LipoParticles: Lipid-Coated PLA Nanoparticles Enhanced In Vitro mRNA Transfection Compared to Liposomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. PLA-NP Synthesis
2.2.2. Microfluidic Production of Liposomes
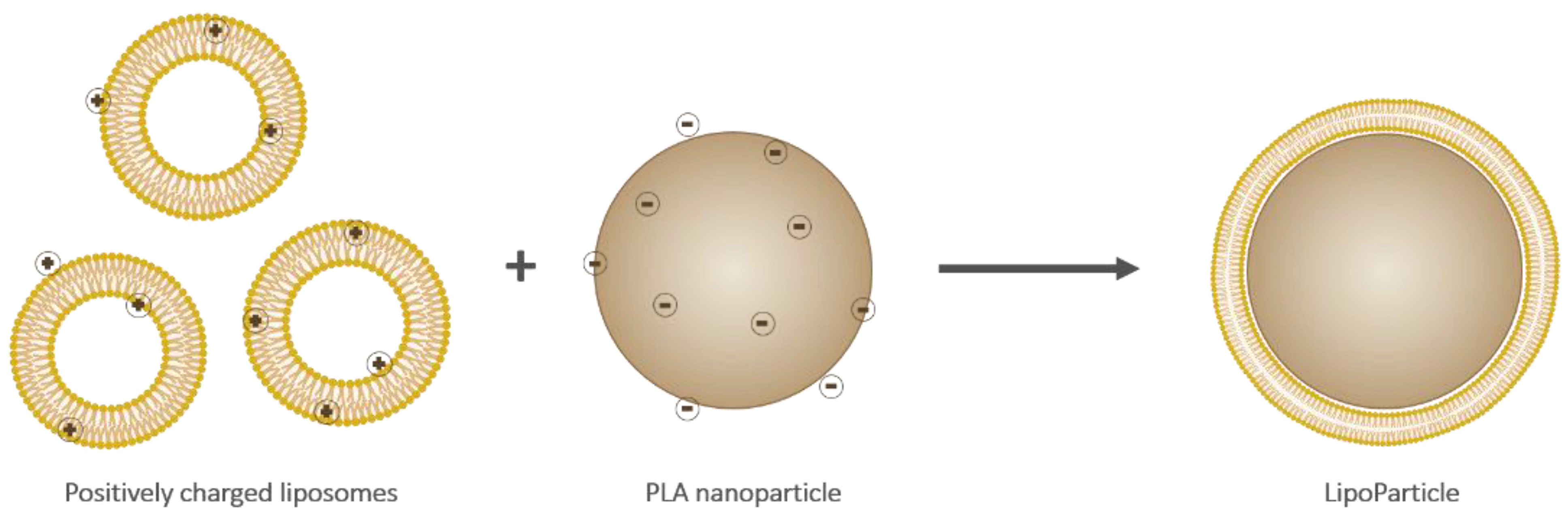
2.2.3. LipoParticle Formulation from PLA-NP and Liposomes
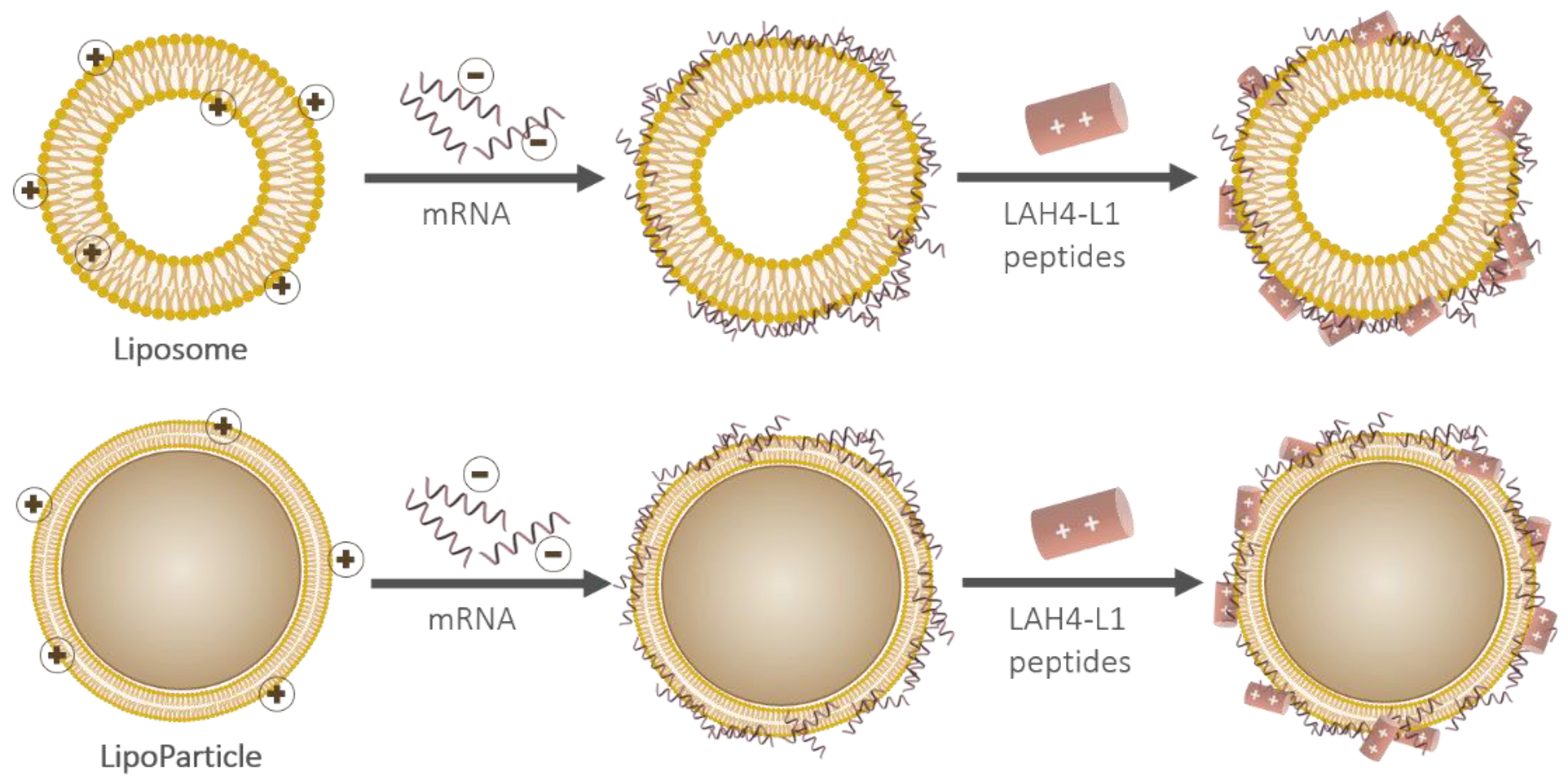
2.2.4. Adsorption of Nucleic Acids and Cationic Cell-Penetrating Peptides Using pLbL Formulation
2.2.5. Size and Zeta Potential Measurements
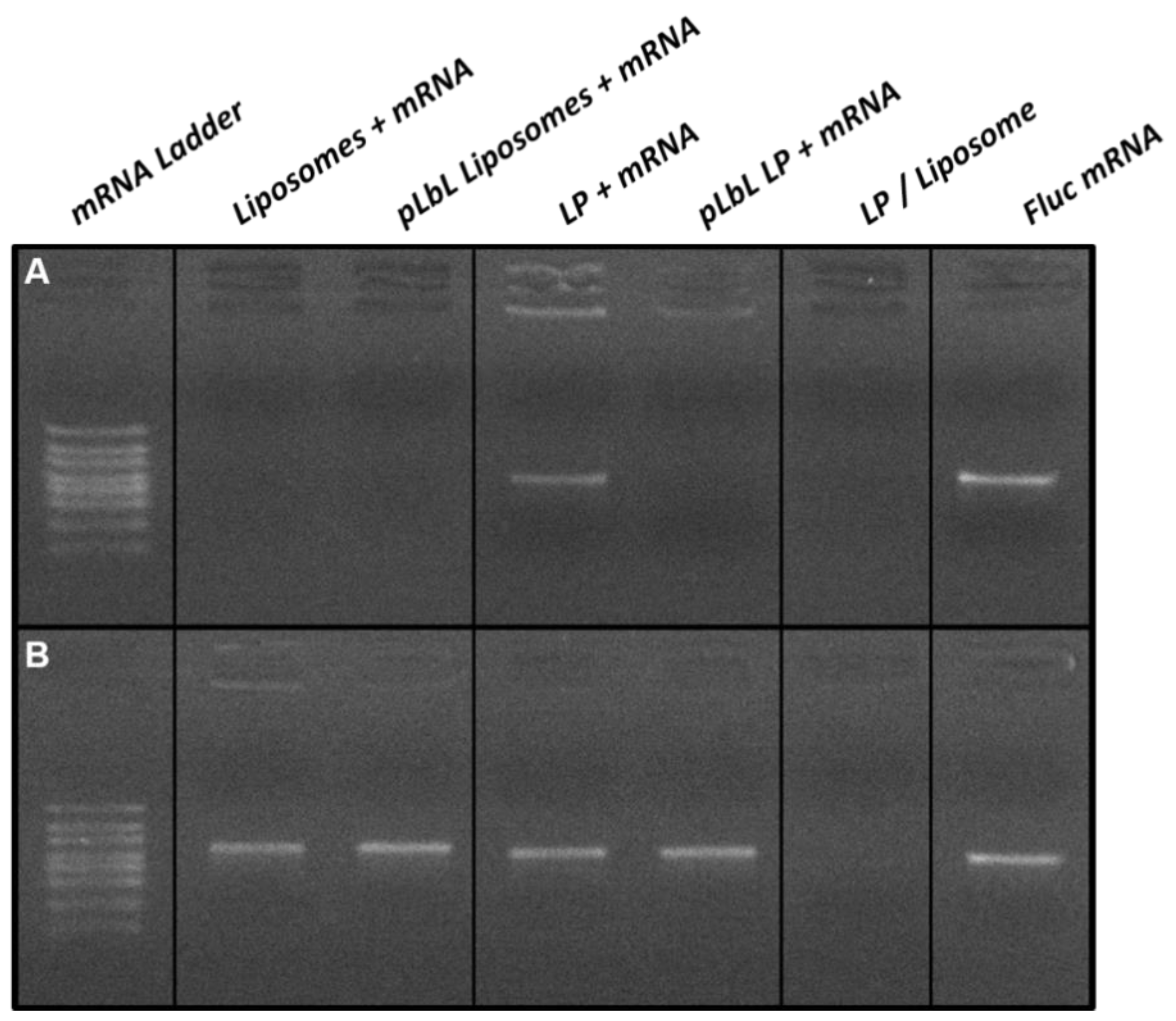
2.2.6. Gel Retardation Assay
2.2.7. Cell Culture
2.2.8. Transfection
2.2.9. Luciferase Assay
2.2.10. Fluorescent Microscopy (eGFP)
2.2.11. Cytotoxicity
2.2.12. Statistical Analysis
3. Results
3.1. Study of the Added Value of a Solid Core within Liposomes
3.1.1. Complexation of mRNA and LAH4-L1 on the Lipid Vectors Using the pLbL Approach
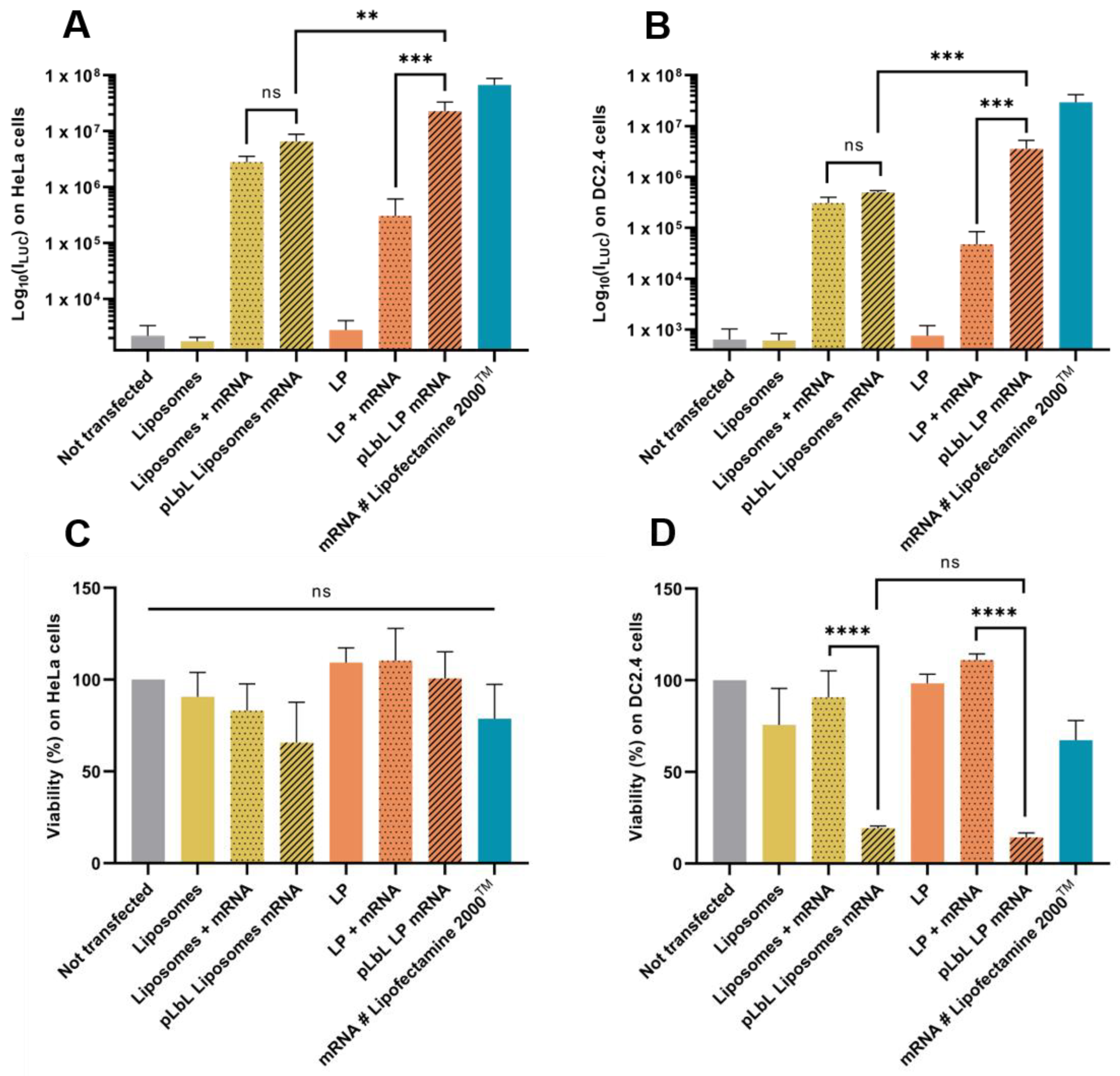
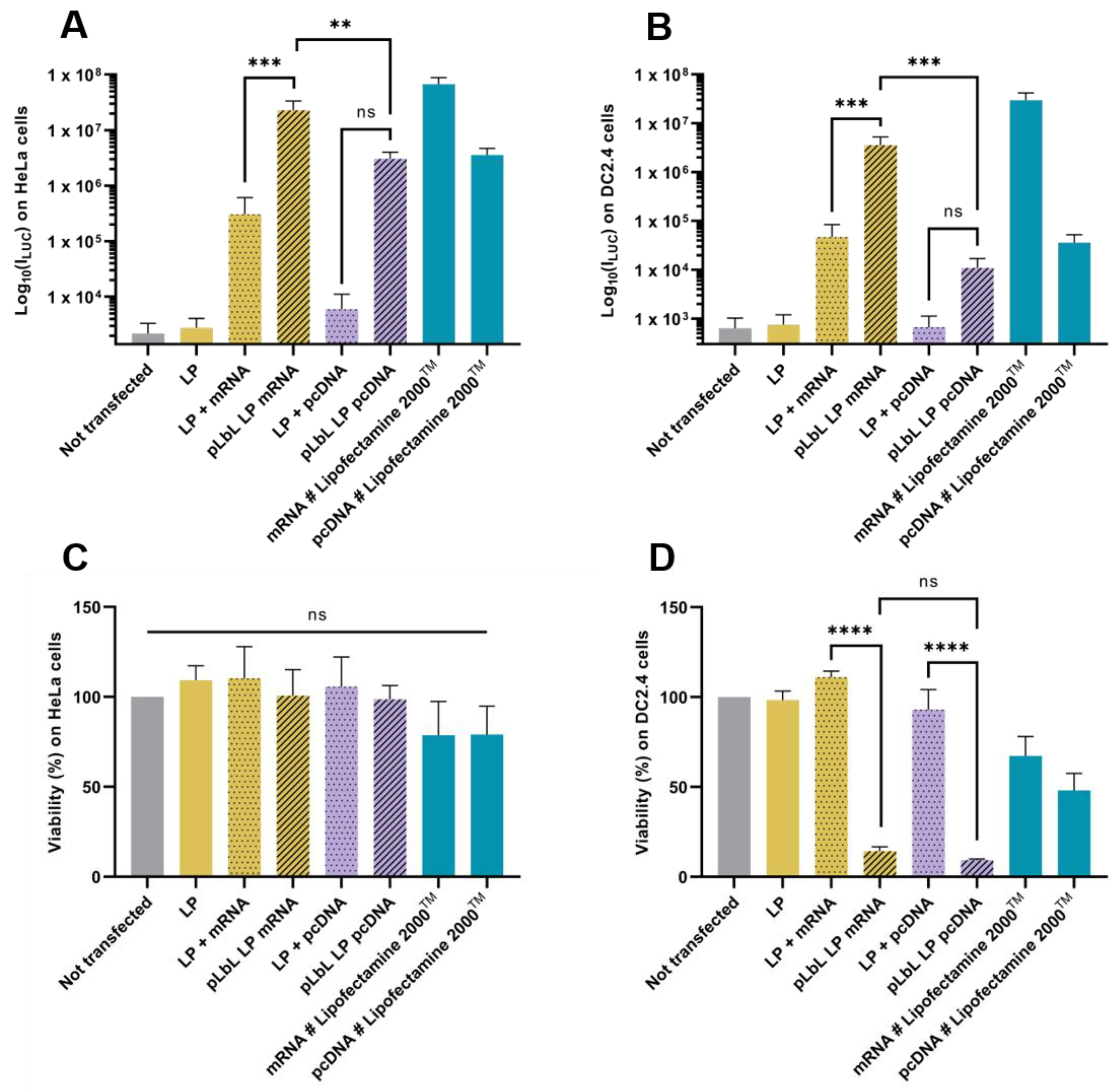
3.1.2. Fluc mRNA Transfection Capacity and In Vitro Cytotoxicity of Each Formulation
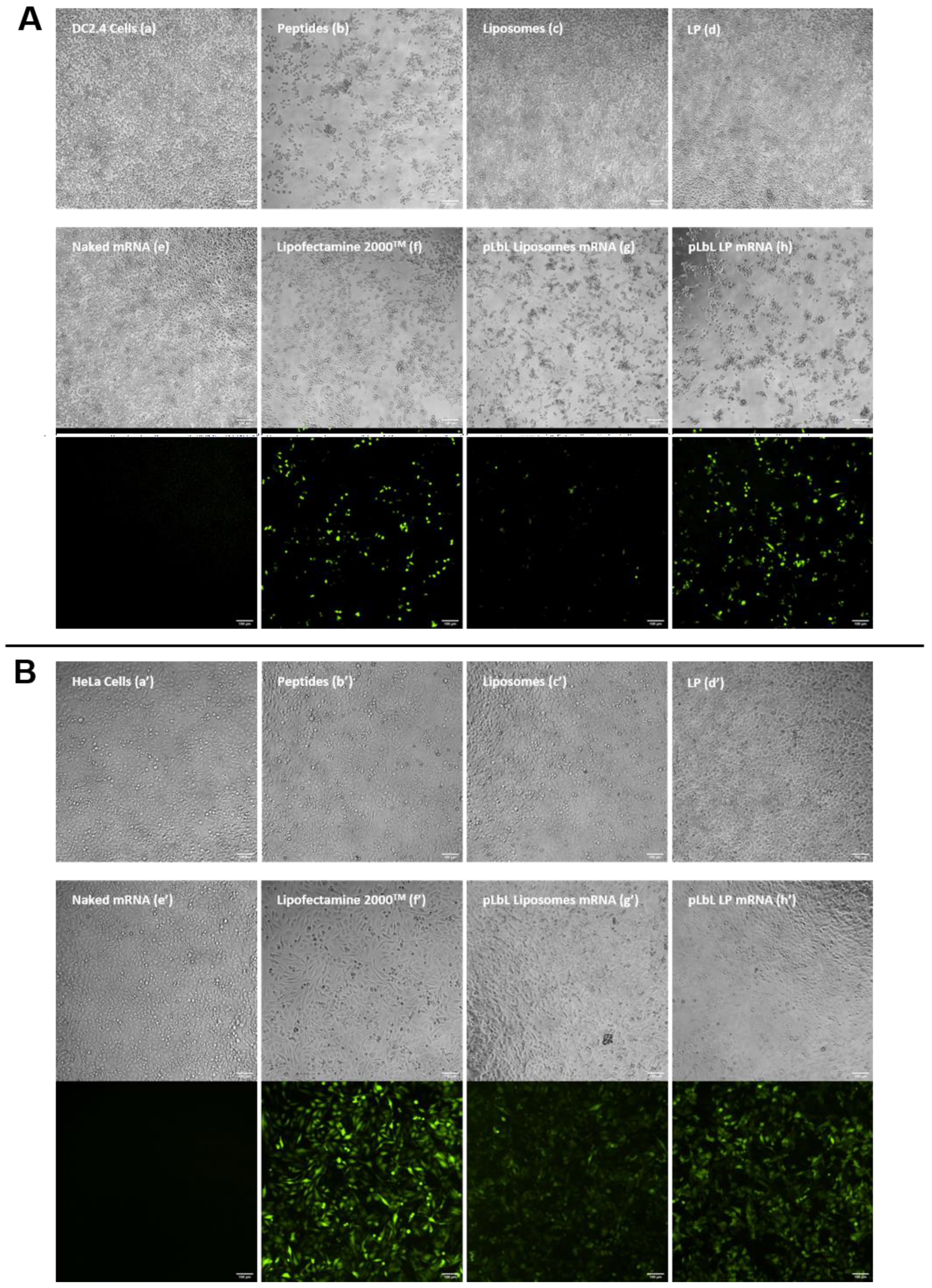
3.1.3. eGFP mRNA Transfection of Lipid Formulations
3.2. Study of the Versatility of the LP as Carrier
3.3. Optimisation Strategy to Reduce Cytotoxicity on Dendritric Cells of the pLbL Approach: Modification of the Nucleic Acids/Peptides Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piyush, R.; Rajarshi, K.; Chatterjee, A.; Khan, R.; Ray, S. Nucleic acid-based therapy for coronavirus disease 2019. Heliyon 2020, 6, e05007. [Google Scholar] [CrossRef]
- Nanomedicine and the COVID-19 vaccines. Nat. Nanotechnol. 2020, 15, 963. [CrossRef]
- Izda, V.; Jeffries, M.A.; Sawalha, A.H. COVID-19: A review of therapeutic strategies and vaccine candidates. Clin. Immunol. 2020, 222, 108634. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines-a new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Hajj, K.A.; Whitehead, K.A. Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017, 2, 1–17. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Weissman, D. Recent advances in mRNA vaccine technology. Curr. Opin. Immunol. 2020, 65, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, A.M.; Oberli, M.A.; Jeklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef]
- Maruggi, G.; Zhang, C.; Li, J.; Ulmer, J.B.; Yu, D. mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Mol. Ther. 2019, 27, 757–772. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef]
- Coolen, A.L.; Lacroix, C.; Mercier-Gouy, P.; Delaune, E.; Monge, C.; Exposito, J.Y.; Verrier, B. Poly(lactic acid) nanoparticles and cell-penetrating peptide potentiate mRNA-based vaccine expression in dendritic cells triggering their activation. Biomaterials 2019, 195, 23–37. [Google Scholar] [CrossRef]
- Xu, S.; Yang, K.; Li, R.; Zhang, L. Mrna vaccine era—Mechanisms, drug platform and clinical prospection. Int. J. Mol. Sci. 2020, 21, 6582. [Google Scholar] [CrossRef]
- Sahin, U.; Karikó, K.; Türeci, Ö. MRNA-based therapeutics-developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Pollard, C.; De Koker, S.; Saelens, X.; Vanham, G.; Grooten, J. Challenges and advances towards the rational design of mRNA vaccines. Trends Mol. Med. 2013, 19, 705–713. [Google Scholar] [CrossRef]
- Linares-Fernández, S.; Lacroix, C.; Exposito, J.Y.; Verrier, B. Tailoring mRNA Vaccine to Balance Innate/Adaptive Immune Response. Trends Mol. Med. 2020, 26, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Anderson, D.G.; Sidik, S.M.; Lourido, S.; Langer, R.; et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. USA 2016, 113, E4133–E4142. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Sloand, J.N.; Gaffey, A.C.; Venkataraman, C.M.; Wang, Z.; Trubelja, A.; Hammer, D.A.; Atluri, P.; Burdick, J.A. Injectable, Guest-Host Assembled Polyethylenimine Hydrogel for siRNA Delivery. Biomacromolecules 2017, 18, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Démoulins, T.; Ebensen, T.; Schulze, K.; Englezou, P.C.; Pelliccia, M.; Guzmán, C.A.; McCullough, K.C.; Ruggli, N. Self-replicating RNA vaccine functionality modulated by fine-tuning of polyplex delivery vehicle structure. J. Control. Release 2017, 266, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Mccarthy, H.O.; McCaffrey, J.; Mccrudden, C.M.; Zholobenko, A.; Ali, A.A.; McBride, J.W.; Massey, A.S.; Pentlavalli, S.; Chen, K.-H.; Cole, G.; et al. Development and characterization of self-assembling nanoparticles using a bio-inspired amphipathic peptide for gene delivery. J. Control. Release 2014, 189, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, C.; Humanes, A.; Coiffier, C.; Gigmes, D.; Verrier, B.; Trimaille, T. Polylactide-Based Reactive Micelles as a Robust Platform for mRNA Delivery. Pharm. Res. 2020, 37. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Geall, A.J.; Verma, A.; Otten, G.R.; Shaw, C.A.; Hekele, A.; Banerjee, K.; Cu, Y.; Beard, C.W.; Brito, L.A.; Krucker, T.; et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci. USA 2012, 109, 14604–14609. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, K.J.; Dorkin, J.R.; Yang, J.H.; Heartlein, M.W.; Derosa, F.; Mir, F.F.; Fenton, O.S.; Anderson, D.G. Optimization of Lipid Nanoparticle Formulations for mRNA Delivery in Vivo with Fractional Factorial and Definitive Screening Designs. Nano Lett. 2015, 15, 7300–7306. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, J.; Troutier, A.L.; Putaux, J.L.; Delair, T.; Ladavière, C. Effect of the polymer nature on the structural organization of lipid/polymer particle assemblies. J. Phys. Chem. B 2008, 112, 13812–13822. [Google Scholar] [CrossRef]
- Troutier, A.L.; Ladavière, C. An overview of lipid membrane supported by colloidal particles. Adv. Colloid Interface Sci. 2007, 133, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, J.; Troutier, A.L.; David, L.; Delair, T.; Ladavière, C. Steric stabilization of lipid/polymer particle assemblies by poly(ethylene glycol)-lipids. Biomacromolecules 2007, 8, 3651–3660. [Google Scholar] [CrossRef]
- Troutier-Thuilliez, A.L.; Thevenot, J.; Delair, T.; Ladavière, C. Adsorption of plasmid DNA onto lipid/polymer particle assemblies. Soft Matter 2009, 5, 4739–4747. [Google Scholar] [CrossRef]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, D.; Exbrayat-Héritier, C.; Rambaud, B.; Megy, S.; Terreux, R.; Verrier, B.; Primard, C. Surface charge modulation of rifampicin-loaded PLA nanoparticles to improve antibiotic delivery in Staphylococcus aureus biofilms. J. Nanobiotechnol. 2021, 19. [Google Scholar] [CrossRef]
- Troutier, A.L.; Véron, L.; Delair, T.; Pichot, C.; Ladavière, C. New insights into self-organization of a model lipid mixture and quantification of its adsorption on spherical polymer particles. Langmuir 2005, 21, 9901–9910. [Google Scholar] [CrossRef]
- Troutier, A.L.; Delair, T.; Pichot, C.; Ladavière, C. Physicochemical and interfacial investigation of lipid/polymer particle assemblies. Langmuir 2005, 21, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Meng, Y.; Zhao, Y.; Zhu, J.; Xu, H.; Zhang, E.; Shi, L.; Du, L.; Liu, G.; Zhang, C.; et al. Toxicological exploration of peptide-based cationic liposomes in siRNA delivery. Colloids Surf. B Biointerfaces 2019, 179, 66–76. [Google Scholar] [CrossRef]
- Knudsen, K.B.; Northeved, H.; Pramod Kumar, E.K.; Permin, A.; Gjetting, T.; Andresen, T.L.; Wegener, K.M.; Lykkesfeldt, J.; Larsen, S.; Jantzen, K.; et al. In vivo toxicity of cationic micelles and liposomes. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 467–477. [Google Scholar] [CrossRef]
- Schöler, N.; Olbrich, C.; Tabatt, K.; Müller, R.H.; Hahn, H.; Liesenfeld, O. Surfactant, but not the size of solid lipid nanoparticles (SLN) influences viability and cytokine production of macrophages. Int. J. Pharm. 2001, 221, 57–67. [Google Scholar] [CrossRef]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Liposomes: From Bangham to Supercritical Fluids. Processes 2020, 8, 1022. [Google Scholar] [CrossRef]
- Lehto, T.; Ezzat, K.; Wood, M.J.; Andaloussi, S.E. Peptides for nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 106, 172–182. [Google Scholar] [CrossRef]
- Teo, S.L.Y.; Rennick, J.J.; Yuen, D.; Al-Wassiti, H.; Johnston, A.P.R.; Pouton, C.W. Unravelling cytosolic delivery of endosomal escape peptides with a quantitative endosomal escape assay (SLEEQ). BioRxiv 2020. [Google Scholar] [CrossRef]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M.; et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, S.; Kumarasinghe, E.S.; Salerno, T.; Mihai, C.; Ketova, T.; Senn, J.J.; Lynn, A.; Bulychev, A.; Chan, J.; Almarsson, Ö.; et al. A Novel Amino Lipid Series for mRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-human Primates. Mol. Ther. 2018, 26, 1509–1519. [Google Scholar] [CrossRef]
- Moulay, G.; Leborgne, C.; Mason, A.J.; Aisenbrey, C.; Kichler, A.; Bechinger, B. Histidine-rich designer peptides of the LAH4 family promote cell delivery of a multitude of cargo. J. Pept. Sci. 2017, 23, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Farkhani, S.M.; Valizadeh, A.; Karami, H.; Mohammadi, S.; Sohrabi, N.; Badrzadeh, F. Cell penetrating peptides: Efficient vectors for delivery of nanoparticles, nanocarriers, therapeutic and diagnostic molecules. Peptides 2014, 57, 78–94. [Google Scholar] [CrossRef]
- Martin, M.E.; Rice, K.G. Peptide-guided gene delivery. AAPS J. 2007, 9, E18. [Google Scholar] [CrossRef] [PubMed]
- Langlet-Bertin, B.; Leborgne, C.; Scherman, D.; Bechinger, B.; Mason, A.J.; Kichler, A. Design and evaluation of histidine-rich amphipathic peptides for siRNA delivery. Pharm. Res. 2010, 27, 1426–1436. [Google Scholar] [CrossRef]
- Kichler, A.; Leborgne, C.; März, J.; Danos, O.; Bechinger, B. Histidine-rich amphipathic peptide antibiotics promote efficient delivery of DNA into mammalian cells. Proc. Natl. Acad. Sci. USA 2003, 100, 1564–1568. [Google Scholar] [CrossRef]
- Legaz, S.; Exposito, J.Y.; Lethias, C.; Viginier, B.; Terzian, C.; Verrier, B. Evaluation of polylactic acid nanoparticles safety using Drosophila model. Nanotoxicology 2016, 10, 1136–1143. [Google Scholar] [CrossRef]
- Vale, N.; Duarte, D.; Silva, S.; Correia, A.S.; Costa, B.; Gouveia, M.J.; Ferreira, A. Cell-penetrating peptides in oncologic pharmacotherapy: A review. Pharmacol. Res. 2020, 162, 105231. [Google Scholar] [CrossRef]
- Gao, W.; Hu, C.-M.J.; Fang, R.H.; Zhang, L. Liposome-like Nanostructures for Drug Delivery. J. Mater. Chem. B 2013, 1, 6569–6585. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, T.; Anam, K.; Ali, N. Influence of phospholipid composition on the adjuvanticity and protective efficacy of liposome-encapsulated Leishmania donovani antigens. J. Parasitol. 2005, 91, 269–274. [Google Scholar] [CrossRef]
- Christensen, D.; Henriksen-Lacey, M.; Kamath, A.T.; Lindenstrøm, T.; Korsholm, K.S.; Christensen, J.P.; Rochat, A.-F.; Lambert, P.-H.; Andersen, P.; Perrie, Y.; et al. A cationic vaccine adjuvant based on a saturated quaternary ammonium lipid have different in vivo distribution kinetics and display a distinct CD4 T cell-inducing capacity compared to its unsaturated analog. J. Control. Release 2012, 160, 468–476. [Google Scholar] [CrossRef]
- Kastner, E.; Schmidt, S.T.; Wilkinson, A.; Christensen, D.; Perrie, Y. The Application of Liposomes as Vaccine Adjuvants. In Subunit Vaccine Delivery; Springer: Berlin/Heidelberg, Germany, 2015; pp. 77–94. [Google Scholar] [CrossRef]

| Type of Vector | Mean Hydrodynamic Diameter (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) |
|---|---|---|---|
| NP-PLA (for LP elaboration) | 151 ± 3 | 0.094 ± 0.013 | −64 ± 3 |
| Liposomes (for LP elaboration) | 81 ± 3 | 0.215 ± 0.009 | 45 ± 4 |
| LipoParticles | 245 ± 14 | 0.135 ± 0.007 | 58 ± 4 |
| Formulation | Mean Hydrodynamic Diameter (nm) | Dispersity (PDI) | Zeta Potential (mV) |
|---|---|---|---|
| Liposomes | 81 ± 3 | 0.215 ± 0.009 | 45 ± 4 |
| Liposomes-mRNA | 108 ± 4 | 0.370 ± 0.048 | 43 ± 5 |
| Liposomes-mRNA-LAH4-L1 (pLbL) | 113 ± 1 | 0.283 ± 0.082 | 44 ± 2 |
| LP | 245 ± 14 | 0.135 ± 0.007 | 58 ± 4 |
| LP-mRNA | 260 ± 27 | 0.229 ± 0.037 | −38 ± 7 |
| LP-mRNA-LAH4-L1 (pLbL) | 305 ± 16 | 0.269 ± 0.026 | 44 ± 2 |
| Formulation | Mean Hydrodynamic Diameter (nm) | Dispersity (PDI) | Zeta Potential (mV) |
|---|---|---|---|
| LP | 245 ± 14 | 0.135 ± 0.007 | 58 ± 4 |
| LP-pcDNA | 307 ± 21 | 0.264 ± 0.039 | −37 ± 6 |
| LP-pcDNA-LAH4-L1 (pLbL) | 306 ± 4 | 0.286 ± 0.013 | 37 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayad, C.; Libeau, P.; Lacroix-Gimon, C.; Ladavière, C.; Verrier, B. LipoParticles: Lipid-Coated PLA Nanoparticles Enhanced In Vitro mRNA Transfection Compared to Liposomes. Pharmaceutics 2021, 13, 377. https://doi.org/10.3390/pharmaceutics13030377
Ayad C, Libeau P, Lacroix-Gimon C, Ladavière C, Verrier B. LipoParticles: Lipid-Coated PLA Nanoparticles Enhanced In Vitro mRNA Transfection Compared to Liposomes. Pharmaceutics. 2021; 13(3):377. https://doi.org/10.3390/pharmaceutics13030377
Chicago/Turabian StyleAyad, Camille, Pierre Libeau, Céline Lacroix-Gimon, Catherine Ladavière, and Bernard Verrier. 2021. "LipoParticles: Lipid-Coated PLA Nanoparticles Enhanced In Vitro mRNA Transfection Compared to Liposomes" Pharmaceutics 13, no. 3: 377. https://doi.org/10.3390/pharmaceutics13030377
APA StyleAyad, C., Libeau, P., Lacroix-Gimon, C., Ladavière, C., & Verrier, B. (2021). LipoParticles: Lipid-Coated PLA Nanoparticles Enhanced In Vitro mRNA Transfection Compared to Liposomes. Pharmaceutics, 13(3), 377. https://doi.org/10.3390/pharmaceutics13030377






