ELISA- and Activity Assay-Based Quantification of BMP-2 Released In Vitro Can Be Biased by Solubility in “Physiological” Buffers and an Interfering Effect of Chitosan
Abstract
1. Introduction
2. Materials and Methods
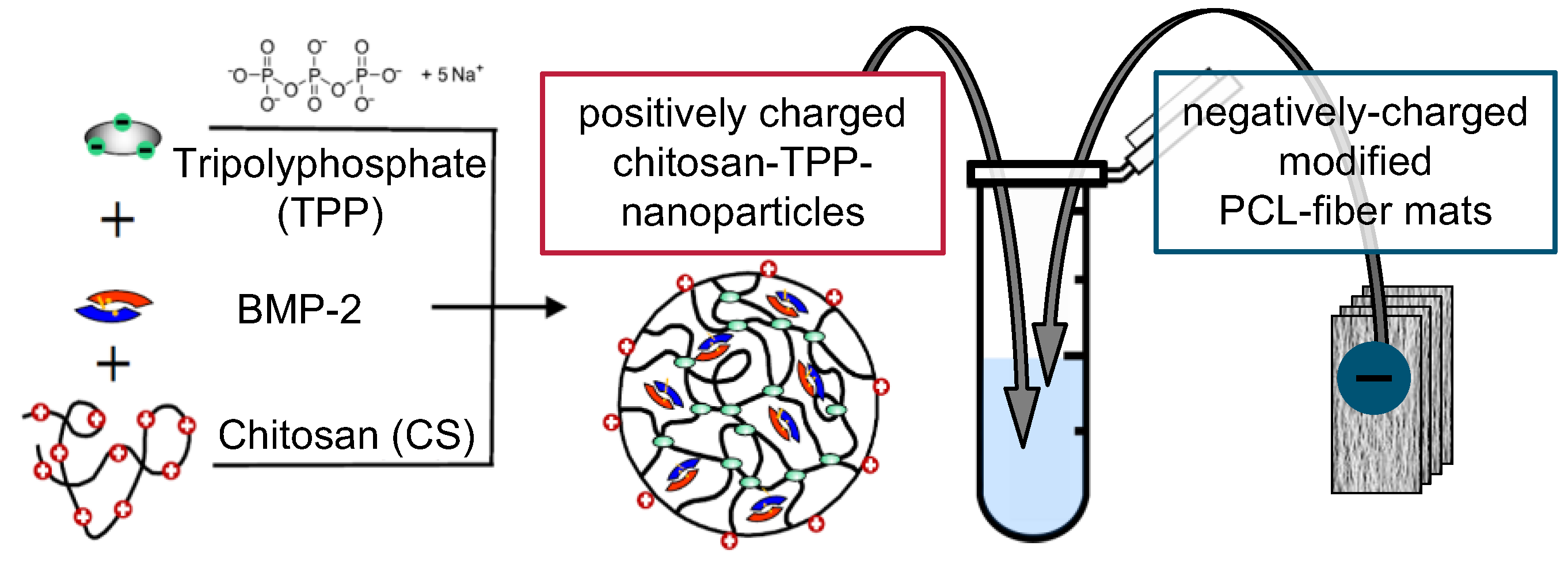
2.1. Implant Prototypes
2.2. In Vitro BMP-2 Release Experiments
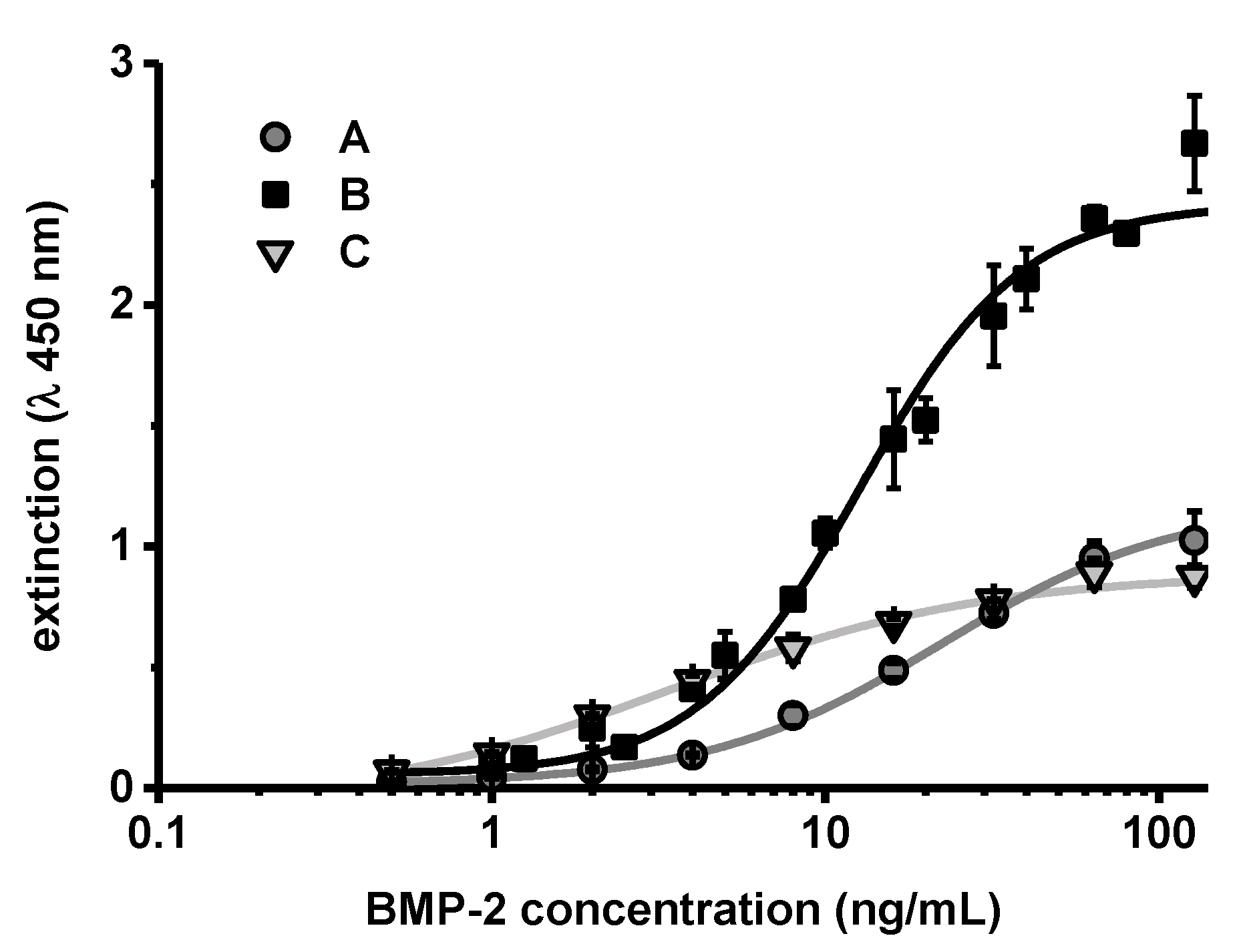
2.3. Quantification of BMP-2 Activity by BRE-Luc Assay
2.4. ELISA Quantification of BMP-2
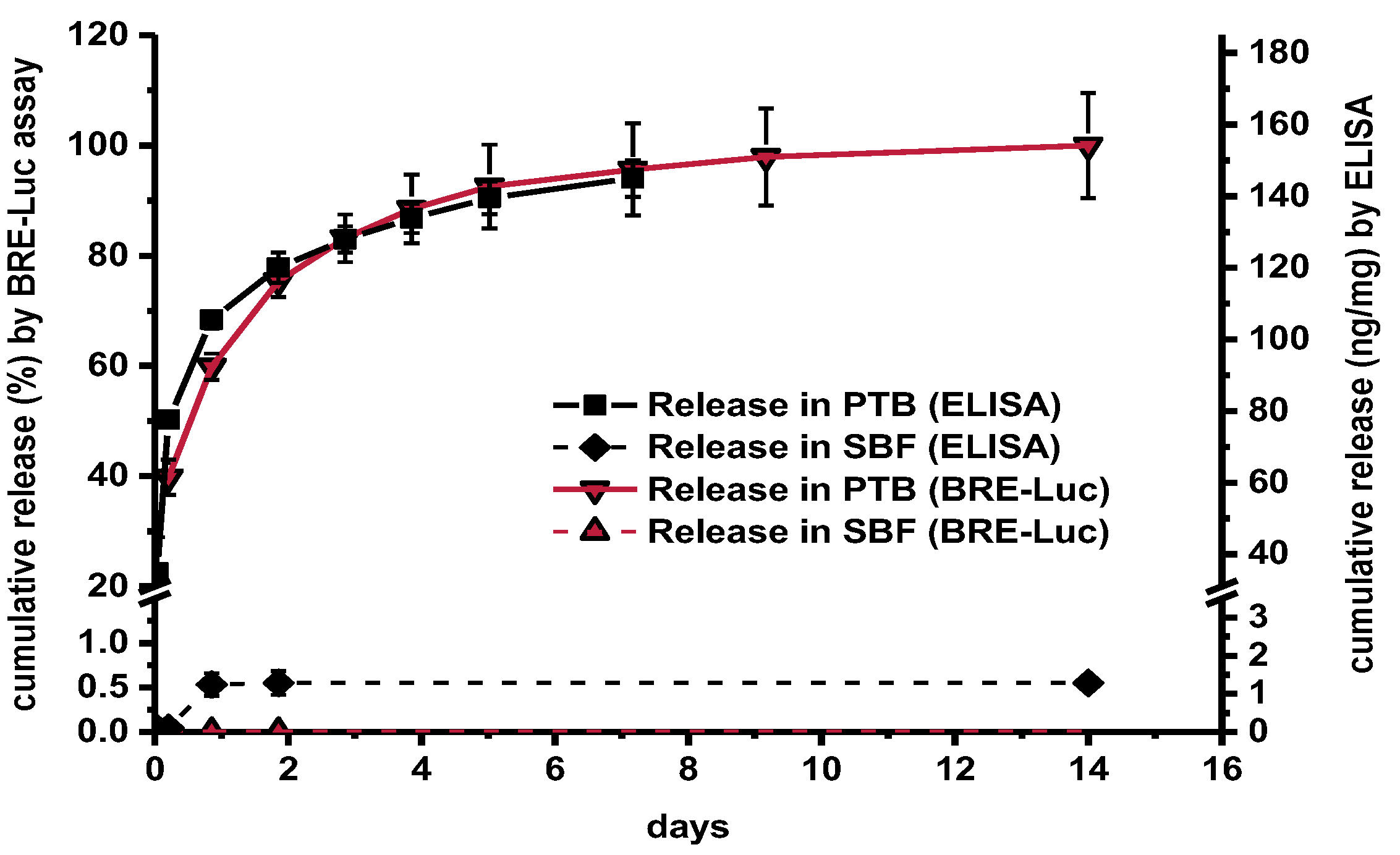
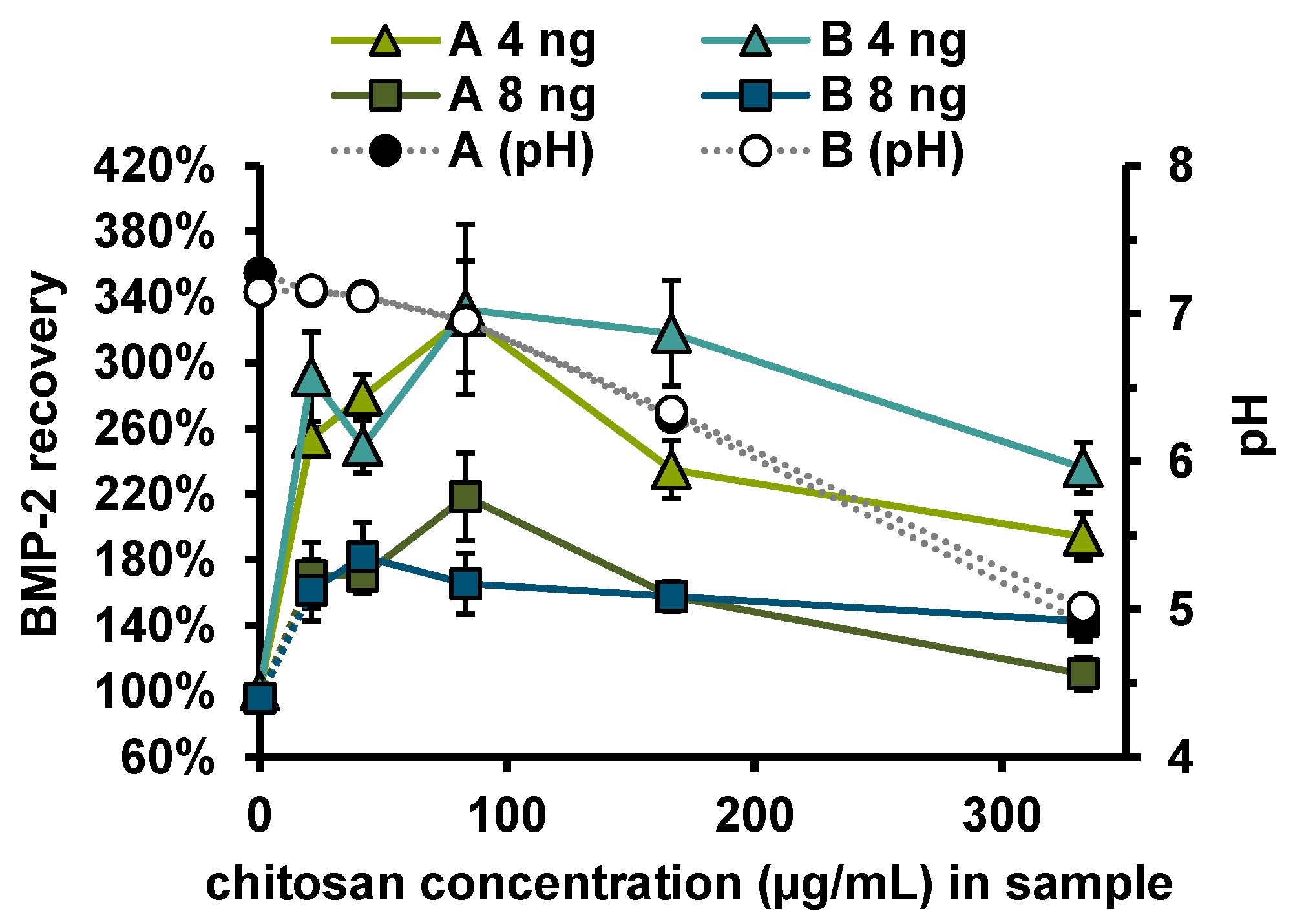
3. Results
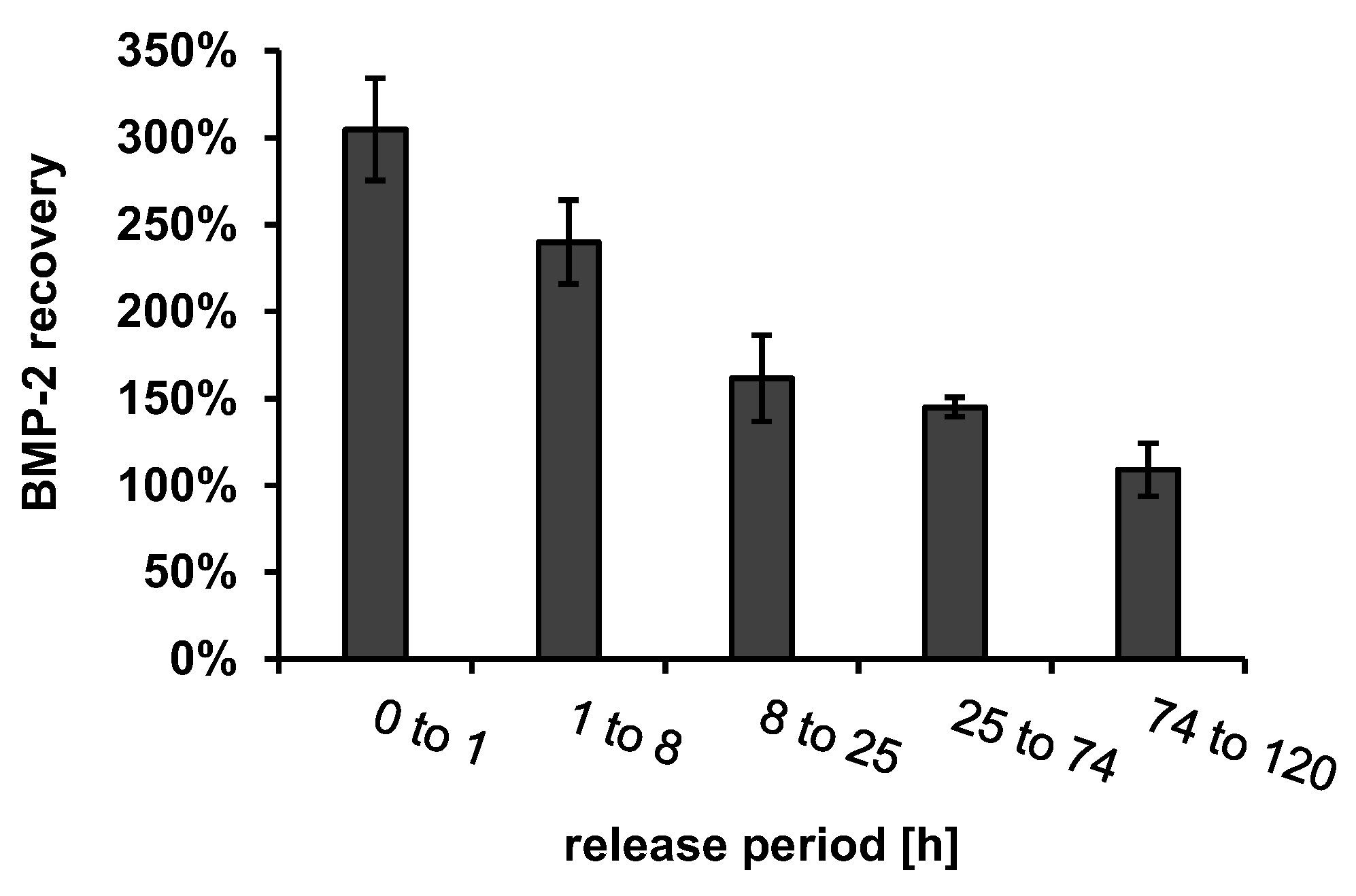
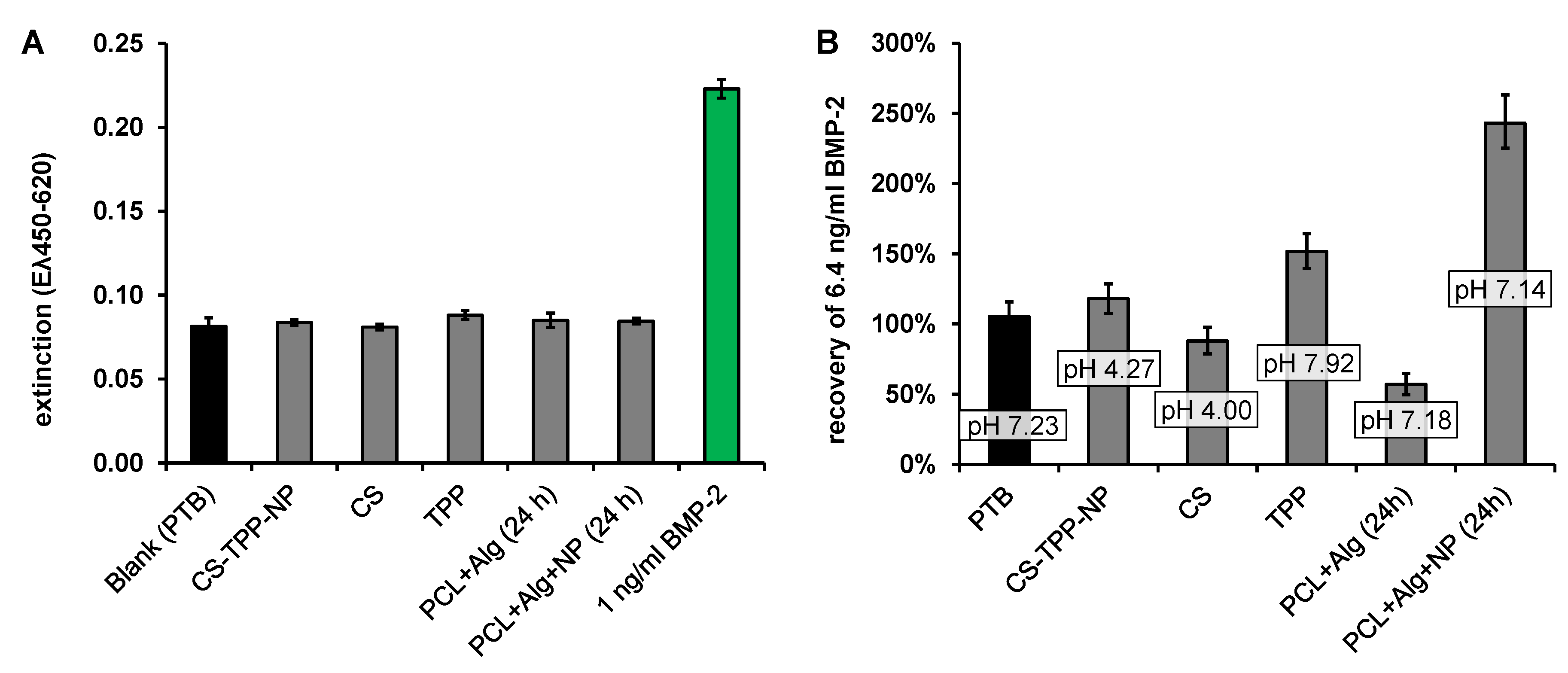
Recovery of Released BMP-2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Begam, H.; Nandi, S.K.; Kundu, B.; Chanda, A. Strategies for delivering bone morphogenetic protein for bone healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 856–869. [Google Scholar] [CrossRef]
- Martinez, M.; Rathbone, M.; Burgess, D.; Huynh, M. In vitro and in vivo considerations associated with parenteral sustained release products: A review based upon information presented and points expressed at the 2007 Controlled Release Society Annual Meeting. J. Control. Release 2008, 129, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Burgess, D.J.; Crommelin, D.J.A.; Hussain, A.S.; Chen, M.-L. Assuring quality and performance of sustained and controlled released parenterals. Eur. J. Pharm. Sci. 2004, 21, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Barr, W.H.; Karnes, H.T. Profiling in vitro drug release from subcutaneous implants: A review of current status and potential implications on drug product development. Biopharm. Drug Dispos. 2006, 27, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Fogh-Andersen, N.; Altura, B.M.; Altura, B.T.; Siggaard-Andersen, O. Composition of interstitial fluid. Clin. Chem. 1995, 41, 1522. [Google Scholar] [CrossRef]
- Marques, M.; Löbenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Loe, F.; Blocki, A.; Peng, Y.; Raghunath, M. Applying macromolecular crowding to enhance extracellular matrix deposition and its remodeling in vitro for tissue engineering and cell-based therapies. Adv. Drug. Deliver. Rev. 2011, 63, 277–290. [Google Scholar] [CrossRef]
- Vroman, L.; Adams, A.L. Identification of rapid changes at plasma-solid interfaces. J. Biomed. Mater. Res. 1969, 3, 43–67. [Google Scholar] [CrossRef]
- Shah, N.J.; Macdonald, M.L.; Beben, Y.M.; Padera, R.F.; Samuel, R.E.; Hammond, P.T. Tunable dual growth factor delivery from polyelectrolyte multilayer films. Biomaterials 2011, 32, 6183–6193. [Google Scholar] [CrossRef]
- Strobel, C.; Bormann, N.; Kadow-Romacker, A.; Schmidmaier, G.; Wildemann, B. Sequential release kinetics of two (gentamicin and BMP-2) or three (gentamicin, IGF-I and BMP-2) substances from a one-component polymeric coating on implants. J. Control. Release 2011, 156, 37–45. [Google Scholar] [CrossRef]
- Korchynskyi, O.; Dijke, P. ten. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J. Biol. Chem. 2002, 277, 4883–4891. [Google Scholar] [CrossRef]
- Stenh, C.; Englund, H.; Lord, A.; Johansson, A.-S.; Almeida, C.G.; Gellerfors, P.; Greengard, P.; Gouras, G.K.; Lannfelt, L.; Nilsson, L.N.G. Amyloid-beta oligomers are inefficiently measured by enzyme-linked immunosorbent assay. Ann. Neurol. 2005, 58, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.-J.; Xia, H.; Chen, L.; Ying, Q.-S.; Yu, X.; Li, L.-H.; Wang, J.-H.; Zhang, Y. Efficient delivery of recombinant human bone morphogenetic protein (rhBMP-2) with dextran sulfate-chitosan microspheres. Exp. Ther. Med. 2018, 15, 3265–3272. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-m.; Wu, Z.-f.; Sun, H.-h.; Wu, H.; Xin, S.-n.; Wang, Q.-t.; Dong, G.-y.; Ma, Z.-w.; Huang, S.; Zhang, Y.-j.; et al. Release of bioactive BMP from dextran-derived microspheres: A novel delivery concept. Int. J. Pharm. 2006, 307, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.S.; Yamamoto, M.; Ueda, H.; Tabata, Y.; Mikos, A.G. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater. 2008, 4, 1126–1138. [Google Scholar] [CrossRef]
- Srouji, S.; Ben-David, D.; Lotan, R.; Livne, E.; Avrahami, R.; Zussman, E. Slow-release human recombinant bone morphogenetic protein-2 embedded within electrospun scaffolds for regeneration of bone defect: In Vitro and In Vivo Evaluation. Tissue Eng. Pt. A 2011, 17, 269–277. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, F.; Zhang, L.; Deng, L.; Sun, H.; Xu, J.; Li, Y.; Xie, X. Sustained dual release of placental growth factor-2 and bone morphogenic protein-2 from heparin-based nanocomplexes for direct osteogenesis. Int. J. Nanomed. 2016, 1147. [Google Scholar] [CrossRef]
- Kang, W.; Lee, D.-S.; Jang, J.-H. Evaluation of sustained BMP-2 release profiles using a novel fluorescence-based retention assay. PLoS ONE 2015, 10, e0123402. [Google Scholar] [CrossRef]
- Ruhé, P.Q.; Boerman, O.C.; Russel, F.G.M.; Mikos, A.G.; Spauwen, P.H.M.; Jansen, J.A. In vivo release of rhBMP-2 loaded porous calcium phosphate cement pretreated with albumin. J. Mater. Sci. Mater. Med. 2006, 17, 919–927. [Google Scholar] [CrossRef]
- Uludag, H.; D’Augusta, D.; Golden, J.; Timony, G.; Li, J.; Riedel, R.M.; Wozney, J. Implantation of recombinant human bone morphogenetic proteins with biomaterial carriers: A correlation between protein pharmacokinetics and osteoinduction in the rat ectopic model. J. Biomed. Mater. Res. 2000, 50, 227–238. [Google Scholar] [CrossRef]
- Quaas, B.; Burmeister, L.; Li, Z.; Satalov, A.; Behrens, P.; Hoffmann, A.; Rinas, U. Stability and biological activity of E. coli derived soluble and precipitated bone morphogenetic protein-2. Pharm. Res. 2019, 36, 184. [Google Scholar] [CrossRef]
- Sundermann, J.; Zagst, H.; Kuntsche, J.; Wätzig, H.; Bunjes, H. Bone morphogenetic protein 2 (BMP-2) aggregates can be solubilized by albumin-investigation of BMP-2 aggregation by light scattering and electrophoresis. Pharmaceutics 2020, 12, 1143. [Google Scholar] [CrossRef]
- Lochmann, A.; Nitzsche, H.; von Einem, S.; Schwarz, E.; Mäder, K. The influence of covalently linked and free polyethylene glycol on the structural and release properties of rhBMP-2 loaded microspheres. J. Control. Release 2010, 147, 92–100. [Google Scholar] [CrossRef]
- Kowalczewski, C.J.; Saul, J.M. Biomaterials for the delivery of growth factors and other therapeutic agents in tissue engineering approaches to bone regeneration. Front. Pharmacol. 2018, 9, 513. [Google Scholar] [CrossRef]
- Sundermann, J.; Oehmichen, S.; Sydow, S.; Burmeister, L.; Quaas, B.; Hänsch, R.; Rinas, U.; Hoffmann, A.; Menzel, H.; Bunjes, H. Varying the sustained release of BMP-2 from chitosan nanogel-functionalized PCL fiber mats by different PCL surface modifications. J. Biomed. Mater. Res. A 2021, 109, 600–614. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, M.; Li, P.; Wang, K.; Fang, L.; Ren, F.; Lu, G.; Lu, X. The interaction of chitosan and BMP-2 tuned by deacetylation degree and pH value. J. Biomed. Mater. Res. A 2019, 107, 769–779. [Google Scholar] [CrossRef]
- Sydow, S.; de Cassan, D.; Hänsch, R.; Gengenbach, T.R.; Easton, C.D.; Thissen, H.; Menzel, H. Layer-by-layer deposition of chitosan nanoparticles as drug-release coatings for PCL nanofibers. Biomater. Sci. 2019, 7. [Google Scholar] [CrossRef]
- de Cassan, D.; Sydow, S.; Schmidt, N.; Behrens, P.; Roger, Y.; Hoffmann, A.; Hoheisel, A.L.; Glasmacher, B.; Hänsch, R.; Menzel, H. Attachment of nanoparticulate drug-release systems on poly(ε-caprolactone) nanofibers via a graftpolymer as interlayer. Colloid. Surf. B 2018, 163, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Poth, N.; Seiffart, V.; Gross, G.; Menzel, H.; Dempwolf, W. Biodegradable chitosan nanoparticle coatings on titanium for the delivery of BMP-2. Biomolecules 2015, 5, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Freier, T.; Koh, H.S.; Kazazian, K.; Shoichet, M.S. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 2005, 26, 5872–5878. [Google Scholar] [CrossRef]
- Lorenz, C.; Hoffmann, A.; Gross, G.; Windhagen, H.; Dellinger, P.; Möhwald, K.; Dempwolf, W.; Menzel, H. Coating of titanium implant materials with thin polymeric films for binding the signaling protein BMP2. Macromol. Biosci. 2011, 11, 234–244. [Google Scholar] [CrossRef]
- Aydin, S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef]
- Wood, W.G. “Matrix effects” in immunoassays. Scand. J. Clin. Lab. Investig. 2009, 51, 105–112. [Google Scholar] [CrossRef]
- Selby, C. Interference in immunoassay. Ann. Clin. Biochem. 1999, 36, 704–721. [Google Scholar] [CrossRef]
- Ruppert, R.; Hoffmann, E.; Sebald, W. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur. J. Biochem. 1996, 237, 295–302. [Google Scholar] [CrossRef]
- Takada, T.; Katagiri, T.; Ifuku, M.; Morimura, N.; Kobayashi, M.; Hasegawa, K.; Ogamo, A.; Kamijo, R. Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J. Biol. Chem. 2003, 278, 43229–43235. [Google Scholar] [CrossRef]
- Peschel, D.; Zhang, K.; Fischer, S.; Groth, T. Modulation of osteogenic activity of BMP-2 by cellulose and chitosan derivatives. Acta Biomater. 2012, 8, 183–193. [Google Scholar] [CrossRef]
- Abbatiello, S.E.; Porter, T.J. Anion-mediated precipitation of recombinant human bone morphogenetic protein (rhBMP-2) is dependent upon the heparin binding N-terminal region. In Proceedings of the Protein Society Meeting, Boston, MA, USA, 13–16 July 1997. [Google Scholar]



| Content | SBF | PTB |
|---|---|---|
| Na+ | 142.0 | 156.9 |
| K+ | 5.0 | 4.4 |
| Mg2+ | 1.5 | - |
| Ca2+ | 2.5 | - |
| Cl− | 125.0 | 139.6 |
| HCO− | 27.0 | - |
| HPO42− | 1.0 | 11.8 |
| SO42− | 0.5 | - |
| Tris | 50.0 | - |
| BSA | - | 0.015 |
| Tween 20 | - | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundermann, J.; Sydow, S.; Burmeister, L.; Hoffmann, A.; Menzel, H.; Bunjes, H. ELISA- and Activity Assay-Based Quantification of BMP-2 Released In Vitro Can Be Biased by Solubility in “Physiological” Buffers and an Interfering Effect of Chitosan. Pharmaceutics 2021, 13, 582. https://doi.org/10.3390/pharmaceutics13040582
Sundermann J, Sydow S, Burmeister L, Hoffmann A, Menzel H, Bunjes H. ELISA- and Activity Assay-Based Quantification of BMP-2 Released In Vitro Can Be Biased by Solubility in “Physiological” Buffers and an Interfering Effect of Chitosan. Pharmaceutics. 2021; 13(4):582. https://doi.org/10.3390/pharmaceutics13040582
Chicago/Turabian StyleSundermann, Julius, Steffen Sydow, Laura Burmeister, Andrea Hoffmann, Henning Menzel, and Heike Bunjes. 2021. "ELISA- and Activity Assay-Based Quantification of BMP-2 Released In Vitro Can Be Biased by Solubility in “Physiological” Buffers and an Interfering Effect of Chitosan" Pharmaceutics 13, no. 4: 582. https://doi.org/10.3390/pharmaceutics13040582
APA StyleSundermann, J., Sydow, S., Burmeister, L., Hoffmann, A., Menzel, H., & Bunjes, H. (2021). ELISA- and Activity Assay-Based Quantification of BMP-2 Released In Vitro Can Be Biased by Solubility in “Physiological” Buffers and an Interfering Effect of Chitosan. Pharmaceutics, 13(4), 582. https://doi.org/10.3390/pharmaceutics13040582







