Studies on Surfactants, Cosurfactants, and Oils for Prospective Use in Formulation of Ketorolac Tromethamine Ophthalmic Nanoemulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Identification and Selection of Potential Surfactants and Cosurfactants Based on HLB Calculations
2.2.2. Determination of Selected Properties and Molecular Descriptors of Cosurfactants
2.2.3. Saturation Solubility Studies of Ketorolac Tromethamine (KT) in Surfactants, Cosurfactants, and Oils
2.2.4. Conjunctival Irritation of Surfactants, Cosurfactants, and Oils by the HET-CAM Assay
2.3. Statistical Analysis
3. Results and Discussion
3.1. Identification and Selection of Potential Surfactants and Cosurfactants Based on HLB Calculations
3.2. Determination of Physicochemical Properties and Molecular Descriptors of Selected Cosurfactants
3.3. Saturation Solubility Studies of Ketorolac Tromethamine in Surfactants, Cosurfactants, and Oils
Effect of Cosurfactants Carbon Chain Length, DEC, log P, and HLB Value on the Solubility of Ketorolac Tromethamine
3.4. Conjunctival Irritation of Surfactants, Cosurfactants, and Oils Determined by the HET-CAM Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, L.; Xin, X.; Zhang, Y. Development and characterization of promising Cremophor EL-stabilized o/w nanoemulsions containing short-chain alcohols as a cosurfactant. RSC Adv. 2017, 7, 19815–19827. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Tao, C.; Lin, X.; Zhang, M.; Zeng, L.; Chen, X.; Song, H. Cationic nanoemulsions with prolonged retention time as promising carriers for ophthalmic delivery of tacrolimus. Eur. J. Pharm. Sci. 2020, 144, 105229. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, A.; Wu, H.; Bi, Y. Development and in vivo evaluation of baicalin-loaded W/O nanoemulsion for lymphatic absorption. Pharm. Dev. Technol. 2019, 24, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Mahboobian, M.M.; Mohammadi, M.; Mansouri, Z. Development of thermosensitive in situ gel nanoemulsions for ocular delivery of acyclovir. J. Drug Deliv. Sci. Technol. 2020, 55, 101400. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Simonazzi, A.; Cid, A.G.; Villegas, M.; Romero, A.I.; Palma, S.D.; Bermúdez, J.M. Nanotechnology applications in drug controlled release. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; William Andrew (Elsevier): Norwich, NY, USA, 2018; pp. 81–116. [Google Scholar] [CrossRef]
- Borrin, T.R.; Georges, E.L.; Moraes, I.C.; Pinho, S.C. Curcumin-loaded nanoemulsions produced by the emulsion inversion point (EIP) method: An evaluation of process parameters and physico-chemical stability. J. Food Eng. 2016, 169, 1–9. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; McClements, D. Influence of nanoemulsion addition on the stability of conventional emulsions. Food Biophys. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Gallarate, M.; Chirio, D.; Bussano, R.; Peira, E.; Battaglia, L.; Baratta, F.; Trotta, M. Development of O/W nanoemulsions for ophthalmic administration of timolol. Int. J. Pharm. 2013, 440, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Subrizi, A.; del Amo, E.M.; Korzhikov-Vlakh, V.; Tennikova, T.; Ruponen, M.; Urtti, A. Design principles of ocular drug delivery systems: Importance of drug payload, release rate, and material properties. Drug Discov. Today 2019, 24, 1446–1457. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M. Nanoemulsion preparation, characterization, and application in the field of biomedicine. In Nanoarchitectonics in Biomedicine; Grumzescu, A.M., Ed.; William Andrew (Elsevier): Amsterdam, The Netherlands, 2019; pp. 169–188. [Google Scholar] [CrossRef]
- Henostroza, M.A.B.; Melo, K.J.C.; Yukuyama, M.N.; Löbenberg, R.; Bou-Chacra, N.A.J.C.; Physicochemical, S.A.; Aspects, E. Cationic rifampicin nanoemulsion for the treatment of ocular tuberculosis. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 597, 124755. [Google Scholar] [CrossRef]
- Dukovski, B.J.; Juretić, M.; Bračko, D.; Randjelović, D.; Savić, S.; Moral, M.C.; Diebold, Y.; Filipović-Grčić, J.; Pepić, I.; Lovrić, J. Functional ibuprofen-loaded cationic nanoemulsion: Development and optimization for dry eye disease treatment. Int. J. Pharm. 2020, 576, 118979. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Nasr, M.; Sammour, O. Nanoemulsion as a feasible and biocompatible carrier for ocular delivery of travoprost: Improved pharmacokinetic/pharmacodynamic properties. Int. J. Pharm. 2020, 583, 119402. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Nair, A.B.; Jacob, S.; Patel, R.K.; Shah, H.; Shehata, T.M.; Morsy, M.A. Nanoemulsion based vehicle for effective ocular delivery of moxifloxacin using experimental design and pharmacokinetic study in rabbits. Pharmaceutics 2019, 11, 230. [Google Scholar] [CrossRef] [PubMed]
- Mahboobian, M.M.; Seyfoddin, A.; Aboofazeli, R.; Foroutan, S.M.; Rupenthal, I.D. Brinzolamide–loaded nanoemulsions: Ex vivo transcorneal permeation, cell viability and ocular irritation tests. Pharm. Dev. Technol. 2019, 24, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Artiga-Artigas, M.; Acevedo-Fani, A.; Martín-Belloso, O. Effect of sodium alginate incorporation procedure on the physicochemical properties of nanoemulsions. Food Hydrocoll. 2017, 70, 191–200. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Kotta, S.; Abdullah, S.T.; Fahmy, U.A.; Alfaleh, M.A.; Asfour, H.Z. Formulation Design, Statistical Optimization, and In Vitro Evaluation of a Naringenin Nanoemulsion to Enhance Apoptotic Activity in A549 Lung Cancer Cells. Pharmaceuticals 2020, 13, 152. [Google Scholar] [CrossRef]
- Alany, R.; Rades, T.; Agatonovic-Kustrin, S.; Davies, N.; Tucker, I. Effects of alcohols and diols on the phase behaviour of quaternary systems. Int. J. Pharm. 2000, 196, 141–145. [Google Scholar] [CrossRef]
- Lawrence, M.J.; Rees, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2000, 45, 89–121. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Karmakar, S.; Biswas, E.; Dey, G.; Barik, R.; Mandal, M.; Pal, T.K. Improvement of cellular uptake, in vitro antitumor activity and sustained release profile with increased bioavailability from a nanoemulsion platform. Int. J. Pharm. 2014, 460, 131–143. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Biswas, N.; Guha, A.; Sahoo, N.; Kuotsu, K. Nonionic surfactant vesicles in ocular delivery: Innovative approaches and perspectives. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Furrer, P.; Plazonnet, B.; Mayer, J.; Gurny, R. Application of in vivo confocal microscopy to the objective evaluation of ocular irritation induced by surfactants. Int. J. Pharm. 2000, 207, 89–98. [Google Scholar] [CrossRef]
- Schmidts, T.; Schlupp, P.; Gross, A.; Dobler, D.; Runkel, F. Required HLB determination of some pharmaceutical oils in submicron emulsions. J. Dispers. Sci. Technol. 2012, 33, 816–820. [Google Scholar] [CrossRef]
- Mithani, S.D.; Bakatselou, V.; TenHoor, C.N.; Dressman, J. Estimation of the increase in solubility of drugs as a function of bile salt concentration. Pharm. Res. 1996, 13, 163–167. [Google Scholar] [CrossRef]
- Goswami, P.; Choudhury, A.; Kumar, D. Microemulsion-A Potential Carrier for Improved Bioavailability. Intern. J. Pharm. Biol. Sci. Arch. 2019, 10, 69–77. [Google Scholar]
- Abraham, M.H.; Chadha, H.S.; Leitao, R.A.; Mitchell, R.C.; Lambert, W.J.; Kaliszan, R.; Nasal, A.; Haber, P. Determination of solute lipophilicity, as log P (octanol) and log P (alkane) using poly (styrene–divinylbenzene) and immobilised artificial membrane stationary phases in reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1997, 766, 35–47. [Google Scholar] [CrossRef]
- Gupta, A.K.; Madan, S.; Majumdar, D.; Maitra, A. Ketorolac entrapped in polymeric micelles: Preparation, characterisation and ocular anti-inflammatory studies. Int. J. Pharm. 2000, 209, 1–14. [Google Scholar] [CrossRef]
- Ahuja, M.; Dhake, A.S.; Sharma, S.K.; Majumdar, D.K. Topical ocular delivery of NSAIDs. AAPS J. 2008, 10, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Orasugh, J.T.; Dutta, S.; Das, D.; Pal, C.; Zaman, A.; Das, S.; Dutta, K.; Banerjee, R.; Ghosh, S.K.; Chattopadhyay, D. Sustained release of ketorolac tromethamine from poloxamer 407/cellulose nanofibrils graft nanocollagen based ophthalmic formulations. Int. J. Biol. Macromol. 2019, 140, 441–453. [Google Scholar] [CrossRef]
- Fathalla, Z.M.; Vangala, A.; Longman, M.; Khaled, K.A.; Hussein, A.K.; El-Garhy, O.H.; Alany, R.G. Poloxamer-based thermoresponsive ketorolac tromethamine in situ gel preparations: Design, characterisation, toxicity and transcorneal permeation studies. Eur. J. Pharm. Biopharm. 2017, 114, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Fathalla, Z.M.; Khaled, K.A.; Hussein, A.K.; Alany, R.G.; Vangala, A. Formulation and corneal permeation of ketorolac tromethamine-loaded chitosan nanoparticles. Drug Dev. Ind. Pharm. 2016, 42, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Perry, H.D.; Donnenfeld, E.D. An update on the use of ophthalmic ketorolac tromethamine 0.4%. Expert Opin. Pharmacother. 2006, 7, 99–107. [Google Scholar] [CrossRef]
- Guo, X.; Rong, Z.; Ying, X. Calculation of hydrophile–lipophile balance for polyethoxylated surfactants by group contribution method. J. Colloidal Interface Sci. 2006, 298, 441–450. [Google Scholar] [CrossRef]
- Davies, J. A quantitative kinetic theory of emulsion type. I. Physical chemistry of the emulsifying agent. Proc. II Int. Congr. Surf. Act. Agents 1957, 1, 426–438. [Google Scholar]
- Mahdi, E.S.; Sakeena, M.H.; Abdulkarim, M.F.; Abdullah, G.Z.; Sattar, M.A.; Noor, A.M. Effect of surfactant and surfactant blends on pseudoternary phase diagram behavior of newly synthesized palm kernel oil esters. Drug Des. Dev. Ther. 2011, 5, 311. [Google Scholar] [CrossRef] [PubMed]
- Kesarla, R.; Tank, T.; Vora, P.A.; Shah, T.; Parmar, S.; Omri, A. Preparation and evaluation of nanoparticles loaded ophthalmic in situ gel. Drug Deliv. 2016, 23, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Rupenthal, I.D.; Green, C.R.; Alany, R.G. Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 2: Precorneal retention and in vivo pharmacodynamic study. Int. J. Pharm. 2011, 411, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Luepke, N. Hen’s egg chorioallantoic membrane test for irritation potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef]
- Yoo, J.; Baskaran, R.; Yoo, B.-K. Self-nanoemulsifying drug delivery system of lutein: Physicochemical properties and effect on bioavailability of warfarin. Biomol. Ther. 2013, 21, 173. [Google Scholar] [CrossRef]
- Legen, I.; Peternel, L.; Novak, S.; Homar, M.; Rozman, P.; Klancar, U. Self-Microemulsifying Drug Delivery System of Abiraterone or Abiraterone Acetate. Patent WO2014009434, 10 July 2013. [Google Scholar]
- Lu, S.; Li, J.; Zhang, S.; Yin, Z.; Xing, T.; Kaplan, D.L. The influence of the hydrophilic–lipophilic environment on the structure of silk fibroin protein. J. Mater. Chem. B 2015, 3, 2599–2606. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, N.; Dupuis, F.; Arab-Tehrany, E.; Denis, F.M.; Paris, C.; Lartaud, I.; Linder, M. Formulation, characterization and pharmacokinetic studies of coenzyme Q10 PUFA’s nanoemulsions. Eur. J. Pharm. Sci. 2012, 47, 305–312. [Google Scholar] [CrossRef]
- Box, K.; Comer, J. Using measured pKa, LogP and solubility to investigate supersaturation and predict BCS class. Curr. Drug Metab. 2008, 9, 869–878. [Google Scholar] [CrossRef]
- Choudhury, H.; Zakaria, N.F.B.; Tilang, P.A.B.; Tzeyung, A.S.; Pandey, M.; Chatterjee, B.; Alhakamy, N.A.; Bhattamishra, S.K.; Kesharwani, P.; Gorain, B. Formulation development and evaluation of rotigotine mucoadhesive nanoemulsion for intranasal delivery. J. Drug Deliv. Sci. Technol. 2019, 54, 101301. [Google Scholar] [CrossRef]
- Rane, S.S.; Anderson, B.D. What determines drug solubility in lipid vehicles: Is it predictable? Adv. Drug Deliv. Rev. 2008, 60, 638–656. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, R.J.; Rawn, J.D. 4-Alkanes and Cycloalkanes: Structures and Reactions. In Organic Chemistry, 2nd ed.; Ouellette, R.J., Rawn, J.D., Eds.; Academic Press: Boston, MA, USA, 2018; pp. 87–133. [Google Scholar] [CrossRef]
- Rose, M.; Palkovits, R. Isosorbide as a Renewable Platform chemical for Versatile Applications—Quo Vadis? ChemSusChem 2012, 5, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Machmudah, S.; Kanda, H.; Goto, M. Hydrolysis of Biopolymers in Near-Critical and Subcritical Water. In Water Extraction of Bioactive Compounds; Gonzalez, H.D., Munoz, M.J.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 69–107. [Google Scholar] [CrossRef]
- Fakhree, M.A.A.; Delgado, D.R.; Martínez, F.; Jouyban, A. The importance of dielectric constant for drug solubility prediction in binary solvent mixtures: Electrolytes and zwitterions in water+ ethanol. AAPS PharmSciTech 2010, 11, 1726–1729. [Google Scholar] [CrossRef]
- Lindsley, C.W. Lipophilicity; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–6. [Google Scholar] [CrossRef]
- Waring, M.J. Lipophilicity in drug discovery. Expert Opin. Drug Discov. 2010, 5, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jiang, S.; Liu, D.; Bi, X.; Wang, F.; Zhang, Q.; Xu, Q. A potential new therapeutic system for glaucoma: Solid lipid nanoparticles containing methazolamide. J. Microencapsul. 2011, 28, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Pierscionek, B.; Carew, M.; Wu, Z.; Alany, R.G. Critical appraisal of alternative irritation models: Three decades of testing ophthalmic pharmaceuticals. Br. Med. Bull. 2015, 113, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Butt, U.; ElShaer, A.; Snyder, L.A.; Al-Kinani, A.A.; Le Gresley, A.; Alany, R.G. Fatty acid based microemulsions to combat ophthalmia neonatorum caused by Neisseria gonorrhoeae and Staphylococcus aureus. J. Nanomater. 2018, 8, 51. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Maria, D.N.; Wang, X.; Simpson, R.N.; Hollingsworth, T.; Jablonski, M.M. Enhanced Corneal Penetration of a Poorly Permeable Drug Using Bioadhesive Multiple Microemulsion Technology. Pharmaceutics 2020, 12, 704. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Li, J.; Liu, R.; Shu, L.; Jin, J. Effects of Labrasol on the corneal drug delivery of baicalin. Drug Deliv. 2009, 16, 399–404. [Google Scholar] [CrossRef]
- Alany, R.; Rades, T.; Nicoll, J.; Tucker, I.; Davies, N. W/O microemulsions for ocular delivery: Evaluation of ocular irritation and precorneal retention. J. Control Release 2006, 111, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Morsi, N.; Mohamed, M.; Refai, H.; El Sorogy, H. Nanoemulsion as a novel ophthalmic delivery system for acetazolamide. Int. J. Pharm. Pharm. Sci. 2014, 6, 227–236. [Google Scholar]
- Liu, Z.; Nie, S.; Guo, H.; Pan, W.; Li, J. Effects of Transcutol P on the corneal permeability of drugs and evaluation of its ocular irritation of rabbit eyes. J. Pharm. Pharmacol. 2006, 58, 45–50. [Google Scholar] [CrossRef] [PubMed]


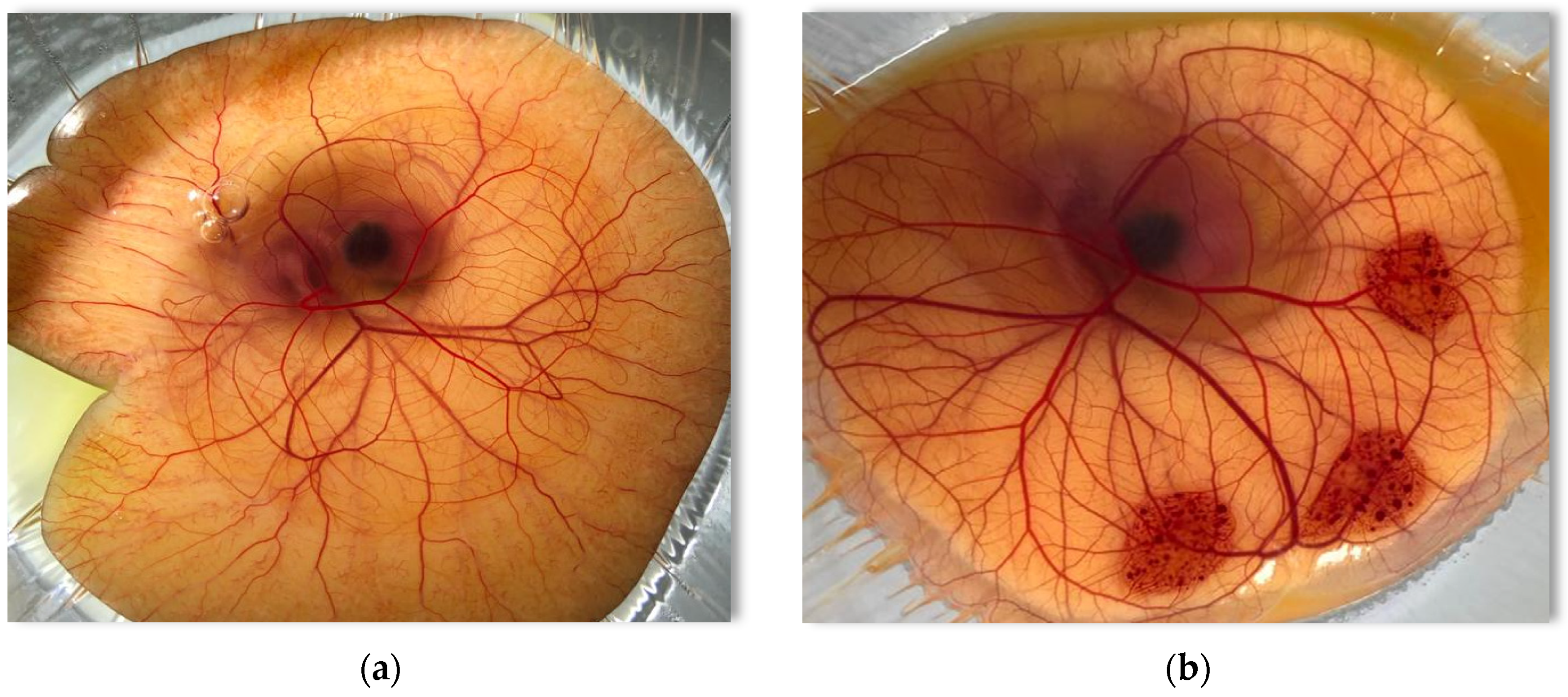
| Effect | Score | ||
|---|---|---|---|
| Time (min) | 0.5 | 2.0 | 5.0 |
| Hyperemia | 5 | 3 | 1 |
| Hemorrhage | 7 | 5 | 3 |
| Clotting or Coagulation | 9 | 7 | 5 |
| Cumulative Score | Irritation Potential |
|---|---|
| 0.0–0.9 | Practically none |
| 1.0–4.9 | Slight |
| 5.0–8.9 | Moderate |
| 9.0–21.0 | Strong |
| Surfactants & Cosurfactants | Calculated HLB | Reported HLB |
|---|---|---|
| Tween 20 | 16.67 | 16.70 [34] |
| Tween 60 | 13.82 | 14.90 [34] |
| Tween 80 | 13.82 | 15.0 [34] |
| Span 20 | 8.67 | 8.60 [34] |
| Span 80 | 5.82 | 4.30 [34] |
| Cremophor RH 40 | 15.65 | 15.0 [41] |
| Cremophor EL | 14.0 | 13.0 [41] |
| Labrasol ALF | 14.69 | 14.0 [40] |
| Labrafil M2125 CS | 5.20 | 4.0 [40] |
| Labrafil M1944 CS | 5.20 | 4.0 [40] |
| Labrafil M2130 CS | 5.20 | 4.0 [40] |
| Ethylene glycol | 9.85 | 9.85 [42] |
| Propylene glycol | 9.37 | 9.38 [42] |
| Butylene glycol | 8.90 | 8.90 [42] |
| Pentylene glycol | 8.42 | 8.43 [19] |
| Hexylene glycol | 7.95 | 7.95 [19] |
| Dimethyl isosorbide | 8.40 | - |
| 2-Butyl-2-ethyl-1,3-propanediol | 6.52 | - |
| 2,3-Butanediol | 8.90 | 8.90 [42] |
| Transcutol P | 8.61 | 4.20 [40] |
| Cosurfactants (Diols) | Chemical Formula | C No 1 | log P 1 | DEC 1 | Mwt 1 (g/mol) | C% 1 |
|---|---|---|---|---|---|---|
| Ethylene glycol (1,2-ethanediol) | C2H6O2 | 2 | −1.69 | 41.4 | 62.07 | 38.70 |
| Propylene glycol (1,2-propanediol) | C3H8O2 | 3 | −1.34 | 32.0 | 76.10 | 47.35 |
| Butylene glycol (1,2-butanediol) | C4H10O2 | 4 | −0.74 | 28.8 | 90.12 | 53.30 |
| Pentylene glycol (1,2-pentanediol) | C5H12O2 | 5 | −0.27 | 18.2 | 104.15 | 57.66 |
| Hexylene glycol (1,2-hexanediol) | C6H14O2 | 6 | 0.58 | 7.70 | 118.17 | 60.98 |
| Excipients | Solubility 1 (mg/mL) | Excipients | Solubility 1 (mg/mL) |
|---|---|---|---|
| Tween 20 | 7.30 ± 0.20 | Pentylene glycol | 16.04 ± 0.95 |
| Tween 60 | 9.89 ± 0.17 | Hexylene glycol | 10.36 ± 0.85 |
| Tween 80 | 3.82 ± 0.41 | Dimethyl isosorbide | 4.54 ± 0.55 |
| Span 20 | 5.01 ± 0.27 | 2-butyl-2-ethyl-1,3-propandiol | 9.55 ± 0.60 |
| Span 80 | 3.18 ± 0.37 | 2,3-butanediol | 22.93 ± 0.24 |
| Cremophor RH 40 | 9.00 ± 0.21 | Transcutol P | 13.32 ± 0.11 |
| Cremophor EL | 4.03 ± 0.38 | Triacetin | 0.06 ± 0.001 |
| Labrasol ALF | 6.64 ± 0.13 | Ethyl Oleate | 0.04 ± 0.01 |
| Labrafil M2125 CS | 0.48 ± 0.06 | Labrafac PG | 0.01 ± 0.002 |
| Labrafil M1944 CS | 0.33 ± 0.03 | Labrafac lipophile WL 1349 | 0.01 ± 0.01 |
| Labrafil M2130 CS | 0.80 ± 0.04 | Isopropyl Myristate | 0.01 ±0.02 |
| Ethylene glycol | 36.84 ± 0.40 | Simulated tear fluid | 199.2 ± 7.40 |
| Propylene glycol | 26.23 ± 0.82 | Deionized water | 217.1 ± 1.13 |
| Butylene glycol | 21.09 ± 1.22 |
| Surfactants, Cosurfactants & Oils | Irritation Potential (Numerical Score) | |||
|---|---|---|---|---|
| Practically Non (0.0–0.9) | Slight (1.0–4.9) | Moderate (5.0–8.9) | Strong (9.0–21.0) | |
| KT solution | √ | |||
| Tween 20 | √ | |||
| Tween 60 | √ | |||
| Tween 80 | √ | |||
| Span 20 | √ | |||
| Span 80 | √ | |||
| Cremophor RH 40 | √ | |||
| Cremophor EL | √ | |||
| Labrasol ALF | √ | |||
| Labrafil M2125 CS | √ | |||
| Labrafil M1944 CS | √ | |||
| Labrafil M2130 CS | √ | |||
| Ethylene glycol | √ | |||
| Propylene glycol | √ | |||
| Butylene glycol | √ | |||
| Pentylene glycol | √ | |||
| Hexylene glycol | √ | |||
| Dimethyl isosorbide | √ | |||
| 2-Butyl-2-ethyl-1,3-propandiol | √ | |||
| 2,3-Butanediol | √ | |||
| Transcutol P | √ | |||
| Triacetin | √ | |||
| Ethyl Oleate | √ | |||
| Labrafac PG | √ | |||
| Labrafac lipophile WL 1349 | √ | |||
| Isopropyl Myristate | √ | |||
| Normal saline | √ | |||
| NaOH (0.1 M) | √ | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smail, S.S.; Ghareeb, M.M.; Omer, H.K.; Al-Kinani, A.A.; Alany, R.G. Studies on Surfactants, Cosurfactants, and Oils for Prospective Use in Formulation of Ketorolac Tromethamine Ophthalmic Nanoemulsions. Pharmaceutics 2021, 13, 467. https://doi.org/10.3390/pharmaceutics13040467
Smail SS, Ghareeb MM, Omer HK, Al-Kinani AA, Alany RG. Studies on Surfactants, Cosurfactants, and Oils for Prospective Use in Formulation of Ketorolac Tromethamine Ophthalmic Nanoemulsions. Pharmaceutics. 2021; 13(4):467. https://doi.org/10.3390/pharmaceutics13040467
Chicago/Turabian StyleSmail, Shahla S., Mowafaq M. Ghareeb, Huner K. Omer, Ali A. Al-Kinani, and Raid G. Alany. 2021. "Studies on Surfactants, Cosurfactants, and Oils for Prospective Use in Formulation of Ketorolac Tromethamine Ophthalmic Nanoemulsions" Pharmaceutics 13, no. 4: 467. https://doi.org/10.3390/pharmaceutics13040467
APA StyleSmail, S. S., Ghareeb, M. M., Omer, H. K., Al-Kinani, A. A., & Alany, R. G. (2021). Studies on Surfactants, Cosurfactants, and Oils for Prospective Use in Formulation of Ketorolac Tromethamine Ophthalmic Nanoemulsions. Pharmaceutics, 13(4), 467. https://doi.org/10.3390/pharmaceutics13040467









