Recent Progress and Challenges for Drug-Resistant Tuberculosis Treatment
Abstract
1. Introduction
2. Progress
2.1. Fluoroquinolone
2.2. Rifamycin
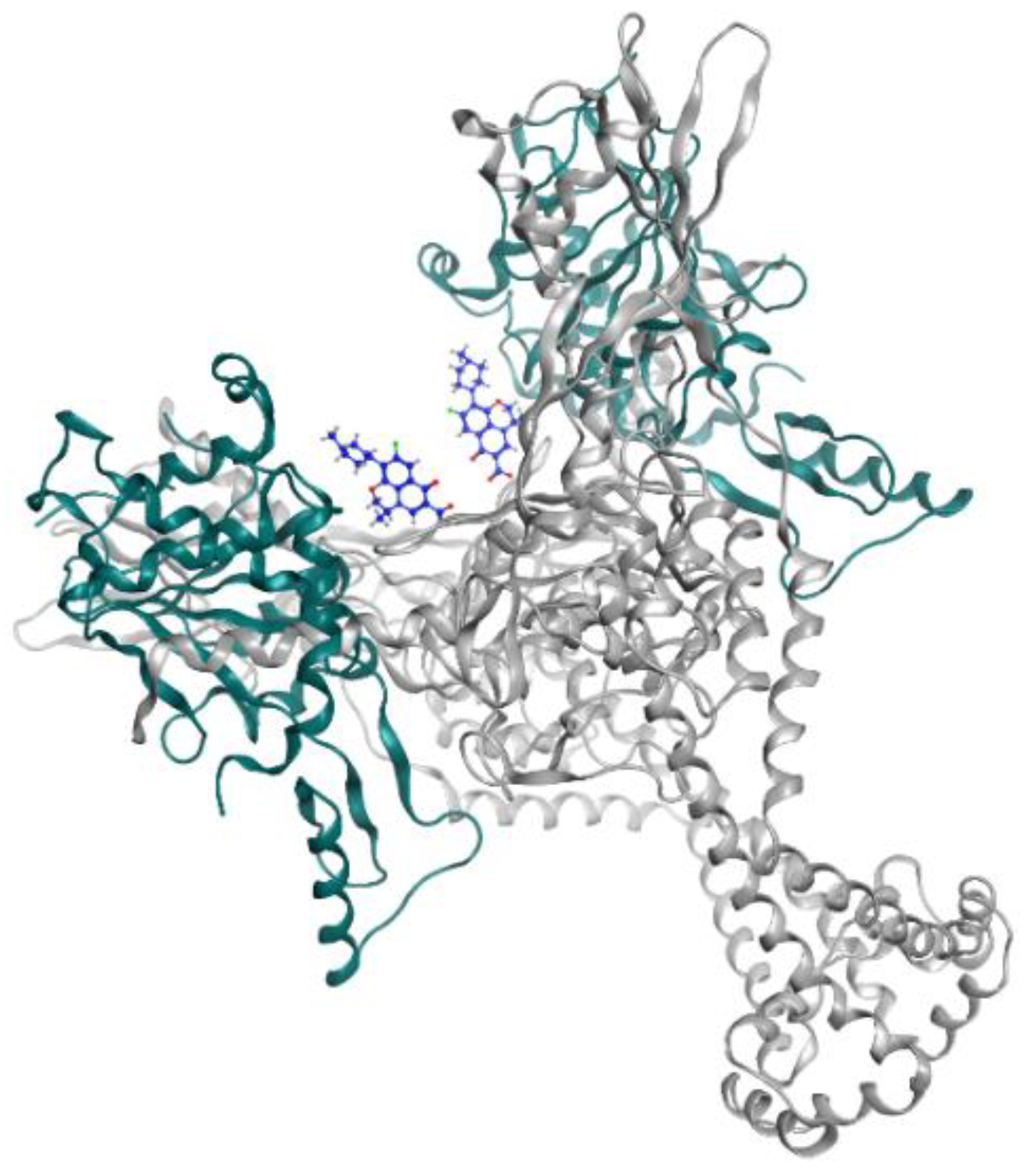
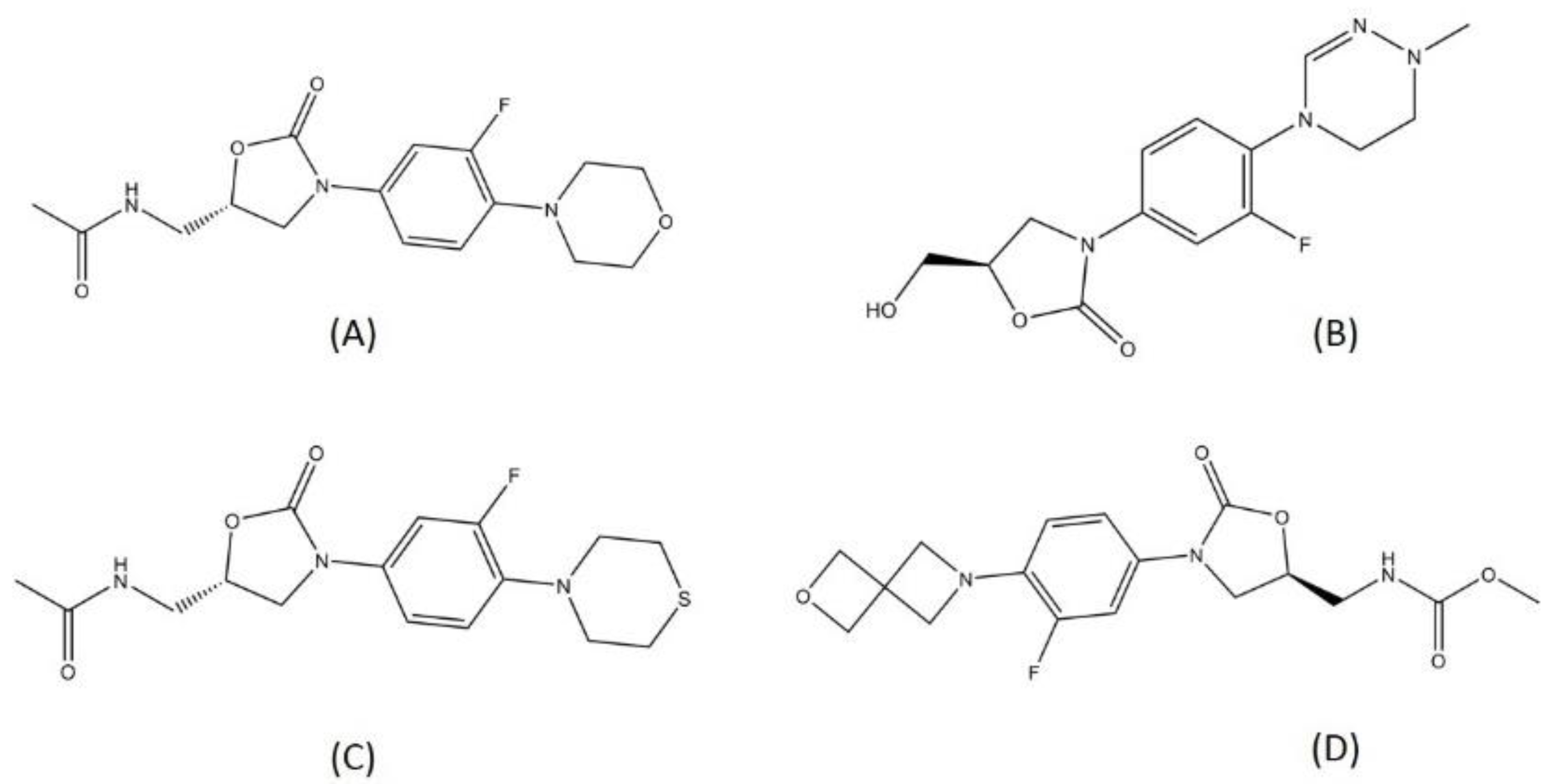
2.3. Oxazolidinone
2.4. Nitroimidazole
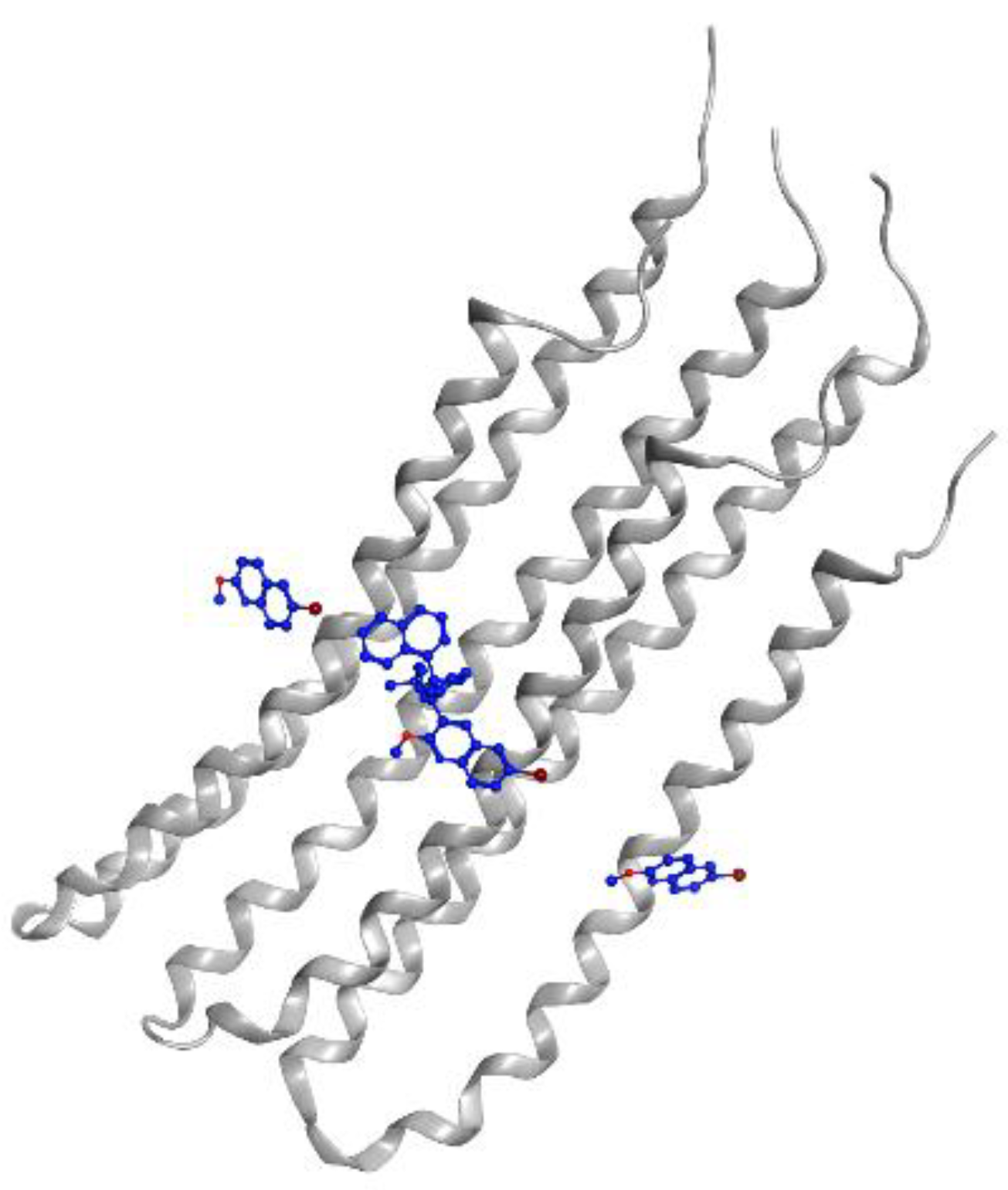
2.5. Diarylquinoline
2.6. Benzothiazinone
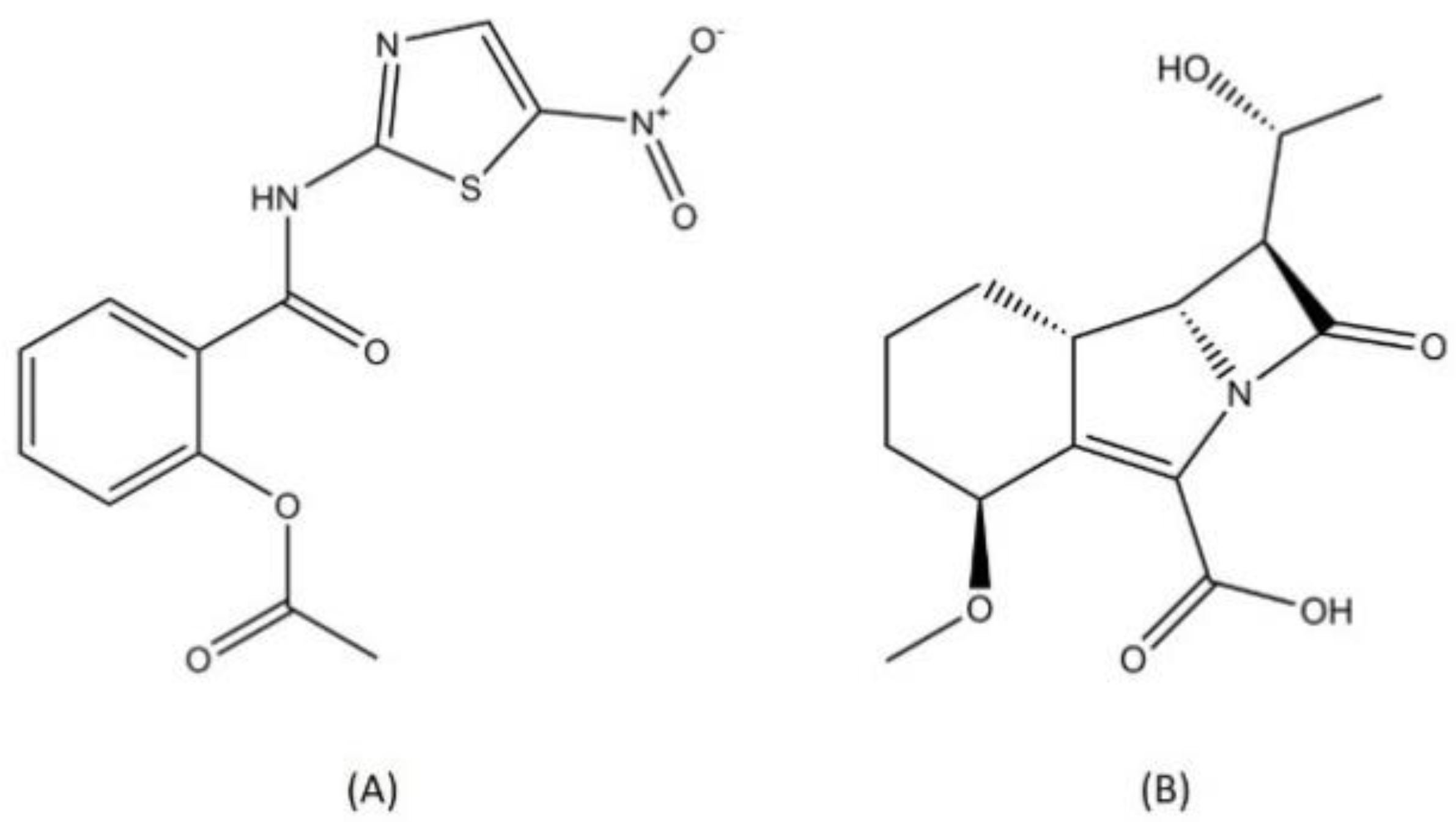
2.7. Other Classes
2.8. Drug Repurposing for TB Treatment
2.9. Host-Directed Therapy
2.10. Tuberculosis (TB) Immunotherapy
3. Challenges
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2018; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Lee, J.Y. Diagnosis and Treatment of Extrapulmonary Tuberculosis. Tuberc. Respir. Dis. (Seoul) 2015, 78, 47–55. [Google Scholar] [CrossRef]
- Esmail, H.; Barry, C.E.; Young, D.B.; Wilkinson, R.J. The Ongoing Challenge of Latent Tuberculosis. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130437. [Google Scholar] [CrossRef]
- Lan, Z.; Bastos, M.; Menzies, D. Treatment of Human Disease Due to Mycobacterium Bovis: A Systematic Review. Eur. Respir. J. 2016, 48, 1500–1503. [Google Scholar] [CrossRef]
- Camus, J.C.; Pryor, M.J.; Médigue, C.; Cole, S.T. Re-Annotation of the Genome Sequence of Mycobacterium Tuberculosis H37Rv. Microbiology 2002. [Google Scholar] [CrossRef] [PubMed]
- Smith, I. Mycobacterium Tuberculosis Pathogenesis and Molecular Determinants of Virulence. Clin. Microbiol. Rev. 2003, 16, 463–496. [Google Scholar] [CrossRef]
- Delogu, G.; Sali, M.; Fadda, G. The Biology of Mycobacterium Tuberculosis Infection. Mediterr. J. Hematol. Infect. Dis. 2013, 5, e2013070. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Rhee, K.Y. Tuberculosis Drug Development: History and Evolution of the Mechanism-Based Paradigm. Cold Spring Harb. Perspect. Med. 2015. [Google Scholar] [CrossRef]
- Zumla, A.; Nahid, P.; Cole, S.T. Advances in the Development of New Tuberculosis Drugs and Treatment Regimens. Nat. Rev. Drug Discov. 2013, 12, 388–404. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Multidrug and Extensively Drug-Resistant TB (M/XDR-TB): 2010 Global Report on Surveillance and Response; WHO: Paris, France, 2010; ISBN 978 92 4 159919 1. [Google Scholar]
- Huynh, J.; Marais, B.J. Multidrug-Resistant Tuberculosis Infection and Disease in Children: A Review of New and Repurposed Drugs. Ther. Adv. Infect. Dis. 2019. [Google Scholar] [CrossRef]
- Nguyen, L. Antibiotic Resistance Mechanisms in M. Tuberculosis: An Update. Arch. Toxicol. 2016, 90, 1585–1604. [Google Scholar] [CrossRef]
- Kurz, S.G.; Furin, J.J.; Bark, C.M. Drug-Resistant Tuberculosis: Challenges and Progress. Infect. Dis. Clin. N. Am. 2016, 30, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, F.; Zhang, W. Bedaquiline and Delamanid in the Treatment of Multidrug-Resistant Tuberculosis: Promising but Challenging. Drug Dev. Res. 2019, 80, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Nunn, A.J.; Phillips, P.P.J.; Meredith, S.K.; Chiang, C.Y.; Conradie, F.; Dalai, D.; Van Deun, A.; Dat, P.T.O.; Lan, N.; Master, I.; et al. A Trial of a Shorter Regimen for Rifampin-Resistant Tuberculosis. N. Engl. J. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sieniawska, E.; Maciejewska-Turska, M.; Światek, L.; Xiao, J. Plant-Based Food Products for Antimycobacterial Therapy. eFood 2020, 1. [Google Scholar] [CrossRef]
- Drlica, K.; Hiasa, H.; Kerns, R.; Malik, M.; Mustaev, A.; Zhao, X. Quinolones: Action and Resistance Updated. Curr. Top. Med. Chem. 2009. [Google Scholar] [CrossRef]
- Aubry, A.; Pan, X.S.; Fisher, L.M.; Jarlier, V.; Cambau, E. Mycobacterium Tuberculosis DNA Gyrase: Interaction with Quinolones and Correlation with Antimycobacterial Drug Activity. Antimicrob. Agents Chemother. 2004. [Google Scholar] [CrossRef] [PubMed]
- García, M.T.; Carreño, D.; Tirado-Vélez, J.M.; Ferrándiz, M.J.; Rodrigues, L.; Gracia, B.; Amblar, M.; Ainsa, J.A.; de la Campa, A.G. Boldine-Derived Alkaloids Inhibit the Activity of DNA Topoisomerase I and Growth of Mycobacterium Tuberculosis. Front. Microbiol. 2018. [Google Scholar] [CrossRef]
- Deweese, J.E.; Osheroff, M.A.; Osheroff, N. DNA Topology and Topoisomerases: Teaching A “knotty” Subject. Biochem. Mol. Biol. Educ. 2009, 37, 2–10. [Google Scholar] [CrossRef]
- Forterre, P.; Gadelle, D. Phylogenomics of DNA Topoisomerases: Their Origin and Putative Roles in the Emergence of Modern Organisms. Nucleic Acids Res. 2009. [Google Scholar] [CrossRef]
- Kumar, R.; Riley, J.E.; Parry, D.; Bates, A.D.; Nagaraja, V. Binding of Two DNA Molecules by Type II Topoisomerases for Decatenation. Nucleic Acids Res. 2012. [Google Scholar] [CrossRef]
- Collin, F.; Karkare, S.; Maxwell, A. Exploiting Bacterial DNA Gyrase as a Drug Target: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2011, 92, 479–497. [Google Scholar] [CrossRef]
- Gillespie, S.H.; Crook, A.M.; McHugh, T.D.; Mendel, C.M.; Meredith, S.K.; Murray, S.R.; Pappas, F.; Phillips, P.P.J.; Nunn, A.J. Four-Month Moxifloxacin-Based Regimens for Drug-Sensitive Tuberculosis. N. Engl. J. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Merle, C.S.; Fielding, K.; Sow, O.B.; Gninafon, M.; Lo, M.B.; Mthiyane, T.; Odhiambo, J.; Amukoye, E.; Bah, B.; Kassa, F.; et al. A Four-Month Gatifloxacin-Containing Regimen for Treating Tuberculosis. N. Engl. J. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Aldred, K.J.; Blower, T.R.; Kerns, R.J.; Berger, J.M.; Osheroff, N. Fluoroquinolone Interactions with Mycobacterium Tuberculosis Gyrase: Enhancing Drug Activity Against Wild-Type and Resistant Gyrase. Proc. Natl. Acad. Sci. USA 2016. [Google Scholar] [CrossRef]
- Rodríguez, J.C.; Ruiz, M.; Climent, A.; Royo, G. In Vitro Activity of Four Fluoroquinolones Against Mycobacterium Tuberculosis. Int. J. Antimicrob. Agents 2001. [Google Scholar] [CrossRef]
- Enna, S.J.; Bylund, D.B. Rifamycin. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Kansas City, KS, USA, 2011; ISBN 9780080552323. [Google Scholar]
- Grosset, J.H. Newer drugs in leprosy. Int. J. Lepr. Other Mycobact. Dis. 2001, 69 (Suppl. 2), S14–S18. [Google Scholar] [PubMed]
- Campbell, E.A.; Korzheva, N.; Mustaev, A.; Murakami, K.; Nair, S.; Goldfarb, A.; Darst, S.A. Structural Mechanism for Rifampicin Inhibition of Bacterial RNA Polymerase. Cell 2001. [Google Scholar] [CrossRef]
- Bortoluzzi, A.; Muskett, F.W.; Waters, L.C.; Addis, P.W.; Rieck, B.; Munder, T.; Schleier, S.; Forti, F.; Ghisotti, D.; Carr, M.D.; et al. Mycobacterium Tuberculosis RNA Polymerase-Binding Protein A (RbpA) and Its Interactions with Sigma Factors. J. Biol. Chem. 2013. [Google Scholar] [CrossRef]
- Jensen, D.; Manzano, A.R.; Rammohan, J.; Stallings, C.L.; Galburt, E.A. CarD and RbpA Modify the Kinetics of Initial Transcription and Slow Promoter Escape of the Mycobacterium Tuberculosis RNA Polymerase. Nucleic Acids Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.P. Resistance to Rifampicin: A Review. J. Antibiot. (Tokyo) 2014, 67, 625–630. [Google Scholar] [CrossRef]
- Koch, A.; Mizrahi, V.; Warner, D.F. The Impact of Drug Resistance on Mycobacterium Tuberculosis Physiology: What Can We Learn from Rifampicin? Emerg. Microbes Infect. 2014, 3, 1–11. [Google Scholar] [CrossRef]
- Kigozi, E.; Kasule, G.W.; Musisi, K.; Lukoye, D.; Kyobe, S.; Katabazi, F.A.; Wampande, E.M.; Joloba, M.L.; Kateete, D.P. Prevalence and Patterns of Rifampicin and Isoniazid Resistance Conferring Mutations in Mycobacterium Tuberculosis Isolates from Uganda. PLoS ONE 2018, 13, e0198091. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, A.; Ortega-Muro, F.; Alameda-Martin, L.; Mitchison, D.; Coates, A. High-Dose Rifampicin Kills Persisters, Shortens Treatment Duration, and Reduces Relapse Rate In Vitro and In Vivo. Front. Microbiol. 2015. [Google Scholar] [CrossRef]
- Boeree, M.J.; Heinrich, N.; Aarnoutse, R.; Diacon, A.H.; Dawson, R.; Rehal, S.; Kibiki, G.S.; Churchyard, G.; Sanne, I.; Ntinginya, N.E.; et al. High-Dose Rifampicin, Moxifloxacin, and SQ109 for Treating Tuberculosis: A Multi-Arm, Multi-Stage Randomised Controlled Trial. Lancet Infect. Dis. 2017. [Google Scholar] [CrossRef]
- Watkins, R.R.; Lemonovich, T.L.; File, T.M. An Evidence-Based Review of Linezolid for the Treatment of Methicillin-Resistant Staphylococcus Aureus (MRSA): Place in Therapy. Core Evid. 2012, 7, 131–143. [Google Scholar] [CrossRef]
- Bozdogan, B.; Appelbaum, P.C. Oxazolidinones: Activity, Mode of Action, and Mechanism of Resistance. Int. J. Antimicrob. Agents 2004, 23, 113–119. [Google Scholar] [CrossRef]
- Sloan, D.J.; Lewis, J.M. Management of Multidrug-Resistant TB: Novel Treatments and Their Expansion to Low Resource Settings. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 163–172. [Google Scholar] [CrossRef]
- Poce, G.; Cocozza, M.; Consalvi, S.; Biava, M. SAR Analysis of New Anti-TB Drugs Currently in Pre-Clinical and Clinical Development. Eur. J. Med. Chem. 2014, 86, 335–351. [Google Scholar] [CrossRef]
- World Health Organization. Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment; WHO: Geneva, Switzerland, 2019; ISBN 9789241550529. [Google Scholar]
- Srivastava, S.; Magombedze, G.; Koeuth, T.; Sherman, C.; Pasipanodya, J.G.; Raj, P.; Wakeland, E.; Deshpande, D.; Gumbo, T. Linezolid Dose that Maximizes Sterilizing Effect While Minimizing Toxicity and Resistance Emergence for Tuberculosis. Antimicrob. Agents Chemother. 2017. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, J.; Cui, P.; Shi, W.; Shi, X.; Niu, H.; Chan, D.; Yew, W.W.; Zhang, W.; Zhang, Y. Mycobacterium Tuberculosis Mutations Associated with Reduced Susceptibility to Linezolid. Antimicrob. Agents Chemother. 2016. [Google Scholar] [CrossRef]
- Zong, Z.; Jing, W.; Shi, J.; Wen, S.; Zhang, T.; Huo, F.; Shang, Y.; Liang, Q.; Huang, H.; Pang, Y. Comparison of In Vitro Activity and MIC Distributions between the Novel Oxazolidinone Delpazolid and Linezolid Against Multidrug-Resistant and Extensively Drug-Resistant Mycobacterium Tuberculosis in China. Antimicrob. Agents Chemother. 2018. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, Y.; Wang, Y.; Liu, C.; Zhao, Y. Beijing Genotype of Mycobacterium Tuberculosis is Significantly Associated with Linezolid Resistance in Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis in China. Int. J. Antimicrob. Agents 2014. [Google Scholar] [CrossRef]
- Wallis, R.S.; Dawson, R.; Friedrich, S.O.; Venter, A.; Paige, D.; Zhu, T.; Silvia, A.; Gobey, J.; Ellery, C.; Zhang, Y.; et al. Mycobactericidal Activity of Sutezolid (PNU-100480) in Sputum (EBA) and Blood (WBA) of Patients with Pulmonary Tuberculosis. PLoS ONE 2014, 9, e94462. [Google Scholar] [CrossRef]
- Ignatius, E.H.; Dooley, K.E. New Drugs for the Treatment of Tuberculosis. Clin. Chest Med. 2019, 40, 811–827. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Zhang, L.; Manjunatha, U.H.; Singh, R.; Patel, S.; Jiricek, J.; Keller, T.H.; Boshoff, H.I.; Barry, C.E.; Dowd, C.S. Structure-Activity Relationships of Antitubercular Nitroimidazoles. 1. Structural Features Associated with Aerobic and Anaerobic Activities of 4 And 5-Nitroimidazoles. J. Med. Chem. 2009. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.Y.L. Metronidazole. In Kucers the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs, Seventh Edition; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781498747967. [Google Scholar]
- Löfmark, S.; Edlund, C.; Nord, C.E. Metronidazole Is Still the Drug of Choice for Treatment of Anaerobic Infections. Clin. Infect. Dis. 2010. [Google Scholar] [CrossRef]
- Bagdasarian, N.; Rao, K.; Malani, P.N. Diagnosis and Treatment of Clostridium Difficile in Adults: A Systematic Review. JAMA J. Am. Med. Assoc. 2015. [Google Scholar] [CrossRef]
- Wilcox, M.H. 147-Nitroimidazoles, Metronidazole, Ornidazole and Tinidazole; and Fidaxomicin. In Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1261–1263. ISBN 9780702062858. [Google Scholar]
- Patterson, S.; Wyllie, S. Nitro Drugs for the Treatment of Trypanosomatid Diseases: Past, Present, and Future Prospects. Trends Parasitol. 2014, 30, 289–298. [Google Scholar] [CrossRef]
- Nepali, K.; Lee, H.Y.; Liou, J.P. Nitro-Group-Containing Drugs. J. Med. Chem. 2019, 62, 2851–2893. [Google Scholar] [CrossRef]
- Hanaki, E.; Hayashi, M.; Matsumoto, M. Delamanid is not Metabolized by Salmonella or Human Nitroreductases: A Possible Mechanism for the Lack of Mutagenicity. Regul. Toxicol. Pharmacol. 2017. [Google Scholar] [CrossRef]
- Stover, C.K.; Warrener, P.; VanDevanter, D.R.; Sherman, D.R.; Arain, T.M.; Langhorne, M.H.; Anderson, S.W.; Towell, J.A.; Yuan, Y.; McMurray, D.N.; et al. A Small-Molecule Nitroimidazopyran Drug Candidate for the Treatment of Tuberculosis. Nature 2000. [Google Scholar] [CrossRef] [PubMed]
- Landge, S.; Ramachandran, V.; Kumar, A.; Neres, J.; Murugan, K.; Sadler, C.; Fellows, M.D.; Humnabadkar, V.; Vachaspati, P.; Raichurkar, A.; et al. Nitroarenes as Antitubercular Agents: Stereoelectronic Modulation to Mitigate Mutagenicity. Chem. Med. Chem. 2016. [Google Scholar] [CrossRef]
- Xavier, A.S.; Lakshmanan, M. Delamanid: A New Armor in Combating Drug-Resistant Tuberculosis. J. Pharmacol. Pharmacother. 2014. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.M.; Sloan, D.J. The Role of Delamanid in the Treatment of Drug-Resistant Tuberculosis. Ther. Clin. Risk Manag. 2015, 11, 779–791. [Google Scholar] [CrossRef]
- Singh, R.; Manjunatha, U.; Boshoff, H.I.M.; Young, H.H.; Niyomrattanakit, P.; Ledwidge, R.; Dowd, C.S.; Ill, Y.L.; Kim, P.; Zhang, L.; et al. PA-824 Kills Nonreplicating Mycobacterium Tuberculosis by Intracellular NO Release. Science 2008. [Google Scholar] [CrossRef]
- Manjunatha, U.; Boshoff, H.I.; Barry, C.E. The Mechanism of Action of PA-824: Novel Insights from Transcriptional Profiling. Commun. Integr. Biol. 2009. [Google Scholar] [CrossRef]
- Haufroid, M.; Wouters, J. Targeting the Serine Pathway: A Promising Approach Against Tuberculosis? Pharmaceuticals 2019, 12, 66. [Google Scholar] [CrossRef]
- Lee, B.M.; Harold, L.K.; Almeida, D.V.; Afriat-Jurnou, L.; Aung, H.L.; Forde, B.M.; Hards, K.; Pidot, S.J.; Ahmed, F.H.; Mohamed, A.E.; et al. Predicting Nitroimidazole Antibiotic Resistance Mutations in Mycobacterium Tuberculosis with Protein Engineering. PLoS Pathog. 2020. [Google Scholar] [CrossRef]
- Samson, I. A New Class of Antimycobacterial Drugs: The Diarylquinolines. Thorax 2005. [Google Scholar] [CrossRef]
- Cholo, M.C.; Mothiba, M.T.; Fourie, B.; Anderson, R. Mechanisms of Action and Therapeutic Efficacies of the Lipophilic Antimycobacterial Agents Clofazimine and Bedaquiline. J. Antimicrob. Chemother. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chahine, E.B.; Karaoui, L.R.; Mansour, H. Bedaquiline: A Novel Diarylquinoline for Multidrug-Resistant Tuberculosis. Ann. Pharmacother. 2014, 48, 107–115. [Google Scholar] [CrossRef]
- Andries, K.; Verhasselt, P.; Guillemont, J.; Göhlmann, H.W.H.; Neefs, J.M.; Winkler, H.; Van Gestel, J.; Timmerman, P.; Zhu, M.; Lee, E.; et al. A Diarylquinoline Drug Active on the ATP Synthase of Mycobacterium Tuberculosis. Science 2005. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.P.; Degani, M.S.; Raju, A.; Anantram, A.; Seervi, M.; Sathaye, S.; Ray, M.; Rajan, M.G.R. Identification of a Novel Class of Quinoline-Oxadiazole Hybrids as Anti-Tuberculosis Agents. Bioorg. Med. Chem. Lett. 2016. [Google Scholar] [CrossRef] [PubMed]
- Segala, E.; Sougakoff, W.; Nevejans-Chauffour, A.; Jarlier, V.; Petrella, S. New Mutations in the Mycobacterial ATP Synthase: New Insights Into the Binding of the Diarylquinoline TMC207 to the ATP Synthase C-Ring Structure. Antimicrob. Agents Chemother. 2012. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.A.; Anthony, R.M.; Bañuls, A.L.; Vu, D.H.; Alffenaar, J.W.C. Bedaquiline Resistance: Its Emergence, Mechanism, and Prevention. Clin. Infect. Dis. 2018. [Google Scholar] [CrossRef]
- Tong, A.S.T.; Choi, P.J.; Blaser, A.; Sutherland, H.S.; Tsang, S.K.Y.; Guillemont, J.; Motte, M.; Cooper, C.B.; Andries, K.; Van Den Broeck, W.; et al. 6-Cyano Analogues of Bedaquiline as Less Lipophilic and Potentially Safer Diarylquinolines for Tuberculosis. ACS Med. Chem. Lett. 2017. [Google Scholar] [CrossRef]
- Sarathy, J.P.; Ganapathy, U.S.; Zimmerman, M.D.; Dartois, V.; Gengenbacher, M.; Dick, T. TBAJ-876, A 3,5-dialkoxypyridine Analogue of Bedaquiline, is Active Against Mycobacterium Abscessus. Antimicrob. Agents Chemother. 2020. [Google Scholar] [CrossRef]
- Makarov, V.; Manina, G.; Mikusova, K.; Möllmann, U.; Ryabova, O.; Saint-Joanis, B.; Dhar, N.; Pasca, M.R.; Buroni, S.; Lucarelli, A.P.; et al. Benzothiazinones Kill Mycobacterium Tuberculosis by Blocking Arabinan Synthesis. Science 2009. [Google Scholar] [CrossRef]
- Crellin, P.K.; Brammananth, R.; Coppel, R.L. Decaprenylphosphoryl-β-D-Ribose 2′-epimerase, the Target of Benzothiazinones and Dinitrobenzamides, is An Essential Enzyme in Mycobacterium Smegmatis. PLoS ONE 2011, 6, e16869. [Google Scholar] [CrossRef]
- Makarov, V.; Lechartier, B.; Zhang, M.; Neres, J.; van der Sar, A.M.; Raadsen, S.A.; Hartkoorn, R.C.; Ryabova, O.B.; Vocat, A.; Decosterd, L.A.; et al. Towards a New Combination Therapy for Tuberculosis with Next Generation Benzothiazinones. EMBO Mol. Med. 2014. [Google Scholar] [CrossRef]
- Foo, C.S.Y.; Lechartier, B.; Kolly, G.S.; Boy-Röttger, S.; Neres, J.; Rybniker, J.; Lupien, A.; Sala, C.; Piton, J.; Cole, S.T. Characterization of DprE1-Mediated Benzothiazinone Resistance in Mycobacterium Tuberculosis. Antimicrob. Agents Chemother. 2016. [Google Scholar] [CrossRef]
- Neres, J.; Pojer, F.; Molteni, E.; Chiarelli, L.R.; Dhar, N.; Boy-Röttger, S.; Buroni, S.; Fullam, E.; Degiacomi, G.; Lucarelli, A.P.; et al. Structural Basis for Benzothiazinone-Mediated Killing of Mycobacterium Tuberculosis. Sci. Transl. Med. 2012. [Google Scholar] [CrossRef]
- Batt, S.M.; Jabeen, T.; Bhowruth, V.; Quill, L.; Lund, P.A.; Eggeling, L.; Alderwick, L.J.; Fuẗterer, K.; Besra, G.S. Structural Basis of Inhibition of Mycobacterium Tuberculosis DprE1 by Benzothiazinone Inhibitors. Proc. Natl. Acad. Sci. USA 2012. [Google Scholar] [CrossRef]
- Piton, J.; Vocat, A.; Lupien, A.; Foo, C.S.; Riabov, O.; Makarov, V.; Cole, S.T. Structure-Based Drug Design and Characterization of Sulfonyl-Piperazine Benzothiazinone Inhibitors of DprE1 from Mycobacterium Tuberculosis. Antimicrob. Agents Chemother. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Peng, C.; Shi, Y.; You, X.; Ran, K.; Xiong, L.; Ye, T.H.; Zhang, L.; Wang, N.; Zhu, Y.; et al. Benzothiazinethione is a Potent Preclinical Candidate for the Treatment of Drug-Resistant Tuberculosis. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; You, X.; Wang, B.; Wei, Z.; Chai, Y.; Wang, B.; Wang, A.; Huang, G.; Liu, M.; Lu, Y. Identification of Better Pharmacokinetic Benzothiazinone Derivatives as New Antitubercular Agents. ACS Med. Chem. Lett. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pethe, K.; Bifani, P.; Jang, J.; Kang, S.; Park, S.; Ahn, S.; Jiricek, J.; Jung, J.; Jeon, H.K.; Cechetto, J.; et al. Discovery of Q203, a Potent Clinical Candidate for the Treatment of Tuberculosis. Nat. Med. 2013. [Google Scholar] [CrossRef]
- De Jager, V.R.; Dawson, R.; Van Niekerk, C.; Hutchings, J.; Kim, J.; Vanker, N.; Van Der Merwe, L.; Choi, J.; Nam, K.; Diacon, A.H. Telacebec (Q203), A New Antituberculosis Agent. N. Engl. J. Med. 2020, 382, 1280–1281. [Google Scholar] [CrossRef]
- Jones, D. Tuberculosis success. Nat. Rev. Drug Discov. 2013. [Google Scholar] [CrossRef]
- O’Malley, T.; Alling, T.; Early, J.V.; Wescott, H.A.; Kumar, A.; Moraski, G.C.; Miller, M.J.; Masquelin, T.; Hipskind, P.A.; Parish, T. Imidazopyridine Compounds Inhibit Mycobacterial Growth by Depleting ATP Levels. Antimicrob. Agents Chemother. 2018. [Google Scholar] [CrossRef]
- Maitra, A.; Bates, S.; Kolvekar, T.; Devarajan, P.V.; Guzman, J.D.; Bhakta, S. Repurposing—A Ray of Hope in Tackling Extensively Drug Resistance in Tuberculosis. Int. J. Infect. Dis. 2015. [Google Scholar] [CrossRef]
- Singh, N.; Narayan, S. Nitazoxanide: A Broad Spectrum Antimicrobial. Med. J. Armed Forces India 2011. [Google Scholar] [CrossRef]
- De Carvalho, L.P.S.; Lin, G.; Jiang, X.; Nathan, C. Nitazoxanide Kills Replicating and Nonreplicating Mycobacterium Tuberculosis and Evades Resistance. J. Med. Chem. 2009. [Google Scholar] [CrossRef] [PubMed]
- Odingo, J.; Bailey, M.A.; Files, M.; Early, J.V.; Alling, T.; Dennison, D.; Bowman, J.; Dalai, S.; Kumar, N.; Cramer, J.; et al. In Vitro Evaluation of Novel Nitazoxanide Derivatives against Mycobacterium tuberculosis. ACS Omega 2017. [Google Scholar] [CrossRef]
- Story-Roller, E.; Lamichhane, G. Have We Realized the Full Potential of β-Lactams for Treating Drug-Resistant TB? IUBMB Life 2018, 70, 881–888. [Google Scholar] [CrossRef]
- Kurz, S.G.; Bonomo, R.A. Reappraising the Use of β-Lactams to Treat Tuberculosis. Expert Rev. Anti. Infect. Ther. 2012, 10, 999–1006. [Google Scholar] [CrossRef]
- Korman, T.M. Ethambutol. In Kucers the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs, Seventh Edition; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781498747967. [Google Scholar]
- Tahlan, K.; Wilson, R.; Kastrinsky, D.B.; Arora, K.; Nair, V.; Fischer, E.; Whitney Barnes, S.; Walker, J.R.; Alland, D.; Barry, C.E.; et al. SQ109 Targets MmpL3, a Membrane Transporter of Trehalose Monomycolate Involved in Mycolic Acid Donation to the Cell Wall Core of Mycobacterium Tuberculosis. Antimicrob. Agents Chemother. 2012. [Google Scholar] [CrossRef] [PubMed]
- Shirude, P.S.; Shandil, R.; Sadler, C.; Naik, M.; Hosagrahara, V.; Hameed, S.; Shinde, V.; Bathula, C.; Humnabadkar, V.; Kumar, N.; et al. Azaindoles: Noncovalent DprE1 Inhibitors from Scaffold Morphing Efforts, Kill Mycobacterium Tuberculosis and Are Efficacious In Vivo. J. Med. Chem. 2013. [Google Scholar] [CrossRef]
- Chatterji, M.; Shandil, R.; Manjunatha, M.R.; Solapure, S.; Ramachandran, V.; Kumar, N.; Saralaya, R.; Panduga, V.; Reddy, J.; Prabhakar, K.R.; et al. 1,4-Azaindole, A Potential Drug Candidate for Treatment of Tuberculosis. Antimicrob. Agents Chemother. 2014. [Google Scholar] [CrossRef]
- Hariguchi, N.; Chen, X.; Hayashi, Y.; Kawano, Y.; Fujiwara, M.; Matsuba, M.; Shimizu, H.; Ohba, Y.; Nakamura, I.; Kitamoto, R.; et al. OPC-167832, A Novel Carbostyril Derivative with Potent Anti-Tuberculosis Activity as a DprE1 inhibitor. Antimicrob. Agents Chemother. 2020. [Google Scholar] [CrossRef]
- Shoen, C.; Pucci, M.; DeStefano, M.; Cynamon, M. Efficacy of SPR720 and SPR750 Gyrase Inhibitors in a Mouse Mycobacterium tuberculosis Infection Model. In Proceedings of the ASM Microbe 2017, New Orleans, LA, USA, 1–5 June 2017. [Google Scholar]
- Xu, J.; Wang, B.; Fu, L.; Zhu, H.; Guo, S.; Huang, H.; Yin, D.; Zhang, Y.; Lu, Y. In Vitro and In Vivo Activities of the Riminophenazine TBI-166 against Mycobacterium Tuberculosis. Antimicrob. Agents Chemother. 2019. [Google Scholar] [CrossRef]
- Madhura, D.B.; Liu, J.; Meibohm, B.; Lee, R.E. Phase II Metabolic Pathways of Spectinamide Antitubercular Agents: A Comparative Study of the Reactivity of 4-Substituted Pyridines to Glutathione Conjugation. Medchemcomm 2016. [Google Scholar] [CrossRef] [PubMed]
- Working Group on New TB Drugs, “Clinical Pipeline,” 2020. Available online: https://www.newtbdrugs.org/pipeline/clinical (accessed on 15 May 2020).
- Caminero, J.A.; Sotgiu, G.; Zumla, A.; Migliori, G.B. Best Drug Treatment for Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis. Lancet Infect. Dis. 2010, 10, 621–629. [Google Scholar] [CrossRef]
- Koh, W.J.; Lee, S.H.; Kang, Y.A.; Lee, C.H.; Choi, J.C.; Lee, J.H.; Jang, S.H.; Yoo, K.H.; Jung, K.H.; Kim, K.U.; et al. Comparison of Levofloxacin Versusmoxifloxacin for Multidrug-Resistant Tuberculosis. Am. J. Respir. Crit. Care Med. 2013. [Google Scholar] [CrossRef]
- Cho, Y.L.; Jang, J. Development of Delpazolid for the Treatment of Tuberculosis. Appl. Sci. 2020, 10, 2211. [Google Scholar] [CrossRef]
- Kaul, G.; Dasgupta, A.; Chopra, S. Delpazolid Oxazolidinone Antibiotic Treatment of Tuberculosis. Drugs Future 2018. [Google Scholar] [CrossRef]
- Mdluli, K.; Cooper, C.; Yang, T.; Lotlikar, M.; Betoudji, F.; Pinn, M.; Converse, P.; Nuermberger, E.; Cho, S.-N.; Oh, T.; et al. TBI-223: A Safer Oxazolidinone in Pre-Clinical Development for Tuberculosis. In Proceedings of the ASM Microbe 2017, New Orleans, LA, USA, 1–5 June 2017. [Google Scholar]
- Gler, M.T.; Skripconoka, V.; Sanchez-Garavito, E.; Xiao, H.; Cabrera-Rivero, J.L.; Vargas-Vasquez, D.E.; Gao, M.; Awad, M.; Park, S.K.; Shim, T.S.; et al. Delamanid for Multidrug-Resistant Pulmonary Tuberculosis. N. Engl. J. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Pretomanid: First Approval. Drugs 2019. [Google Scholar] [CrossRef]
- Pym, A.S.; Diacon, A.H.; Tang, S.J.; Conradie, F.; Danilovits, M.; Chuchottaworn, C.; Vasilyeva, I.; Andries, K.; Bakare, N.; De Marez, T.; et al. Bedaquiline in the Treatment of Multidrug and Extensively Drugresistant Tuberculosis. Eur. Respir. J. 2016. [Google Scholar] [CrossRef]
- Sutherland, H.S.; Tong, A.S.T.; Choi, P.J.; Conole, D.; Blaser, A.; Franzblau, S.G.; Cooper, C.B.; Upton, A.M.; Lotlikar, M.U.; Denny, W.A.; et al. Structure-Activity Relationships for Analogs of the Tuberculosis Drug Bedaquiline with the Naphthalene Unit Replaced by Bicyclic Heterocycles. Bioorg. Med. Chem. 2018. [Google Scholar] [CrossRef]
- Sarathy, J.P.; Ragunathan, P.; Shin, J.; Cooper, C.B.; Upton, A.M.; Grüber, G.; Dick, T. TBAJ-876 Retains Bedaquiline’s Activity against Subunits c and ε of Mycobacterium Tuberculosis F-ATP Synthase. Antimicrob. Agents Chemother. 2019. [Google Scholar] [CrossRef]
- Lupien, A.; Vocat, A.; Foo, C.S.Y.; Blattes, E.; Gillon, J.Y.; Makarov, V.; Cole, S.T. Optimized Background Regimen for Treatment of Active Tuberculosis with the Next-Generation Benzothiazinone Macozinone (PBTZ169). Antimicrob. Agents Chemother. 2018. [Google Scholar] [CrossRef]
- Makarov, V.; Mikušová, K. Development of Macozinone for TB Treatment: An Update. Appl. Sci. 2020, 10, 2269. [Google Scholar] [CrossRef]
- De Carvalho, L.P.S.; Darby, C.M.; Rhee, K.Y.; Nathan, C. Nitazoxanide Disrupts Membrane Potential and Intrabacterial pH Homeostasis of Mycobacterium Tuberculosis. ACS Med. Chem. Lett. 2011. [Google Scholar] [CrossRef]
- Harausz, E.P.; Chervenak, K.A.; Good, C.E.; Jacobs, M.R.; Wallis, R.S.; Sanchez-Felix, M.; Boom, W.H. Activity of Nitazoxanide and Tizoxanide Against Mycobacterium Tuberculosis in Vitro and in Whole Blood Culture. Tuberculosis 2016. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. In Case Medical Research. Early Bactericidal Activity of TBA-7371 in Pulmonary Tuberculosis. Case Med. Res. 2019. [CrossRef]
- Hariguchi, N.; Chen, X.; Matsuba, M.; Hayashi, Y.; Fujiwara, M.; Ohba, Y.; Kawano, Y.; Shimizu, H.; Matsumoto, M.; Inagaki, K. OPC-167832, A Newly Synthesized Carbostyril Derivative, is A Promising Anti-Tuberculosis Drug Candidate to Improve Tuberculosis Treatment. In Proceedings of the ASM Microbe 2018, Atlanta, GA, USA, 8–10 June 2018. [Google Scholar]
- Vilchèze, C. Mycobacterial Cell Wall: A Source of Successful Targets for Old and New Drugs. Appl. Sci. 2020, 10, 2278. [Google Scholar] [CrossRef]
- Zumla, A.; Rao, M.; Wallis, R.S.; Kaufmann, S.H.E.; Rustomjee, R.; Mwaba, P.; Vilaplana, C.; Yeboah-Manu, D.; Chakaya, J.; Ippolito, G.; et al. Host-Directed Therapies for Infectious Diseases: Current Status, Recent Progress, and Future Prospects. Lancet Infect. Dis. 2016, 16, 47–63. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Chung, C.; Jo, E.K. Autophagy: A New Strategy for Host-Directed Therapy of Tuberculosis. Virulence 2019, 10, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.E.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-Directed Therapies for Bacterial and Viral Infections. Nat. Rev. Drug Discov. 2018, 17, 35–56. [Google Scholar] [CrossRef]
- Gomez, G.B.; Dowdy, D.W.; Bastos, M.L.; Zwerling, A.; Sweeney, S.; Foster, N.; Trajman, A.; Islam, M.A.; Kapiga, S.; Sinanovic, E.; et al. Cost and Cost-Effectiveness of Tuberculosis Treatment Shortening: A Model-Based Analysis. BMC Infect. Dis. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Tuberculosis Infection and Latent Tuberculosis. Tuberc. Respir. Dis. (Seoul) 2016, 79, 201–206. [Google Scholar] [CrossRef]
- de Martino, M.; Lodi, L.; Galli, L.; Chiappini, E. Immune Response to Mycobacterium tuberculosis: A Narrative Review. Front. Pediatr. 2019. [Google Scholar] [CrossRef]
- Palucci, I.; Delogu, G. Host Directed Therapies for Tuberculosis: Futures Strategies for an Ancient Disease. Chemotherapy 2018, 63, 172–180. [Google Scholar] [CrossRef]
- Keravis, T.; Lugnier, C. Cyclic Nucleotide Phosphodiesterase (PDE) Isozymes as Targets of the Intracellular Signalling Network: Benefits of PDE Inhibitors in Various Diseases and Perspectives for Future Therapeutic Developments. Br. J. Pharmacol. 2012, 165, 1288–1305. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Leung, B.; Poole, P. Phosphodiesterase 4 Inhibitors for Chronic Obstructive Pulmonary Disease. Cochrane Database Syst. Rev. 2017, 9, CD002309. [Google Scholar] [CrossRef] [PubMed]
- Dorhoi, A.; Kaufmann, S.H.E. Perspectives on Host Adaptation in Response to Mycobacterium Tuberculosis: Modulation of Inflammation. Semin. Immunol. 2014, 26, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Subbian, S.; Tsenova, L.; Holloway, J.; Peixoto, B.; O’Brien, P.; Dartois, V.; Khetani, V.; Zeldis, J.B.; Kaplan, G. Adjunctive Phosphodiesterase-4 Inhibitor Therapy Improves Antibiotic Response to Pulmonary Tuberculosis in a Rabbit Model. EBioMedicine 2016. [Google Scholar] [CrossRef] [PubMed]
- Subbian, S.; Koo, M.S.; Tsenova, L.; Khetani, V.; Zeldis, J.B.; Fallows, D.; Kaplan, G. Pharmacologic Inhibition of Host Phosphodiesterase-4 Improves Isoniazid-Mediated Clearance of Mycobacterium Tuberculosis. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef]
- Fatonah, A.; Satrio Wicaksono, I.; Tambunan, U.S.F. Strategies of Tuberculosis-HIV Vaccines Design Using Immunoinformatic Approach. Online J. Biol. Sci. 2019, 19, 110–116. [Google Scholar] [CrossRef]
- Hasskarl, J. Everolimus. Recent Results Cancer Res. 2018, 211, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, D.M. Twenty-Five Years of mTOR: Uncovering the Link from Nutrients to Growth. Proc. Natl. Acad. Sci. USA 2017, 114, 11818–11825. [Google Scholar] [CrossRef]
- Cerni, S.; Shafer, D.; To, K.; Venketaraman, V. Investigating the Role of Everolimus in mTOR Inhibition and Autophagy Promotion as a Potential Host-Directed Therapeutic Target in Mycobacterium tuberculosis Infection. J. Clin. Med. 2019, 8, 232. [Google Scholar] [CrossRef]
- Jeon, S.Y.; Yhim, H.Y.; Lee, N.R.; Song, E.K.; Kwak, J.Y.; Yim, C.Y. Everolimus-Induced Activation of Latent Mycobacterium Tuberculosis Infection in a Patient with Metastatic Renal Cell Carcinoma. Korean J. Intern. Med. 2017, 32, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.S.; Zumla, A. Vitamin D as Adjunctive Host-Directed Therapy in Tuberculosis: A Systematic Review. Open Forum Infect. Dis. 2016. [Google Scholar] [CrossRef]
- Soeharto, D.A.; Rifai, D.A.; Marsudidjadja, S.; Roekman, A.E.; Assegaf, C.K.; Louisa, M. Vitamin D as an Adjunctive Treatment to Standard Drugs in Pulmonary Tuberculosis Patients: An Evidence-Based Case Report. Adv. Prev. Med. 2019. [Google Scholar] [CrossRef]
- Baindara, P. Host-Directed Therapies to Combat Tuberculosis and Associated Non-Communicable Diseases. Microb. Pathog. 2019, 130, 156–168. [Google Scholar] [CrossRef]
- Workineh, M.; Mathewos, B.; Moges, B.; Gize, A.; Getie, S.; Stendahl, O.; Schon, T.; Abate, E. Vitamin D Deficiency Among Newly Diagnosed Tuberculosis Patients and Their Household Contacts: A Comparative Cross-Sectional Study. Arch. Public Health 2017. [Google Scholar] [CrossRef]
- Salahuddin, N.; Ali, F.; Hasan, Z.; Rao, N.; Aqeel, M.; Mahmood, F. Vitamin D Accelerates Clinical Recovery from Tuberculosis: Results of the SUCCINCT Study [Supplementary Cholecalciferol in Recovery from Tuberculosis]. A Randomized, Placebo-Controlled, Clinical Trial of Vitamin D Supplementation in Patients with Pulmonar. BMC Infect. Dis. 2013. [Google Scholar] [CrossRef] [PubMed]
- Bruchfeld, J.; Correia-Neves, M.; Kallenius, G. Tuberculosis and HIV Coinfection. Cold Spring Harb. Perspect. Med. 2015, 5. [Google Scholar] [CrossRef]
- Hoft, D.; Abate, G. Immunotherapy for Tuberculosis: Future Prospects. ImmunoTargets Ther. 2016. [Google Scholar] [CrossRef]
- Yang, X.Y.; Chen, Q.F.; Li, Y.P.; Wu, S.M. Mycobacterium Vaccae as Adjuvant Therapy to Anti-Tuberculosis Chemotherapy in Never-Treated Tuberculosis Patients: A Meta-Analysis. PLoS ONE 2011, 6, e23826. [Google Scholar] [CrossRef]
- Butov, D.A.; Efremenko, Y.V.; Prihoda, N.D.; Zaitzeva, S.I.; Yurchenko, L.V.; Sokolenko, N.I.; Butova, T.S.; Stepanenko, A.L.; Kutsyna, G.A.; Jirathitikal, V.; et al. Randomized, Placebo-Controlled Phase II Trial of Heat-Killed Mycobacterium Vaccae (Immodulon Batch) Formulated as an Oral Pill (V7). Immunotherapy 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Nell, A.S.; D’Lom, E.; Bouic, P.; Sabaté, M.; Bosser, R.; Picas, J.; Amat, M.; Churchyard, G.; Cardona, P.J. Safety, Tolerability, and Immunogenicity of the Novel Antituberculous Vaccine RUTI: Randomized, Placebo-Controlled Phase II Clinical Trial in Patients with Latent Tuberculosis Infection. PLoS ONE 2014, 9, e89612. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana, C.; Montané, E.; Pinto, S.; Barriocanal, A.M.; Domenech, G.; Torres, F.; Cardona, P.J.; Costa, J. Double-Blind, Randomized, Placebo-Controlled Phase I Clinical Trial of the Therapeutical Antituberculous Vaccine RUTI®. Vaccine 2010, 28. [Google Scholar] [CrossRef] [PubMed]
- Skrahin, A.; Ahmed, R.K.; Ferrara, G.; Rane, L.; Poiret, T.; Isaikina, Y.; Skrahina, A.; Zumla, A.; Maeurer, M.J. Autologous Mesenchymal Stromal Cell Infusion as Adjunct Treatment in Patients with Multidrug and Extensively Drug-Resistant Tuberculosis: An Open-Label Phase 1 Safety Trial. Lancet Respir. Med. 2014, 2. [Google Scholar] [CrossRef]
- Arjanova, O.V.; Prihoda, N.D.; Yurchenko, L.V.; Sokolenko, N.I.; Frolov, V.M.; Tarakanovskaya, M.G.; Batdelger, D.; Jirathitikal, V.; Bourinbaiar, A.S. Adjunct Oral Immunotherapy in Patients with Re-Treated, Multidrug-Resistant or HIV-Coinfected TB. Immunotherapy 2011, 3. [Google Scholar] [CrossRef]
- Shen, H.; Min, R.; Tan, Q.; Xie, W.; Wang, H.; Pan, H.; Zhang, L.; Xu, H.; Zhang, X.; Dai, J. The Beneficial Effects of Adjunctive Recombinant Human Interleukin-2 for Multidrug Resistant Tuberculosis. Arch. Med. Sci. 2015, 11. [Google Scholar] [CrossRef]
- Johnson, B.J.; Bekker, L.G.; Rickman, R.; Brown, S.; Lesser, M.; Ress, S.; Willcox, P.; Steyn, L.; Kaplan, G. rhuIL-2 Adjunctive Therapy in Multidrug Resistant Tuberculosis: A Comparison of Two Treatment Regimens and Placebo. Tuber. Lung Dis. 1997, 78. [Google Scholar] [CrossRef]
- Johnson, J.L.; Ssekasanvu, E.; Okwera, A.; Mayanja, H.; Hirsch, C.S.; Nakibali, J.G.; Jankus, D.D.; Eisenach, K.D.; Boom, W.H.; Ellner, J.J.; et al. Randomized Trial of Adjunctive Interleukin-2 in Adults with Pulmonary Tuberculosis. Am. J. Respir. Crit. Care Med. 2003, 168. [Google Scholar] [CrossRef] [PubMed]
- Hamasur, B.; Haile, M.; Pawlowski, A.; Schröder, U.; Källenius, G.; Svenson, S.B. A Mycobacterial Lipoarabinomannan Specific Monoclonal Antibody and its F(ab′)2 Fragment Prolong Survival of Mice Infected with Mycobacterium Tuberculosis. Clin. Exp. Immunol. 2004, 138. [Google Scholar] [CrossRef] [PubMed]
- Giosuè, S.; Casarini, M.; Ameglio, F.; Zangrilli, P.; Palla, M.; Altieri, A.M.; Bisetti, A. Aerosolized Interferon-Alpha Treatment in Patients with Multi-Drug-Resistant Pulmonary Tuberculosis. Eur. Cytokine Netw. 2000, 11, 11. [Google Scholar]
- Wallis, R.S.; Kyambadde, P.; Johnson, J.L.; Horter, L.; Kittle, R.; Pohle, M.; Ducar, C.; Millard, M.; Mayanja-Kizza, H.; Whalen, C.; et al. A Study of the Safety, Immunology, Virology, and Microbiology of Adjunctive Etanercept in HIV-1-Associated Tuberculosis. AIDS 2004, 18. [Google Scholar] [CrossRef] [PubMed]
- Prabowo, S.A.; Painter, H.; Zelmer, A.; Smith, S.G.; Seifert, K.; Amat, M.; Cardona, P.J.; Fletcher, H.A. RUTI Vaccination Enhances Inhibition of Mycobacterial Growth Ex Vivo and Induces a Shift of Monocyte Phenotype in Mice. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Cox, H.; Mizrahi, V. Drug-Resistant Tuberculosis: Challenges and Opportunities for Diagnosis and Treatment. Curr. Opin. Pharmacol. 2018, 42, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kendall, E.A.; Azman, A.S.; Cobelens, F.G.; Dowdy, D.W. MDR-TB Treatment as Prevention: The Projected Population-Level Impact of Expanded Treatment for Multidrug-Resistant Tuberculosis. PLoS ONE 2017, 12, e0172748. [Google Scholar] [CrossRef]
- Nguyen, T.N.A.; Berre, V.A.; Le Bañuls, A.L.; Nguyen, T.V.A. Molecular Diagnosis of Drug-Resistant Tuberculosis; A Literature Review. Front. Microbiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Tollefson, D.; Ngari, F.; Mwakala, M.; Gethi, D.; Kipruto, H.; Cain, K.; Bloss, E. Under-Reporting of Sputum Smear-Positive Tuberculosis Cases in Kenya. Int. J. Tuberc. Lung Dis. 2016. [Google Scholar] [CrossRef]
- Manjelievskaia, J.; Erck, D.; Piracha, S.; Schrager, L. Drug-Resistant TB: Deadly, Costly and in Need of a Vaccine. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 186–191. [Google Scholar] [CrossRef]
- Gebreweld, F.H.; Kifle, M.M.; Gebremicheal, F.E.; Simel, L.L.; Gezae, M.M.; Ghebreyesus, S.S.; Mengsteab, Y.T.; Wahd, N.G. Factors Influencing Adherence to Tuberculosis Treatment in Asmara, Eritrea: A Qualitative Study. J. Health Popul. Nutr. 2018. [Google Scholar] [CrossRef]
- Alipanah, N.; Jarlsberg, L.; Miller, C.; Linh, N.N.; Falzon, D.; Jaramillo, E.; Nahid, P. Adherence Interventions and Outcomes of Tuberculosis Treatment: A Systematic Review and Meta-Analysis of Trials and Observational Studies. PLoS Med. 2018. [Google Scholar] [CrossRef]
- Vernon, A.; Fielding, K.; Savic, R.; Dodd, L.; Nahid, P. The Importance of Adherence in Tuberculosis Treatment Clinical Trials and its Relevance in Explanatory and Pragmatic Trials. PLoS Med. 2019, 16, e1002884. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, H.S.; Azagew, A.W. Non-adherence to Anti-Tuberculosis Treatment, Reasons and Associated Factors among TB Patients Attending at Gondar Town Health Centers, Northwest Ethiopia. BMC Res. Notes 2018. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.W.; Park, H.O.; Jang, H.N.; Yang, J.H.; Kim, S.H.; Moon, S.H.; Byun, J.H.; Lee, C.E.; Kim, J.W.; Kang, D.H. Side Effects Associated with the Treatment of Multidrug-Resistant Tuberculosis at a Tuberculosis Referral Hospital in South Korea. Medicine (United States) 2017. [Google Scholar] [CrossRef]
- Machado, D.; Girardini, M.; Viveiros, M.; Pieroni, M. Challenging the Drug-Likeness Dogma for New Drug Discovery in Tuberculosis. Front. Microbiol. 2018, 9, 1367. [Google Scholar] [CrossRef] [PubMed]





| Chemical Class | Compound | Progress | Mode of Action | Reference(s) |
|---|---|---|---|---|
| Fluoroquinolone | Levofloxacin | Phase 2 | DNA gyrase inhibitor | [102,103] |
| Rifamycin | Rifampicin (high dose) | Phase 2 | RpoB inhibitor | [37] |
| Oxazolidinone | Delpazolid | Phase 2 | Inhibition of protein synthesis | [104,105] |
| Sutezolid | Phase 2 | Inhibition of protein synthesis | [47] | |
| TBI-223 | Phase 1 | Inhibits the binding of N-formylmethionyl tRNA to ribosome | [106] | |
| Nitroimidazole | Delamanid | Phase 3—approved | Inhibits cell wall synthesis | [107] |
| Pretomanid | Phase 3—approved | Inhibits cell wall synthesis | [108] | |
| Diarylquinoline | Bedaquiline | Phase 3—accepted | Inhibits mycobacterial ATP synthase | [109] |
| TBAJ-587 | Preclinical trial | Inhibits mycobacterial ATP synthase and hERG potassium channel | [110] | |
| TBAJ-876 | Preclinical trial | Inhibits mycobacterial ATP synthase | [73,111] | |
| Benzothiazinone | Macozinone | Phase 2 | DprE1 inhibitor | [112,113] |
| BTZ-043 | Phase 1 | DprE1 inhibitor | [74] | |
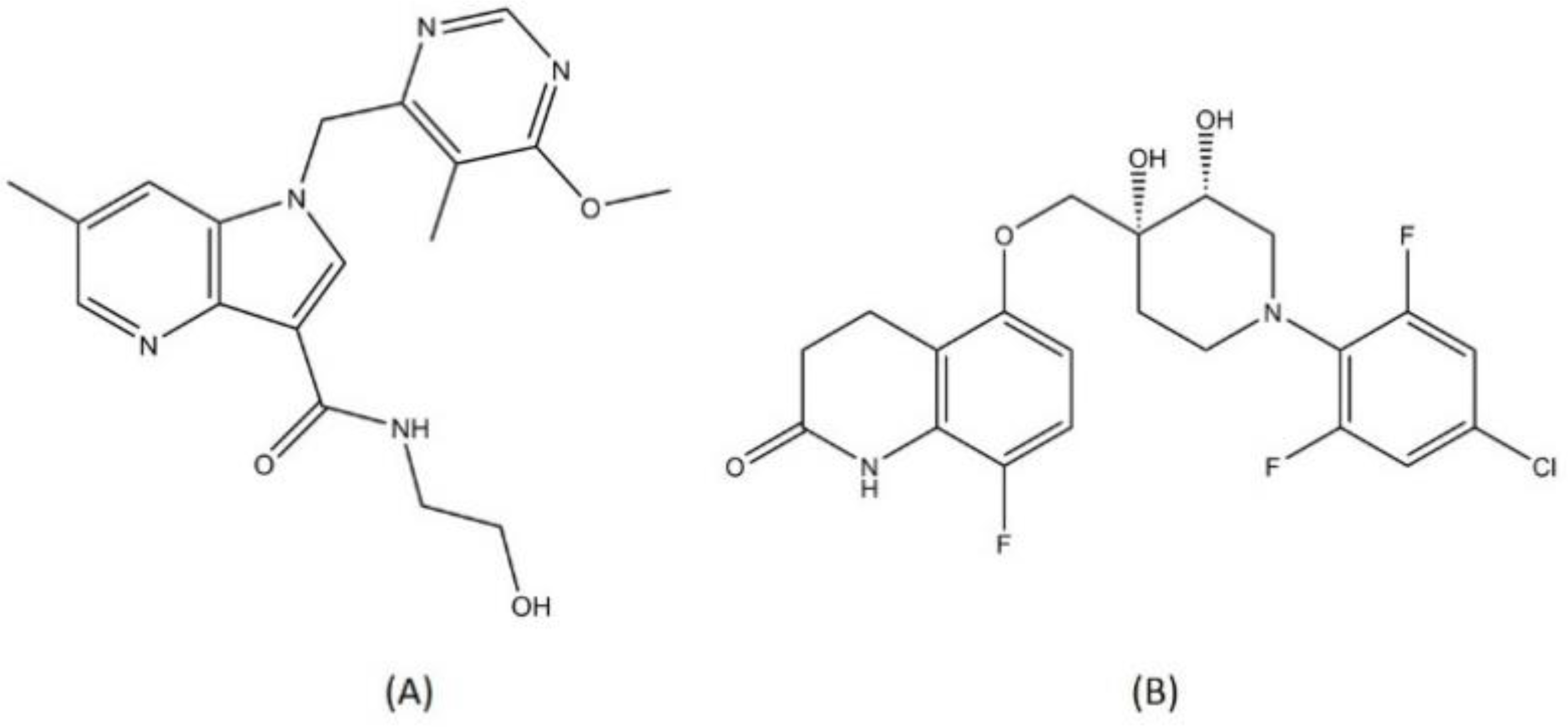
| Other classes | Telacebec (imidazopyridine) | Phase 2 | QcrB inhibitor | [84] |
| Nitazoxanide (nitrothiazolyl-salicylamide derivate) | Phase 2 | Disruption of membrane potential and pH homeostasis | [114,115] | |
| SQ109 (ethylenediamine) | Phase 2 | MmpL3 inhibitor | [37,94] | |
| TBA-7371 (1,4-azaindole) | Phase 2 | DprE1 inhibitor | [116] | |
| OPC-167832 (3,4-dihydrocarbostyril derivate) | Phase 2 | DprE1 inhibitor | [117] | |
| SPR-720 (ethyl urea benzimidazole) | Phase 1 | GyrB inhibitor | [98] | |
| TBI-166 (riminophenazine) | Phase 1 | Membrane destabilization | [99] | |
| Sanfetrinem (beta-lactam) | Preclinical trial | Inhibits peptidoglycan synthesis | [118] | |
| Spectinamide-1810 (spectinamide) | Preclinical trial | Selective ribosomal inhibition | [100] |
| Therapeutics | Composition | Target (Outcome) | References |
|---|---|---|---|
| Mycobacterium vaccae | Killed, interdermal | Meta-analysis of 54-studies on newly diagnosed pulmonary TB (improved sputum conversion and X-ray changes | [143,144] |
| Capsule | Faster smear conversion | [143,145] | |
| RUTI® | Detoxified cellular fragments of Mycobacterium tuberculosis | Phase I and II clinical trials on LTBI cases or healthy volunteers (immunogenic, reasonable tolerability) | [143,146,147] |
| Autologous MSC | MSC | MDR or XDR patients (with radiologic improvement) | [143,148] |
| V5 immunitor | Inactivated pooled blood | Re-treatment or proven MDR (higher rate of sputum conversion) | [143,149] |
| Cytokines and cytokine inhibitors | IL-2 | MDR-TB patients (better sputum conversion rate), MDR-TB patients (decrease AFB smear counts with daily IL-2 compared to control or pulse IL-2), new TB patients (significant delays in culture conversion) | [143,150] |
| IFN-γ | MDR-TB patients (all smear negative/improved, MDR-TB cases (no marked microbiologic effect), HIV-positive TB cases (more rapid culture conversion compared to historical control) | [143,151] | |
| Drugs/compounds | High-dose steroid | HIV-positive TB cases (increased culture conversion at 1 month) | [143,152] |
| Levamisole | Newly diagnosed pulmonary TB patients (improved radiology, but no effect on smear conversion) | [143,153] | |
| Albendazole | New pulmonary TB patients (no effect on clinical, radiologic, and microbiologic outcome) | [143,154] | |
| Thalidomide | HIV-positive (clinical improvement), HIV-positive (no clinical difference) | [143,155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stephanie, F.; Saragih, M.; Tambunan, U.S.F. Recent Progress and Challenges for Drug-Resistant Tuberculosis Treatment. Pharmaceutics 2021, 13, 592. https://doi.org/10.3390/pharmaceutics13050592
Stephanie F, Saragih M, Tambunan USF. Recent Progress and Challenges for Drug-Resistant Tuberculosis Treatment. Pharmaceutics. 2021; 13(5):592. https://doi.org/10.3390/pharmaceutics13050592
Chicago/Turabian StyleStephanie, Filia, Mutiara Saragih, and Usman Sumo Friend Tambunan. 2021. "Recent Progress and Challenges for Drug-Resistant Tuberculosis Treatment" Pharmaceutics 13, no. 5: 592. https://doi.org/10.3390/pharmaceutics13050592
APA StyleStephanie, F., Saragih, M., & Tambunan, U. S. F. (2021). Recent Progress and Challenges for Drug-Resistant Tuberculosis Treatment. Pharmaceutics, 13(5), 592. https://doi.org/10.3390/pharmaceutics13050592






