In Silico Selection and Evaluation of Pugnins with Antibacterial and Anticancer Activity Using Skin Transcriptome of Treefrog (Boana pugnax)
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatic Analysis
2.1.1. Transcriptome
2.1.2. Databases
2.1.3. Alignments from the Databases
2.1.4. Obtaining Physicochemical Parameters of Peptides
2.1.5. In Silico Prediction of Antimicrobial Activity, Cell-Penetrating Peptide and Anticancer Using Support Vector Machine
2.1.6. Filter for the Selection of Candidates with High Probability of Presenting Combined Antibacterial-Anticancer Activity (ABC) and Being Cationic with Helical Structure
2.1.7. Phylogenetic Analysis and Search for De Novo Motifs in Candidate Sequences after the Filter
2.1.8. Molecular Modeling of Candidate ABC Peptides
2.2. Molecular Dynamics
2.2.1. Water Box System Construction
2.2.2. Gram-Negative and Gram-Positive Bacterial Membrane Models Construction
2.2.3. Molecular Dynamics of Candidate ABC Peptides
2.3. Synthesis, Characterization and Circular Dichroism of Peptides
2.4. Antimicrobial Test
2.5. Hemolytic Test
2.6. Cell Lines
Cytotoxicity Test (MTT) 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide
2.7. Flow Cytometry
2.7.1. Evaluation of Mitochondrial Membrane Potential and Cytoplasmic Membrane Integrity
2.7.2. Apoptosis Analysis
2.7.3. Cell Cycle Analyses
2.8. Statistics
3. Results
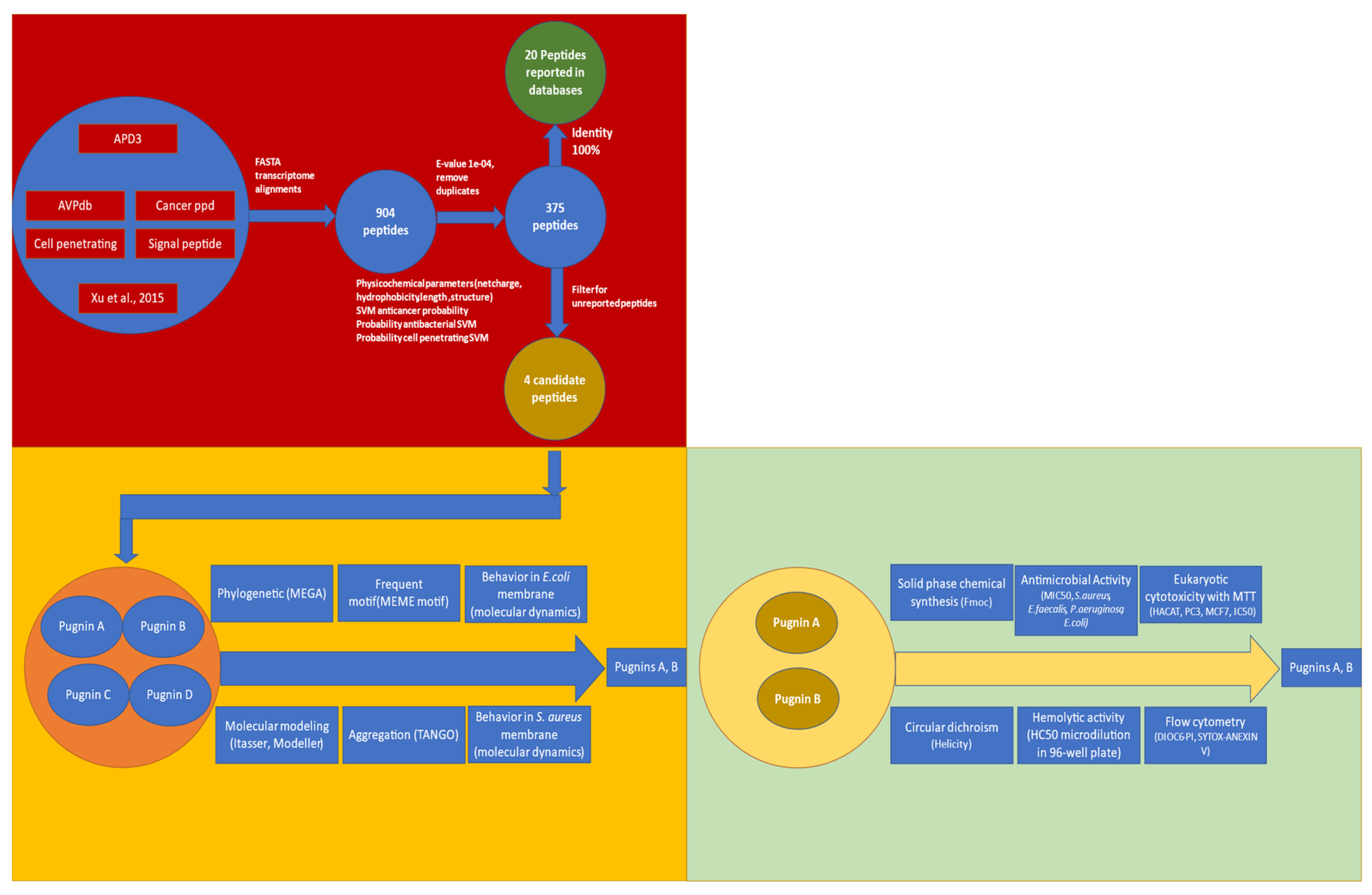
3.1. Searching ABC Peptides from B. pugnax Transcriptome
3.1.1. Alignment Transcriptome to Peptides Databases
3.1.2. Filtering the 375 Peptides to Obtain Peptide Candidates for Chemical Synthesis
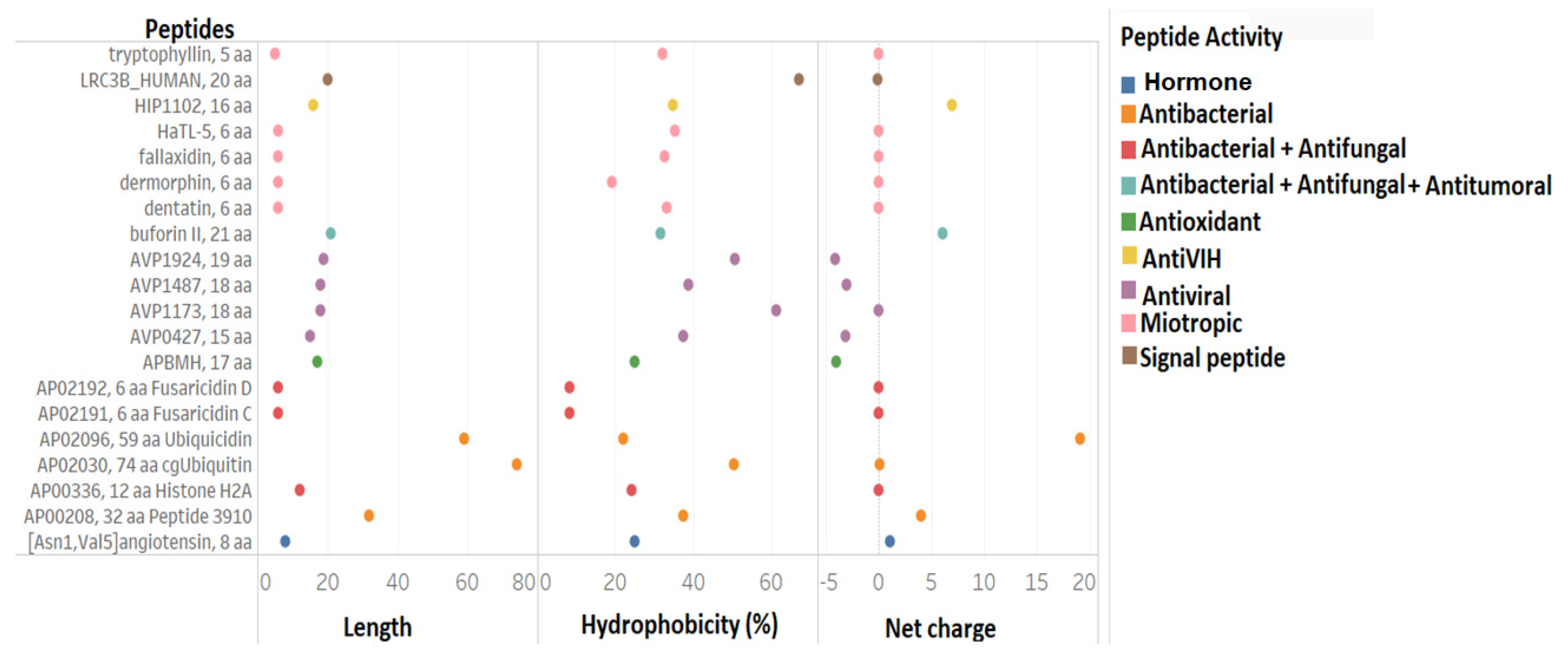
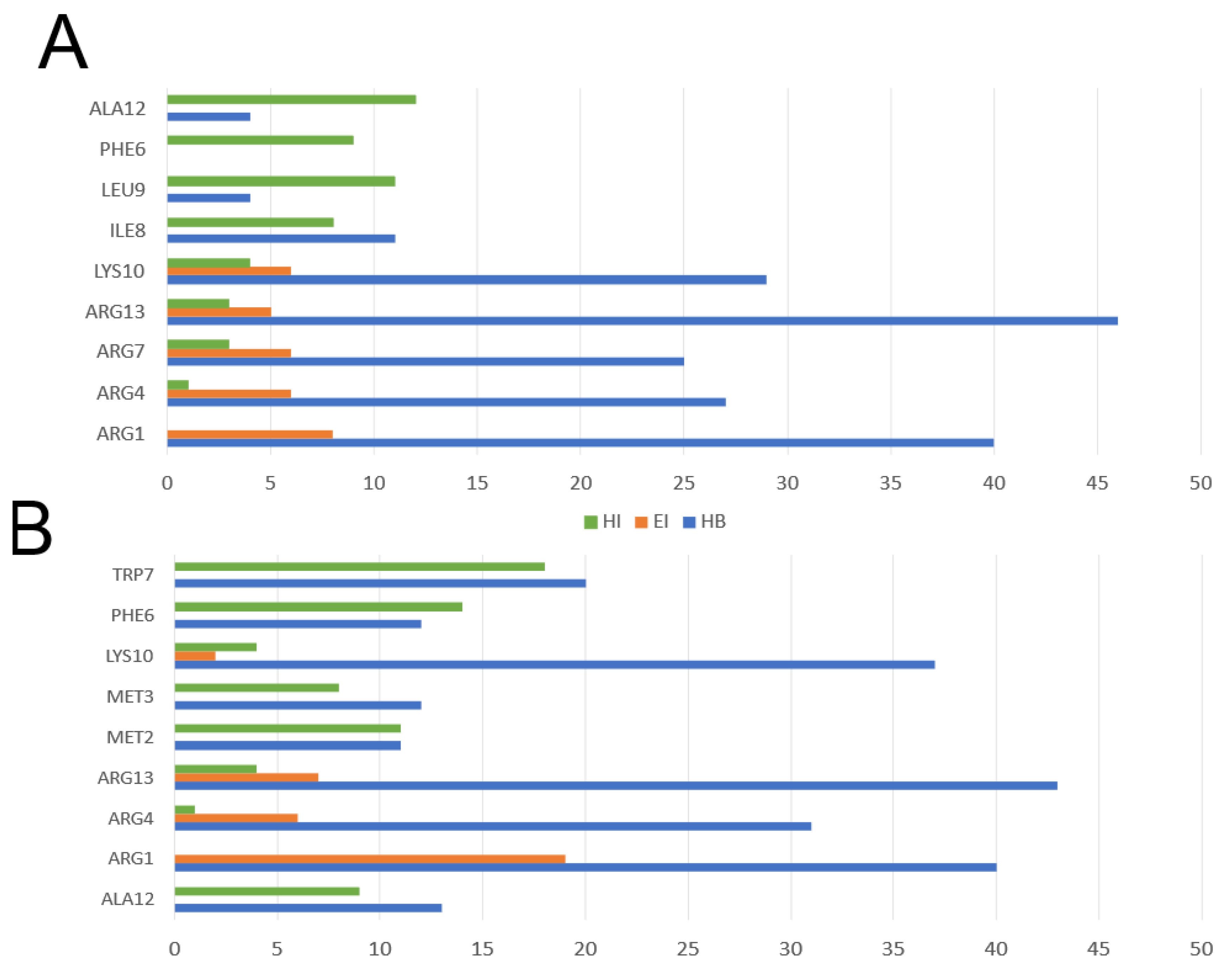
3.2. Comparison of Physicochemical and Structural Characteristics between Helical Cationic Peptides with Probable Combined Antibacterial–Anticancer Activities
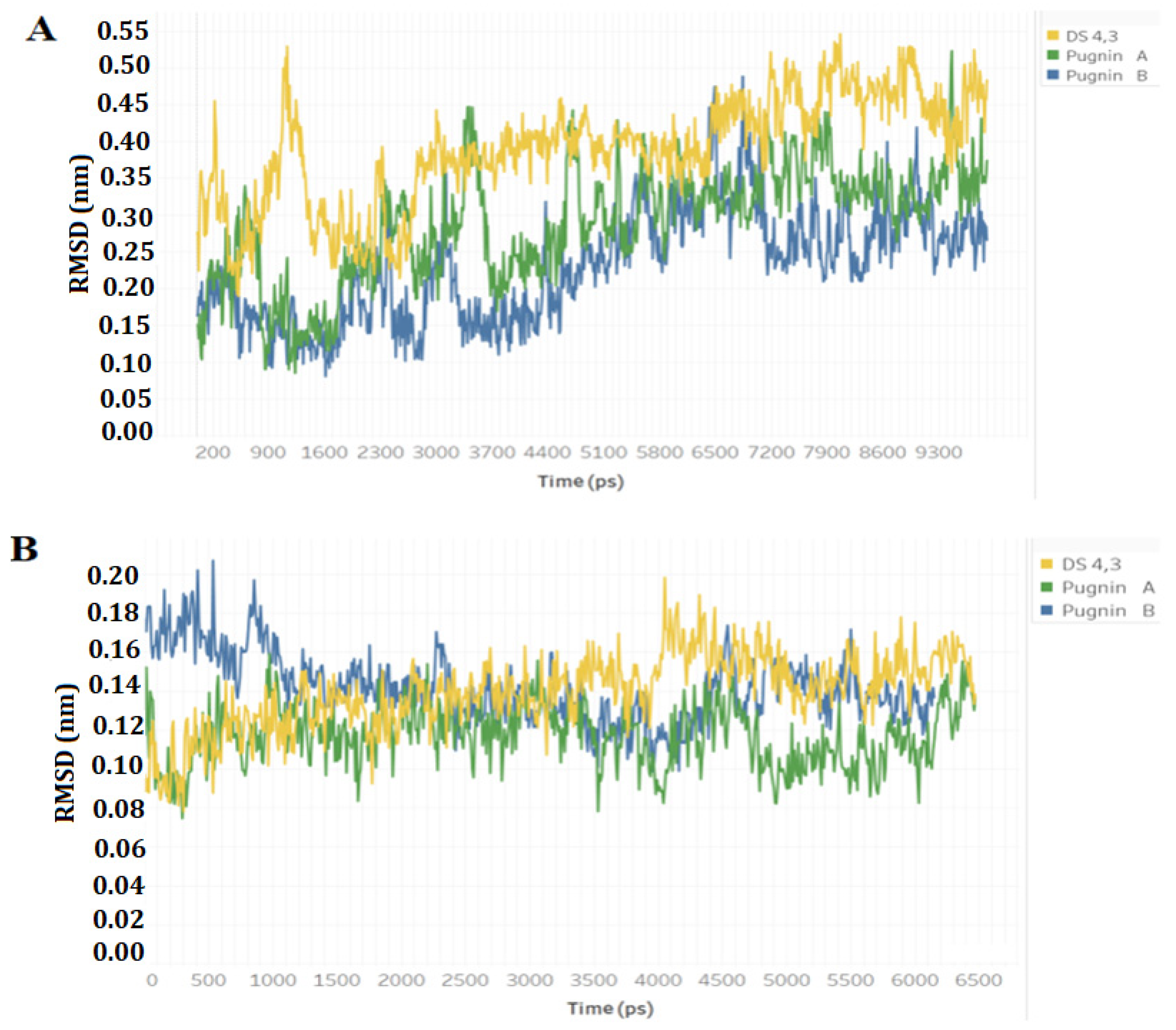
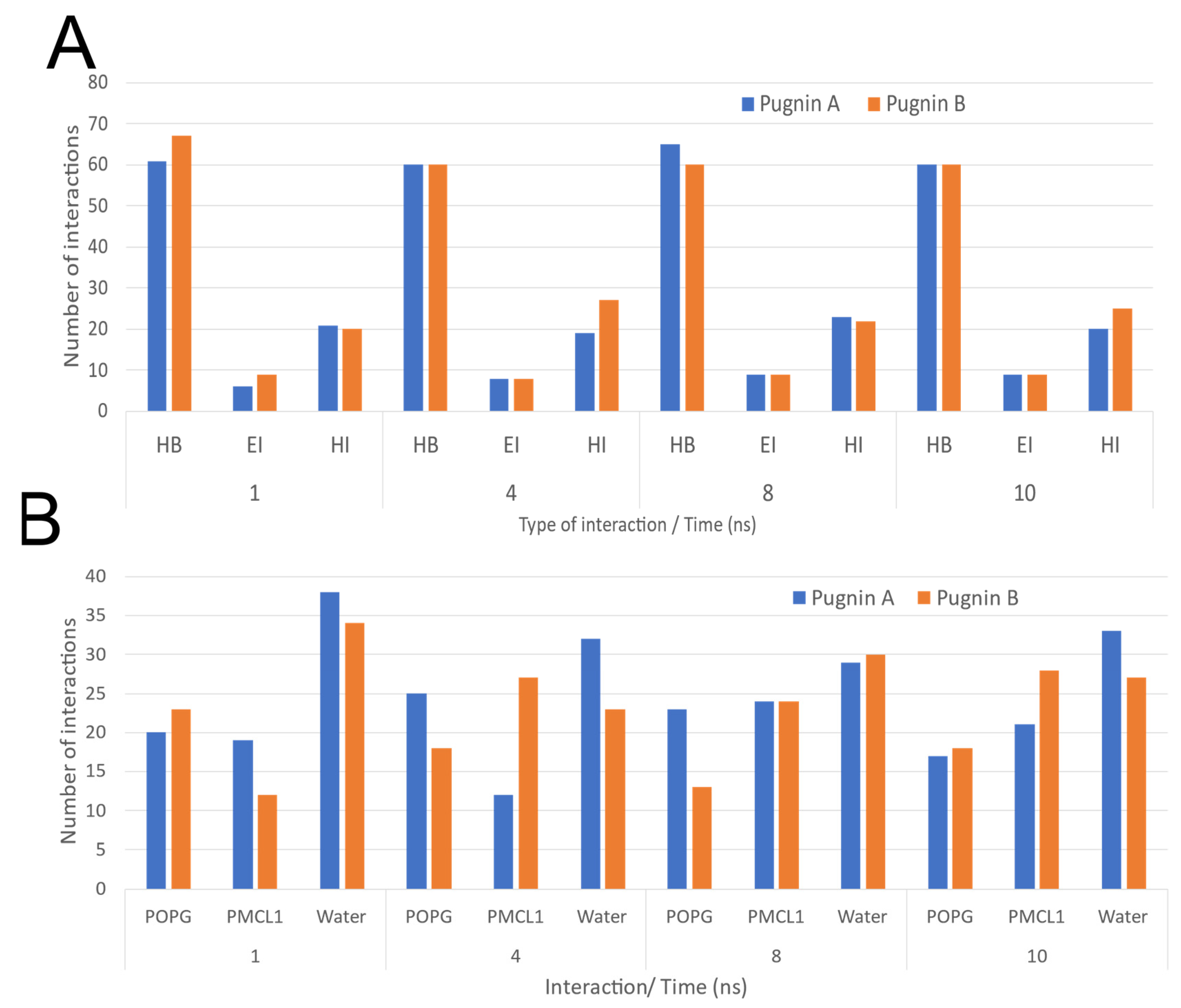
3.3. Pugnins Modeling and Molecular Dynamics Analysis
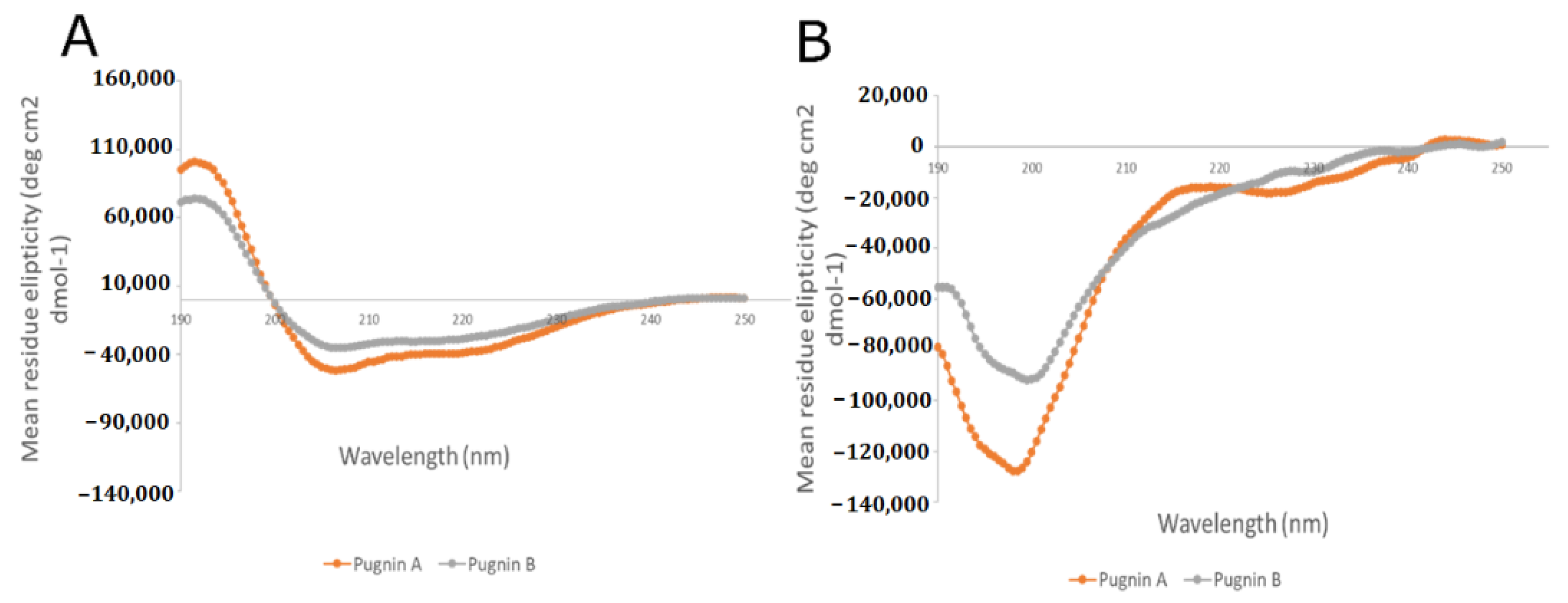
3.4. RP- HPLC Chromatography, Mass Spectromety, and Circular Dichroism
3.5. Antibacterial Test
3.6. Hemolytic Test
3.7. MTT Cytoxicity Test
3.8. Evaluation of Retention of DIOC6 and Incorporation of Propidium Iodide by Flow Cytometry
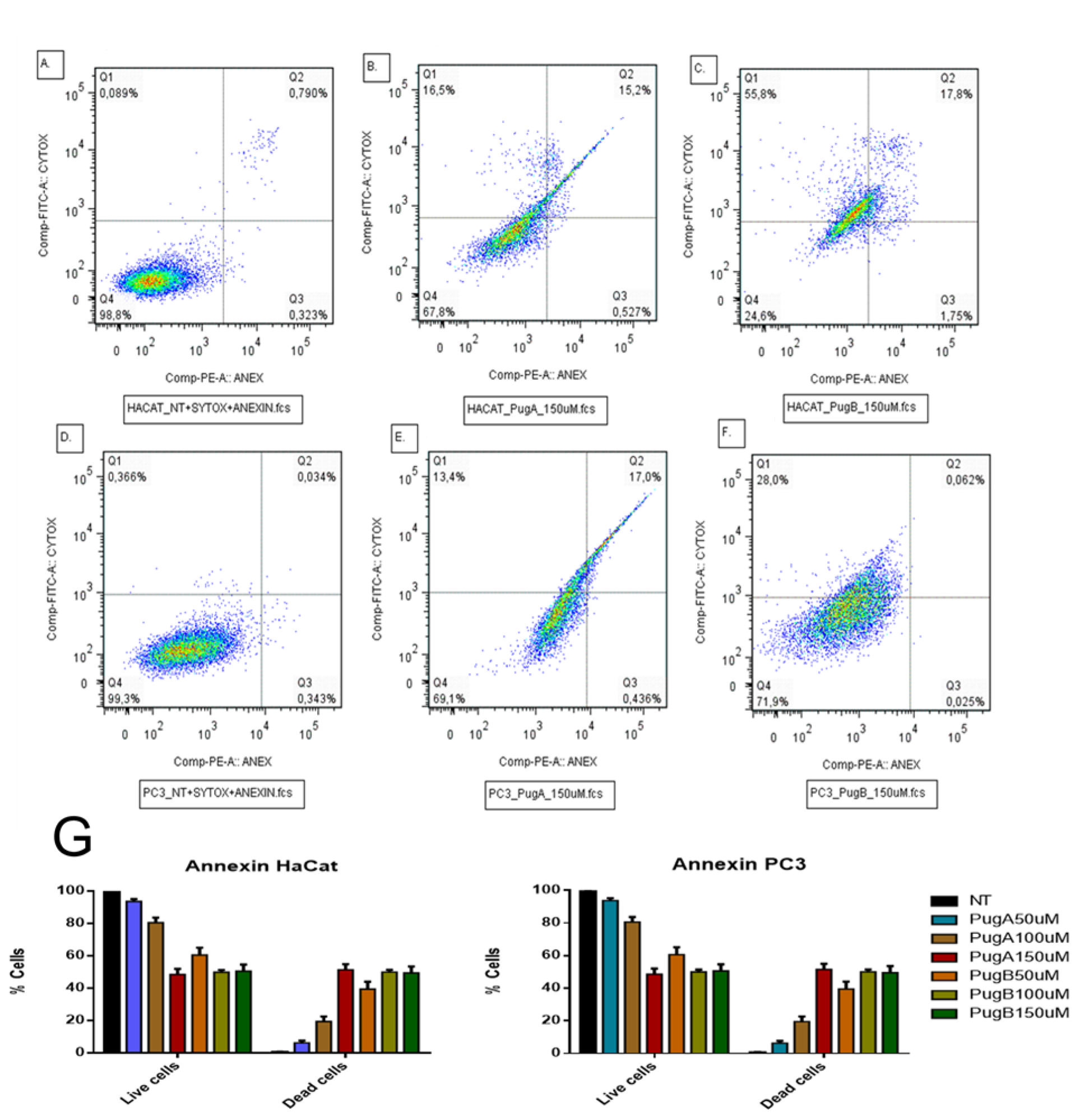
3.9. Effect on Apoptosis Induction Evaluated by AnnexinV-PE/SYTOX in the HaCaT and PC3 Cell Line
3.10. Analysis of the Cell Cycle of HaCaT and PC3 Cell Lines Treated with Pugnin A and B
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guilfoile, P.; Alcamo, I.E. Antibiotic-Resistant Bacteria; Deadly diseases and epidemics; Chelsea House: New York, NY, USA, 2007; ISBN 978-0-7910-9188-3. [Google Scholar]
- Arbab, I.A.; Looi, C.Y.; Abdul, A.B.; Cheah, F.K.; Wong, W.F.; Sukari, M.A.; Abdullah, R.; Mohan, S.; Syam, S.; Arya, A.; et al. Dentatin Induces Apoptosis in Prostate Cancer Cells via Bcl-2, Bcl-XL, Survivin Downregulation, Caspase-9, -3/7 Activation, and NF- κ B Inhibition. Evid. Based Complement. Altern. Med. 2012, 2012, 856029. [Google Scholar] [CrossRef]
- Peters, G.J. Cancer Drug Resistance: A New Perspective. CDR 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Gottler and Ramamoorthy Structure, Membrane Orientation, Mechanism, and Function of Pexiganan–A Highly Potent Antimicrobial Peptide Designed from Magainin. Biochim. Biophys. 2009, 1788, 1680–1686. [CrossRef] [PubMed]
- Lakshmaiah Narayana, J.; Chen, J.-Y. Antimicrobial Peptides: Possible Anti-Infective Agents. Peptides 2015, 72, 88–94. [Google Scholar] [CrossRef]
- Uhlig, T.; Kyprianou, T.; Martinelli, F.G.; Oppici, C.A.; Heiligers, D.; Hills, D.; Calvo, X.R.; Verhaert, P. The Emergence of Peptides in the Pharmaceutical Business: From Exploration to Exploitation. EuPA Open Proteom. 2014, 4, 58–69. [Google Scholar] [CrossRef]
- Liscano, Y.; Oñate-Garzón, J.; Delgado, J.P. Peptides with Dual Antimicrobial–Anticancer Activity: Strategies to Overcome Peptide Limitations and Rational Design of Anticancer Peptides. Molecules 2020, 25, 4245. [Google Scholar] [CrossRef]
- Conlon, J.M. Structural Diversity and Species Distribution of Host-Defense Peptides in Frog Skin Secretions. Cell. Mol. Life Sci. 2011, 68, 2303–2315. [Google Scholar] [CrossRef] [PubMed]
- König, E.; Bininda-Emonds, O.R.P.; Shaw, C. The Diversity and Evolution of Anuran Skin Peptides. Peptides 2015, 63, 96–117. [Google Scholar] [CrossRef]
- Lupo, A.; Cesaro, E.; Montano, G.; Zurlo, D.; Izzo, P.; Costanzo, P. KRAB-Zinc Finger Proteins: A Repressor Family Displaying Multiple Biological Functions. Curr. Genom. 2013, 14, 268–278. [Google Scholar] [CrossRef]
- Yeung, A.T.Y.; Gellatly, S.L.; Hancock, R.E.W. Multifunctional Cationic Host Defence Peptides and Their Clinical Applications. Cell Mol. Life Sci. 2011, 68, 2161–2176. [Google Scholar] [CrossRef]
- Duellman, W.E.; Zug, G.R. Tree Frog|Amphibian, Family Hylidae. Available online: https://www.britannica.com/animal/tree-frog-amphibian-Hylidae-family (accessed on 22 October 2019).
- Xu, X.; Lai, R. The Chemistry and Biological Activities of Peptides from Amphibian Skin Secretions. Chem. Rev. 2015, 115, 1760–1846. [Google Scholar] [CrossRef]
- Liscano Martinez, Y.; Arenas Gómez, C.M.; Smith, J.; Delgado, J.P. A Tree Frog (Boana Pugnax) Dataset of Skin Transcriptome for the Identification of Biomolecules with Potential Antimicrobial Activities. Data Brief 2020, 32, 106084. [Google Scholar] [CrossRef] [PubMed]
- Biodiversidad en Cifras 2019. SiB Colombia 2019. Available online: https://sibcolombia.net/biodiversidad-en-cifras-2019/ (accessed on 22 October 2019).
- Ortiz-Yusty, C.; Daza, J.; Paez, V.; Bock, B. The Collection of the Herpetological Museum of the University of Antioquia (Northwestern Colombia). Biodivers. Data J. 2015, 3, e1325. [Google Scholar] [CrossRef]
- Escalona, M.; Prieto-Torres, D.; Rojas-Runjaic, F.J.M. Unveiling the Geographic Distribution of Boana Pugnax (Schmidt, 1857) (Anura, Hylidae) in Venezuela: New State Records, Range Extension, and Potential Distribution. Check List 2017, 13, 671–681. [Google Scholar] [CrossRef]
- Márquez, M.; Nava-González, F.; Sánchez, D.; Calcagno, M.; Lampo, M. Immmunological Clearance of Batrachochytrium Dendrobatidis Infection at a Pathogen-Optimal Temperature in the Hylid Frog Hypsiboas Crepitans. EcoHealth 2010, 7, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Zompra, A.A.; Galanis, A.S.; Werbitzky, O.; Albericio, F. Manufacturing Peptides as Active Pharmaceutical Ingredients. Future Med. Chem. 2009, 1, 361–377. [Google Scholar] [CrossRef]
- Lee, D.; Hahm, K.-S.; Park, Y.; Kim, H.-Y.; Lee, W.; Lim, S.-C.; Seo, Y.-K.; Choi, C.-H. Functional and Structural Characteristics of Anticancer Peptide Pep27 Analogues. Cancer Cell Int. 2005, 5, 21. [Google Scholar] [CrossRef][Green Version]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with Dual Antimicrobial and Anticancer Activities. Front. Chem. 2017, 5. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6. [Google Scholar] [CrossRef]
- Koutsopoulos Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-08-100736-5.
- Gaspar, D.; Veiga, A.S.; Castanho, M.A.R.B. From Antimicrobial to Anticancer Peptides. A Review. Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef]
- Bharath, E.N.; Manjula, S.N.; Vijaychand, A. In Silico Drug Design-tool for Overcoming the Innovation Deficit in the Drug Discovery Process. Int. J. Pharm. Pharm. 2011, 3, 5. [Google Scholar]
- Mustata, G.; Muftuoglu, Y. Computational Strategies in Cancer Drug Discovery. In Advances in Cancer Management; Mohan, R., Ed.; InTech Open: London, UK, 2012; ISBN 978-953-307-870-0. [Google Scholar]
- E-kobon, T.; Thongararm, P.; Roytrakul, S.; Meesuk, L.; Chumnanpuen, P. Prediction of Anticancer Peptides against MCF-7 Breast Cancer Cells from the Peptidomes of Achatina Fulica Mucus Fractions. Comput. Struct. Biotechnol. J. 2016, 14, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De Novo Transcript Sequence Reconstruction from RNA-Seq Using the Trinity Platform for Reference Generation and Analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Thakur, N.; Tandon, H.; Kumar, M. AVPdb: A Database of Experimentally Validated Antiviral Peptides Targeting Medically Important Viruses. Nucleic Acids Res. 2014, 42, D1147–D1153. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The Antimicrobial Peptide Database as a Tool for Research and Education. Nucleic Acids Res 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Tyagi, A.; Tuknait, A.; Anand, P.; Gupta, S.; Sharma, M.; Mathur, D.; Joshi, A.; Singh, S.; Gautam, A.; Raghava, G.P.S. CancerPPD: A Database of Anticancer Peptides and Proteins. Nucleic Acids Res. 2015, 43, D837–D843. [Google Scholar] [CrossRef]
- Gautam, A.; Singh, H.; Tyagi, A.; Chaudhary, K.; Kumar, R.; Kapoor, P.; Raghava, G.P.S. CPPsite: A Curated Database of Cell Penetrating Peptides. Database 2012, 2012, bas015. [Google Scholar] [CrossRef]
- Signal Peptide Database. Available online: http://www.signalpeptide.de/?m=myprotein (accessed on 1 November 2019).
- Chung, E.M.C.; Dean, S.N.; Propst, C.N.; Bishop, B.M.; van Hoek, M.L. Komodo Dragon-Inspired Synthetic Peptide DRGN-1 Promotes Wound-Healing of a Mixed-Biofilm Infected Wound. NPJ Biofilms Microbiomes 2017, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Tang, J.; Liu, H.; Shen, C.; Rong, M.; Zhang, Z.; Lai, R. A Potential Wound-Healing-Promoting Peptide from Salamander Skin. Faseb J. 2014, 28, 3919–3929. [Google Scholar] [CrossRef]
- Mangoni, M.L.; McDermott, A.M.; Zasloff, M. Antimicrobial Peptides and Wound Healing: Biological and Therapeutic Considerations. Exp. Derm. 2016, 25, 167–173. [Google Scholar] [CrossRef]
- Tang, J.; Liu, H.; Gao, C.; Mu, L.; Yang, S.; Rong, M.; Zhang, Z.; Liu, J.; Ding, Q.; Lai, R. A Small Peptide with Potential Ability to Promote Wound Healing. PLoS ONE 2014, 9, e92082. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Reis, L.A.; Feric, N.; Knee, E.J.; Gu, J.; Cao, S.; Laschinger, C.; Londono, C.; Antolovich, J.; McGuigan, A.P.; et al. Diabetic Wound Regeneration Using Peptide-Modified Hydrogels to Target Re-Epithelialization. Proc. Natl. Acad. Sci. USA 2016, 113, E5792–E5801. [Google Scholar] [CrossRef] [PubMed]
- Pearson, W.R. Finding Protein and Nucleotide Similarities with FASTA. In Current Protocols in Bioinformatics; Bateman, A., Pearson, W.R., Stein, L.D., Stormo, G.D., Yates, J.R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 3.9.1–3.9.25. ISBN 978-0-471-25095-1. [Google Scholar]
- Schaffer, A.A.; Wolf, Y.I.; Ponting, C.P.; Koonin, E.V.; Aravind, L.; Altschul, S.F. IMPALA: Matching a Protein Sequence against a Collection of PSI-BLAST-Constructed Position-Specific Score Matrices. Bioinformatics 1999, 15, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Madden, T. The BLAST sequence analysis tool. In The NCBI Handbook, 2nd ed.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2013. [Google Scholar]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A Web Server to Screen Sequences with Specific -Helical Properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef]
- Lear, S.; Cobb, S.L. Pep-Calc.Com: A Set of Web Utilities for the Calculation of Peptide and Peptoid Properties and Automatic Mass Spectral Peak Assignment. J. Comput. Aided Mol. Des. 2016, 30, 271–277. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. Peptide Synthesis and Proteotypic Peptide Analyzing Tool. Available online: https://www.thermofisher.com/ht/en/home/life-science/protein-biology/peptides-proteins/custom-peptide-synthesis-services/peptide-analyzing-tool.html (accessed on 2 November 2019).
- Guermeur, Y.; Geourjon, C.; Gallinari, P.; Deleage, G. Improved Performance in Protein Secondary Structure Prediction by Inhomogeneous Score Combination. Bioinformatics 1999, 15, 413–421. [Google Scholar] [CrossRef]
- Urbanc, B. Protein Actions: Principles and Modeling by I. Bahar, R.L. Jernigan, and K.A. Dill. J. Biol. Phys. 2017, 43, 1–5. [Google Scholar] [CrossRef]
- Feder, R.; Dagan, A.; Mor, A. Structure-Activity Relationship Study of Antimicrobial Dermaseptin S4 Showing the Consequences of Peptide Oligomerization on Selective Cytotoxicity. J. Biol. Chem. 2000, 275, 4230–4238. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Escamilla, A.-M.; Rousseau, F.; Schymkowitz, J.; Serrano, L. Prediction of Sequence-Dependent and Mutational Effects on the Aggregation of Peptides and Proteins. Nat. Biotechnol. 2004, 22, 1302–1306. [Google Scholar] [CrossRef]
- Ntwasa, M.; Goto, A.; Kurata, S. Coleopteran Antimicrobial Peptides: Prospects for Clinical Applications. Int. J. Microbiol. 2012, 2012, 101989. [Google Scholar] [CrossRef]
- Zelezetsky, I.; Tossi, A. Alpha-Helical Antimicrobial Peptides—Using a Sequence Template to Guide Structure–Activity Relationship Studies. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 1436–1449. [Google Scholar] [CrossRef]
- Park, I.Y.; Cho, J.H.; Kim, K.S.; Kim, Y.-B.; Kim, M.S.; Kim, S.C. Helix Stability Confers Salt Resistance upon Helical Antimicrobial Peptides. J. Biol. Chem. 2004, 279, 13896–13901. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Saitou, N. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Long, S.B.; Tao, X.; Campbell, E.B.; MacKinnon, R. Atomic Structure of a Voltage-Dependent K+ Channel in a Lipid Membrane-like Environment. Nature 2007, 450, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-J.; Tani, K.; Irie, K.; Hiroaki, Y.; Shimomura, T.; McMillan, D.G.; Cook, G.M.; Schertler, G.F.X.; Fujiyoshi, Y.; Li, X.-D. Two Alternative Conformations of a Voltage-Gated Sodium Channel. J. Mol. Biol. 2013, 425, 4074–4088. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER: Comparative Protein Structure Modeling Using Modeller. In Current Protocols in Bioinformatics; Bateman, A., Pearson, W.R., Stein, L.D., Stormo, G.D., Yates, J.R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 5.6.1–5.6.37. ISBN 978-0-471-25095-1. [Google Scholar]
- Lovell, S.C.; Davis, I.W.; Arendall, W.B.; de Bakker, P.I.W.; Word, J.M.; Prisant, M.G.; Richardson, J.S.; Richardson, D.C. Structure Validation by Cα Geometry: ϕ,ψ and Cβ Deviation. Proteins 2003, 50, 437–450. [Google Scholar] [CrossRef]
- Wiederstein, M.; Sippl, M.J. ProSA-Web: Interactive Web Service for the Recognition of Errors in Three-Dimensional Structures of Proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.B.; Arendall, W.B.; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-Atom Structure Validation for Macromolecular Crystallography. Acta Cryst. D Biol. Cryst. 2010, 66, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A Web-Based Graphical User Interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M.; Epand, R.F. Lipid Domains in Bacterial Membranes and the Action of Antimicrobial Agents. Biochim. Biophys. Acta (BBA) Biomembr. 2009, 1788, 289–294. [Google Scholar] [CrossRef]
- Liscano, Y.; Salamanca, C.H.; Vargas, L.; Cantor, S.; Laverde-Rojas, V.; Oñate-Garzón, J. Increases in Hydrophilicity and Charge on the Polar Face of Alyteserin 1c Helix Change Its Selectivity towards Gram-Positive Bacteria. Antibiotics 2019, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable Molecular Dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmüller, H.; MacKerell, A.D. CHARMM36m: An Improved Force Field for Folded and Intrinsically Disordered Proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Legge, F.S.; Treutlein, H.; Howlett, G.J.; Yarovsky, I. Molecular Dynamics Simulations of a Fibrillogenic Peptide Derived from Apolipoprotein C-II. Biophys. Chem. 2007, 130, 102–113. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A Message-Passing Parallel Molecular Dynamics Implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Ciccotti, G.; Ryckaert, J.P. Molecular Dynamics Simulation of Rigid Molecules. Comput. Phys. Rep. 1986, 4, 346–392. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Heinig, M.; Frishman, D. STRIDE: A Web Server for Secondary Structure Assignment from Known Atomic Coordinates of Proteins. Nucleic Acids Res. 2004, 32, W500–W502. [Google Scholar] [CrossRef] [PubMed]
- McGibbon, R.T.; Beauchamp, K.A.; Harrigan, M.P.; Klein, C.; Swails, J.M.; Hernández, C.X.; Schwantes, C.R.; Wang, L.-P.; Lane, T.J.; Pande, V.S. MDTraj: A Modern Open Library for the Analysis of Molecular Dynamics Trajectories. Biophys. J. 2015, 109, 1528–1532. [Google Scholar] [CrossRef] [PubMed]
- Luna, O.F.; Gomez, J.; Cárdenas, C.; Albericio, F.; Marshall, S.H.; Guzmán, F. Deprotection Reagents in Fmoc Solid Phase Peptide Synthesis: Moving Away from Piperidine? Molecules 2016, 21, 1542. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: M07-A10; Approved Standard, 10th ed.; Clinical and Laboratory Standards Institute, Ed.; Documents/Clinical and Laboratory Standards Institute; Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2015; ISBN 978-1-56238-987-1. [Google Scholar]
- De Boer, M.; Heuer, C.; Hussein, H.; McDougall, S. Minimum Inhibitory Concentrations of Selected Antimicrobials against Escherichia Coli and Trueperella Pyogenes of Bovine Uterine Origin. J. Dairy Sci. 2015, 98, 12. [Google Scholar] [CrossRef]
- Yamazhan, T.; Aydemir, Ş.; Tünger, A.; Serter, D.; Gökengin, D. In Vitro Activities of Various Antimicrobials against Brucella Melitensis Strains in the Aegean Region in Turkey. Med. Princ. Pract. 2005, 14, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, A.; Cirioni, O.; Barchiesi, F.; Del Prete, M.S.; Fortuna, M.; Caselli, F.; Scalise, G. In Vitro Susceptibility Tests for Cationic Peptides: Comparison of Broth Microdilution Methods for Bacteria That Grow Aerobically. Antimicrob. Agents Chemother 2000, 44, 1694–1696. [Google Scholar] [CrossRef]
- Santa-Gonzalez, G.A.; Gomez-Molina, A.; Arcos-Burgos, M.; Meyer, J.N.; Camargo, M. Distinctive Adaptive Response to Repeated Exposure to Hydrogen Peroxide Associated with Upregulation of DNA Repair Genes and Cell Cycle Arrest. Redox Biol. 2016, 9, 124–133. [Google Scholar] [CrossRef]
- Zapata-Patiño, M.A.; Delgado-Charris, J.P. Evaluación In Vitro de Tres Péptidos Multifuncionales (Figaininas) Extraídos de Secreciones Cutáneas de Hypsiboas Raniceps Sobre Líneas Celulares Tumorales; Universidad de Antioquia: Medellín, Colombia, 2014; p. 34. [Google Scholar]
- Leppink, J.; O’Sullivan, P.; Winston, K. The Bridge between Design and Analysis. Perspect. Med. Educ. 2017, 6, 265–269. [Google Scholar] [CrossRef][Green Version]
- Kim, T.K. Understanding One-Way ANOVA Using Conceptual Figures. Korean J. Anesth. 2017, 70, 22. [Google Scholar] [CrossRef]
- Brown, M.B.; Forsythe, A.B. Robust Tests for the Equality of Variances. J. Am. Stat. Assoc. 1974, 69, 364–367. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.K. What Is the Proper Way to Apply the Multiple Comparison Test? Korean J. Anesth. 2018, 71, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Orr, D.F.; O’Rourke, M.; McLynn, C.; Bjourson, A.J.; McClean, S.; Hirst, D.; Rao, P.; Shaw, C. Pachymedusa Dacnicolor Tryptophyllin-1: Structural Characterization, Pharmacological Activity and Cloning of Precursor CDNA. Regul. Pept. 2004, 117, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Marenah, L.; Flatt, P.R.; Orr, D.F.; McClean, S.; Shaw, C.; Abdel-Wahab, Y.H.A. Skin Secretion of the Toad Bombina Variegata Contains Multiple Insulin-Releasing Peptides Including Bombesin and Entirely Novel Insulinotropic Structures. Biol. Chem. 2004, 385. [Google Scholar] [CrossRef] [PubMed]
- Montecucchi, P.C. Isolation and Primary Structure Determination of Amphibian Skin Tryptophyllins. Peptides 1985, 6, 187–195. [Google Scholar] [CrossRef]
- Erspamer, V.; Corsi, R.; Severini, C.; BARRAt, D.; SIMMACOt, M.; Kreil, G. Deltorphins: A Family of Naturally Occurring Peptides with High Affinity and Selectivity for 6 Opioid Binding Sites. Proc. Natl. Acad. Sci. USA 1989, 86, 5188–5192. [Google Scholar] [CrossRef] [PubMed]
- Melchiorri, P.; Negri, L. The Dermorphin Peptide Family. Gen. Pharmacol. Vasc. Syst. 1996, 27, 1099–1107. [Google Scholar] [CrossRef]
- AL-Abboodi, A.; Rasedee, A.; Abdul, A.B.; Taufiq-Yap, Y.H.; Alkaby, W.; Ghaji, M.; Waziri, P.; Al-Qubaisi, M. Anticancer effect of dentatin and dentatin-hydroxypropyl-β-cyclodextrin complex on human colon cancer (HT-29) cell line. Drug Des. Dev. Ther. 2017, 11, 3309–3319. [Google Scholar] [CrossRef][Green Version]
- Park, C.B.; Kim, H.S.; Kim, S.C. Mechanism of Action of the Antimicrobial Peptide Buforin II: Buforin II Kills Microorganisms by Penetrating the Cell Membrane and Inhibiting Cellular Functions. Biochem. Biophys. Res. Commun. 1998, 244, 253–257. [Google Scholar] [CrossRef]
- Park, C.B.; Yi, K.-S.; Matsuzaki, K.; Kim, M.S.; Kim, S.C. Structure-Activity Analysis of Buforin II, a Histone H2A-Derived Antimicrobial Peptide: The Proline Hinge Is Responsible for the Cell-Penetrating Ability of Buforin II. Proc. Natl. Acad. Sci. USA 2000, 97, 8245–8250. [Google Scholar] [CrossRef]
- Seo, J.-K.; Lee, M.J.; Go, H.-J.; Kim, G.D.; Jeong, H.D.; Nam, B.-H.; Park, N.G. Purification and Antimicrobial Function of Ubiquitin Isolated from the Gill of Pacific Oyster, Crassostrea Gigas. Mol. Immunol. 2013, 53, 88–98. [Google Scholar] [CrossRef]
- Kajimura, Y.; Kaneda, M. Fusaricidin A, a New Depsipeptide Antibiotic Produced by Bacillus Polymyxa KT-8 Taxonomy, Fermentation, Isolation, Structure Elucidation and Biological Activity. J. Antibiot. 1996, 49, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Agerberth, B.; Boman, A.; Andersson, M.; Jornvall, H.; Mutt, V.; Boman, H.G. Isolation of Three Antibacterial Peptides from Pig Intestine: Gastric Inhibitory Polypeptide(7-42), Diazepam-Binding Inhibitor(32-86) and a Novel Factor, Peptide 3910. Eur. J. Biochem. 1993, 216, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Budge, P.J.; Lebowitz, J.; Graham, B.S. Antiviral Activity of RhoA-Derived Peptides against Respiratory Syncytial Virus Is Dependent on Formation of Peptide Dimers. Antimicrob. Agents Chemother. 2003, 47, 3470–3477. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Androphy, E.; Chen, J.; Baleja, J.D. Design and Characterization of Helical Peptides That Inhibit the E6 Protein of Papillomavirus †,‡. Biochemistry 2004, 43, 7421–7431. [Google Scholar] [CrossRef]
- Si, Y.; Liu, S.; Liu, X.; Jacobs, J.L.; Cheng, M.; Niu, Y.; Jin, Q.; Wang, T.; Yang, W. A Human Claudin-1-Derived Peptide Inhibits Hepatitis C Virus Entry. Hepatology 2012, 56, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Derossi, D.; Calvet, S.; Trembleau, A.; Brunissen, A.; Chassaing, G.; Prochiantz, A. Cell Internalization of the Third Helix of the Antennapedia Homeodomain Is Receptor-Independent. J. Biol. Chem. 1996, 271, 18188–18193. [Google Scholar] [CrossRef]
- Habault, J.; Poyet, J.-L. Recent Advances in Cell Penetrating Peptide-Based Anticancer Therapies. Molecules 2019, 24, 927. [Google Scholar] [CrossRef] [PubMed]
- Kilk, K.; Magzoub, M.; Pooga, M.; Eriksson, L.E.G.; Langel, Ü.; Gräslund, A. Cellular Internalization of a Cargo Complex with a Novel Peptide Derived from the Third Helix of the Islet-1 Homeodomain. Comparison with the Penetratin Peptide. Bioconjug. Chem. 2001, 12, 911–916. [Google Scholar] [CrossRef]
- Mizuguchi, T.; Ohashi, N.; Nomura, W.; Komoriya, M.; Hashimoto, C.; Yamamoto, N.; Murakami, T.; Tamamura, H. Anti-HIV Screening for Cell-Penetrating Peptides Using Chloroquine and Identification of Anti-HIV Peptides Derived from Matrix Proteins. Bioorg. Med. Chem. 2015, 23, 4423–4427. [Google Scholar] [CrossRef]
- Je, J.-Y.; Qian, Z.-J.; Kim, S.-K. Antioxidant Peptide Isolated from Muscle Protein of Bullfrog, Rana Catesbeiana Shaw. J. Med. Food 2007, 10, 401–407. [Google Scholar] [CrossRef]
- Jeong, J.-H.; Kim, K.; Lim, D.; Jeong, K.; Hong, Y.; Nguyen, V.H.; Kim, T.-H.; Ryu, S.; Lim, J.-A.; Kim, J.I.; et al. Anti-Tumoral Effect of the Mitochondrial Target Domain of Noxa Delivered by an Engineered Salmonella Typhimurium. PLoS ONE 2014, 9, e80050. [Google Scholar] [CrossRef]
- Zou, R.; Zhu, X.; Tu, Y.; Wu, J.; Landry, M.P. Activity of Antimicrobial Peptide Aggregates Decreases with Increased Cell Membrane Embedding Free Energy Cost. Biochemistry 2018, 57, 2606–2610. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.; Shivangi; Thakur, S.C.; Meena, L.S. Structural Prediction and Mutational Analysis of Rv3906c Gene of Mycobacterium Tuberculosis H 37 Rv to Determine Its Essentiality in Survival. Adv. Bioinform. 2018, 2018, 6152014. [Google Scholar] [CrossRef]
- Dixit, S.B.; Ponomarev, S.Y.; Beveridge, D.L. Root Mean Square Deviation Probability Analysis of Molecular Dynamics Trajectories on DNA. J. Chem. Inf. Model. 2006, 46, 1084–1093. [Google Scholar] [CrossRef]
- Wieprecht, T.; Beyermann, M.; Seelig, J. Thermodynamics of the Coil–a-Helix Transition of Amphipathic Peptides in a Membrane Environment: The Role of Vesicle Curvature. Biophys. Chem. 2002, 96, 191–201. [Google Scholar] [CrossRef]
- Bondar, A.-N.; White, S.H. Hydrogen Bond Dynamics in Membrane Protein Function. Biochim. Biophys. Acta (BBA) Biomembr. 2012, 1818, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L. Automatic Identification of Mobile and Rigid Substructures in Molecular Dynamics Simulations and Fractional Structural Fluctuation Analysis. PLoS ONE 2015, 10, e0119264. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.M.R.P.S.; Landemberger, M.C.; Walz, R.; Carlotti, C.G.; Huang, N.; Cunha, D.R.; Moura, R.; Caballero, O.L.; Sakamoto, A.C.; Nitrini, R.; et al. High Capacity and Low Cost Detection of Prion Protein Gene Variant Alleles by Denaturing HPLC. J. Neurosci. Methods 2004, 139, 263–269. [Google Scholar] [CrossRef]
- Cherry, M.A.; Higgins, S.K.; Melroy, H.; Lee, H.-S.; Pokorny, A. Peptides with the Same Composition, Hydrophobicity, and Hydrophobic Moment Bind to Phospholipid Bilayers with Different Affinities. J. Phys. Chem. B 2014, 118, 12462–12470. [Google Scholar] [CrossRef]
- Deber, C.M.; Li, S.-C. Peptides in Membranes: Helicity and Hydrophobicity. Biopolymers 1995, 37, 295–318. [Google Scholar] [CrossRef]
- Pacor, S.; Grillo, A.; Đorđević, L.; Zorzet, S.; Lucafò, M.; Da Ros, T.; Prato, M.; Sava, G. Effects of Two Fullerene Derivatives on Monocytes and Macrophages. Biomed. Res. Int. 2015, 2015, 915130. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, U.; Venkatesan, T.; Radhakrishnan, V.; Samuel, S.; Rathinavelu, A. Differential Mechanisms of Cell Death Induced by HDAC Inhibitor SAHA and MDM2 Inhibitor RG7388 in MCF-7 Cells. Cells 2018, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Vasil, A.I.; Hale, J.D.; Hancock, R.E.W.; Vasil, M.L.; Hodges, R.S. Effects of Net Charge and the Number of Positively Charged Residues on the Biological Activity of Amphipathic α-Helical Cationic Antimicrobial Peptides. Biopolymers 2008, 90, 369–383. [Google Scholar] [CrossRef]
- Lee, J.; Choi, H.; Cho, J.; Lee, D.G. Effects of Positively Charged Arginine Residues on Membrane Pore Forming Activity of Rev–NIS Peptide in Bacterial Cells. Biochim. Biophys. Acta (BBA) Biomembr. 2011, 1808, 2421–2427. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.I.; Prenner, E.J.; Vogel, H.J. Tryptophan- and Arginine-Rich Antimicrobial Peptides: Structures and Mechanisms of Action. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 1184–1202. [Google Scholar] [CrossRef]
- Zhou, H.X.; Lyu, P.C.; Wemmer, D.E.; Kallenbach, N.R. Structure of a C-Terminal.Alpha.-Helix Cap in a Synthetic Peptide. J. Am. Chem. Soc. 1994, 116, 1139–1140. [Google Scholar] [CrossRef]
- Forood, B.; Feliciano, E.J.; Nambiar, K.P. Stabilization of A-Helical Structures in Short Peptides via End Capping. Proc. Natl. Acad. Sci. USA 1993, 90, 838–842. [Google Scholar] [CrossRef]
- Glukhov, E.; Stark, M.; Burrows, L.L.; Deber, C.M. Basis for Selectivity of Cationic Antimicrobial Peptides for Bacterial Versus Mammalian Membranes. J. Biol. Chem. 2005, 280, 33960–33967. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharm. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Chen, Y.; Guarnieri, M.T.; Vasil, A.I.; Vasil, M.L.; Mant, C.T.; Hodges, R.S. Role of Peptide Hydrophobicity in the Mechanism of Action of -Helical Antimicrobial Peptides. Antimicrob. Agents Chemother. 2007, 51, 1398–1406. [Google Scholar] [CrossRef]
- Lhor, M.; Bernier, S.C.; Horchani, H.; Bussières, S.; Cantin, L.; Desbat, B.; Salesse, C. Comparison between the Behavior of Different Hydrophobic Peptides Allowing Membrane Anchoring of Proteins. Adv. Colloid Interface Sci. 2014, 207, 223–239. [Google Scholar] [CrossRef]
- Polozov, I.V.; Polozova, A.I.; Molotkovsky, J.G.; Epand, R.M. Amphipathic Peptide Affects the Lateral Domain Organization of Lipid Bilayers. Biochim. Biophys. Acta (BBA) Biomembr. 1997, 1328, 125–139. [Google Scholar] [CrossRef][Green Version]
- Kumariya, R.; Sood, S.K.; Rajput, Y.S.; Saini, N.; Garsa, A.K. Increased Membrane Surface Positive Charge and Altered Membrane Fluidity Leads to Cationic Antimicrobial Peptide Resistance in Enterococcus Faecalis. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Nešuta, O.; Buděšínský, M.; Hadravová, R.; Monincová, L.; Humpoličková, J.; Čeřovský, V. How Proteases from Enterococcus Faecalis Contribute to Its Resistance to Short α-Helical Antimicrobial Peptides. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef]
- Vaezi, Z.; Bortolotti, A.; Luca, V.; Perilli, G.; Mangoni, M.L.; Khosravi-Far, R.; Bobone, S.; Stella, L. Aggregation Determines the Selectivity of Membrane-Active Anticancer and Antimicrobial Peptides: The Case of KillerFLIP. Biochim. Biophys. Acta (BBA) Biomembr. 2019, 183107. [Google Scholar] [CrossRef] [PubMed]
- Maturana, P.; Martinez, M.; Noguera, M.E.; Santos, N.C.; Disalvo, E.A.; Semorile, L.; Maffia, P.C.; Hollmann, A. Lipid Selectivity in Novel Antimicrobial Peptides: Implication on Antimicrobial and Hemolytic Activity. Colloids Surf. B Biointerfaces 2017, 153, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Platre, M.P. Anionic Lipids and the Maintenance of Membrane Electrostatics in Eukaryotes. Plant Signal. Behav. 2017, 12, e1282022. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.A.; Cheng, K.H.; Somerharju, P. Phospholipid Composition of the Mammalian Red Cell Membrane Can Be Rationalized by a Superlattice Model. Proc. Natl. Acad. Sci. USA 1998, 95, 4964–4969. [Google Scholar] [CrossRef]
- Schweizer, F. Cationic Amphiphilic Peptides with Cancer-Selective Toxicity. Eur. J. Pharmacol. 2009, 625, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Kustanovich, I.; Shalev, D.E.; Mikhlin, M.; Gaidukov, L.; Mor, A. Structural Requirements for Potent Versus Selective Cytotoxicity for Antimicrobial Dermaseptin S4 Derivatives. J. Biol. Chem. 2002, 277, 16941–16951. [Google Scholar] [CrossRef]











| Inhibitory Concentration (µM) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptides | S. aureus ATCC 25923 | E. faecalis ATCC 29212 | P. aeruginosa ATCC 27853 | E. coli ATCC 25922 | ||||||||
| MIC50 | MIC90 | MBC | MIC50 | MIC90 | MBC | MIC50 | MIC90 | MBC | MIC50 | MIC90 | MBC | |
| Pugnin A | 11.0 | 148.9 | 183.4 | 0.70 | 107.2 | 150.0 | 4.1 | 28.5 | 34.6 | 14.2 | 69.4 | 83.3 |
| Pugnin B | 9.20 | 549.7 | 684.8 | 18.0 | 111.2 | 134.5 | 8.9 | 158.4 | 195.7 | 0.10 | 57.8 | 72.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liscano, Y.; Medina, L.; Oñate-Garzón, J.; Gúzman, F.; Pickholz, M.; Delgado, J.P. In Silico Selection and Evaluation of Pugnins with Antibacterial and Anticancer Activity Using Skin Transcriptome of Treefrog (Boana pugnax). Pharmaceutics 2021, 13, 578. https://doi.org/10.3390/pharmaceutics13040578
Liscano Y, Medina L, Oñate-Garzón J, Gúzman F, Pickholz M, Delgado JP. In Silico Selection and Evaluation of Pugnins with Antibacterial and Anticancer Activity Using Skin Transcriptome of Treefrog (Boana pugnax). Pharmaceutics. 2021; 13(4):578. https://doi.org/10.3390/pharmaceutics13040578
Chicago/Turabian StyleLiscano, Yamil, Laura Medina, Jose Oñate-Garzón, Fanny Gúzman, Monica Pickholz, and Jean Paul Delgado. 2021. "In Silico Selection and Evaluation of Pugnins with Antibacterial and Anticancer Activity Using Skin Transcriptome of Treefrog (Boana pugnax)" Pharmaceutics 13, no. 4: 578. https://doi.org/10.3390/pharmaceutics13040578
APA StyleLiscano, Y., Medina, L., Oñate-Garzón, J., Gúzman, F., Pickholz, M., & Delgado, J. P. (2021). In Silico Selection and Evaluation of Pugnins with Antibacterial and Anticancer Activity Using Skin Transcriptome of Treefrog (Boana pugnax). Pharmaceutics, 13(4), 578. https://doi.org/10.3390/pharmaceutics13040578







