Development and Evaluation of 1′-Acetoxychavicol Acetate (ACA)-Loaded Nanostructured Lipid Carriers for Prostate Cancer Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NLCs
2.3. Characterization of NLCs
2.3.1. Particle Size, Polydispersity Index (PDI), and Zeta Potential
2.3.2. Entrapment Efficiency
2.3.3. Coating Efficiency
2.3.4. Transmission Electron Microscopy (TEM)
2.3.5. Differential Scanning Calorimetry (DSC)
2.3.6. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.7. In Vitro Drug Release
2.4. Cell Culture
2.5. Cytotoxicity Assay
2.6. Cellular Uptake Study
2.7. Migration Assay
2.8. Invasion Assay
2.9. In Vivo Anti-Tumor Efficacy of the NLCs
2.10. Immunohistochemistry (IHC) Analysis of Tumor Biopsies
3. Results and Discussion
3.1. AMD3100 Surface-Modified ACA-NLC Was Successfully Formulated and Characterized
3.2. Rate of ACA Release from the NLC Is Sustained
3.3. AMD-ACA-NLC Demonstrated Time-Dependent and Enhanced Cellular Uptake via Different Endocytic Pathways
3.4. AMD-ACA-NLC Showed Time-Based and Improved Cytotoxicity against PC-3 Cell Lines
3.5. AMD-ACA-NLC Exhibited Anti-Migration and Anti-Invasion Properties in PC-3 Cells
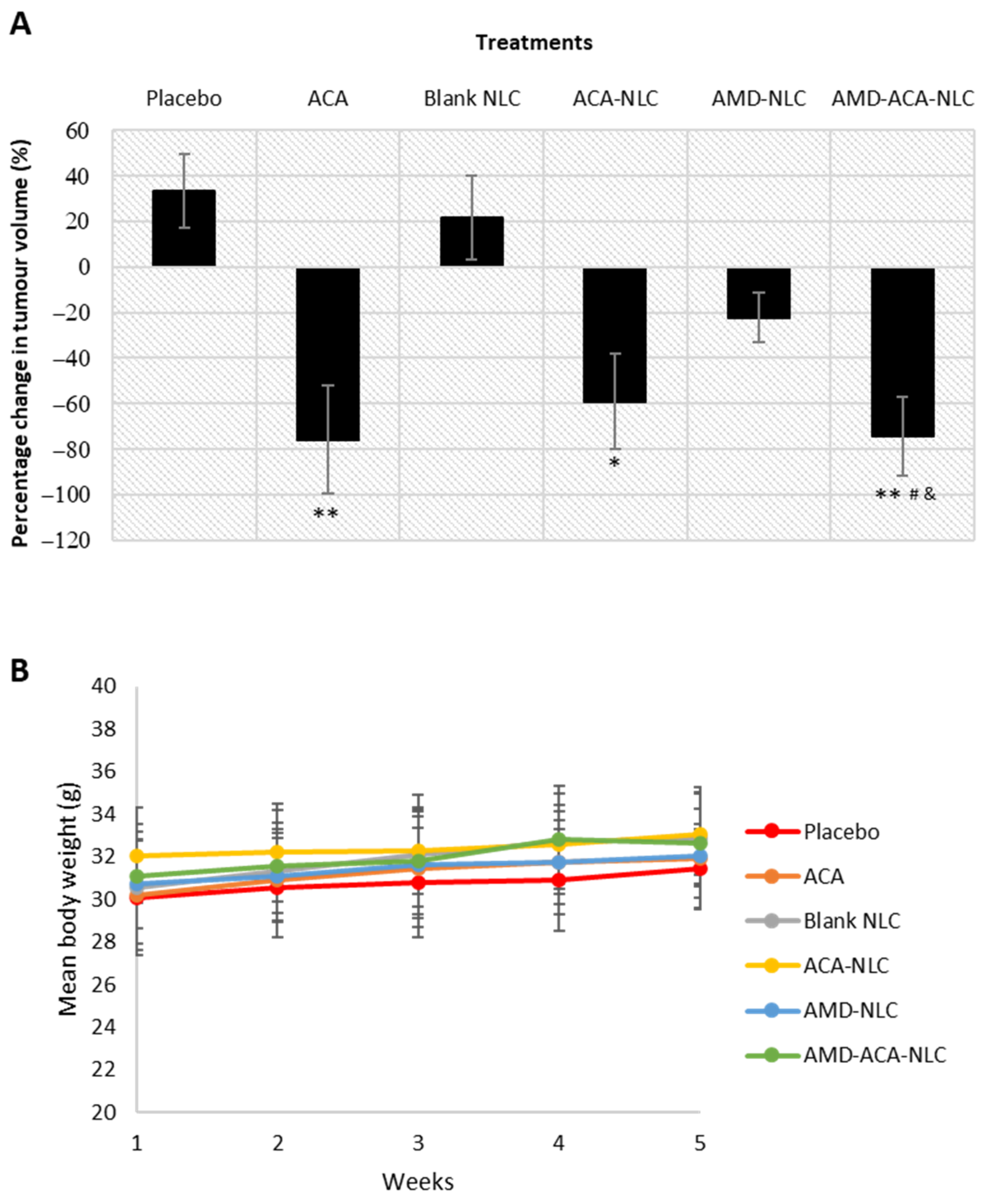
3.6. AMD-ACA-NLC Showed Regression of Tumor Growth
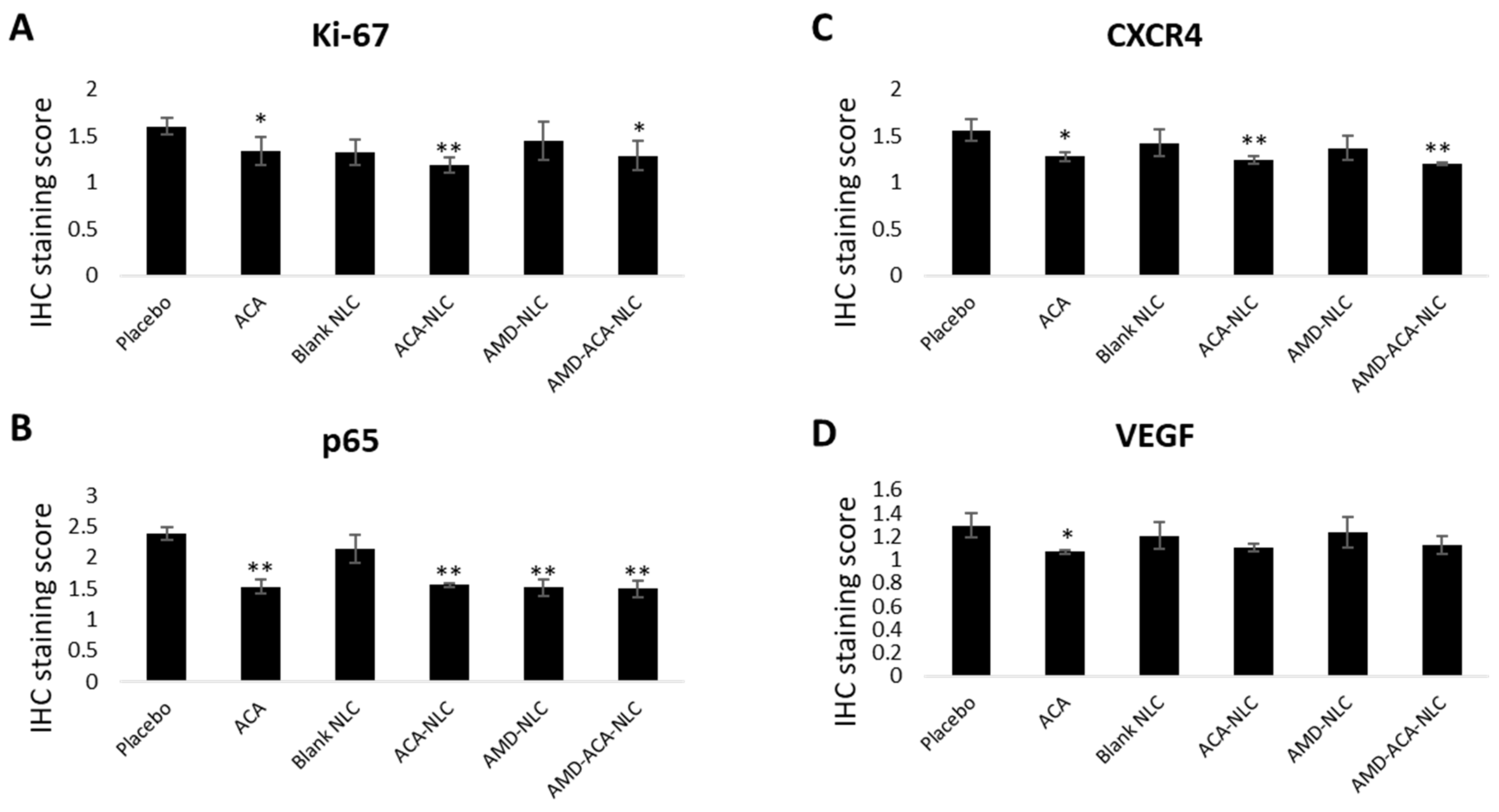
3.7. AMD-ACA-NLC Downregulated Tumor Marker Expressions
4. Conclusions
5. Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer. Global Cancer Observatory [Homepage on the Internet]. 2020. Available online: https://gco.iarc.fr (accessed on 30 December 2020).
- Picus, J.; Schultz, M. Docetaxel (Taxotere) as monotherapy in the treatment of hormone-refractory prostate cancer: Preliminary results. Semin. Oncol. 1999, 26, 14–18. [Google Scholar] [PubMed]
- Antonarakis, E.S.; Paller, C.J. Cabazitaxel: A novel second-line treatment for metastatic castration-resistant prostate cancer. Drug Des. Dev. Ther. 2011, 5, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Kondo, A.; Ohigashi, H.; Murakami, A.; Suratwadee, J.; Koshimizu, K. 1′-acetoxychavicol acetate as a potent inhibitor of tumor promoter-induced Epstein-Barr Virus activation from Languas galanga, a traditional Thai condiment. Biosci. Biotechnol. Biochem. 1993, 57, 1344–1345. [Google Scholar] [CrossRef]
- Awang, K.; Azmi, M.N.; Aun, L.I.; Aziz, A.N.; Ibrahim, H.; Nagoor, N.H. The Apoptotic Effect of 1′S-1′-Acetoxychavicol Acetate from Alpinia Conchigera on Human Cancer Cells. Molecules 2010, 15, 8048–8059. [Google Scholar] [CrossRef] [PubMed]
- Arshad, N.M.; In, L.L.; Tchen Lin Soh, M.N.; Ibrahim, H.; Awang, K.; Dudich, E.; Tatulov, E.; Nagoor, N.H. Recombinant human alpha fetoprotein synergistically potentiates the anti-cancer effects of 1′-S-1′-acetoxychavicol acetate when used as a complex against human tumours harbouring AFP-receptors. Oncotarget 2015, 6, 16151–16167. [Google Scholar] [CrossRef]
- La, L.L.; Arshad, N.M.; Ibrahim, H.; Azmi, M.N.; Awang, K.; Nagoor, N.H. 1′-Acetoxychavicol acetate inhibits growth of human oral carcinoma xenograft in mice and potentiates cisplatin effect via proinflammatory microenvironment alterations. BMC Complement. Altern. Med. 2012, 12, 179. [Google Scholar] [CrossRef]
- Blanco, E.; Hsiao, A.; Ruiz-Esparza, G.U.; Landry, M.G.; Meric-Bernstam, F.; Ferrari, M. Molecular-targeted nanotherapies in cancer: Enabling treatment specificity. Mol. Oncol. 2011, 5, 492–503. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Li, S. Polymeric Micelles: Nanocarriers for Cancer-Targeted Drug Delivery. AAPS PharmSciTech 2014, 15, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007, 9, E128–E147. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2016, 24, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Radtke, M.; Wissing, S. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 9–24. [Google Scholar] [CrossRef]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorg. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef]
- Sundar, D.S.; Antoniraj, M.G.; Kumar, C.S.; Mohapatra, S.S.; Houreld, N.N.; Ruckmani, K. Recent Trends of Biocompatible and Biodegradable Nanoparticles in Drug Delivery: A Review. Curr. Med. Chem. 2016, 23, 3730–3751. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhu, Z.; Wang, H.; Jin, S.; Yang, X.; Han, C.; Pan, W. Polyarginine and PEG-AEYLR comodified nanostructured lipid carrier: 10 mol% uncleavable PEG-AEYLR showed no shielding effect to polyarginine in vitro while maintaining good tumor targeting in vivo. Artif. Cells Nanomed. Biotechnol. 2017, 46, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, M.; Li, N.; Yang, X.; Han, C.; Pan, W. Redox sensitive PEG controlled octaarginine and targeting peptide co-modified nanostructured lipid carriers for enhanced tumour penetrating and targeting in vitro and in vivo. Artif. Cells Nanomed. Biotechnol. 2017, 46, 313–322. [Google Scholar] [CrossRef]
- Kong, F.; Han, Y.; Zhang, Y.; Li, D.; Chen, Y.; Sun, J. Transferrin-modified nanostructured lipid carriers as multifunctional nanomedicine for codelivery of DNA and doxorubicin. Int. J. Nanomed. 2014, 9, 4107–4116. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, J.; Kebebe, D.; Wu, Y.; Zhang, B.; Liu, Z. Cell penetrating peptides functionalized gambogic acid-nanostructured lipid carrier for cancer treatment. Drug Deliv. 2018, 25, 757–765. [Google Scholar] [CrossRef]
- Liles, W.C.; Broxmeyer, H.E.; Rodger, E.; Wood, B.; Hübel, K.; Cooper, S.; Hangoc, G.; Bridger, G.J.; Henson, G.W.; Calandra, G.; et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood 2003, 102, 2728–2730. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Wang, J.; Shelburne, C.E.; Lopatin, D.E.; Chinnaiyan, A.M.; Rubin, M.A.; Pienta, K.J.; Taichman, R.S. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J. Cell. Biochem. 2003, 89, 462–473. [Google Scholar] [CrossRef]
- Taichman, R.S.; Cooper, C.; Keller, E.T.; Pienta, K.J.; Taichman, N.S.; McCauley, L.K. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002, 62, 1832–1837. [Google Scholar]
- Aiuti, A.; Webb, I.; Bleul, C.; Springer, T.; Gutierrez-Ramos, J. The Chemokine SDF-1 Is a Chemoattractant for Human CD34+ Hematopoietic Progenitor Cells and Provides a New Mechanism to Explain the Mobilization of CD34+ Progenitors to Peripheral Blood. J. Exp. Med. 1997, 185, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Azad, B.B.; Nimmagadda, S. The Intricate Role of CXCR4 in Cancer. Adv. Cancer Res. 2014, 124, 31–82. [Google Scholar] [CrossRef]
- Slettenaar, V.I.; Wilson, J.L. The chemokine network: A target in cancer biology? Adv. Drug Deliv. Rev. 2006, 58, 962–974. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Yang, X.; Zhou, Y.; Ping, Q.; Oupicky, D.; Sun, M. Dual-function nanostructured lipid carriers to deliver IR780 for breast cancer treatment: Anti-metastatic and photothermal anti-tumor therapy. Acta Biomater. 2017, 53, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Domanska, U.M.; Timmer-Bosscha, H.; Nagengast, W.B.; Oude Munnink, T.H.; Kruizinga, R.C.; Ananias, H.J.; Kliphuis, N.M.; Huls, G.; De Vries, E.G.; de Jong, I.J.; et al. CXCR4 Inhibition with AMD3100 Sensitizes Prostate Cancer to Docetaxel Chemotherapy. Neoplasia 2012, 14, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Wientjes, M.G.; Au, J.L. Delivery of nanomedicines to extracellular and intracellular compartments of a solid tumor. Adv. Drug Deliv. Rev. 2012, 64, 29–39. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, Y.; Smith, E. Experimental Design for the Optimization of Lipid Nanoparticles. J. Pharm. Sci. 2009, 98, 1813–1819. [Google Scholar] [CrossRef]
- Das, S.; Chaudhury, A. Recent Advances in Lipid Nanoparticle Formulations with Solid Matrix for Oral Drug Delivery. AAPS PharmSciTech 2011, 12, 62–76. [Google Scholar] [CrossRef]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lipid nanocarriers for dermal delivery of lutein: Preparation, characterization, stability and performance. Int. J. Pharm. 2011, 414, 267–275. [Google Scholar] [CrossRef]
- Subramaniam, B.; Siddik, Z.H.; Nagoor, N.H. Optimization of nanostructured lipid carriers: Understanding the types, designs, and parameters in the process of formulations. J. Nanopart. Res. 2020, 22, 1–29. [Google Scholar] [CrossRef]
- Jenning, V.; Thünemann, A.F.; Gohla, S.H. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int. J. Pharm. 2000, 199, 167–177. [Google Scholar] [CrossRef]
- Pornputtapitak, W.; Pantakitcharoenkul, J.; Teeranachaideekul, V.; Sinthiptharakoon, K.; Sapcharoenkun, C.; Meemuk, B. Effect of Oil Content on Physiochemical Characteristics of γ-Oryzanol-Loaded Nanostructured Lipid Carriers. J. Oleo Sci. 2019, 68, 699–707. [Google Scholar] [CrossRef]
- Makoni, P.A.; Kasongo, K.W.; Walker, R.B. Short Term Stability Testing of Efavirenz-Loaded Solid Lipid Nanoparticle (SLN) and Nanostructured Lipid Carrier (NLC) Dispersions. Pharmaceutics 2019, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.C.; Borah, A.; Jindal, A.; Jacob, E.M.; Yamamoto, Y.; Kumar, D.S. BioPerine Encapsulated Nanoformulation for Overcoming Drug-Resistant Breast Cancers. Asian J. Pharm. Sci. 2020, 15, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Ghate, V.M.; Lewis, S.A.; Prabhu, P.; Dubey, A.; Patel, N. Nanostructured lipid carriers for the topical delivery of tretinoin. Eur. J. Pharm. Biopharm. 2016, 108, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Pathak, K. Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: Comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int. J. Pharm. 2011, 415, 232–243. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. Drug Res. 2010, 67, 217–223. [Google Scholar]
- Perrie, Y.; Rades, T. FASTtrack Pharmaceutics: Drug Delivery and Targeting; Pharmaceutical Press: London, UK; Chicago, IL, USA, 2012. [Google Scholar]
- Finke, J.H.; Richter, C.; Gothsch, T.; Kwade, A.; Büttgenbach, S.; Müller-Goymann, C.C. Coumarin 6 as a fluorescent model drug: How to identify properties of lipid colloidal drug delivery systems via fluorescence spectroscopy? Eur. J. Lipid Sci. Technol. 2014, 116, 1234–1246. [Google Scholar] [CrossRef]
- Xu, P.; Yin, Q.; Shen, J.; Chen, L.; Yu, H.; Zhang, Z.; Li, Y. Synergistic inhibition of breast cancer metastasis by silibinin-loaded lipid nanoparticles containing TPGS. Int. J. Pharm. 2013, 454, 21–30. [Google Scholar] [CrossRef]
- Aleanizy, F.S.; Alqahtani, F.Y.; Setó, S.; Al Khalil, N.; AlEshaiwi, L.; Alghamdi, M.; AlQuadeib, B.; Alkahtani, H.; Aldarwesh, A.; Alqahtani, Q.H.; et al. Trastuzumab Targeted Neratinib Loaded Poly-Amidoamine Dendrimer Nanocapsules for Breast Cancer Therapy. Int. J. Nanomed. 2020, 15, 5433–5443. [Google Scholar] [CrossRef]
- Zhu, W.B.; Zhao, Z.F.; Zhou, X. AMD3100 inhibits epithelial-mesenchymal transition, cell invasion, and metastasis in the liver and the lung through blocking the SDF-1α/CXCR4 signaling pathway in prostate cancer. J. Cell. Physiol. 2019, 234, 11746–11759. [Google Scholar] [CrossRef] [PubMed]
- Emami, J.; Rezazadeh, M.; Varshosaz, J.; Tabbakhian, M.; Aslani, A. Formulation of LDL Targeted Nanostructured Lipid Carriers Loaded with Paclitaxel: A Detailed Study of Preparation, Freeze Drying Condition, andIn VitroCytotoxicity. J. Nanomater. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Bui, N.Q.; Lim, I.G.; Oh, J. Paclitaxel-loaded chitosan oligosaccharide-stabilized gold nanoparticles as novel agents for drug delivery and photoacoustic imaging of cancer cells. Int. J. Pharm. 2016, 511, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.S.; Yoon, S.J.; Lee, J.Y.; Cho, N.H.; Choi, Y.D.; Song, Y.S.; Hong, S.J. Inhibition of tumor growth and histopathological changes following treatment with a chemokine receptor CXCR4 antagonist in a prostate cancer xenograft model. Oncol. Lett. 2013, 6, 933–938. [Google Scholar] [CrossRef]
- Nordin, N.; Yeap, S.K.; Rahman, H.S.; Zamberi, N.R.; Abu, N.; Mohamad, N.E.; How, C.W.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. In vitro cytotoxicity and anticancer effects of citral nanostructured lipid carrier on MDA MBA-231 human breast cancer cells. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pei, J.; Ma, Z.; Fu, J.; Chen, F.; Du, S. Docetaxel-loaded ultrasmall nanostructured lipid carriers for cancer therapy: In vitro and in vivo evaluation. Cancer Chemother. Pharmacol. 2020, 85, 731–739. [Google Scholar] [CrossRef]
- Li, W.; Fu, J.; Ding, Y.; Liu, D.; Jia, N.; Chen, D.; Hu, H. Low density lipoprotein-inspired nanostructured lipid nanoparticles containing pro-doxorubicin to enhance tumor-targeted therapeutic efficiency. Acta Biomater. 2019, 96, 456–467. [Google Scholar] [CrossRef]
- Stewart, M.P.; Sharei, A.R.; Ding, X.S.; Sahay, G.; Langer, R.S.; Jensen, K.F. In vitro and ex vivo strategies for intracellular delivery. Nature 2016, 538, 183–192. [Google Scholar] [CrossRef]
- Rahman, H.S.; Rasedee, A.; Othman, H.H.; Chartrand, M.S.; Namvar, F.; Yeap, S.K.; Samad, N.A.; Andas, R.J.; Nadzri, N.M.; Anasamy, T.; et al. Acute Toxicity Study of Zerumbone-Loaded Nanostructured Lipid Carrier on BALB/c Mice Model. BioMed Res. Int. 2014, 2014, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.; Yeap, S.K.; Zamberi, N.R.; Abu, N.; Mohamad, N.E.; Rahman, H.S.; How, C.W.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. Characterization and toxicity of citral incorporated with nanostructured lipid carrier. PeerJ 2018, 6, e3916. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Gatter, K. Ki67 protein: The immaculate deception? Histopathology 2002, 40, 2–11. [Google Scholar] [CrossRef]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 is a promising molecular target in the diagnosis of cancer (Review). Mol. Med. Rep. 2015, 11, 1566–1572. [Google Scholar] [CrossRef]
- Meteoglu, I.; Erdoğdu, I.H.; Tuncyurek, P.; Coşkun, A.; Culhaci, N.; Erkus, M.; Barutca, S. Nuclear Factor Kappa B, Matrix Metalloproteinase-1, p53, and Ki-67 Expressions in the Primary Tumors and the Lymph Node Metastases of Colorectal Cancer Cases. Gastroenterol. Res. Pract. 2015, 2015, 945392-9. [Google Scholar] [CrossRef]
- Singh, S.; Singh, U.P.; Grizzle, W.E.; Lillard, J.W. CXCL12-CXCR4 interactions modulate prostate cancer cell migration, metalloproteinase expression and invasion. Lab. Investig. 2004, 84, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cheng, G.; Hao, M.; Zheng, J.; Zhou, X.; Zhang, J.; Taichman, R.S.; Pienta, K.J.; Wang, J. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010, 29, 709–722. [Google Scholar] [CrossRef]
- Zhi, Y.; Lu, H.; Duan, Y.; Sun, W.; Guan, G.; Dong, Q.; Yang, C. Involvement of the nuclear factor-κB signaling pathway in the regulation of CXC chemokine receptor-4 expression in neuroblastoma cells induced by tumor necrosis factor-α. Int. J. Mol. Med. 2015, 35, 349–357. [Google Scholar] [CrossRef]
- Helbig, G.; Christopherson, K.W., 2nd; Bhat-Nakshatri, P.; Kumar, S.; Kishimoto, H.; Miller, K.D.; Broxmeyer, H.E.; Nakshatri, H. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J. Biol. Chem. 2003, 278, 21631-8. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an Active Player in Human Cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, C.; Shen, J.; Wang, D.; Hao, J.; Hu, Z. SDF-1/CXCR4 axis induces apoptosis of human degenerative nucleus pulposus cells via the NF-κB pathway. Mol. Med. Rep. 2016, 14, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Verheul, H.M.; Pinedo, H.M. The Role of Vascular Endothelial Growth Factor (VEGF) in Tumor Angiogenesis and Early Clinical Development of VEGFReceptor Kinase Inhibitors. Clin. Breast Cancer 2000, 1, S80–S84. [Google Scholar] [CrossRef]
- Xie, T.-X.; Xia, Z.; Zhang, N.; Gong, W.; Huang, S. Constitutive NF-κB activity regulates the expression of VEGF and IL-8 and tumor angiogenesis of human glioblastoma. Oncol. Rep. 2010, 23, 725–732. [Google Scholar] [PubMed]
- Kiriakidis, S.; Andreakos, E.; Monaco, C.; Foxwell, B.; Feldmann, M.; Paleolog, E. VEGF expression in human macrophages is NF-κB-dependent: Studies using adenoviruses expressing the endogenous NF-κB inhibitor IκBα and a kinase-defective form of the IκB kinase 2. J. Cell Sci. 2003, 116, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhang, H.; Gao, D.; Wang, H.; Li, Y.; Liu, G. Significance of VEGF and NF-kB expression in thyroid carcinoma. Chin. Clin. Oncol. 2006, 3, 166–171. [Google Scholar] [CrossRef]
- Nordin, N.; Yeap, S.K.; Rahman, H.S.; Zamberi, N.R.; Mohamad, N.E.; Abu, N.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. Antitumor and Anti-Metastatic Effects of Citral-Loaded Nanostructured Lipid Carrier in 4T1-Induced Breast Cancer Mouse Model. Molecules 2020, 25, 2670. [Google Scholar] [CrossRef]

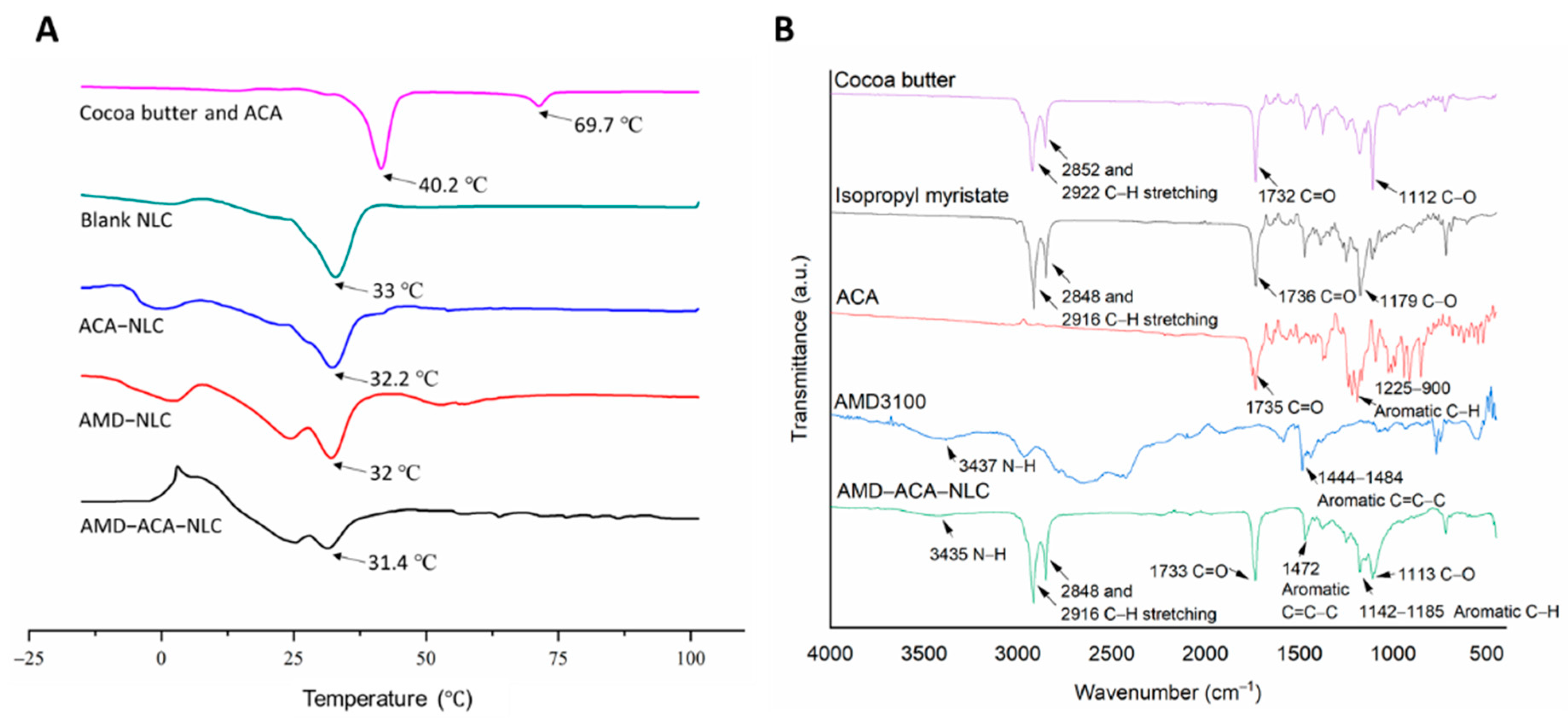

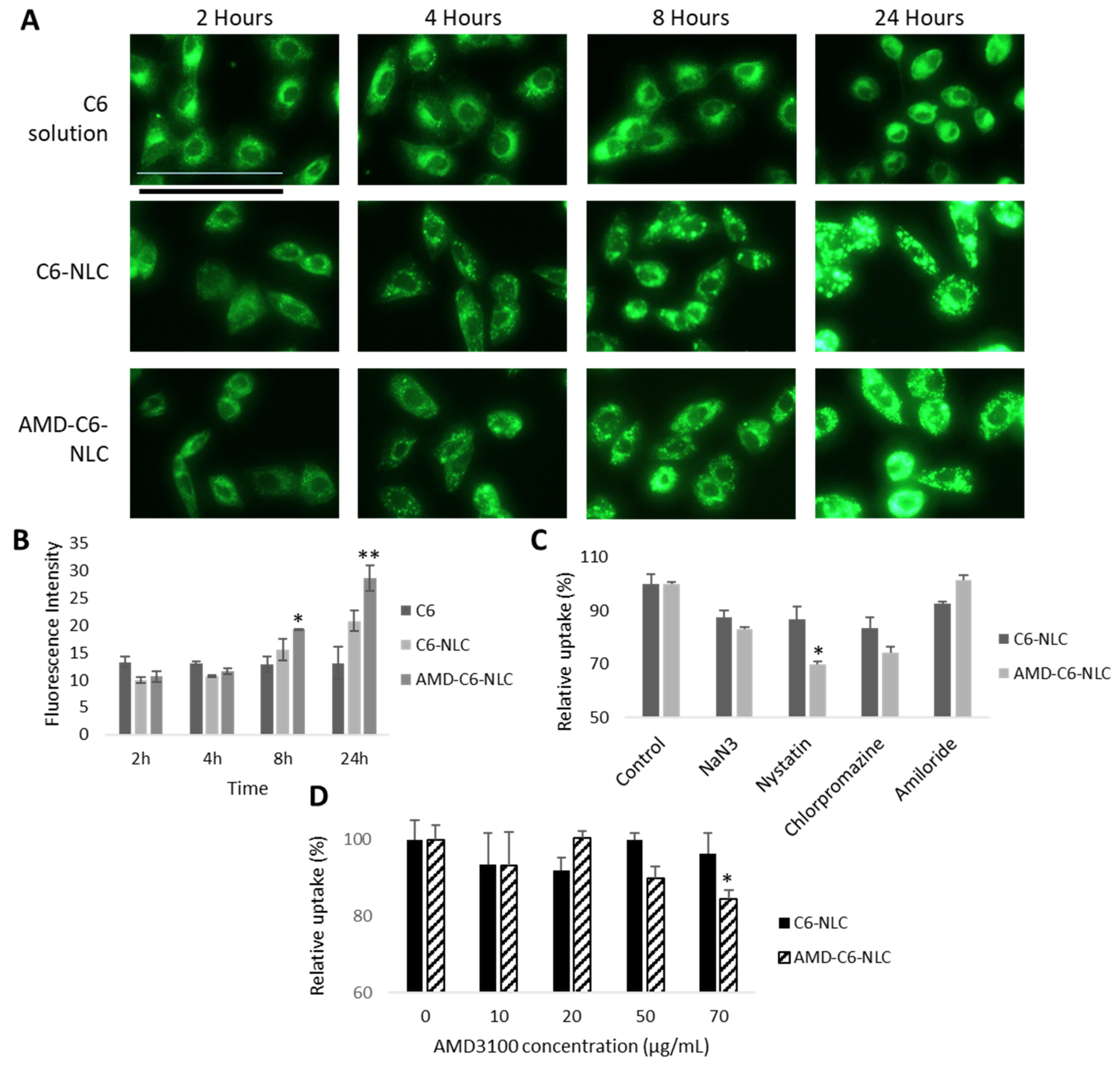
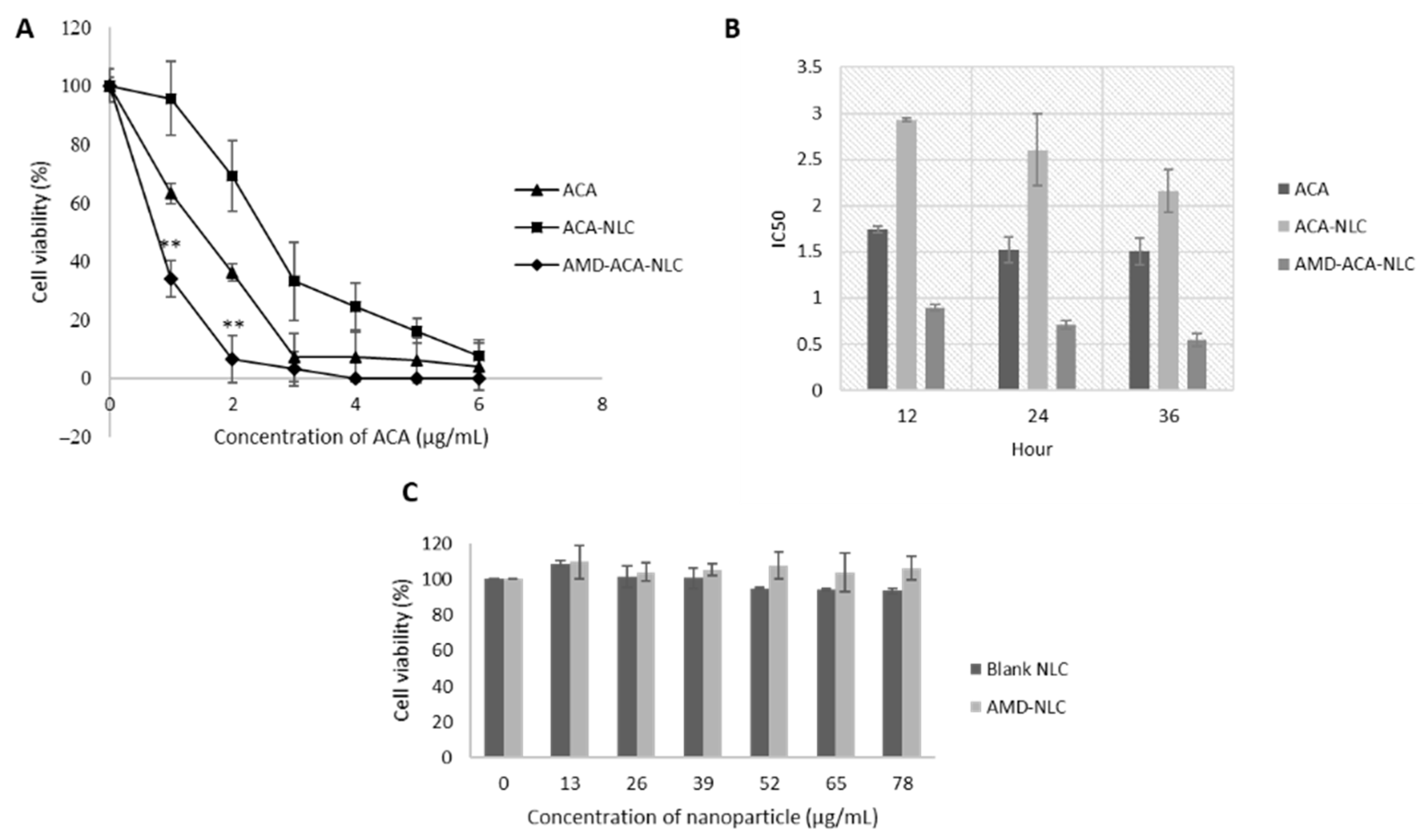
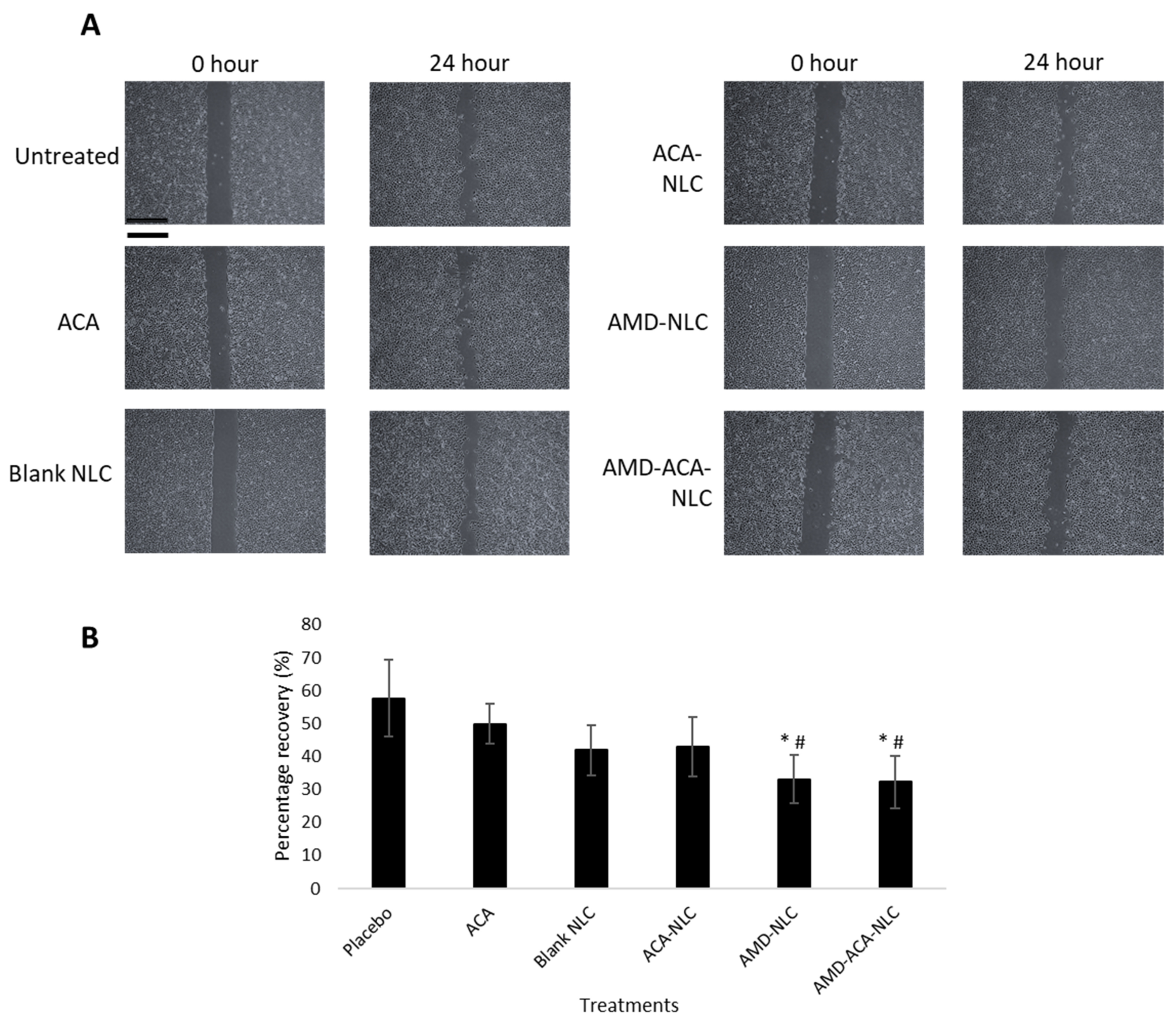

| Samples | Particle Size (nm) | PDI | Zeta Potential (mV) | Entrapment Efficiency (ACA) | Coating Efficiency (AMD3100) |
|---|---|---|---|---|---|
| Blank NLC | 116.9 ± 0.60 | 0.204 ± 0.01 | −30.9 ± 1.41 | - | - |
| ACA-NLC | 115.0 ± 1.27 | 0.185 ± 0.01 | −23.1 ± 0.78 | 94.0 ± 3.77 | - |
| AMD-NLC | 115.2 ± 0.36 | 0.145 ± 0.02 | −12.0 ± 0.94 | - | 77.3 ± 3.21 |
| AMD-ACA-NLC | 120.7 ± 2.07 | 0.145 ± 0.02 | −11.9 ± 0.27 | 91.3 ± 2.54 | 74.9 ± 5.17 |
| Cell Line | Treatments | IC50 (µg/mL) | |
|---|---|---|---|
| 12 h | 24 h | ||
| RWPE-1 | ACA | >6 | >6 |
| ACA-NLC | 4.81 ± 0.11 | 4.47 ± 0.66 | |
| AMD-ACA-NLC | 4.96 ± 0.01 | 5.12 ± 0.50 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramaniam, B.; Arshad, N.M.; Malagobadan, S.; Misran, M.; Nyamathulla, S.; Mun, K.S.; Nagoor, N.H. Development and Evaluation of 1′-Acetoxychavicol Acetate (ACA)-Loaded Nanostructured Lipid Carriers for Prostate Cancer Therapy. Pharmaceutics 2021, 13, 439. https://doi.org/10.3390/pharmaceutics13040439
Subramaniam B, Arshad NM, Malagobadan S, Misran M, Nyamathulla S, Mun KS, Nagoor NH. Development and Evaluation of 1′-Acetoxychavicol Acetate (ACA)-Loaded Nanostructured Lipid Carriers for Prostate Cancer Therapy. Pharmaceutics. 2021; 13(4):439. https://doi.org/10.3390/pharmaceutics13040439
Chicago/Turabian StyleSubramaniam, Bavani, Norhafiza M. Arshad, Sharan Malagobadan, Misni Misran, Shaik Nyamathulla, Kein Seong Mun, and Noor Hasima Nagoor. 2021. "Development and Evaluation of 1′-Acetoxychavicol Acetate (ACA)-Loaded Nanostructured Lipid Carriers for Prostate Cancer Therapy" Pharmaceutics 13, no. 4: 439. https://doi.org/10.3390/pharmaceutics13040439
APA StyleSubramaniam, B., Arshad, N. M., Malagobadan, S., Misran, M., Nyamathulla, S., Mun, K. S., & Nagoor, N. H. (2021). Development and Evaluation of 1′-Acetoxychavicol Acetate (ACA)-Loaded Nanostructured Lipid Carriers for Prostate Cancer Therapy. Pharmaceutics, 13(4), 439. https://doi.org/10.3390/pharmaceutics13040439






