Abstract
The blood–brain barrier (BBB) is a natural obstacle for drug delivery into the human brain, hindering treatment of central nervous system (CNS) disorders such as acute ischemic stroke, brain tumors, and human immunodeficiency virus (HIV)-1-associated neurocognitive disorders. Poly(lactic-co-glycolic acid) (PLGA) is a biocompatible polymer that is used in Food and Drug Administration (FDA)-approved pharmaceutical products and medical devices. PLGA nanoparticles (NPs) have been reported to improve drug penetration across the BBB both in vitro and in vivo. Poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA), and poloxamer (Pluronic) are widely used as excipients to further improve the stability and effectiveness of PLGA formulations. Peptides and other linkers can be attached on the surface of PLGA to provide targeting delivery. With the newly published guidance from the FDA and the progress of current Good Manufacturing Practice (cGMP) technologies, manufacturing PLGA NP-based drug products can be achieved with higher efficiency, larger quantity, and better quality. The translation from bench to bed is feasible with proper research, concurrent development, quality control, and regulatory assurance.
1. Blood–Brain Barrier (BBB) and Drug Delivery
Compared with other therapeutic areas, drug development is more challenging for brain diseases such as brain cancers, Alzheimer’s diseases (AD), acute ischemic stroke, and human immunodeficiency virus (HIV)-1-associated neurocognitive disorders (HAND) [1,2,3,4]. Many systemically administered drug products cannot pass the BBB [5]. The BBB restricts the entry of compounds into the central nervous system (CNS) through the presence of brain microvascular endothelial cells, pericytes, perivascular astrocytes, and tight junctions. In addition, the presence of efflux transporters at the BBB has been recognized as a key element to poor drug penetration [6,7]. ATP-binding cassette (ABC) membrane-associated transporters, such as P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and multidrug resistance-associated protein (MRP1) show significant expressions at the BBB, protecting the brain from potential harmful endogenous and exogenous substances [6,7]. As a result, the BBB only selectively transports molecules such as certain amino acids, sugars, and gaseous molecules (e.g., oxygen and carbon dioxide) into the brain [8]. For example, antiretroviral drugs (ARVs) have shown to be effective in managing HIV-1 [9]. However, due to the inability of ARVs to cross the BBB, they are not highly recommended clinically for the treatment of HAND. Studies showed that, upon boosting with a pharmaco-enhancer, i.e., ritonavir, ARVs including indinavir, elvitegravir, and lopinavir reached therapeutic concentrations in plasma but did not reach therapeutic concentration in the brain, indicating the challenges of delivering drugs to the CNS [10,11].
2. Strategies to Cross BBB
Several strategies have been used to improve drug delivery to the brain. Efforts have been made for the development of inhibitors for ABC transporters due to their high expressions on the BBB [2,12,13]. Studies showed that blocking ABC transporters may significantly improve drug penetrations across the BBB. However, this method has not been used clinically due to the wide distribution of ABC transporters throughout the body, the potential toxicity of inhibitors, and unexpected drug–drug interactions [12]. Another approach is the “BBB opening” approach. Opening the BBB can be achieved by using a hyperosmotic solution to shrink the endothelial cells or using certain cytotoxic agents to disrupt the BBB tight junctions [12,13]. However, opening the tight junctions of the BBB is risky clinically because it may also allow the entry of harmful components into the brain and cause side-effects such as seizures and other long-term neurological complications [12]. Moreover, the development of prodrugs to increase their capacity to penetrate the BBB is another potential delivery approach [12]. Prodrugs can be synthesized with sufficient lipophilicity to facilitate the crossing of the endothelial cell membrane and release the parent ARVs into the brain. However, developing prodrugs as a delivery strategy needs a full evaluation of toxicity, cost, and efficacy, as prodrugs are considered to be a separate chemical entity.
A nanoparticle (NP)-based drug delivery system is considered a promising option to improve drug delivery to the brain [3]. NP-based formulations are usually a colloidal system made of polymers, lipids, or other large macromolecules such as albumin. A therapeutic agent may be released through diffusion or erosion of the matrix [14]. The NP-based delivery system can cross the BBB through membrane transcytosis, bypass efflux transporters, and effectively deliver the therapeutic molecule to the CNS [3]. NPs that have been studied for brain delivery include polymeric NPs such as poly(d,l-lactide-co-glycolide) (PLGA) [15,16] and poly(butyl-cyanoacrylate) (PBCA) NPs [17,18], magnetic NPs (MNPs) composed of an iron oxide core [18], lipid-based nanoformulations such as solid lipid nanoparticles (SLN) and liposomes [12,18], and polymeric micelles-based nanoformulations such as Pluronic micelles [12]. Extracellular vesicles (EVs), liposome-like natural carriers, have drawn attention for delivering drugs into the brain as a potential alternative to NPs [19,20,21].
3. Introduction of Physical, Chemical, and Biological Characteristics of PLGA Polymer
3.1. Synthesis of PLGA Polymer
PLGA is a synthetic copolymer composed of lactic and glycolic acid polyesters. Synthesis of PLGA is commonly achieved either through ring-opening polymerization reactions of lactide and glycolide [22,23] or through polycondensation reactions of lactic acid and glycolic acid to form PLA and PGA block polymers [24,25]. Ring-opening polymerization processes can be used to generate high-molecular-weight PLGA polymers [26], while polycondensation processes are more suitable for the synthesis of low-molecular-weight polymers [27].
3.2. Physicochemical and Biomolecular Characteristics of PLGA
PLA can exist in d- or l-lactic acid, as well as in d,l-lactic acid, configurations. Homo isomeric PLAs are more crystalline due to the uniform spatial arrangement leading to tighter packing of the polymer chains [28,29]. On the other hand, glycolic acid has no asymmetric carbon; thus, PGA exists only in a highly crystalline form. PLA is more hydrophobic than PGA due to its methyl side groups. As a result, the hydrophobicity and crystallinity of PLGA can be controlled through the ratio of lactide to glycolide. PLGA physicochemical properties, such as mechanical strength, solubility, rate of hydration, rate of hydrolysis, and glass transition temperature (Tg), are heavily influenced by crystallinity and hydrophobicity [30,31,32]. PLGA with a high degree of crystallinity will have a higher Tg and mechanical strength, as well as a decreased rate of hydration and hydrolysis.
In general, PLGA NPs are susceptible to clearance by the reticuloendothelial system (RES) through opsonin-mediated phagocytosis [33]. RES elimination and biodistribution of PLGA NPs depend on size, hydrophobicity, and surface charge. Cytotoxicity of PLGA NPs was investigated in vitro by monitoring the cell viability of Caco-2 and HeLa cell lines [34,35]. The study indicates that PLGA NPs at the concentration tested were not toxic to cells as both cell lines retained over 75% viability. According to histopathology assays, orally administered PLGA nanoparticles did not elicit adverse effects in mice [35]. Tissue distribution analysis in mice indicated that most of the PLGA NPs were detected in the liver, kidney, heart, and brain, with small amounts detected in plasma [35]. In aqueous conditions, PLGA undergoes hydrolysis of its ester bonds. Hydrolytic biodegradation of PLGA leads to nontoxic byproducts [36,37]. Several studies have investigated the factors that affect PLGA biodegradation, including intrinsic properties such as hydration rate, hydrophobicity/hydrophilicity, polymer chemical composition, molecular weight, crystallinity, and Tg [38]. Moreover, external factors such as pH and chemical additives were also shown to influence hydrolytic degradation [39].
4. PLGA NPs as a Brain Drug Delivery System
PLGA is a highly investigated polymer due to its ability to form NPs, micelles, and microspheres, as it possesses the properties of biocompatibility, biodegradability, and tolerability [40]. As drug delivery systems, PLGA NPs can be used to prepare controlled-release dosage forms of small-molecule drugs, peptides, and nucleic acids [41]. Through proper copolymerization with PEG and surface modifications with linkers, PLGA NPs have been demonstrated as promising carriers for drug delivery across the BBB.
4.1. PLGA NPs Modifications and Mechanisms
PLGA NPs can be prepared through various processing methods including (1) double-emulsion solvent evaporation [42], (2) single-emulsion solvent evaporation [43], (3) phase separation [44], (4) spray-drying [45,46,47], (5) salting out [48,49], and (6) nanoprecipitation [50,51]. Even though the names are different, processing methods focus on the self-assembly characteristics of PLGA in aqueous solutions to finish the drug encapsulation [52,53,54]. Details of processing methods and potential scale-up technologies are discussed in Section 5.
PLGA NPs can cross the BBB passively or through active endocytosis mechanisms as shown in Figure 1. Unmodified PLGA NPs cross the BBB primarily through passive internalization based on size, which was found to have low brain uptake. Several strategies have been developed to improve the penetration of NPs into the brain. These strategies modify NPs with components designed to take advantage of BBB endocytosis pathways. Modified PLGA NPs have been designed to cross the BBB through adsorption-mediated transcytosis (AMT) [55], carrier-mediated transport (CMT), and receptor-mediated transcytosis (RMT) [56].
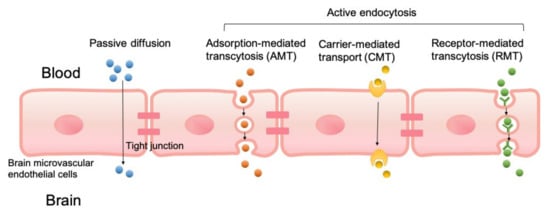
Figure 1.
Transport mechanisms of poly(lactic-co-glycolic acid) (PLGA) nanoparticles (NPs) to cross the blood–brain barrier (BBB), including passive diffusion, adsorption-mediated transcytosis (AMT), carrier-mediated transport (CMT), and receptor-mediated transcytosis (RMT).
PLGA NP surfaces are modified with positive charges that electrostatically interact with negatively charged regions of the luminal surfaces, which helps PLGA to cross the BBB. Several cationic modifications of PLGA NPs have been demonstrated to utilize the AMT concepts to improve brain uptake. In CMT systems, PLGA NPs are modified with membrane-permeable molecules such as amino acids, nutrients, and membranotropic peptides, and they are able to transport cargo across the BBB endothelium. CMT systems also include designs that take advantage of ABC transporters. With RMT, PLGA NPs are modified or covalently connected with ligands that target specific cell surface receptors known to be BBB transport pathways. A search of the PubMed database with the keywords “PLGA nanoparticles”, “BBB”, and “drug delivery” from 2015 to date returned 133 publications. From the search results, only research articles that included BBB permeability studies of control “unmodified” PLGA NPs and modified PLGA NPs are summarized in Table 1. Tandem systems that utilized multiple modifications have been reported. Guarnieri et al. [57] demonstrated the cooperative effects of glycoprotein H 625, a CMT modification with iron-mimicking protein CRT and RMT modification, in enhancing PLGA NP permeation of BBB. Liu et al. [58] developed a PLGA NP drug delivery system modified with angiopep-2 (RMT) and 1, 2-Dioleoyl-3-trimethylammonium-propane (AMT), for gefitinib and Golgi phosphoprotein 3 for the treatment of glioblastoma. Intranasal [59,60,61] or subcutaneous [62] administration of PLGA NP drug systems can bypass the BBB and avoid issues associated with systemic administration.

Table 1.
Summary of BBB permeability studies of unmodified and modified PLGA NPs.
4.2. PLGA–PEG Co-Polymeric NPs, Modifications, and Applications
To overcome its short half-life, PLGA is combined with polyethylene glycol (PEG) to form PLGA–PEG copolymer NPs [63,64,65]. The PLGA–PEG copolymer is widely used in pharmaceutical products and devices. Recently, several advancements have been made to modify the surface of the PLGA–PEG NPs to further increase their ability to cross the BBB and deliver drugs into the brain.
The favorable chemistry of the PLGA–PEG NPs makes them amenable for conjugation with various peptides and linkers for use in the treatment of various neurodegenerative diseases and glioma. Memantine is commonly used for the treatment of mild and moderate AD. Encapsulating it in PLGA–PEG NPs using a double-emulsion method increased the delivery to the target tissue with ameliorated pathological markers compared to free memantine [66].
Pioglitazone-loaded PLGA–PEG NPs produced by the solvent displacement technique reduced amyloid burden and decreased memory impairment by increasing the rate of transcytosis across BBB and slowly releasing pioglitazone in the target tissue [78]. Selegiline- or donepezil-loaded PLGA–PEG NPs produced by the solvent evaporation method destabilized the beta-amyloid formation in vitro [79,80]. Various natural compounds and drugs encapsulated in PLGA–PEG NPs were shown to be effective in reducing AD pathology in in vitro models [81]. The antinociceptive effect of loperamide was increased by two- to threefold by encapsulation in a PLGA–PEG–PLGA triblock polymeric NPs coated with poloxamer 188 or polysorbate 80 compared to unmodified NPs alone [82]. With peptides as a modification on PLGA–PEG NPs, Hoyos-Ceballos et al. [35] showed that angiopep-2 conjugated to PLGA–PEG NPs increased their ability to cross the BBB in C57/BL6 mice. PLGA–PEG NPs conjugated with B6 peptide increased the delivery of curcumin into the CNS in an AD mouse model, showing a reduced expression of hallmark AD pathological markers, including amyloid-beta, presenilin-1, phosphorylated tau, and beta-secretase 1, compared to curcumin alone or NPs without B6 peptide [83].
Surnar et al. [84] added a targeting function to PLGA–PEG NPs through conjugating with a lipophilic triphenylphosphonium cation on the surface using a butylene linker. This NP system, when loaded with either coenzyme Q10 or aspirin, was able to cross the mitochondrial double membrane in endothelial cells and astrocytes to reduce the oxidative stress, which is extremely valuable to treat HAND [84]. Yu et al. [85] optimized the development of PLGA–PEG polymersomes conjugated on the surface with lactoferrin as a brain targeted delivery system for peptides. They loaded the NPs with S14G-humanin peptides which exerted a protective effect by decreasing the caspase-3 and bax expression in the rat hippocampus neurons treated with amyloid-beta. Similarly, Bi et al. [72] showed that PLGA–PEG NPs modified with lactoferrin on their surface were able to deliver rotigotine into the striatum by intranasal administration for potential use in Parkinson’s disease (PD). Lectin-conjugated PLGA–PEG NPs were able to deliver the basic fibroblast growth factor peptide cargo across the BBB after intranasal administration [86]. Similarly, odrranalectin-conjugated PLGA–PEG NPs were able to efficiently deliver the encapsulated urocortin peptide into the brain as a treatment for PD [86]. Recently, Amanda et al. [87] showed that a modified PLGA–PEG NP system encapsulating epigallocatechin gallate was able to ameliorate neurological deficits induced by 3-nitropropionic acid in Huntington’s disease mouse model.
Glioma is an invasive carcinoma of the brain with an average life expectancy of approximately 12–14 months and has poor survival rates [88]. NP systems were used to deliver drug into the brain. Receptor-mediated transcytosis provides a chance to target specific receptors expressed on the surface of cancer cells through ligand–receptor interactions. Cui and coworkers [89] developed a novel dual-targeting PLGA–PEG-based magnetic NP system that crosses the BBB by conjugating transferrin receptor-binding peptide T7 on the surface and encapsulating curcumin and paclitaxel into the NP hydrophobic core. Compared to free drugs, the mice with orthotopic glioma survived with the NP system. Similarly, doxorubicin and tetrahydrocurcumin encapsulated in transferrin-modified PLGA–PEG NPs showed effectiveness in reducing the glioma tumor volume in combination with radiotherapy [90]. Lactoferrin-conjugated PLGA–PEG NPs increased the brain concentrations of shikonin, a naphthoquinone pigment for the potential use in the treatment of glioma [91]. The iNGR-conjugated PLGA–PEG-based NP system designed to target the glioma tumor vessel was effective in delivering the paclitaxel to the glioma parenchyma. Moreover, this NP system was able to travel deeper into the glioma to increase survival rates [92]. Farnesyl thiosalicylic acid, an inhibitor of Ras oncoprotein, was shown to be effective against glioblastoma when administered as PLGA–PEG-based hybrid NPs, which contained 1,2-distearoyl-glycerol-3-phosphoethanolamine and 1,2-dioleoyl-3-trimethylammonium-propane [93].
Another strategy to treat glioma is to target the genes overexpressed in malignant glioma cells. Cyclic hexapeptide-conjugated PLGA–PEG NPs were able to deliver curcumin to the glioma by binding to integrins that are upregulated on the glial cell surface [94]. A nine amino acid linear peptide (Pep-1) targeting the interleukin 13 receptor α2 overexpressed on the surface of gliomas was conjugated to PLGA–PEG NPs [95]. Modification with CGKRK peptide, which targets the heparan sulfate expressed on neovascular endothelial cells, resulted in a dual-targeted approach and increased the median survival time in intracranial glioma mice [96]. A biodegradable PLGA–PEG polymer NP system targeting the Fn14 receptor overexpressed on the brain tumor cells was generated by conjugating PLGA–PEG NPs with ITEM4 monoclonal antibody. The half-life of these NPs was more than doubled compared to nontargeted PLGA–PEG NPs [97].
4.3. PLGA NPs for Theranostic Applications
Theranostic represents a novel and powerful emerging platform that integrates targeted therapeutic entities with noninvasive imaging and has the great potential to personalize and advance medicine. Several nanosized delivery vehicles, including gold and iron-oxide nanoparticles, as well as quantum dots, have been extensively studied for theranostic applications [98,99,100]. Given safety concerns, off-target effects, and slow excretion kinetics from the body, risks may limit its use in the course of diseases. PLGA NP-based theranostic applications can deliver a therapeutic agent while simultaneously monitoring therapy response in real time [101,102]. Contrast agents, such as the radionuclide or fluorophore, play a critical role in enabling visualization of a target with conventional imaging techniques, e.g., magnetic resonance imaging (MRI), optical imaging, and X-ray computed tomography. Contrast agents including superparamagnetic iron oxide (SPIO) and gadolinium were shown to be encapsulated with polymeric nanoparticles [103]. For example, SPIO and chemotherapy drug docetaxel can both be directly encapsulated with PLGA [104]. Similarly, an human epidermal growth factor receptor 2 (HER2)-targeted PLGA–PEG block copolymer nanoparticle, upon encapsulation with MnFe2O4 and doxorubicin, was designed to target breast cancer in vivo [105]. Encapsulation of anticancer drug N′-(2-Methoxybenzylidene)-3-methyl-1-phenyl-H-Thieno[2,3-c]Pyrazole-5-Carbohyd-razide (MTPC) with plasmonic gold nanorods in PLGA-b-PEG polymeric nanospheres enhanced both biodistribution and pharmacokinetics of the MTPC in tumor-bearing mice [106]. Similar to MRI, radionuclide imaging has high sensitivity with no tissue-penetration limitations. Several radionuclide compounds have been extensively studied along with PLGA with the goal of formulating a robust nano delivery system [107]. For instance, Wang and colleagues [108] designed PLGA-lipid hybrid nanocarriers for theranostic therapy, encapsulating an anticancer agent in the matrix, while the lipid shell was chelated with indium-111 or yttrium-90 as radiotherapy agents. Shao et al. [107] demonstrated the therapeutic effect of 32P-CP-PLGA brachytherapy for glioma with the integrin αvβ3-targeted radiotracer 68Ga-3PRGD2. Press and colleagues [109] demonstrated the cell-type-specific delivery of short interfering RNAs by covalent conjugation of DY-635 fluorescent dye with known hepatobiliary clearance to a PLGA, which allowed them to monitor distribution, uptake, and clearance of short hairpin RNA from the target organ. The major hurdle for the treatment of neurodegenerative diseases is to design therapeutic molecules in a way that it can cross the BBB. Zhang et al. [86] showed that the lectin-modified PLGA nanoparticle encapsulated with basic fibroblast growth factor enhanced drug delivery to the brain, supporting the role of the PLGA NP-based drug delivery system for CNS disorders. Therefore, nanocarriers based on PLGA offer biocompatibility, good stability, and regulated drug release rate and represent an excellent and emerging platform in theranostic medicine.
5. From Research and Development (R&D) to cGMP: Technologies for Scale-Up
To promote promising formulations from the R&D stage to clinical trials, drug products need to be manufactured under certain guidelines. Current Good Manufacturing Practice (cGMP) are regulations enforced by the FDA to ensure the quality of pharmaceutical products [110]. Most PLGA NP-based pharmaceutical products or medical devices are either injectable or implantable [111]. Therefore, sterility and potency are among the top-quality aspects to be considered for PLGA NPs. Per cGMP regulations, sterile products must be manufactured in a registered sterile facility [19]. Furthermore, facilities need to show that a sterile environment is properly maintained with validated sanitization [112]. PLGA NP-based pharmaceutical products have several steps during production: (1) dissolution and mixture of the active pharmaceutical ingredient (API) and PLGA; (2) stabilization of the mixture and removal of organic solvents; (3) separation of free API from PLGA–API product; (4) sterilization; (5) fill-finish. Table 2 presents selected publications showing processing details in the R&D stage.

Table 2.
Processing details for PLGA NP-based systems from publications.
For scale-up, dissolution of API/PLGA is achieved using stainless-steel tanks with temperature control through double jacketing. Mechanical motors are built into most tanks for light agitation. Since PLGA is not water-soluble, while lipophilic APIs can be dissolved concurrently, hydrophilic ones need to be dissolved separately from PLGA. To stabilize the formulation, PVA is mostly used as a surfactant. Moreover, poloxamer (also named Pluronic), polysorbate, sodium cholate, and D-α-tocopheryl pol-yethylene glycol succinate (TPGS) are also reported as promising alternatives. Subsequently, aggressive agitation is needed to decrease the mixture droplets’ particle size. High-pressure homogenization is widely used in cGMP production, and this technology is also reported with details in Table 2. To remove organic solvents, most publications took advantage of the different boiling points in water and organic solvents through overnight stirring. While this method is useful on the R&D scale, GMP-scale production uses vacuum-assisted rotary evaporation, which is more efficient and easier to validate.
Since PLGA NP-based pharmaceutical products represent a self-assembly drug delivery system, it is critical to separate free APIs from encapsulated ones. The encapsulation rate is one key quality control aspect due to the concern of toxicity. In Table 2, most publications used centrifugation, assisted by filters and membranes. In cGMP production, diafiltration is the technology preferred due to its higher recovery rate and better cost-effectiveness compared to centrifugation. Diafiltration uses a semipermeable membrane to separate the free drug from encapsulated drug based on the difference in cutoff molecular weight. Furthermore, diafiltration can form a closed system to return encapsulated products to the container for reprocessing until a target encapsulation rate is reached with the highest yield [113].
Among popular terminal sterilization methods, steam sterilization (autoclave) and gamma irradiation were reported to cause degradation of PLGA [114]. Vaporized ethylene oxide is commonly used for sterile gowning materials, but its residual is toxic if injected [115]. Electron beam technology is less aggressive than gamma irradiation but can still cause degradation of PLGA [116]. According to the available technologies for cGMP production and the particle size distribution reported from literature, sterile filtration is the best option for PLGA NP-based products. However, a filter compatibility study needs to be performed for validation purposes to minimize API retaining during filtration.
Depending on the concentration of PLGA NP-based pharmaceutical products, the solution may be free-flowing or with high viscosity. High viscosity is a problem for both sterile filtration and automatic filling systems. Filters may be clotted during filtration, the filling accuracy may be compromised, and rejection of vials may occur frequently. Therefore, an engineering fill-finish of placebo is strongly recommended as an industry standard for PLGA NP-based drug products. According to the stability data, lyophilization is recommended for extended shelf life. As presented in Table 2, lyophilization was reported using sugar molecules as cryoprotection. In cGMP production, lyophilization is considered a sterile product and is performed in the last step. The difficult part of lyophilization is the programming of cycles to minimize the moisture content (<5%, w/w).
Figure 2 is a concept flowchart for cGMP production of PLGA NP-based pharmaceutical products. The steps in Figure 2 are provided on the basis of the details in Table 2 and the industry standards under cGMP guidelines.
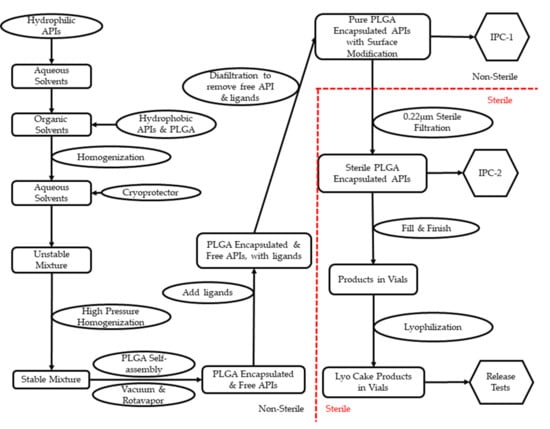
Figure 2.
Concept flowchart of cGMP operations for PLGA NP-based drug products.
6. Quality and Regulatory Assurance
The quality of nano-based pharmaceutical products is a learning curve for both manufacturers and regulatory agencies. In 2006, the FDA initialed the Nanotechnology Task Force to improve the service for nano-based products. After its first public report in 2007, FDA published the second report in 2020, emphasizing its commitment to nano-based products. Among the five published guidance for industry regarding nanomaterials, two of them were designed for pharmaceutical and medical products. Another draft guidance is under review for drug products and biological products. On the basis of the above documents, FDA will not accept or reject nanotechnology products according to category. Instead, FDA will conduct a comprehensive review to make a science-focused decision.
Size distribution is the FDA’s first consideration among all factors. Dynamic light scattering (DLS) technology is widely used for quality control purposes. Furthermore, a chromatography-based assay of APIs and encapsulation rate is critical to avoid toxicity. United States Pharmacopeia (USP) monograph “Goserelin Implants” also requests HPLC results to provide the retention time of PLGA, which is not rare for quality control of polymers [128]. Table 3 is a summary of the most needed in-process tests and final release tests.

Table 3.
In-process and release tests for PLGA NP-based drug products.
During the Covid-19 pandemic, several liposome-based vaccines were approved for emergency use [129]. Liposome-based pharmaceutical products have similar self-assembly characteristics compared to PLGA NP-based ones. Therefore, their quality and regulatory focus should be similar. Furthermore, the success of vaccines also revealed the possibility of PLGA NP-based products to be approved through facilitated regulatory pathways, especially for emergency purposes under global regulatory systems [130,131].
7. Conclusions
This review presented recent progress in PLGA NPs as a vehicle to deliver drug to the brain in a controllable and targeted manner. Unlike most NPs, PLGA NPs show a promising future to become a clinically and commercially feasible drug delivery system. PLGA has been approved for both pharmaceutical products and medical devices, which provides clear quality control, quality assurance, and regulatory requirements for future products. Furthermore, the improvement of cGMP technologies, especially those in nanomedicines and sterile injectables, has also removed most obstacles for PLGA NPs. Even though safety is not the major concern, clinical trials are needed to monitor the efficacy and toxicity of PLGA NPs. Uncertainties, such as drug encapsulation rate, assembly stability, particle size distribution stability, and in vivo pharmacokinetics, may be the focus for future research and development.
Author Contributions
All the authors contributed to writing and/or editing the manuscripts.
Funding
The authors acknowledge financial support from the Plough Center for Sterile Drug Delivery Solutions and National Institutes of Health (CA213232).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Pardridge, W.M. Alzheimer’s disease drug development and the problem of the blood-brain barrier. Alzheimers Dement. 2009, 5, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Zepeda, D.; Taghi, M.; Scherrmann, J.M.; Decleves, X.; Menet, M.C. ABC Transporters at the Blood-Brain Interfaces, Their Study Models, and Drug Delivery Implications in Gliomas. Pharmaceutics 2019, 12, 20. [Google Scholar] [CrossRef]
- Wong, H.L.; Wu, X.Y.; Bendayan, R. Nanotechnological advances for the delivery of CNS therapeutics. Adv. Drug Deliv. Rev. 2012, 64, 686–700. [Google Scholar] [CrossRef]
- Bertrand, L.; Nair, M.; Toborek, M. Solving the Blood-Brain Barrier Challenge for the Effective Treatment of HIV Replication in the Central Nervous System. Curr. Pharm. Des. 2016, 22, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2005, 2, 86–98. [Google Scholar] [CrossRef]
- Mahringer, A.; Ott, M.; Reimold, I.; Reichel, V.; Fricker, G. The ABC of the blood-brain barrier—Regulation of drug efflux pumps. Curr. Pharm. Des. 2011, 17, 2762–2770. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Saylor, D.; Dickens, A.M.; Sacktor, N.; Haughey, N.; Slusher, B.; Pletnikov, M.; Mankowski, J.L.; Brown, A.; Volsky, D.J.; McArthur, J.C. HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat. Rev. Neurol. 2016, 12, 234–248. [Google Scholar] [CrossRef]
- Decloedt, E.H.; Rosenkranz, B.; Maartens, G.; Joska, J. Central nervous system penetration of antiretroviral drugs: Pharmacokinetic, pharmacodynamic and pharmacogenomic considerations. Clin. Pharm. 2015, 54, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.W.; Johnson, B.; Nicotera, J.; Bailey, V.L.; Harris, V.L.; Bowles, F.B.; Raffanti, S.; Schranz, J.; Finn, T.S.; Saah, A.J.; et al. Effects of ritonavir on indinavir pharmacokinetics in cerebrospinal fluid and plasma. Antimicrob. Agents Chemother. 2003, 47, 2131–2137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nair, M.; Jayant, R.D.; Kaushik, A.; Sagar, V. Getting into the brain: Potential of nanotechnology in the management of NeuroAIDS. Adv. Drug Deliv. Rev. 2016, 103, 202–217. [Google Scholar] [CrossRef]
- Haluska, M.; Anthony, M.L. Osmotic blood-brain barrier modification for the treatment of malignant brain tumors. Clin. J. Oncol. Nurs. 2004, 8, 263–267. [Google Scholar] [CrossRef]
- Zhi, K.; Lebo, D.B. A preformulation strategy for the selection of controlled-release components to simulate a subcutaneous implant. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2020, 19, 344–356. [Google Scholar] [CrossRef]
- Gong, Y.; Chowdhury, P.; Nagesh, P.K.B.; Rahman, M.A.; Zhi, K.; Yallapu, M.M.; Kumar, S. Novel elvitegravir nanoformulation for drug delivery across the blood-brain barrier to achieve HIV-1 suppression in the CNS macrophages. Sci. Rep. 2020, 10, 3835. [Google Scholar] [CrossRef]
- Gong, Y.; Zhi, K.; Nagesh, P.K.B.; Sinha, N.; Chowdhury, P.; Chen, H.; Gorantla, S.; Yallapu, M.M.; Kumar, S. An Elvitegravir Nanoformulation Crosses the Blood-Brain Barrier and Suppresses HIV-1 Replication in Microglia. Viruses 2020, 12, 564. [Google Scholar] [CrossRef]
- Patel, T.; Zhou, J.; Piepmeier, J.M.; Saltzman, W.M. Polymeric nanoparticles for drug delivery to the central nervous system. Adv. Drug. Deliv. Rev. 2012, 64, 701–705. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control Release 2018, 270, 290–303. [Google Scholar] [CrossRef]
- Zhi, K.; Kumar, A.; Raji, B.; Kochat, H.; Kumar, S. Formulation, manufacturing and regulatory strategies for extracellular vesicles-based drug products for targeted therapy of central nervous system diseases. Expert Rev. Precis. Med. Drug Dev. 2020, 5, 469–481. [Google Scholar] [CrossRef]
- Kumar, S.; Zhi, K.; Mukherji, A.; Gerth, K. Repurposing antiviral protease inhibitors using extracellular vesicles for potential therapy of COVID-19. Viruses 2020, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zhou, L.; Zhi, K.; Raji, B.; Pernell, S.; Tadrous, E.; Kodidela, S.; Nookala, A.; Kochat, H.; Kumar, S. Challenges in Biomaterial-Based Drug Delivery Approach for the Treatment of Neurodegenerative Diseases: Opportunities for Extracellular Vesicles. Int. J. Mol. Sci. 2020, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Gilding, D.K.; Reed, A.M. Biodegradable polymers for use in surgery—polyglycolic/poly(actic acid) homo- and copolymers: 1. Polymer 1979, 20, 1459–1464. [Google Scholar] [CrossRef]
- Deasy, P.B.; Finan, M.P.; Meegan, M.J. Preparation and characterization of lactic/glycolic acid polymers and copolymers. J. Microencapsul. 1989, 6, 369–378. [Google Scholar] [CrossRef]
- Gao, Q.; Lan, P.; Shao, H.; Hu, X. Direct Synthesis with Melt Polycondensation and Microstructure Analysis of Poly(L-lactic acid-co-glycolic acid). Polym. J. 2002, 34, 786–793. [Google Scholar] [CrossRef]
- Fukuzaki, H.; Yoshida, M.; Asano, M.; Kumakura, M. Synthesis of copoly(d,l-lactic acid) with relatively low molecular weight and in vitro degradation. Eur. Polym. J. 1989, 25, 1019–1026. [Google Scholar] [CrossRef]
- Bendix, D. Chemical synthesis of polylactide and its copolymers for medical applications. Polym. Degrad. Stab. 1998, 59, 129–135. [Google Scholar] [CrossRef]
- Lunt, J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym. Degrad. Stab. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Sarasua, J.-R.; Prud’homme, R.E.; Wisniewski, M.; Le Borgne, A.; Spassky, N. Crystallization and Melting Behavior of Polylactides. Macromolecules 1998, 31, 3895–3905. [Google Scholar] [CrossRef]
- Sarasua, J.R.; López-Rodríguez, N.; Zuza, E.; Petisco, S.; Castro, B.; del Olmo, M.; Palomares, T.; Alonso-Varona, A. Crystallinity assessment and in vitro cytotoxicity of polylactide scaffolds for biomedical applications. J. Mater. Sci. Mater. Med. 2011, 22, 2513–2523. [Google Scholar] [CrossRef]
- Tsuji, H.; Miyauchi, S. Poly(l-lactide): VI Effects of crystallinity on enzymatic hydrolysis of poly(l-lactide) without free amorphous region. Polym. Degrad. Stab. 2001, 71, 415–424. [Google Scholar] [CrossRef]
- Wang, N.; Wu, X.S.; Li, C.; Feng, M.F. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers: I. Synthesis and characterization. J. Biomater. Sci. Polym. Ed. 2000, 11, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.E., 3rd; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Hoyos-Ceballos, G.P.; Ruozi, B.; Ottonelli, I.; Da Ros, F.; Vandelli, M.A.; Forni, F.; Daini, E.; Vilella, A.; Zoli, M.; Tosi, G.; et al. PLGA-PEG-ANG-2 Nanoparticles for Blood-Brain Barrier Crossing: Proof-of-Concept Study. Pharmaceutics 2020, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Erbetta, C.D.A.C.; Alves, R.J.; Magalh, J.; de Souza Freitas, R.F.; de Sousa, R.G. Synthesis and characterization of poly (D, L-lactide-co-glycolide) copolymer. J. Biomater. Nanobiotechnol. 2012, 3, 208–225. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.S.; Wang, N. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers. Part II: Biodegradation. J. Biomater. Sci. Polym. Ed. 2001, 12, 21–34. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Locatelli, E.; Franchini, M.C. Biodegradable PLGA-b-PEG polymeric nanoparticles: Synthesis, properties, and nanomedical applications as drug delivery system. J. Nanoparticle Res. 2012, 14, 1–17. [Google Scholar] [CrossRef]
- Gong, Y.; Chowdhury, P.; Midde, N.M.; Rahman, M.A.; Yallapu, M.M.; Kumar, S. Novel elvitegravir nanoformulation approach to suppress the viral load in HIV-infected macrophages. Biochem. Biophys. Rep. 2017, 12, 214–219. [Google Scholar] [CrossRef]
- Kluge, J.; Fusaro, F.; Casas, N.; Mazzotti, M.; Muhrer, G. Production of PLGA micro- and nanocomposites by supercritical fluid extraction of emulsions: I. Encapsulation of lysozyme. J. Supercrit. Fluids 2009, 50, 327–335. [Google Scholar] [CrossRef]
- Arshady, R. Preparation of biodegradable microspheres and microcapsules: 2. Polyactides and related polyesters. J. Control. Release 1991, 17, 1–21. [Google Scholar] [CrossRef]
- Edelman, R.; Russell, R.G.; Losonsky, G.; Tall, B.D.; Tacket, C.O.; Levine, M.M.; Lewis, D.H. Immunization of rabbits with enterotoxigenic E. coli colonization factor antigen (CFA/I) encapsulated in biodegradable microspheres of poly (lactide-co-glycolide). Vaccine 1993, 11, 155–158. [Google Scholar] [CrossRef]
- Mu, L.; Feng, S.S. Fabrication, characterization and in vitro release of paclitaxel (Taxol) loaded poly (lactic-co-glycolic acid) microspheres prepared by spray drying technique with lipid/cholesterol emulsifiers. J. Control Release 2001, 76, 239–254. [Google Scholar] [CrossRef]
- Jensen, D.M.; Cun, D.; Maltesen, M.J.; Frokjaer, S.; Nielsen, H.M.; Foged, C. Spray drying of siRNA-containing PLGA nanoparticles intended for inhalation. J. Control Release 2010, 142, 138–145. [Google Scholar] [CrossRef]
- Arpagaus, C. PLA/PLGA nanoparticles prepared by nano spray drying. J. Pharm. Investig. 2019, 49, 405–426. [Google Scholar] [CrossRef]
- Ibrahim, H.; Bindschaedler, C.; Doelker, E.; Buri, P.; Gurny, R. Aqueous nanodispersions prepared by a salting-out process. Int. J. Pharm. 1992, 87, 239–246. [Google Scholar] [CrossRef]
- Allémann, E.; Gurny, R.; Doelker, E. Preparation of aqueous polymeric nanodispersions by a reversible salting-out process: Influence of process parameters on particle size. Int. J. Pharm. 1992, 87, 247–253. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, C. Tuning the Size of Poly(lactic-co-glycolic Acid) (PLGA) Nanoparticles Fabricated by Nanoprecipitation. Biotechnol. J. 2018, 13, 1700203. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Astete, C.E.; Sabliov, C.M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289. [Google Scholar] [CrossRef]
- Ding, D.; Zhu, Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 1041–1060. [Google Scholar] [CrossRef]
- Hervé, F.; Ghinea, N.; Scherrmann, J.M. CNS delivery via adsorptive transcytosis. AAPS J. 2008, 10, 455–472. [Google Scholar] [CrossRef]
- Pulgar, V.M. Transcytosis to Cross the Blood Brain Barrier, New Advancements and Challenges. Front. Neurosci. 2018, 12, 1019. [Google Scholar] [CrossRef]
- Falanga, A.P.; Melone, P.; Cagliani, R.; Borbone, N.; D’Errico, S.; Piccialli, G.; Netti, P.A.; Guarnieri, D. Design, Synthesis and Characterization of Novel Co-Polymers Decorated with Peptides for the Selective Nanoparticle Transport across the Cerebral Endothelium. Molecules 2018, 23, 1655. [Google Scholar] [CrossRef]
- Ye, C.; Pan, B.; Xu, H.; Zhao, Z.; Shen, J.; Lu, J.; Yu, R.; Liu, H. Co-delivery of GOLPH3 siRNA and gefitinib by cationic lipid-PLGA nanoparticles improves EGFR-targeted therapy for glioma. J. Mol. Med. 2019, 97, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, J.; Kamal, Z.; Guo, P.; Wu, X.; Lu, L.; Wu, H.; Qiu, M. Odorranalectin modified PEG-PLGA/PEG-PBLG curcumin-loaded nanoparticle for intranasal administration. Drug Dev. Ind. Pharm. 2020, 46, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Sarolia, J.; Vyas, B.; Wagh, P.; Ankur, K.; Kumar, M.A. PLGA nanoparticles for nose to brain delivery of Clonazepam: Formulation, optimization by 32 Factorial design, in vitro and in vivo evaluation. Curr. Drug Deliv. 2020. [Google Scholar] [CrossRef]
- Chatzitaki, A.T.; Jesus, S.; Karavasili, C.; Andreadis, D.; Fatouros, D.G.; Borges, O. Chitosan-coated PLGA nanoparticles for the nasal delivery of ropinirole hydrochloride: In vitro and ex vivo evaluation of efficacy and safety. Int. J. Pharm. 2020, 589, 119776. [Google Scholar] [CrossRef]
- Zhao, P.; Le, Z.; Liu, L.; Chen, Y. Therapeutic Delivery to the Brain via the Lymphatic Vasculature. Nano Lett. 2020, 20, 5415–5420. [Google Scholar] [CrossRef]
- Jusu, S.M.; Obayemi, J.D.; Salifu, A.A.; Nwazojie, C.C.; Uzonwanne, V.; Odusanya, O.S.; Soboyejo, W.O. Drug-encapsulated blend of PLGA-PEG microspheres: In vitro and in vivo study of the effects of localized/targeted drug delivery on the treatment of triple-negative breast cancer. Sci. Rep. 2020, 10, 14188. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG-PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control Release 2014, 183, 77–86. [Google Scholar] [CrossRef]
- Rafiei, P.; Haddadi, A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: Pharmacokinetics and biodistribution profile. Int. J. Nanomed. 2017, 12, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C.; Camins, A.; Carmona, N.; Silva, A.M.; Souto, E.B.; et al. Memantine loaded PLGA PEGylated nanoparticles for Alzheimer’s disease: In vitro and in vivo characterization. J. Nanobiotechnol. 2018, 16, 32. [Google Scholar] [CrossRef]
- Wang, Z.H.; Wang, Z.Y.; Sun, C.S.; Wang, C.Y.; Jiang, T.Y.; Wang, S.L. Trimethylated chitosan-conjugated PLGA nanoparticles for the delivery of drugs to the brain. Biomaterials 2010, 31, 908–915. [Google Scholar] [CrossRef]
- Wang, L.; Hao, Y.; Li, H.; Zhao, Y.; Meng, D.; Li, D.; Shi, J.; Zhang, H.; Zhang, Z.; Zhang, Y. Co-delivery of doxorubicin and siRNA for glioma therapy by a brain targeting system: Angiopep-2-modified poly(lactic-co-glycolic acid) nanoparticles. J. Drug Target 2015, 23, 832–846. [Google Scholar] [CrossRef]
- Fornaguera, C.; Dols-Perez, A.; Calderó, G.; García-Celma, M.J.; Camarasa, J.; Solans, C. PLGA nanoparticles prepared by nano-emulsion templating using low-energy methods as efficient nanocarriers for drug delivery across the blood-brain barrier. J. Control Release 2015, 211, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Chen, Y.C. Targeting delivery of etoposide to inhibit the growth of human glioblastoma multiforme using lactoferrin- and folic acid-grafted poly(lactide-co-glycolide) nanoparticles. Int. J. Pharm. 2015, 479, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.A.; Gomes, B.; Fricker, G.; Coelho, M.A.N.; Rocha, S.; Pereira, M.C. Cellular uptake of PLGA nanoparticles targeted with anti-amyloid and anti-transferrin receptor antibodies for Alzheimer’s disease treatment. Colloids Surf. B Biointerfaces 2016, 145, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Wang, A.; Chu, Y.; Liu, S.; Mu, H.; Liu, W.; Wu, Z.; Sun, K.; Li, Y. Intranasal delivery of rotigotine to the brain with lactoferrin-modified PEG-PLGA nanoparticles for Parkinson’s disease treatment. Int. J. Nanomed. 2016, 11, 6547–6559. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, X.; Mu, H.; Meng, Q.; Jiang, Y.; Wang, Y.; Lu, X.; Wang, A.; Liu, S.; Zhang, Y.; et al. RVG29-modified docetaxel-loaded nanoparticles for brain-targeted glioma therapy. Int. J. Pharm. 2018, 543, 179–189. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Sevin, E.; Gosselet, F.; Lima, J.; Coelho, M.A.N.; Loureiro, J.A.; Pereira, M.C. Receptor-mediated PLGA nanoparticles for glioblastoma multiforme treatment. Int. J. Pharm. 2018, 545, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Muniswamy, V.J.; Raval, N.; Gondaliya, P.; Tambe, V.; Kalia, K.; Tekade, R.K. ‘Dendrimer-Cationized-Albumin’ encrusted polymeric nanoparticle improves BBB penetration and anticancer activity of doxorubicin. Int. J. Pharm. 2019, 555, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Davoodi, P.; Zhan, W.; Chow, P.K.; Wang, C.H. Development of Nanoparticles for Drug Delivery to Brain Tumor: The Effect of Surface Materials on Penetration Into Brain Tissue. J. Pharm. Sci. 2019, 108, 1736–1745. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J.; Xu, J.; Song, X.; Huang, H.; Feng, Y.; Fu, C. Rhynchophylline Loaded-mPEG-PLGA Nanoparticles Coated with Tween-80 for Preliminary Study in Alzheimer’s Disease. Int. J. Nanomed. 2020, 15, 1149–1160. [Google Scholar] [CrossRef]
- Silva-Abreu, M.; Calpena, A.C.; Andrés-Benito, P.; Aso, E.; Romero, I.A.; Roig-Carles, D.; Gromnicova, R.; Espina, M.; Ferrer, I.; García, M.L.; et al. PPARγ agonist-loaded PLGA-PEG nanocarriers as a potential treatment for Alzheimer’s disease: In vitro and in vivo studies. Int. J. Nanomed. 2018, 13, 5577–5590. [Google Scholar] [CrossRef]
- Baysal, I.; Yabanoglu-Ciftci, S.; Tunc-Sarisozen, Y.; Ulubayram, K.; Ucar, G. Interaction of selegiline-loaded PLGA-b-PEG nanoparticles with beta-amyloid fibrils. J. Neural Transm. 2013, 120, 903–910. [Google Scholar] [CrossRef]
- Baysal, I.; Ucar, G.; Gultekinoglu, M.; Ulubayram, K.; Yabanoglu-Ciftci, S. Donepezil loaded PLGA-b-PEG nanoparticles: Their ability to induce destabilization of amyloid fibrils and to cross blood brain barrier in vitro. J. Neural Transm. 2017, 124, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.U.; Shah, S.A.; Badshah, H.; Khan, M.; Kim, M.O. Anthocyanins encapsulated by PLGA@PEG nanoparticles potentially improved its free radical scavenging capabilities via p38/JNK pathway against Aβ(1-42)-induced oxidative stress. J. Nanobiotechnology 2017, 15, 12. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsieh, W.Y.; Lee, W.F.; Zeng, D.T. Effects of surface modification of PLGA-PEG-PLGA nanoparticles on loperamide delivery efficiency across the blood-brain barrier. J. Biomater. Appl. 2013, 27, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zheng, Y.; Liu, X.; Fang, W.; Chen, X.; Liao, W.; Jing, X.; Lei, M.; Tao, E.; Ma, Q.; et al. Curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide for potential use in Alzheimer’s disease. Drug Deliv. 2018, 25, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Surnar, B.; Basu, U.; Banik, B.; Ahmad, A.; Marples, B.; Kolishetti, N.; Dhar, S. Nanotechnology-mediated crossing of two impermeable membranes to modulate the stars of the neurovascular unit for neuroprotection. Proc. Natl. Acad. Sci. USA 2018, 115, E12333–E12342. [Google Scholar] [CrossRef]
- Yu, Y.; Pang, Z.; Lu, W.; Yin, Q.; Gao, H.; Jiang, X. Self-assembled polymersomes conjugated with lactoferrin as novel drug carrier for brain delivery. Pharm. Res. 2012, 29, 83–96. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Feng, C.; Shao, X.; Liu, Q.; Zhang, Q.; Pang, Z.; Jiang, X. Intranasal nanoparticles of basic fibroblast growth factor for brain delivery to treat Alzheimer’s disease. Int. J. Pharm. 2014, 461, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Ettcheto, M.; Espina, M.; Auladell, C.; Folch, J.; Kühne, B.A.; Barenys, M.; Sánchez-López, E.; Souto, E.B.; García, M.L.; et al. Epigallocatechin-3-gallate PEGylated poly(lactic-co-glycolic) acid nanoparticles mitigate striatal pathology and motor deficits in 3-nitropropionic acid intoxicated mice. Nanomedicine 2021, 16, 19–35. [Google Scholar] [CrossRef]
- Price, R.L.; Chiocca, E.A. Evolution of malignant glioma treatment: From chemotherapy to vaccines to viruses. Neurosurgery 2014, 61 (Suppl. 1), 74–83. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, M.; Zeng, F.; Jin, H.; Xu, Q.; Huang, Y. Dual-Targeting Magnetic PLGA Nanoparticles for Codelivery of Paclitaxel and Curcumin for Brain Tumor Therapy. Acs Appl. Mater. Interfaces 2016, 8, 32159–32169. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, L.; Zhai, G.; Ji, J.; Liu, A. Multifunctional Polyethylene Glycol (PEG)-Poly (Lactic-Co-Glycolic Acid) (PLGA)-Based Nanoparticles Loading Doxorubicin and Tetrahydrocurcumin for Combined Chemoradiotherapy of Glioma. Med. Sci. Monit. 2020, 25, 9737. [Google Scholar] [CrossRef]
- Li, H.; Tong, Y.; Bai, L.; Ye, L.; Zhong, L.; Duan, X.; Zhu, Y. Lactoferrin functionalized PEG-PLGA nanoparticles of shikonin for brain targeting therapy of glioma. Int. J. Biol. Macromol. 2018, 107, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Gao, X.; Hu, Q.; Jiang, D.; Feng, X.; Zhang, X.; Song, Q.; Yao, L.; Huang, M.; Jiang, X.; et al. iNGR-modified PEG-PLGA nanoparticles that recognize tumor vasculature and penetrate gliomas. Biomaterials 2014, 35, 4319–4332. [Google Scholar] [CrossRef]
- Kaffashi, A.; Lüle, S.; Bozdağ Pehlivan, S.; Sarısözen, C.; Vural, İ.; Koşucu, H.; Demir, T.; Buğdaycı, K.E.; Söylemezoğlu, F.; Karlı Oğuz, K.; et al. Farnesylthiosalicylic acid-loaded lipid-polyethylene glycol-polymer hybrid nanoparticles for treatment of glioblastoma. J. Pharm. Pharm. 2017, 69, 1010–1021. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Hua, H.; Wang, A.; Liu, W.; Li, Y.; Fu, F.; Shi, Y.; Sun, K. Cyclic hexapeptide-conjugated nanoparticles enhance curcumin delivery to glioma tumor cells and tissue. Int. J. Nanomed. 2017, 12, 5717–5732. [Google Scholar] [CrossRef]
- Wang, B.; Lv, L.; Wang, Z.; Zhao, Y.; Wu, L.; Fang, X.; Xu, Q.; Xin, H. Nanoparticles functionalized with Pep-1 as potential glioma targeting delivery system via interleukin 13 receptor α2-mediated endocytosis. Biomaterials 2014, 35, 5897–5907. [Google Scholar] [CrossRef]
- Lv, L.; Jiang, Y.; Liu, X.; Wang, B.; Lv, W.; Zhao, Y.; Shi, H.; Hu, Q.; Xin, H.; Xu, Q.; et al. Enhanced Antiglioblastoma Efficacy of Neovasculature and Glioma Cells Dual Targeted Nanoparticles. Mol. Pharm. 2016, 13, 3506–3517. [Google Scholar] [CrossRef]
- Wadajkar, A.S.; Dancy, J.G.; Roberts, N.B.; Connolly, N.P.; Strickland, D.K.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Decreased non-specific adhesivity, receptor targeted (DART) nanoparticles exhibit improved dispersion, cellular uptake, and tumor retention in invasive gliomas. J. Control Release 2017, 267, 144–153. [Google Scholar] [CrossRef]
- Elgqvist, J. Nanoparticles as Theranostic Vehicles in Experimental and Clinical Applications-Focus on Prostate and Breast Cancer. Int. J. Mol. Sci. 2017, 18, 1102. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Mody, N.; Agrawal, U.; Vyas, S.P. Theranostic Nanomedicine; A Next Generation Platform for Cancer Diagnosis and Therapy. Mini Rev. Med. Chem. 2017, 17, 1746–1757. [Google Scholar] [CrossRef]
- Chen, F.; Ehlerding, E.B.; Cai, W. Theranostic nanoparticles. J. Nucl. Med. 2014, 55, 1919–1922. [Google Scholar] [CrossRef] [PubMed]
- Mieszawska, A.J.; Kim, Y.; Gianella, A.; van Rooy, I.; Priem, B.; Labarre, M.P.; Ozcan, C.; Cormode, D.P.; Petrov, A.; Langer, R.; et al. Synthesis of polymer-lipid nanoparticles for image-guided delivery of dual modality therapy. Bioconjug. Chem. 2013, 24, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Liu, T.; Yang, C. Development of PLGA-lipid nanoparticles with covalently conjugated indocyanine green as a versatile nanoplatform for tumor-targeted imaging and drug delivery. Int. J. Nanomed. 2016, 11, 5807–5821. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sun, J.; Sun, L.; Dai, Y.; Liu, L.; Li, X.; Wang, J.; Weng, J.; Jia, W.; Zhang, Z. Preparation and characterization of interferon-loaded magnetic biodegradable microspheres. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 189–196. [Google Scholar] [CrossRef]
- Ling, Y.; Wei, K.; Luo, Y.; Gao, X.; Zhong, S. Dual docetaxel/superparamagnetic iron oxide loaded nanoparticles for both targeting magnetic resonance imaging and cancer therapy. Biomaterials 2011, 32, 7139–7150. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, C.H.; Ko, H.J.; Suh, J.S.; Yoon, H.G.; Lee, K.; Huh, Y.M.; Haam, S. Multifunctional magneto-polymeric nanohybrids for targeted detection and synergistic therapeutic effects on breast cancer. Angew. Chem. Int. Ed. Engl. 2007, 46, 8836–8839. [Google Scholar] [CrossRef]
- Darwish, W.M.A.; Bayoumi, N.A. Gold nanorod-loaded (PLGA-PEG) nanocapsules as near-infrared controlled release model of anticancer therapeutics. Lasers Med. Sci. 2020, 35, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.; Wang, Y.; Liu, X.; Zhao, M.; Song, J.; Huang, P.; Wang, F.; Wang, Z. Investigation of Newly Prepared Biodegradable (32)P-chromic Phosphate-polylactide-co-glycolide Seeds and Their Therapeutic Response Evaluation for Glioma Brachytherapy. Contrast Media Mol. Imaging 2018, 2018, 2630480. [Google Scholar] [CrossRef]
- Wang, A.Z.; Yuet, K.; Zhang, L.; Gu, F.X.; Huynh-Le, M.; Radovic-Moreno, A.F.; Kantoff, P.W.; Bander, N.H.; Langer, R.; Farokhzad, O.C. ChemoRad nanoparticles: A novel multifunctional nanoparticle platform for targeted delivery of concurrent chemoradiation. Nanomedicine 2010, 5, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Press, A.T.; Traeger, A.; Pietsch, C.; Mosig, A.; Wagner, M.; Clemens, M.G.; Jbeily, N.; Koch, N.; Gottschaldt, M.; Bézière, N.; et al. Cell type-specific delivery of short interfering RNAs by dye-functionalised theranostic nanoparticles. Nat. Commun. 2014, 5, 5565. [Google Scholar] [CrossRef]
- US-FDA. Current Good Manufacturing Practice (CGMP) Regulations. Available online: https://www.fda.gov/drugs/pharmaceutical-quality-resources/current-good-manufacturing-practice-cgmp-regulations (accessed on 18 February 2021).
- Park, K.; Skidmore, S.; Hadar, J.; Garner, J.; Park, H.; Otte, A.; Soh, B.K.; Yoon, G.; Yu, D.; Yun, Y.; et al. Injectable, long-acting PLGA formulations: Analyzing PLGA and understanding microparticle formation. J. Control. Release 2019, 304, 125–134. [Google Scholar] [CrossRef]
- Polarine, J.; Chai, R.; Kochat, H.; Pulliam, P.J.; Zhi, K.; Brooks, K. In-Situ Disinfectant Validation Case Study. Am. Pharm. Rev. 2021, 1–6. [Google Scholar] [CrossRef]
- Wasalathanthri, D.P.; Feroz, H.; Puri, N.; Hung, J.; Lane, G.; Holstein, M.; Chemmalil, L.; Both, D.; Ghose, S.; Ding, J. Real-time monitoring of quality attributes by inline Fourier transform infrared spectroscopic sensors at ultrafiltration and diafiltration of bioprocess. Biotechnol. Bioeng. 2020, 117, 3766–3774. [Google Scholar] [CrossRef] [PubMed]
- Yaman, A. Alternative methods of terminal sterilization for biologically active macromolecules. Curr. Opin. Drug Discov. Devel. 2001, 4, 760–763. [Google Scholar] [PubMed]
- Lü, J.M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef]
- Loo, J.; Ooi, C.; Boey, F. Degradation of poly (lactide-co-glycolide)(PLGA) and poly (L-lactide)(PLLA) by electron beam radiation. Biomaterials 2005, 26, 1359–1367. [Google Scholar] [CrossRef]
- Malinovskaya, Y.; Melnikov, P.; Baklaushev, V.; Gabashvili, A.; Osipova, N.; Mantrov, S.; Ermolenko, Y.; Maksimenko, O.; Gorshkova, M.; Balabanyan, V.; et al. Delivery of doxorubicin-loaded PLGA nanoparticles into U87 human glioblastoma cells. Int. J. Pharm. 2017, 524, 77–90. [Google Scholar] [CrossRef]
- Chang, J.; Paillard, A.; Passirani, C.; Morille, M.; Benoit, J.P.; Betbeder, D.; Garcion, E. Transferrin adsorption onto PLGA nanoparticles governs their interaction with biological systems from blood circulation to brain cancer cells. Pharm. Res. 2012, 29, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Semete, B.; Booysen, L.; Lemmer, Y.; Kalombo, L.; Katata, L.; Verschoor, J.; Swai, H.S. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomedicine 2010, 6, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Wohlfart, S.; Khalansky, A.S.; Gelperina, S.; Maksimenko, O.; Bernreuther, C.; Glatzel, M.; Kreuter, J. Efficient chemotherapy of rat glioblastoma using doxorubicin-loaded PLGA nanoparticles with different stabilizers. PloS ONE 2011, 6, e19121. [Google Scholar] [CrossRef]
- Rigon, L.; Salvalaio, M.; Pederzoli, F.; Legnini, E.; Duskey, J.T.; D’Avanzo, F.; De Filippis, C.; Ruozi, B.; Marin, O.; Vandelli, M.A.; et al. Targeting Brain Disease in MPSII: Preclinical Evaluation of IDS-Loaded PLGA Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2014. [Google Scholar] [CrossRef] [PubMed]
- Seju, U.; Kumar, A.; Sawant, K.K. Development and evaluation of olanzapine-loaded PLGA nanoparticles for nose-to-brain delivery: In vitro and in vivo studies. Acta Biomater. 2011, 7, 4169–4176. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Fukuda, T.; Nagaoka, Y.; Hasumura, T.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Venugopal, K.; Kumar, D.S. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PloS ONE 2012, 7, e32616. [Google Scholar] [CrossRef] [PubMed]
- Monge, M.; Fornaguera, C.; Quero, C.; Dols-Perez, A.; Calderó, G.; Grijalvo, S.; García-Celma, M.J.; Rodríguez-Abreu, C.; Solans, C. Functionalized PLGA nanoparticles prepared by nano-emulsion templating interact selectively with proteins involved in the transport through the blood-brain barrier. Eur. J. Pharm. Biopharm. 2020, 156, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Sowmya, S.; Cinu, T.A.; Aleykutty, N.A.; Thomas, S.; Souto, E.B. Surface modified PLGA nanoparticles for brain targeting of Bacoside-A. Eur. J. Pharm. Sci. 2014, 63, 29–35. [Google Scholar] [CrossRef]
- Orunoğlu, M.; Kaffashi, A.; Pehlivan, S.B.; Şahin, S.; Söylemezoğlu, F.; Oğuz, K.K.; Mut, M. Effects of curcumin-loaded PLGA nanoparticles on the RG2 rat glioma model. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, A.; Chakravarti, S.; Ghosh, A.; Shaw, R.; Bhandary, S.; Bhattacharyya, S.; Sen, P.C.; Ghosh, M.K. Anti-SSTR2 peptide based targeted delivery of potent PLGA encapsulated 3,3′-diindolylmethane nanoparticles through blood brain barrier prevents glioma progression. Oncotarget 2017, 8, 65339–65358. [Google Scholar] [CrossRef]
- USP-NF. Goserelin Implants. Available online: https://online.uspnf.com/uspnf/document/1_GUID-556307CA-0AAB-4ED2-94D6-6878DCEAF168_1_en-US?source=Search%20Results&highlight=PLGA (accessed on 21 February 2021).
- US-FDA. COVID-19 Vaccines. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (accessed on 23 February 2021).
- Liberti, L.; Breckenridge, A.; Hoekman, J.; Leufkens, H.; Lumpkin, M.; McAuslane, N.; Stolk, P.; Zhi, K.; Rägo, L. Accelerating access to new medicines: Current status of facilitated regulatory pathways used by emerging regulatory authorities. J. Public Health Policy 2016, 37, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Liberti, L.; Breckenridge, A.; Hoekman, J.; Leufkens, H.; Lumpkin, M.; McAuslane, N.; Stolk, P.; Zhi, K.; Rägo, L. Practical aspects of developing, implementing and using facilitated regulatory pathways in the emerging markets. In Proceedings of the Poster Drug Information Association Annual Meeting, Philadelphia, PA, USA, 26–30 June 2016. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).