New In Vitro Coculture Model for Evaluating Intestinal Absorption of Different Lipid Nanocapsules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Caco-2 and HMEC-1 Cells Culture
2.3. Caco-2/HMEC-1 Monoculture and Coculture on Transwell®
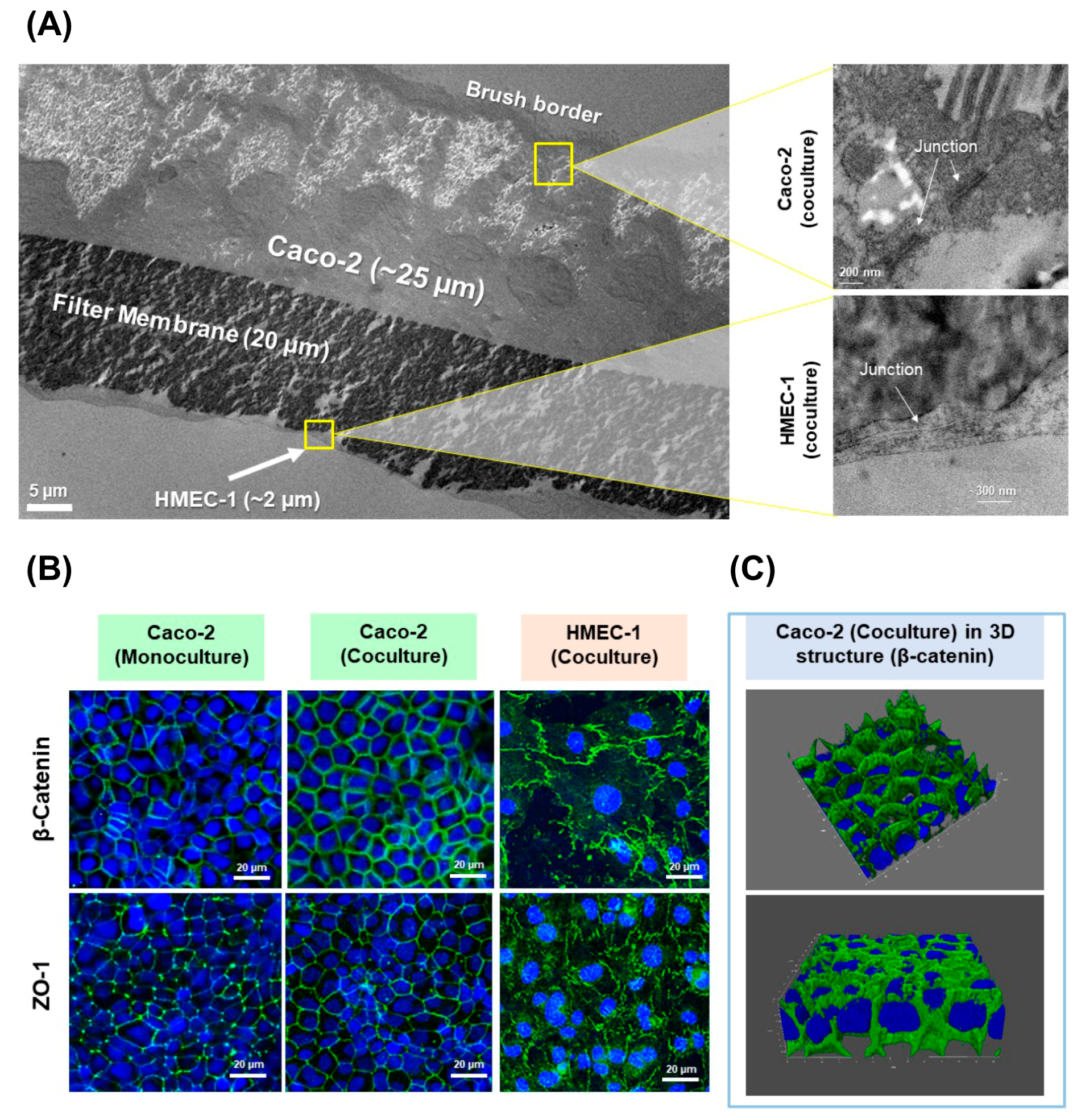
2.4. Membrane Morphology
2.4.1. Transmission Electron Microscopy (TEM)
2.4.2. Confocal Fluorescence Microscopy
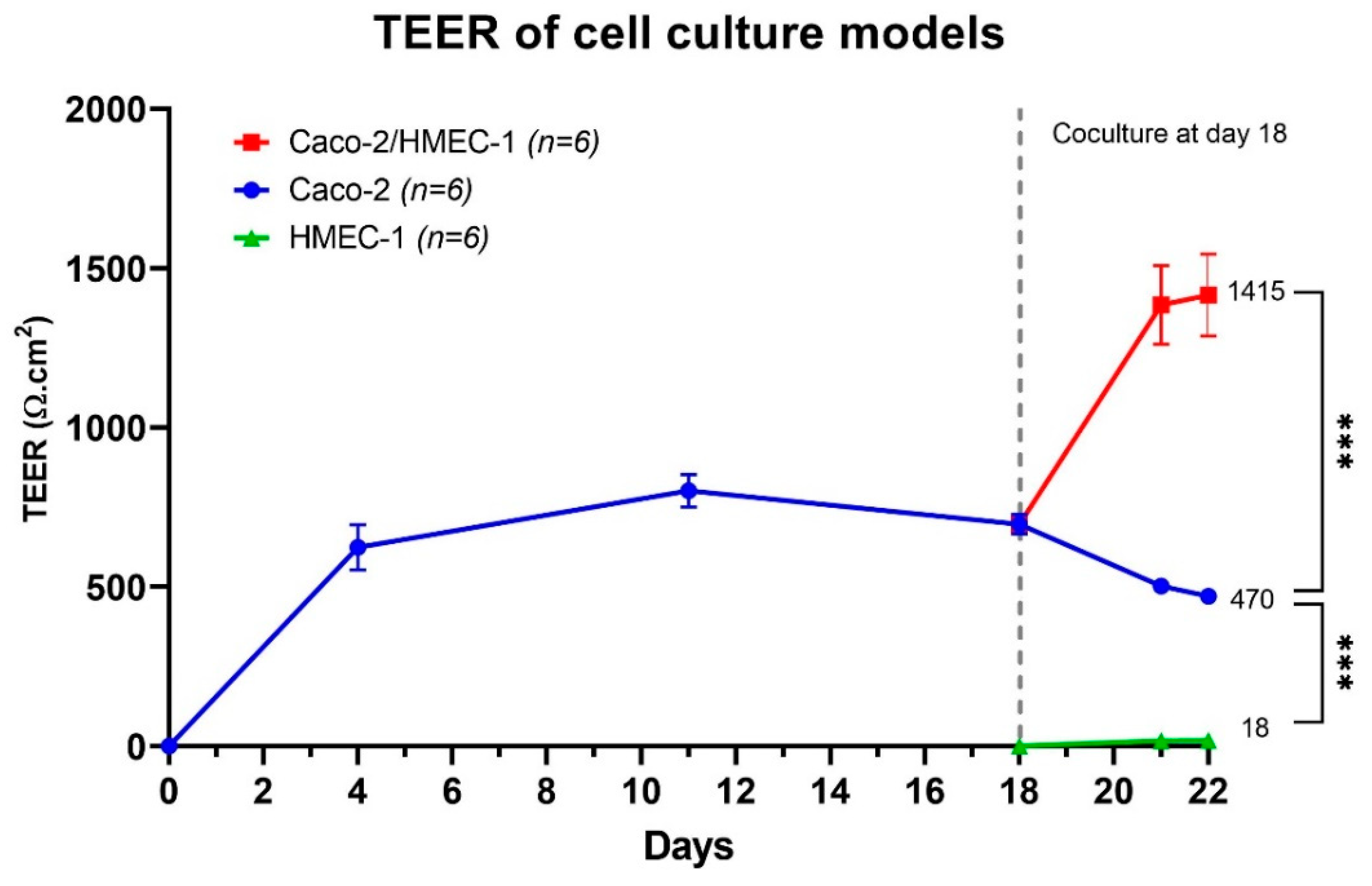
2.4.3. Trans-Epithelial Electrical Resistance (TEER)
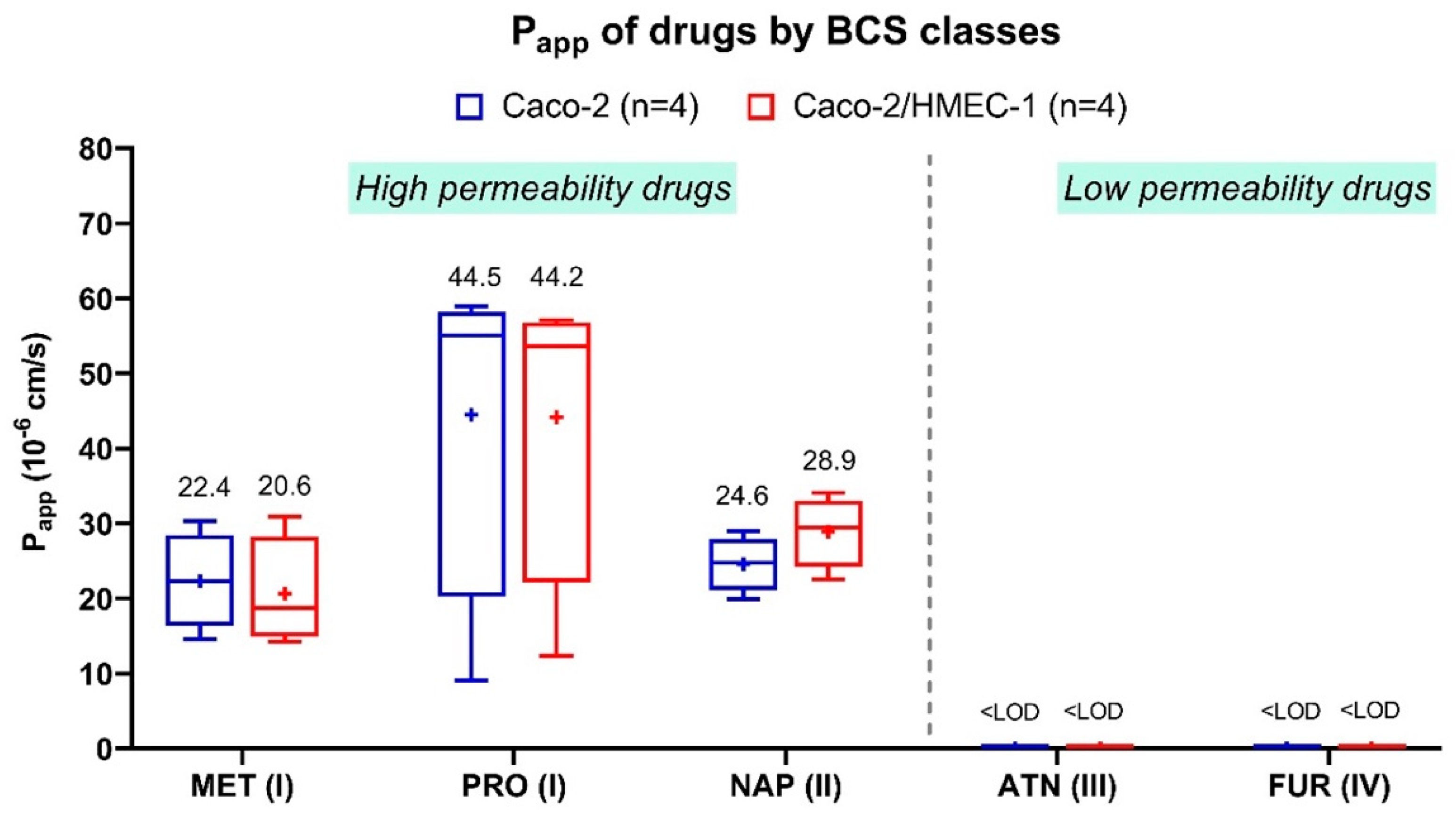
2.5. Transport Assay of the Free Drugs
2.5.1. Transport Assay Experiment
2.5.2. Drug Analysis by HPLC-UV
2.5.3. Apparent Permeability Calculation
2.6. Formulation of FRET-LNCs
2.6.1. Synthesis of DiI- and DiD-TPB
2.6.2. Formulation of FRET Lipid Nanocapsules (FRET-LNCs)
2.7. Characterization of FRET-LNCs
2.8. Transport Assay of Intact LNCs across Membranes
2.8.1. Transport Assay of FRET-LNCs
2.8.2. Quantitative FRET Fluorimetry of Intact LNCs
2.8.3. Transport Efficiency of FRET-LNCs
2.9. Statistical Analysis
3. Results and Discussions
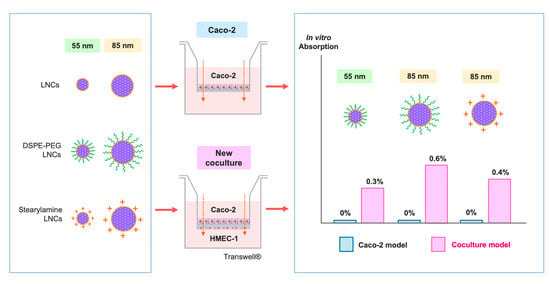
3.1. Development of the New In Vitro Coculture Model
3.2. Transport Assay of Intact LNCs across Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Sample Preparation for Drug Assay by HPLC-UV
Appendix A.1. Sample Preparation for Metoprolol and Propranolol
Appendix A.2. Sample Preparation for Naproxen
Appendix A.3. Sample Preparation for Atenolol
Appendix A.4. Sample Preparation for Furosemide
References
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human Intestinal Barrier Function in Health and Disease. Clin. Trans. Gastroenterol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Groschwitz, K.R.; Hogan, S.P. Intestinal Barrier Function: Molecular Regulation and Disease Pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal Permeability—A New Target for Disease Prevention and Therapy. BMC Gastroenterol. 2014, 14, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Heurtault, B.; Saulnier, P.; Pech, B.; Proust, J.E.; Benoit, J.P. A Novel Phase Inversion-Based Process for the Preparation of Lipid Nanocarriers. Pharm. Res. 2002, 19, 875–880. [Google Scholar] [CrossRef]
- Heurtault, B.; Saulnier, P.; Pech, B.; Venier-Julienne, M.C.; Proust, J.E.; Phan-Tan-Luu, R.; Benoît, J.P. The Influence of Lipid Nanocapsule Composition on Their Size Distribution. Eur. J. Pharm. Sci. 2003, 18, 55–61. [Google Scholar] [CrossRef]
- Lainé, A.L.; Gravier, J.; Henry, M.; Sancey, L.; Béjaud, J.; Pancani, E.; Wiber, M.; Texier, I.; Coll, J.L.; Benoit, J.P.; et al. Conventional versus Stealth Lipid Nanoparticles: Formulation and in Vivo Fate Prediction through FRET Monitoring. J. Control. Release 2014, 188, 1–8. [Google Scholar] [CrossRef]
- Ramadan, A.; Lagarce, F.; Pierre, L.; Tessier-Marteau, A.; Thomas, O.; Macchi, L.; Saulnier, P.; Benoit, J.-P. Oral Fondaparinux: Use of Lipid Nanocapsules as Nanocarriers and in Vivo Pharmacokinetic Study. Int. J. Nanomed. 2011, 6, 2941–2951. [Google Scholar] [CrossRef]
- Messaoudi, K.; Saulnier, P.; Boesen, K.; Benoit, J.; Lagarce, F. Anti-Epidermal Growth Factor Receptor SiRNA Carried by Chitosan-Transacylated Lipid Nanocapsules Increases Sensitivity of Glioblastoma Cells to Temozolomide. Int. J. Nanomed. 2014, 9, 1479–1490. [Google Scholar]
- Eissa, M.M.; El-Moslemany, R.M.; Ramadan, A.A.; Amer, E.I.; El-Azzouni, M.Z.; El-Khordagui, L.K. Miltefosine Lipid Nanocapsules for Single Dose Oral Treatment of Schistosomiasis Mansoni: A Preclinical Study. PLoS ONE 2015, 10, e0141788. [Google Scholar] [CrossRef]
- Roger, E.; Lagarce, F.; Garcion, E.; Benoit, J.P. Lipid Nanocarriers Improve Paclitaxel Transport throughout Human Intestinal Epithelial Cells by Using Vesicle-Mediated Transcytosis. J. Control. Release 2009, 140, 174–181. [Google Scholar] [CrossRef]
- Peltier, S.; Oger, J.; Couet, W.; Benoı, J. Enhanced Oral Paclitaxel Bioavailability After Administration of Paclitaxel-Loaded Lipid Nanocapsules. Pharm. Res. 2006, 23, 1243–1250. [Google Scholar] [CrossRef]
- Pensel, P.E.; Ullio, G.; Fabbri, J.; Ceballos, L.; Sanchez, S.; Alvarez, L.I.; Allemandi, D.; Pierre, J.; Palma, S.D.; Elissondo, M.C. Acta Tropica Cystic Echinococcosis Therapy: Albendazole-Loaded Lipid Nanocapsules Enhance the Oral Bioavailability and Efficacy in Experimentally Infected Mice. Acta Trop. 2015, 152, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Amara, R.O.; Ramadan, A.A.; El-Moslemany, R.M.; Eissa, M.M.; El-Azzouni, M.Z.; El-Khordagui, L.K. Praziquantel—Lipid Nanocapsules: An Oral Nanotherapeutic with Potential Schistosoma Mansoni Tegumental Targeting. Int. J. Nanomed. 2018, 13, 4493–4505. [Google Scholar] [CrossRef]
- Abozaid, D.; Ramadan, A.; Barakat, H.; Khalafallah, N. Acyclovir Lipid Nanocapsules Gel for Oromucosal Delivery: A Preclinical Evidence of Efficacy in the Chicken Pouch Membrane Model. Eur. J. Pharm. Sci. 2018, 121, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Briot, T.; Roger, E.; Verger, A.; Clavreul, A.; Lagarce, F. Development and in Vitro Evaluations of New Decitabine Nanocarriers for the Treatment of Acute Myeloid Leukemia. Int. J. Nanomed. 2017, 12, 8427–8442. [Google Scholar] [CrossRef][Green Version]
- Varshosaz, J.; Taymouri, S.; Jahanian-najafabadi, A.; Alizadeh, A. Efavirenz Oral Delivery via Lipid Nanocapsules: Formulation, Optimisation, and Ex-Vivo Gut Permeation Study. IET Nanobiotechnol. 2018, 12, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Roger, E.; Lagarce, F.; Benoit, J. Development and Characterization of a Novel Lipid Nanocapsule Formulation of Sn38 for Oral Administration. Eur. J. Pharm. Biopharm. 2011, 79, 181–188. [Google Scholar] [CrossRef]
- Sahoo, H. Förster resonance energy transfer—A spectroscopic nanoruler: Principle and applications. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 20–30. [Google Scholar] [CrossRef]
- Wallace, B.; Atzberger, P.J. Förster resonance energy transfer: Role of diffusion of fluorophore orientation and separation in observed shifts of FRET efficiency. PLoS ONE 2017, 12, e0177122. [Google Scholar] [CrossRef]
- Lagarce, F.; Groo, A.-C.; Saulnier, P.; Gimel, J.-C.; Gravier, J.; Ailhas, C.; Benoit, J.-P. Fate of paclitaxel lipid nanocapsules in intestinal mucus in view of their oral delivery. Int. J. Nanomed. 2013, 8, 4291–4302. [Google Scholar] [CrossRef]
- Roger, E.; Gimel, J.-C.; Bensley, C.; Klymchenko, A.S.; Benoit, J.-P. Lipid nanocapsules maintain full integrity after crossing a human intestinal epithelium model. J. Control. Release 2017, 253, 11–18. [Google Scholar] [CrossRef]
- Billat, P.-A.; Roger, E.; Faure, S.; Lagarce, F. Models for drug absorption from the small intestine: Where are we and where are we going? Drug Discov. Today 2017, 22, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the Human Colon Carcinoma Cell Line (Caco-2) as a Model System for Intestinal Epithelial Permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Han, C.; Wang, B. Factors That Impact the Developability of Drug Candidates; Wiley: Hoboken, NJ, USA, 2016; ISBN 9781118833322. [Google Scholar]
- Van Breemen, R.B.; Li, Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef]
- Rinaki, E.; Valsami, G.; Macheras, P. Classification System: The Central Role of Dose/Solubility Ratio. Pharm. Res. 2003, 20, 1917–1925. [Google Scholar] [CrossRef]
- The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ICH Harmonised Guideline. Biopharmaceutics Classification System-Based Biowaivers. M9. Available online: https://database.ich.org/sites/default/files/M9_Guideline_Step4_2019_1116.pdf (accessed on 16 April 2021).
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1995, 12, 413–420. [Google Scholar]
- Costa, J.; Ahluwalia, A. Advances and Current Challenges in Intestinal in Vitro Model Engineering: A Digest. Front. Bioeng. Biotechnol. 2019, 7, 1–14. [Google Scholar] [CrossRef]
- Beloqui, A.; Brayden, D.J.; Artursson, P.; Préat, V.; Des Rieux, A. A Human Intestinal M-Cell-like Model for Investigating Particle, Antigen and Microorganism Translocation. Nat. Protoc. 2017, 12, 1387–1399. [Google Scholar] [CrossRef]
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; Di Sabatino, A.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A Gut-Vascular Barrier Controls the Systemic Dissemination of Bacteria. Science 2015, 350, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Sorribas, M.; de Gottardi, A.; Moghadamrad, S.; Hassan, M.; Spadoni, I.; Rescigno, M.; Wiest, R. Isoproterenol Disrupts Intestinal Barriers Activating Gut-Liver-Axis: Effects on Intestinal Mucus and Vascular Barrier as Entry Sites. Digestion 2020, 101, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, F.; Tacconi, C.; D’Alessio, S. Beyond Intestinal Barrier: The Blood Endothelium as a Second Wall of Defense Against Bacterial Invasion. Gastroenterology 2016, 150, 1678–1680. [Google Scholar] [CrossRef][Green Version]
- Deshmukh, R.; Bandyopadhyay, N.; Abed, S.N.; Bandopadhyay, S.; Pal, Y.; Deb, P.K. Strategies for Pulmonary Delivery of Drugs; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128145081. [Google Scholar]
- Kasper, J.Y.; Hermanns, M.I.; Cavelius, C.; Kraegeloh, A.; Jung, T.; Danzebrink, R.; Unger, R.E.; Kirkpatrick, C.J. The Role of the Intestinal Microvasculature in Inflammatory Bowel Disease: Studies with a Modified Caco-2 Model Including Endothelial Cells Resembling the Intestinal Barrier in Vitro. Int. J. Nanomed. 2016, 11, 6353–6364. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of Drug Permeability and Prediction of Drug Absorption in Caco-2 Monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]
- Kilin, V.N.; Anton, H.; Anton, N.; Steed, E.; Vermot, J.; Vandamme, T.F.; Mely, Y.; Klymchenko, A.S. Counterion-Enhanced Cyanine Dye Loading into Lipid Nano-Droplets for Single-Particle Tracking in Zebrafish. Biomaterials 2014, 35, 4950–4957. [Google Scholar] [CrossRef]
- Young, B.; O’Dowd, G.; Woodford, P. Membrane Specialisations of Epithelia. In Wheater’s Functional Histology: A Text and Colour Atlas; Churchill Livingstone: London, UK, 2013; pp. 88–91. ISBN 9780702047473. [Google Scholar]
- Briske-Anderson, M.J.; Finley, J.W.; Newman, S.M. The Influence of Culture Time and Passage Number on the Morphological and Physiological Development of Caco-2 Cells. Proc. Soc. Exp. Biol. Med. 1997, 214, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Hollander, D.; Tran, L.T.; Nguyen, D.; Hoa, N.; Bhalla, D. Cytoskeletal Regulation of Caco-2 Intestinal Monolayer Paracellular Permeability. J. Cell. Physiol. 1995, 164, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; O’Dowd, G.; Woodford, P. The microcirculation. In Wheater’s Functional Histology: A Text and Colour Atlas; Churchill Livingstone: London, UK, 2013; pp. 150–153. ISBN 9780702047473. [Google Scholar]
- Rüffer, C.; Strey, A.; Janning, A.; Kim, K.S.; Gerke, V. Cell−Cell Junctions of Dermal Microvascular Endothelial Cells Contain Tight and Adherens Junction Proteins in Spatial Proximity. Biochemistry 2004, 43, 5360–5369. [Google Scholar] [CrossRef] [PubMed]
- Hilgendorf, C.; Spahn-Langguth, H.; Regårdh, C.G.; Lipka, E.; Amidon, G.L.; Langguth, P. Caco-2 versus Caco-2/HT29-MTX Co-Cultured Cell Lines: Permeabilities via Diffusion, inside- and Outside-Directed Carrier-Mediated Transport. J. Pharm. Sci. 2000, 89, 63–75. [Google Scholar] [CrossRef]
- Artursson, P.; Karlsson, J. Correlation between Oral Drug Absorption in Humans and Apparent Drug Permeability Coefficients in Human Intestinal Epithelial (Caco-2) Cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Aungst, B.J.; Nguyen, N.H.; Bulgarelli, J.P.; Oates-lenz, K. The Influence of Donor and Reservoir Additives on Caco-2 Permeability and Secretory Transport of HIV Protease Inhibitors and Other Lipophilic Compounds. Pharm. Res. 2000, 17, 1–5. [Google Scholar] [CrossRef]
- Jung, S.J.; Choi, S.O.; Um, S.Y.; Kim, J.I.; Choo, H.Y.P.; Choi, S.Y.; Chung, S.Y.; Youn, S. Prediction of the Permeability of Drugs through Study on Quantitative Structure—Permeability Relationship. J. Pharm. Biomed. Anal. 2006, 41, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Magnusson, C. Epithelial Transport of Drugs in Cell Culture. II: Effect of Extracel I u Lar Calcium Concent Rat Ion on the Paracel I u Lar Transport of Drugs of Different Lipophilicities across Monolayers of Intestinal Epithelial (Caco-2) Cells. J. Pharm. Sci. 1990, 79, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P. Epithelial Transport of Drugs in Cell Culture. I: A Model for Studying the Passive Diffusion of Drugs over Intestinal. J. Pharm. Sci. 1990, 79, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Volpe, D.A.; Faustino, P.J.; Ciavarella, A.B.; Asafu-Adjaye, E.B.; Ellison, C.D.; Yu, L.X.; Hussain, A.S. Classification of Drug Permeability with a Caco-2 Cell Monolayer Assay. Clin. Res. Regul. Aff. 2007, 24, 39–47. [Google Scholar] [CrossRef]
- Roger, E.; Lagarce, F.; Garcion, E.; Benoit, J.P. Biopharmaceutical Parameters to Consider in Order to Alter the Fate of Nanocarriers after Oral Delivery. Nanomedicine 2010, 5, 287–306. [Google Scholar] [CrossRef]
- Groo, A.C.; Bossiere, M.; Trichard, L.; Legras, P.; Benoit, J.P.; Lagarce, F. In Vivo Evaluation of Paclitaxel-Loaded Lipid Nanocapsules after Intravenous and Oral Administration on Resistant Tumor. Nanomedicine 2015, 10, 589–601. [Google Scholar] [CrossRef]
- Mccann, J.J.; Choi, U.B.; Zheng, L.; Weninger, K.; Bowen, M.E. Optimizing Methods to Recover Absolute FRET Efficiency from Immobilized Single Molecules. Biophys. J. 2010, 99, 961–970. [Google Scholar] [CrossRef]
- Nir, E.; Michalet, X.; Hamadani, K.M.; Laurence, T.A.; Neuhauser, D. Shot-Noise Limited Single-Molecule FRET Histograms: Comparison between Theory and Experiments. J. Phys. Chem. 2006, 110, 22103–22124. [Google Scholar] [CrossRef]
- Bannunah, A.M.; Vllasaliu, D.; Lord, J.; Stolnik, S. Mechanisms of Nanoparticle Internalization and Transport Across an Intestinal Epithelial Cell Model: E Ff Ect of Size and Surface Charge. Mol. Pharm. 2014, 11, 4363–4373. [Google Scholar] [CrossRef]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in Oral Nano-Delivery Systems for Colon Targeted Drug Delivery in Inflammatory Bowel Disease: Selective Targeting to Diseased versus Healthy Tissue. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1117–1132. [Google Scholar] [CrossRef]
- Furst, T.; Dakwar, G.R.; Zagato, E.; Lechanteur, A.; Remaut, K.; Evrard, B.; Braeckmans, K.; Piel, G. Freeze-Dried Mucoadhesive Polymeric System Containing Pegylated Lipoplexes: Towards a Vaginal Sustained Released System for SiRNA. J. Controll. Rel. 2016, 236, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Paillard, A.; Hindré, F.; Vignes-colombeix, C.; Benoit, J.; Garcion, E. The Importance of Endo-Lysosomal Escape with Lipid Nanocapsules for Drug Subcellular Bioavailability. Biomaterials 2010, 31, 7542–7554. [Google Scholar] [CrossRef] [PubMed]
- Garcion, E.; Lamprecht, A.; Paillard, A.; Aubert-Pouessel, A.; Menei, P.; Benoı, J. A New Generation of Anticancer, Drug-Loaded, Colloidal Vectors Reverses Multidrug Resistance in Glioma and Reduces Tumor Progression in Rats. Mol. Cancer Ther. 2006, 5, 1710–1723. [Google Scholar] [CrossRef] [PubMed]
- Hamelers, I.H.L.; Staffhorst, R.W.H.M.; Voortman, J.; De Kruijff, B.; Reedijk, J.; Van Bergen, P.M.P.; Kroon, A.I.P.M. De Cancer Therapy: Preclinical High Cytotoxicity of Cisplatin Nanocapsules in Ovarian Carcinoma Cells Depends on Uptake by Caveolae-Mediated Endocytosis. Clin. Cancer Res. 2009, 15, 1259–1269. [Google Scholar] [CrossRef] [PubMed]

| BCS Classes | Solubility | Permeability | Drugs |
|---|---|---|---|
| I | High | High | Metoprolol tartrate, Propranolol HCl |
| II | Low | High | Naproxen |
| III | High | Low | Atenolol |
| IV | Low | Low | Furosemide |
| Compositions | Quantity (% w/w) | |||||
|---|---|---|---|---|---|---|
| F1 | F1-DSPE-PEG | F1-SA | F2 | F2-DSPE-PEG | F2-SA | |
| Captex® 8000 (2% w/w DiI-TPB) | 5.5 | 5.5 | 5.5 | 8.5 | 8.5 | 8.5 |
| Captex® 8000 (2% w/w DiD-TPB) | 5.5 | 5.5 | 5.5 | 8.5 | 8.5 | 8.5 |
| Kolliphor® HS-15 | 11.5 | 11.5 | 11.5 | 9.3 | 9.3 | 9.3 |
| Purified water | 21.3 | 21.3 | 21.3 | 17.5 | 17.5 | 17.5 |
| DSPE-mPEG (2000) | - | 0.6 | - | - | 0.6 | - |
| Stearylamine | - | - | 0.1 | - | - | 0.1 |
| Lipoid® S75-3 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 |
| NaCl | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| Purified water (2 °C) | 54.7 | 54.7 | 54.7 | 54.7 | 54.7 | 54.7 |
| Formulas | Particle Size (nm) | Particle Size Distribution | Particle Concentration (×1014 Particles/mL) | Zeta Potential (mV) | FRET Proximity Ratio | |||
|---|---|---|---|---|---|---|---|---|
| D10 (nm) | D50 (nm) | D90 (nm) | Span | |||||
| F1 (n = 3) | 57.8 ± 9.7 | 45.3 ± 5.0 | 54.2 ± 7.7 | 65.6 ± 12.5 | 0.37 ± 0.08 | 7.7 ± 1.2 | 4.1 ± 0.8 | 0.89 ± 0.04 |
| F1-DSPE-PEG (n = 2) | 53.1 | 42.7 | 50.9 | 62.5 | 0.38 | 7.9 | −4.9 | 0.89 |
| F1-SA (n = 3) | 56.0 ± 5.6 | 45.3 ± 4.5 | 53.5 ± 5.1 | 65.6 ± 7.2 | 0.38 ± 0.04 | 8.0 ± 3.1 | 15.4 ± 2.1 | 0.89 ± 0.04 |
| F2 (n = 3) | 92.6 ± 10.0 | 67.1 ± 6.2 | 88.5 ± 8.9 | 120.4 ± 15.3 | 0.60 ± 0.04 | 5.5 ± 2.7 | 16.3 ± 3.7 | 0.93 ± 0.02 |
| F2-DSPE-PEG (n = 2) | 83.3 | 61.3 | 77.3 | 109.9 | 0.63 | 6.6 | 3.2 | 0.92 |
| F2-SA (n = 3) | 82.6 ± 5.8 | 62.3 ± 3.7 | 78.7 ± 5.2 | 104.7 ± 9.9 | 0.54 ± 0.07 | 6.8 ± 2.3 | 27.3 ± 2.8 | 0.93 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaeokhamloed, N.; Roger, E.; Béjaud, J.; Lautram, N.; Manero, F.; Perrot, R.; Briet, M.; Abbara, C.; Legeay, S. New In Vitro Coculture Model for Evaluating Intestinal Absorption of Different Lipid Nanocapsules. Pharmaceutics 2021, 13, 595. https://doi.org/10.3390/pharmaceutics13050595
Kaeokhamloed N, Roger E, Béjaud J, Lautram N, Manero F, Perrot R, Briet M, Abbara C, Legeay S. New In Vitro Coculture Model for Evaluating Intestinal Absorption of Different Lipid Nanocapsules. Pharmaceutics. 2021; 13(5):595. https://doi.org/10.3390/pharmaceutics13050595
Chicago/Turabian StyleKaeokhamloed, Norraseth, Emillie Roger, Jérôme Béjaud, Nolwenn Lautram, Florence Manero, Rodolphe Perrot, Marie Briet, Chadi Abbara, and Samuel Legeay. 2021. "New In Vitro Coculture Model for Evaluating Intestinal Absorption of Different Lipid Nanocapsules" Pharmaceutics 13, no. 5: 595. https://doi.org/10.3390/pharmaceutics13050595
APA StyleKaeokhamloed, N., Roger, E., Béjaud, J., Lautram, N., Manero, F., Perrot, R., Briet, M., Abbara, C., & Legeay, S. (2021). New In Vitro Coculture Model for Evaluating Intestinal Absorption of Different Lipid Nanocapsules. Pharmaceutics, 13(5), 595. https://doi.org/10.3390/pharmaceutics13050595