Effects of Magnesium, Calcium, and Aluminum Chelation on Fluoroquinolone Absorption Rate and Bioavailability: A Computational Study
Abstract
1. Introduction
2. Methods
2.1. Oral Absorption Rate Constant Calculations
2.2. Physicochemical Descriptor Calculations
2.3. Molecular Modeling
2.3.1. Metal Chelate Construction
2.3.2. Conformational Search
2.3.3. Quantum Mechanical Computations
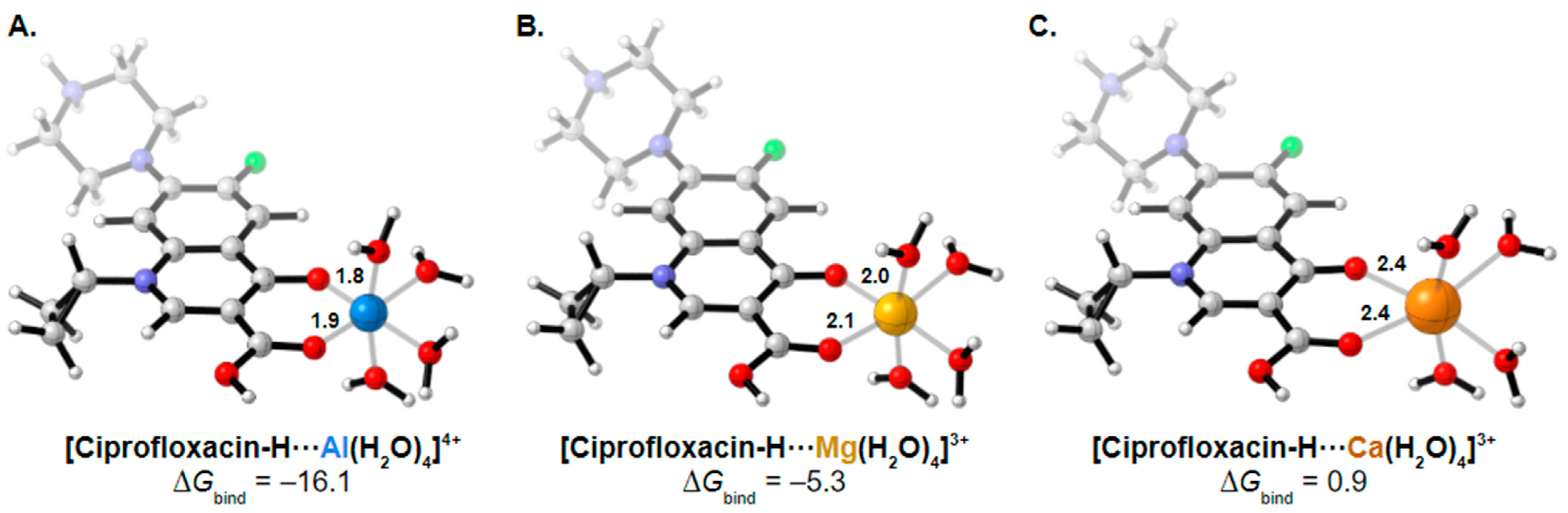
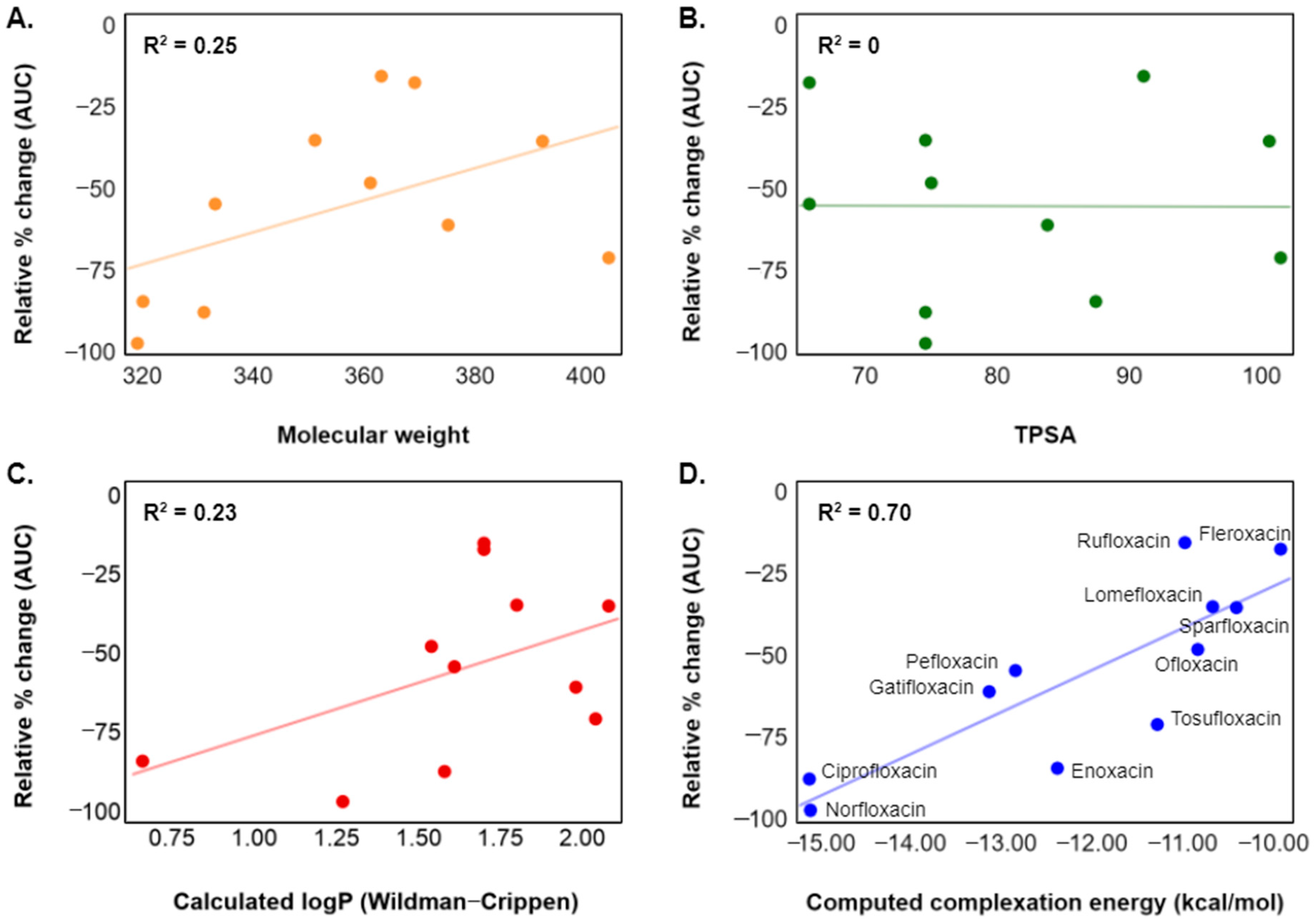
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ClinCalc, Ciprofloxacin, Drug Usage Statistics, United States, 2008–2018. Available online: https://clincalc.com/DrugStats/Drugs/Ciprofloxacin (accessed on 5 February 2021).
- Andre, V.; Da Silva, A.R.F.; Fernandes, A.; Frade, R.; Garcia, C.; Rijo, P.; Antunes, A.M.M.; Rocha, J.; Duarte, M.T. Mg- and Mn-MOFs Boost the Antibiotic Activity of Nalidixic Acid. ACS Appl. Bio. Mater. 2019, 2, 2347–2354. [Google Scholar] [CrossRef]
- Emmerson, A.M. The quinolones: Decades of development and use. J. Antimicrob. Chemother. 2003, 51, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. MedChemComm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Idowu, T.; Schweizer, F. Ubiquitous Nature of Fluoroquinolones: The Oscillation between Antibacterial and Anticancer Activities. Antibiotics 2017, 6, 26. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Ennis, K.; Vercaigne, L.; Walkty, A.; Gin, A.S.; Embil, J.; Smith, H.; Hoban, D.J. A Critical Review of the Fluoroquinolones. Drugs 2002, 62, 13–59. [Google Scholar] [CrossRef]
- Ball, P. Quinolone generations: Natural history or natural selection? J. Antimicrob. Chemother. 2000, 46, 17–24. [Google Scholar] [CrossRef]
- Pitman, S.K.; Hoang, U.T.P.; Wi, C.H.; Alsheikh, M.; Hiner, D.A.; Percival, K.M. Wi Revisiting Oral Fluoroquinolone and Multivalent Cation Drug-Drug Interactions: Are They Still Relevant? Antibiotics 2019, 8, 108. [Google Scholar] [CrossRef]
- Nix, D.E.; Watson, W.A.; Lener, M.E.; Frost, R.W.; Krol, G.; Goldstein, H.R.; Lettieri, J.T.; Schentag, J.J. Effects of aluminum and magnesium antacids and ranitidine on the absorption of ciprofloxacin. Clin. Pharmacol. Ther. 1989, 46, 700–705. [Google Scholar] [CrossRef]
- Garrelts, J.C.; Godley, P.J.; Peterie, J.D.; Gerlach, E.H.; Yakshe, C.C. Sucralfate significantly reduces ciprofloxacin concentrations in serum. Antimicrob. Agents Chemother. 1990, 34, 931–933. [Google Scholar] [CrossRef]
- Sahai, J.; Gallicano, K.; Oliveras, L.; Khaliq, S.; Hawley-Foss, N.; Garber, G. Cations in the didanosine tablet reduce ciprofloxacin bioavailability. Clin. Pharmacol. Ther. 1993, 53, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Sahai, J.; Healy, D.P.; Stotka, J.; Polk, R.E. The influence of chronic administration of calcium carbonate on the bioavailability of oral ciprofloxacin. Br. J. Clin. Pharmacol. 1993, 35, 302–304. [Google Scholar] [PubMed]
- Lee, L.J.; Hafkin, B.; Lee, I.D.; Hoh, J.; Dix, R. Effects of food and sucralfate on a single oral dose of 500 milligrams of levofloxacin in healthy subjects. Antimicrob. Agents Chemother. 1997, 41, 2196–2200. [Google Scholar] [CrossRef] [PubMed]
- Shiba, K.; Sakai, O.; Shimada, J.; Okazaki, O.; Aoki, H.; Hakusui, H. Effects of antacids, ferrous sulfate, and ranitidine on absorption of DR-3355 in humans. Antimicrob. Agents Chemother. 1992, 36, 2270–2274. [Google Scholar] [CrossRef] [PubMed]
- Grasela, T.H.; Schentag, J.J.; Sedman, A.J.; Wilton, J.H.; Thomas, D.J.; Schultz, R.W.; Lebsack, M.E.; Kinkel, A.W. Inhibition of enoxacin absorption by antacids or ranitidine. Antimicrob. Agents Chemother. 1989, 33, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Shimada, J.; Shiba, K.; Oguma, T.; Miwa, H.; Yoshimura, Y.; Nishikawa, T.; Okabayashi, Y.; Kitagawa, T.; Yamamoto, S. Effect of antacid on absorption of the quinolone lomefloxacin. Antimicrob. Agents Chemother. 1992, 36, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Jaehde, U.; Sörgel, F.; Stephan, U.; Schunack, W. Effect of an antacid containing magnesium and aluminum on absorption, metabolism, and mechanism of renal elimination of pefloxacin in humans. Antimicrob. Agents Chemother. 1994, 38, 1129–1133. [Google Scholar] [CrossRef]
- Lazzaroni, M.; Imbimbo, B.P.; Bargiggia, S.; Sangaletti, O.; Bo, L.D.; Broccali, G.; Porro, G.B. Effects of magnesium-aluminum hydroxide antacid on absorption of rufloxacin. Antimicrob. Agents Chemother. 1993, 37, 2212–2216. [Google Scholar] [CrossRef]
- Campbell, N.R.; Kara, M.; Hasinoff, B.B.; Haddara, W.M.; McKay, D.W. Norfloxacin interaction with antacids and minerals. Br. J. Clin. Pharmacol. 1992, 33, 115–116. [Google Scholar] [CrossRef]
- Lehto, P.; Kivisto, K.T. Effect of sucralfate on absorption of norfloxacin and ofloxacin. Antimicrob. Agents Chemother. 1994, 38, 248–251. [Google Scholar] [CrossRef]
- Nix, D.; Wilton, J.H.; Ronald, B.; Distlerath, L.; Williams, V.C.; Norman, A. Inhibition of norfloxacin absorption by antacids. Antimicrob. Agents Chemother. 1990, 34, 432–435. [Google Scholar] [CrossRef]
- Parpia, S.H.; E Nix, D.; Hejmanowski, L.G.; Goldstein, H.R.; Wilton, J.H.; Schentag, J.J. Sucralfate reduces the gastrointestinal absorption of norfloxacin. Antimicrob. Agents Chemother. 1989, 33, 99–102. [Google Scholar] [CrossRef]
- Flor, S.; Guay, D.R.; A Opsahl, J.; Tack, K.; Matzke, G.R. Effects of magnesium-aluminum hydroxide and calcium carbonate antacids on bioavailability of ofloxacin. Antimicrob. Agents Chemother. 1990, 34, 2436–2438. [Google Scholar] [CrossRef] [PubMed]
- Lubowski, T.J.; Nightingale, C.H.; Sweeney, K.; Quintiliani, R. Effect of sucralfate on pharmacokinetics of fleroxacin in healthy volunteers. Antimicrob. Agents Chemother. 1992, 36, 2758–2760. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, L.; Wong, H. Food Effects on Oral Drug Absorption: Application of Physiologically-Based Pharmacoki-netic Modeling as a Predictive Tool. Pharmaceutics 2020, 12, 672. [Google Scholar] [CrossRef] [PubMed]
- Barton, T.D.; Fishman, N.O.; Weiner, M.G.; LaRosa, L.A.; Lautenbach, E. High Rate of Coadministration of Di- or Tri-valent Cation-Containing Compounds With Oral Fluoroquinolones: Risk Factors and Potential Implications. Infect. Control. Hosp. Epidemiol. 2005, 26, 93–99. [Google Scholar] [CrossRef]
- Mallet, L.; Huang, A. Coadministration of Gatifloxacin and Multivitamin Preparation Containing Minerals: Potential Treatment Failure in an Elderly Patient. Ann. Pharmacother. 2005, 39, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Spivey, J.M.; Cummings, D.M.; Pierson, N.R. Failure of Prostatitis Treatment Secondary to Probable Ciprofloxa-cin-Sucralfate Drug Interaction. Pharmacotherapy 1996, 16, 314–316. [Google Scholar] [CrossRef]
- Garey, K.W.; Suda, K.J.; Danziger, L.H. Treatment failures secondary to drug interactions with divalent cations and fluoroquinolone. Pharm. World Sci. 2005, 27, 81–82. [Google Scholar] [CrossRef]
- Cohen, K.A.; Lautenbach, E.; Weiner, M.G.; Synnestvedt, M.; Gasink, L.B. Coadministration of Oral Levofloxacin with Agents That Impair Absorption: Impact on Antibiotic Resistance. Infect. Control. Hosp. Epidemiology 2008, 29, 975–977. [Google Scholar] [CrossRef]
- Drlica, K. The mutant selection window and antimicrobial resistance. J. Antimicrob. Chemother. 2003, 52, 11–17. [Google Scholar] [CrossRef]
- Quain, R.D.; Barton, T.D.; Fishman, N.O.; Weiner, M.G.; Lautenbach, E. Coadministration of oral levofloxacin with agents that impair its absorption: Potential impact on emergence of resistance. Int. J. Antimicrob. Agents 2005, 26, 327–330. [Google Scholar] [CrossRef]
- Vitorino, G.P.; Barrera, G.D.; Mazzieri, M.R.; Binning, R.; Bacelo, D.E. A DFT study of hydration in neutral and zwitterionic norfloxacin. Chem. Phys. Lett. 2006, 432, 538–544. [Google Scholar] [CrossRef]
- Pavez, P.; Encinas, M.V.; Toro-Labbé, A. Photophysics and Photochemistry of Nalidixic Acid†. Photochem. Photobiol. 2006, 82, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Musa, K.A.K.; Eriksson, L.A. Theoretical Assessment of Norfloxacin Redox and Photochemistry. J. Phys. Chem. A 2009, 113, 10803–10810. [Google Scholar] [CrossRef] [PubMed]
- Aristilde, L.; Sposito, G. Molecular modeling of metal complexation by a fluoroquinolone antibiotic. Environ. Toxicol. Chem. 2008, 27, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
- Bridle, M.J.; Janesko, B.G. Computational study of fluoroquinolone binding to Mg(H2O)N2+ and its applicability to future drug design. Int. J. Quantum Chem. 2017, 117, e25428. [Google Scholar] [CrossRef]
- Khalil, T.E.; El-Dissouky, A.; Al-Wahaib, D.; Abrar, N.M.; El-Sayed, D.S. Synthesis, characterization, antimicrobial activity, 3D-QSAR, DFT, and molecular docking of some ciprofloxacin derivatives and their copper(II) complexes. Appl. Organomet. Chem. 2020, 34, 5998. [Google Scholar] [CrossRef]
- Dorotíková, S.; Plevová, K.; Bučinský, L.; Malček, M.; Herich, P.; Kucková, L.; Bobeničová, M.; Šoralová, S.; Kožíšek, J.; Fronc, M.; et al. Conformational, Spectroscopic, and Molecular Dynamics DFT Study of Precursors for New Potential Antibacterial Fluoroquinolone Drugs. J. Phys. Chem. A 2014, 118, 9540–9551. [Google Scholar] [CrossRef]
- Li, X.; Zhang, R.; Yang, X. QSAR study on fluoroquinolones as antibacterial agents active for Pseudomonas aeruginosa. Front. Chem. China 2010, 5, 80–87. [Google Scholar] [CrossRef]
- Syahri, J.; Suwantono; Nasution, H.; Nurohmah, B.A.; Yuanita, E. QSAR study on fluoroquinolone derivatives as potential antibacterial agents. In Proceedings of the 8th International Conference of the Indonesian Chemical Society (ICICS), Bogor, Indonesia, 6–7 August 2019. [Google Scholar]
- Barry, A.L. In vitro activity of the fluoroquinolone compounds. Antimicrob. Newsl. 1988, 5, 69–76. [Google Scholar] [CrossRef]
- Baynes, R.; Dix, K.; Riviere, J. Chapter 6 Distribution and Pharmacokinetics Models. Pestic. Biotransform. Dispos. 2012, 117–147. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast Calculation of Molecular Polar Surface Area as a Sum of Fragment-Based Contributions and Its Application to the Prediction of Drug Transport Properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Wildman, S.A.; Crippen, G.M. Prediction of Physicochemical Parameters by Atomic Contributions. J. Chem. Inf. Comput. Sci. 1999, 39, 868–873. [Google Scholar] [CrossRef]
- Landrum, G. RDkit: Open-Source Cheminformatics. 2006. Available online: http://www.rdkit.org (accessed on 5 February 2021).
- Moriwaki, H.; Tian, Y.-S.; Kawashita, N.; Takagi, T. Mordred: A molecular descriptor calculator. J. Cheminformatics 2018, 10, 1–14. [Google Scholar] [CrossRef]
- Popović, G.; Milovanović, L.; Kapetanović, V. Study of acid–base equilibria of fleroxacin. J. Pharm. Biomed. Anal. 1998, 18, 859–863. [Google Scholar] [CrossRef]
- Takács-Novák, K.; Noszál, B.; Hermecz, I.; Keresztúri, G.; Podányi, B.; Szasz, G. Protonation Equilibria of Quinolone Antibacterials. J. Pharm. Sci. 1990, 79, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Cuprys, A.; Pulicharla, R.; Brar, S.K.; Drogui, P.; Verma, M.; Surampalli, R.Y. Fluoroquinolones metal complexation and its environmental impacts. Co-ord. Chem. Rev. 2018, 376, 46–61. [Google Scholar] [CrossRef]
- Decktor, D.L.; Robinson, M.; Maton, P.N.; Lanza, F.L.; Gottlieb, S. Effects of aluminum/magnesium hydroxide and calcium carbonate on esophageal and gastric ph in subjects with heartburn. Am. J. Ther. 1995, 2, 546–552. [Google Scholar] [CrossRef]
- Drevenšek, P.; Košmrlj, J.; Giester, G.; Skauge, T.; Sletten, E.; Sepčić, K.; Turel, I. X-Ray crystallographic, NMR and antimicrobial activity studies of magnesium complexes of fluoroquinolones—Racemic ofloxacin and its S-form, levofloxacin. J. Inorg. Biochem. 2006, 100, 1755–1763. [Google Scholar] [CrossRef]
- Sagdinc, S.; Bayarı, S. Spectroscopic studies on the interaction of ofloxacin with metals. J. Mol. Struct. 2004, 691, 107–113. [Google Scholar] [CrossRef]
- Park, H.-R.; Kim, T.H.; Bark, K.-M. Physicochemical properties of quinolone antibiotics in various environments. Eur. J. Med. Chem. 2002, 37, 443–460. [Google Scholar] [CrossRef]
- Ross, D.L.; Riley, C.M. Physicochemical properties of the fluoroquinolone antimicrobials. III. Complexation of lomefloxacin with various metal ions and the effect of metal ion complexation on aqueous solubility. Int. J. Pharm. 1992, 87, 203–213. [Google Scholar] [CrossRef]
- Turel, I. The interactions of metal ions with quinolone antibacterial agents. Co-ord. Chem. Rev. 2002, 232, 27–47. [Google Scholar] [CrossRef]
- Uivarosi, V. Metal Complexes of Quinolone Antibiotics and Their Applications: An Update. Molecules 2013, 18, 11153–11197. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 2764, Ciprofloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ciprofloxacin (accessed on 14 February 2021).
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Sinha, R.K.; Biswas, P. Structural elucidation of Levofloxacin and Ciprofloxacin using density functional theory and Raman spectroscopy with inexpensive lab-built setup. J. Mol. Struct. 2020, 1222, 128946. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Smith, D.G.A.; Burns, L.A.; Simmonett, A.C.; Parrish, R.M.; Schieber, M.C.; Galvelis, R.; Kraus, P.; Kruse, H.; Di Remigio, R.; Alenaizan, A.; et al. Psi4 1.4: Open-source software for high-throughput quantum chemistry. J. Chem. Phys. 2020, 152, 184108. [Google Scholar] [CrossRef]
- Legault, C.Y. CYLview 1.0b. Université de Sherbrooke, 2009. Available online: http://www.cylview.org (accessed on 14 February 2021).
- Shiba, K.; Sakamoto, M.; Nakazawa, Y.; Sakai, O. Effects of Antacid on Absorption and Excretion of New Quinolones. Drugs 1995, 49, 360–361. [Google Scholar] [CrossRef]
- Frost, R.W.; Lasseter, K.C.; Noe, A.J.; Shamblen, E.C.; Lettieri, J.T. Effects of aluminum hydroxide and calcium carbonate antacids on the bioavailability of ciprofloxacin. Antimicrob. Agents Chemother. 1992, 36, 830–832. [Google Scholar] [CrossRef]
- Lober, S.; Ziege, S.; Rau, M.; Schreiber, G.; Mignot, A.; Koeppe, P.; Lode, H. Pharmacokinetics of Gatifloxacin and Interaction with an Antacid Containing Aluminum and Magnesium. Antimicrob. Agents Chemother. 1999, 43, 1067–1071. [Google Scholar] [CrossRef]
- Stass, H.; Schühly, U.; Möller, J.-G.; Delesen, H. Effects of Sucralfate on the Oral Bioavailability of Moxifloxacin, a Novel 8-Methoxyfluoroquinolone, in Healthy Volunteers. Clin. Pharma. 2001, 40, 49–55. [Google Scholar] [CrossRef]
- Stass, H.; Wandel, C.; Delesen, H.; Möller, J.-G. Effect of Calcium Supplements on the Oral Bioavailability of Moxifloxacin in Healthy Male Volunteers. Clin. Pharm. 2001, 40, 27–32. [Google Scholar] [CrossRef]
- Huang, S.; Du, P.; Min, C.; Liao, Y.; Sun, H.; Jiang, Y. Poly(1-amino-5-chloroanthraquinone): Highly Selective and Ultrasensitive Fluorescent Chemosensor For Ferric Ion. J. Fluoresc. 2013, 23, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A BOILED-Egg to Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Talevi, A.; Goodarzi, M.; Ortiz, E.V.; Duchowicz, P.R.; Bellera, C.L.; Pesce, G.; Castro, E.A.; Bruno-Blanch, L.E. Prediction of drug intestinal absorption by new linear and non-linear QSPR. Eur. J. Med. Chem. 2011, 46, 218–228. [Google Scholar] [CrossRef]
- Nix, D.E.; Watson, W.A.; Handy, L.; Frost, R.W.; Rescott, D.L.; Goldstein, H.R. The Effect of Sucralfate Pretreatment on the Pharmacokinetics of Ciprofloxacin. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1989, 9, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- Breda, S.A.; Jimenez-Kairuz, A.F.; Manzo, R.H.; Olivera, M.E. Solubility behavior and biopharmaceutical classification of novel high-solubility ciprofloxacin and norfloxacin pharmaceutical derivatives. Int. J. Pharm. 2009, 371, 106–113. [Google Scholar] [CrossRef]
- Simon, Ž.; Katja, B.; Darko, U.; Marjan, V.; Albin, K. Metal cation–fluoroquinolone complexes do not permeate through the intestinal absorption barrier. J. Pharm. Biomed. Anal. 2010, 53, 655–659. [Google Scholar] [CrossRef]
- Imaoka, A.; Hattori, M.; Akiyoshi, T.; Ohtani, H. Decrease in Ciprofloxacin Absorption by Polyvalent Metal Cations Is Not Fully Attributable to Chelation or Adsorption. Drug Metab. Pharm. 2014, 29, 414–418. [Google Scholar] [CrossRef]
- Tanaka, M.; Kurata, T.; Fujisawa, C.; Ohshima, Y.; Aoki, H.; Okazaki, O.; Hakusui, H. Mechanistic study of inhibition of levofloxacin absorption by aluminum hydroxide. Antimicrob. Agents Chemother. 1993, 37, 2173–2178. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Cheng, Z.; Xie, F. Evaluation of Pharmacokinetic Drug–Drug Interactions: A Review of the Mechanisms, In Vitro and In Silico Approaches. Metabolites 2021, 11, 75. [Google Scholar] [CrossRef]
- Chakravarty, K.; Antontsev, V.; Khotimchenko, M.; Gupta, N.; Jagarapu, A.; Bundey, Y.; Hou, H.; Maharao, N.; Varshney, J. Accelerated Repurposing and Drug Development of Pulmonary Hypertension Therapies for COVID-19 Treatment Using an AI-Integrated Biosimulation Platform. Molecules 2021, 26, 1912. [Google Scholar] [CrossRef] [PubMed]
- Maharao, N.; Antontsev, V.; Wright, M.; Varshney, J. Entering the era of computationally driven drug development. Drug Metab. Rev. 2020, 52, 283–298. [Google Scholar] [CrossRef] [PubMed]
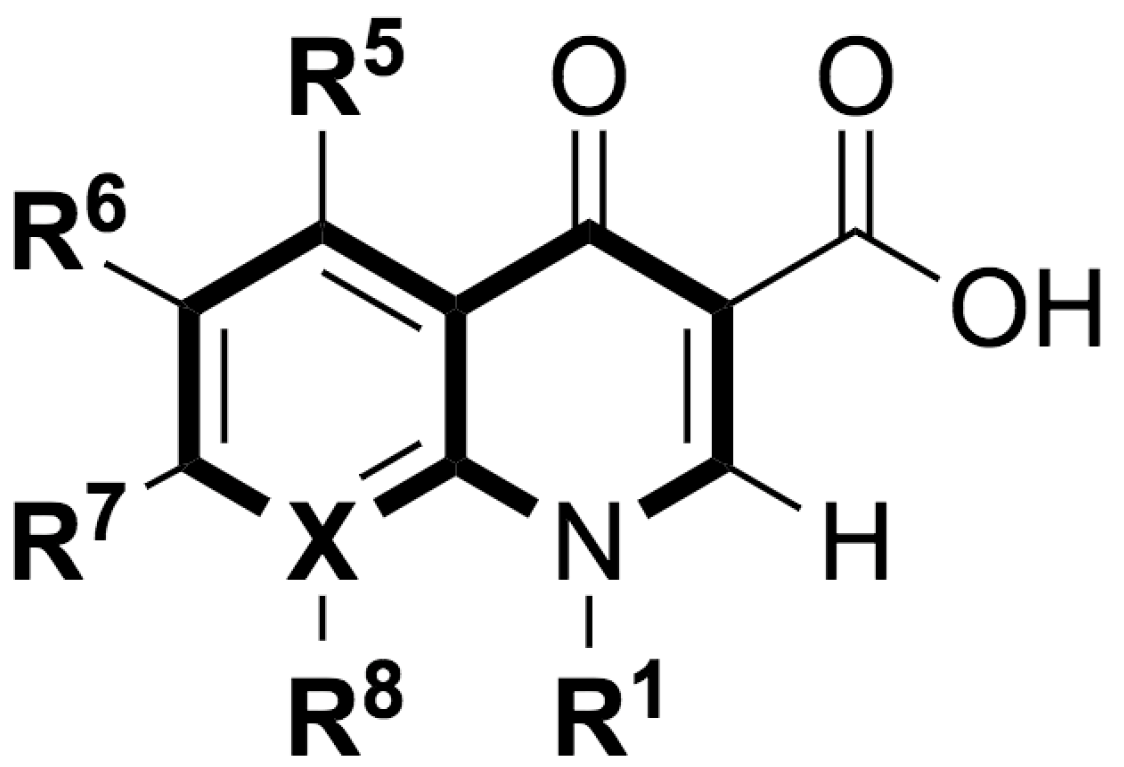
| Generation | Fluoroquinolone | Structure | MW | TPSA | Calculated logP |
|---|---|---|---|---|---|
| Second | Ciprofloxacin | 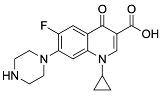 | 331.13 | 74.57 | 1.58 |
| Second | Enoxacin | 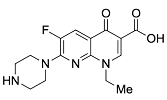 | 320.13 | 87.46 | 0.66 |
| Second | Fleroxacin | 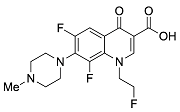 | 369.13 | 65.78 | 1.70 |
| Second | Lomefloxacin | 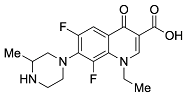 | 351.14 | 74.57 | 1.80 |
| Second | Norfloxacin | 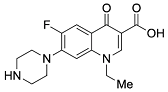 | 319.13 | 74.57 | 1.27 |
| Second | Ofloxacin | 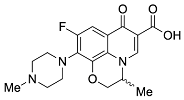 | 361.14 | 75.01 | 1.54 |
| Second | Pefloxacin | 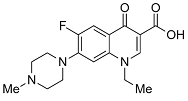 | 333.15 | 65.78 | 1.61 |
| Second | Rufloxacin | 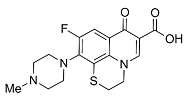 | 363.11 | 91.08 | 1.70 |
| Third | Levofloxacin | 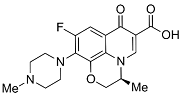 | 361.14 | 75.01 | 1.54 |
| Third | Sparfloxacin | 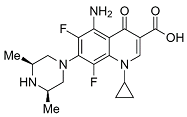 | 392.17 | 100.59 | 2.08 |
| Third | Tosufloxacin | 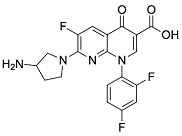 | 404.11 | 101.45 | 2.04 |
| Fourth | Gatifloxacin | 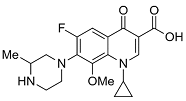 | 375.16 | 83.8 | 1.98 |
| Fourth | Moxifloxacin | 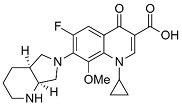 | 401.18 | 83.8 | 2.37 |
| Relative Change (%) | ||||||
|---|---|---|---|---|---|---|
| Fluoroquinolone | Multivalent Metal | Metal Source | Cmax | AUC | Calculated ka | Reference |
| Ciprofloxacin | Aluminum | Aluminum hydroxide | −81.1 | −84.6 | −14.3 | [72] |
| Aluminum | Aluminum hydroxide | −84.6 | −87.5 | −60.6 | [71] | |
| Aluminum | Sucralfate | −90.0 | −87.5 | N/A | [10] | |
| Aluminum/Magnesium | Maalox | −80.1 | −84.9 | 157.9 | [9] | |
| Aluminum/Magnesium | Didanosine | −92.6 | −98.3 | 49.2 | [11] | |
| Calcium | Titralac | −37.9 | −41.1 | 5.9 | [12] | |
| Enoxacin | Aluminum | Aluminum hydroxide | −78.3 | −84.2 | N/A | [71] |
| Aluminum/Magnesium | Maalox | −70.0 | −73.2 | −52.1 | [15] | |
| Fleroxacin | Aluminum | Aluminum hydroxide | −25.0 | −17.2 | −47.6 | [71] |
| Aluminum | Sucralfate | −26.4 | −24.0 | −40.3 | [24] | |
| Lomefloxacin | Aluminum | Aluminum hydroxide | −54.5 | −34.8 | −55.1 | [71] |
| Aluminum | Kolantyl | −46.1 | −40.8 | 25.6 | [16] | |
| Norfloxacin | Aluminum | Aluminum hydroxide | −93.3 | −97.0 | N/A | [71] |
| Aluminum | Sucralfate | −92.2 | −91.3 | −23.8 | [20] | |
| Aluminum/Magnesium | Maalox | −95.1 | N/A | −6.1 | [21] | |
| Calcium | Titralac | −65.9 | −62.6 | −80.2 | [21] | |
| Ofloxacin | Aluminum | Aluminum hydroxide | −59.4 | −47.9 | −65.0 | [71] |
| Aluminum | Sucralfate | −69.5 | −61.0 | −46.2 | [20] | |
| Pefloxacin | Aluminum/Magnesium | Maalox | −60.8 | −54.3 | −57.5 | [17] |
| Rufloxacin | Aluminum/Magnesium | Maalox | 6.1 | −15.2 | −34.0 | [18] |
| Levofloxacin | Aluminum | Aluminum hydroxide | −66.7 | −45.2 | −55.5 | [71] |
| Sparfloxacin | Aluminum | Aluminum hydroxide | −22.2 | −35.1 | 47.1 | [71] |
| Tosufloxacin | Aluminum | Aluminum hydroxide | −66.7 | −70.8 | −67.4 | [71] |
| Gatifloxacin | Aluminum/Magnesium | Maalox | −68.4 | −60.8 | −13.5 | [73] |
| Moxifloxacin | Aluminum | Sucralfate | −79.5 | −59.9 | −80.7 | [74] |
| Calcium | Calcium-Sandoz | −15.5 | −2.4 | −74.1 | [75] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walden, D.M.; Khotimchenko, M.; Hou, H.; Chakravarty, K.; Varshney, J. Effects of Magnesium, Calcium, and Aluminum Chelation on Fluoroquinolone Absorption Rate and Bioavailability: A Computational Study. Pharmaceutics 2021, 13, 594. https://doi.org/10.3390/pharmaceutics13050594
Walden DM, Khotimchenko M, Hou H, Chakravarty K, Varshney J. Effects of Magnesium, Calcium, and Aluminum Chelation on Fluoroquinolone Absorption Rate and Bioavailability: A Computational Study. Pharmaceutics. 2021; 13(5):594. https://doi.org/10.3390/pharmaceutics13050594
Chicago/Turabian StyleWalden, Daniel M., Maksim Khotimchenko, Hypatia Hou, Kaushik Chakravarty, and Jyotika Varshney. 2021. "Effects of Magnesium, Calcium, and Aluminum Chelation on Fluoroquinolone Absorption Rate and Bioavailability: A Computational Study" Pharmaceutics 13, no. 5: 594. https://doi.org/10.3390/pharmaceutics13050594
APA StyleWalden, D. M., Khotimchenko, M., Hou, H., Chakravarty, K., & Varshney, J. (2021). Effects of Magnesium, Calcium, and Aluminum Chelation on Fluoroquinolone Absorption Rate and Bioavailability: A Computational Study. Pharmaceutics, 13(5), 594. https://doi.org/10.3390/pharmaceutics13050594






