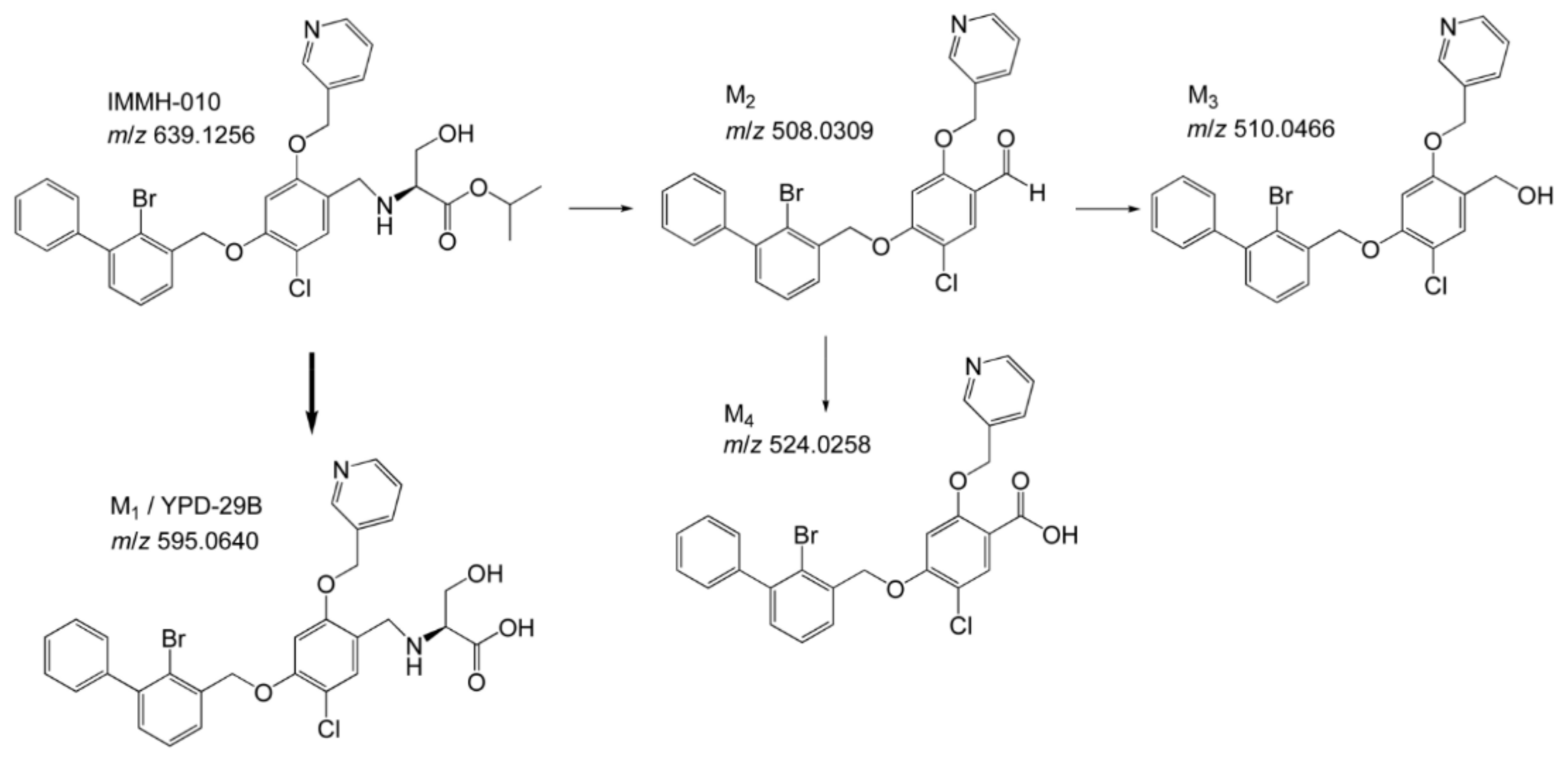
Blocking the PD-1/PD-L1 interaction is a powerful strategy in cancer immunotherapy and much research has focused on developing effective PD-1/PD-L1 inhibitors. IMMH-010, which was designed as a prodrug of potent PD-1/PD-L1 inhibitor YPD-29B, is currently in a phase I clinical trial. A pharmacokinetic study of IMMH-010 helped to reveal the mode of action of this type of PD-1/PD-L1 inhibitor and provided useful information for drug development and clinical applications. In the present study, we analyzed the metabolites of IMMH-010 in vivo and in vitro and confirmed that YPD-29B is the main metabolite of IMMH-010. We also used various recombinant esterases to hydrolyze IMMH-010 to YPD-29B and found that IMMH-010 hydrolysis is catalyzed by CES1. In summary, our metabolism research showed that our prodrug strategy worked as expected.
We explored IMMH-010 metabolism in detail, including the major metabolic sites and the species variation. There are species and tissue differences in esterase activities [
11,
15]. These differences determined which tissue is the primary metabolic site for IMMH-010 and the interspecies differences in IMMH-010 metabolism. The major classes of esterases involved in drug metabolism include butyrylcholinesterases, CESs, paraoxonases, and AADACs [
16,
17,
18,
19,
20]. The expressions of these esterases have been reported. Butyrylcholinesterase activity is mainly observed in the plasma of mice, rats, dogs, monkeys, and humans, but the activity is low in rats [
11]. CES activity is observed in the blood of mice and rats but not in the blood of dogs, monkeys, or humans. CES is also present in the liver and intestine of these species. Mouse, rat, and monkey intestine show higher CES activity than the human intestine. Paraoxonase activity is mainly found in the plasma and liver of mice, rats, dogs, monkeys, and humans, but it is lower in monkey plasma and liver. AADAC is expressed in the liver and gastrointestinal tract.
In the present study, human, monkey, rat, and mouse plasma were used to study the in vitro metabolism of IMMH-010. IMMH-010 was found to be rapidly metabolized in rat and mouse plasma, but it was stable in monkey and human plasma. This result is consistent with the distribution of esterases in various species; CES activity is observed in the blood of mice and rats, but not in the blood of dogs, monkeys, or humans. Thus, this result also indicated that IMMH-010 was mainly hydrolyzed to active metabolite YPD-29B by CES1.
CES is a member of the serine hydrolase superfamily. Human CES is classified into five families: CES1, CES2, CES3, CES4A, and CES5A. CES1 and CES2 hydrolyze a number of clinically used prodrugs and drugs [
21]. Except for oxybutinin, CES1 substrates have a large acyl group and small alcohol group, whereas a small acyl group and large alcohol group are common features of CES2 substrates [
18]. AADAC is a microsomal serine esterase. Similar to CES2, AADAC prefers substrates with a small acyl group and large alcohol group. We performed inhibition studies by using inhibitors to estimate the contribution of CES1 and CES2 to IMMH-010 hydrolysis in HLM. Shimizu et al. [
22] reported that digitonin is a specific inhibitor of CES1, but due to the interference of certain proteins, its inhibitory effect in HLM is weak, and telmisartan is a strong inhibitor of CES2 in both HLM and recombinants. IMMH-010 contains a large acyl group and a small alcohol group. We observed that digitonin (100 μM) inhibited the formation of YPD-29B by 35.8% in HLM, whereas telmisartan (50 μM) showed no inhibition, indicating that CES1 may be involved in IMMH-010 hydrolysis. To evaluate further the contribution of CES1, CES2, and AADAC to IMMH-010 metabolism, recombinant human CES1, CES2, and AADAC were used. The results confirmed that CES1 rather than CES2 and AADAC catalyzed IMMH-010 hydrolysis.
We compared the relative contribution and species difference of the intestine and liver to IMMH-010 bioactivation. Because liver S9 fractions are better than hepatocytes for predicting the hepatic metabolic clearance of ester prodrugs [
23], we compared IMMH-010 metabolism in human, monkey, dog, and rat liver S9 fractions. Furthermore, IMMH-010 hydrolysis in rat liver and intestinal S9 fractions and in human liver and intestinal microsomes was compared. The results showed that IMMH-010 was hydrolyzed rapidly in all species in a similar manner, and the intestine did not contribute much to the first-pass metabolism of IMMH-010. Consistently, CES1 is predominantly expressed in the liver of all species, whereas CES2 is expressed in the gastrointestinal tract. Thus, CES1, but not CES2, was further considered to have the major role in IMMH-010 bioactivation.
Because IMMH-010 bioactivation in humans and monkeys was similar in our in vitro studies, we speculated that the PK of IMMH-010 in monkeys might be similar to that in humans. Considering the transformation ratio of IMMH-010 to YPD-29B in human liver S9 fractions was slightly higher than that of monkeys (53.5% vs. 42.6%), the exposure of YPD-29B in humans may be higher than that in monkeys.
B16F10 melanoma cells and MC38 colon cells are widely used in anti-PD-L1 cancer immunotherapy because they express PD-L1 [
24]. In this study, B16F10 melanoma and MC38 colon tumor models were established. The antitumor activity and the PK characteristics of IMMH-010 were evaluated in both models. Significant reductions in tumor growth were observed in both models without weight loss. Therefore, IMMH-010 demonstrated robust antitumor activity. The PK of active metabolite YPD-29B in the two tumor xenograft mice models was similar. YPD-29B was eliminated slowly in tumors. The t
1/2β of YPD-29B in tumors was 7.6–25 times longer than that of plasma, which may be related to its receptor binding ability, resulting in higher drug exposure in tumors, which is beneficial for treatment. Compared to normal tissues, the expression and activity of drug-metabolizing enzymes are significantly dysregulated in tumor tissues [
25]. The higher exposure of YPD-29B in tumors may be related to the changes in the activity of drug-metabolizing enzymes in tumor tissues and the combination of YPD-29B and PD-L1.