Safety and Tolerability of Topical Ophthalmic Triamcinolone Acetonide-Loaded Liposomes Formulation and Evaluation of Its Biologic Activity in Patients with Diabetic Macular Edema
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of TALF
2.2. Evaluation of Safety and Tolerability of TALF in Animals
2.3. Evaluation of Safety and Tolerability in Healthy Volunteers
2.4. Evaluation of the Biologic Activity of TALF in Patients with Diabetic Macular Edema
2.5. Ethical Considerations
2.6. Statistical Analysis
3. Results
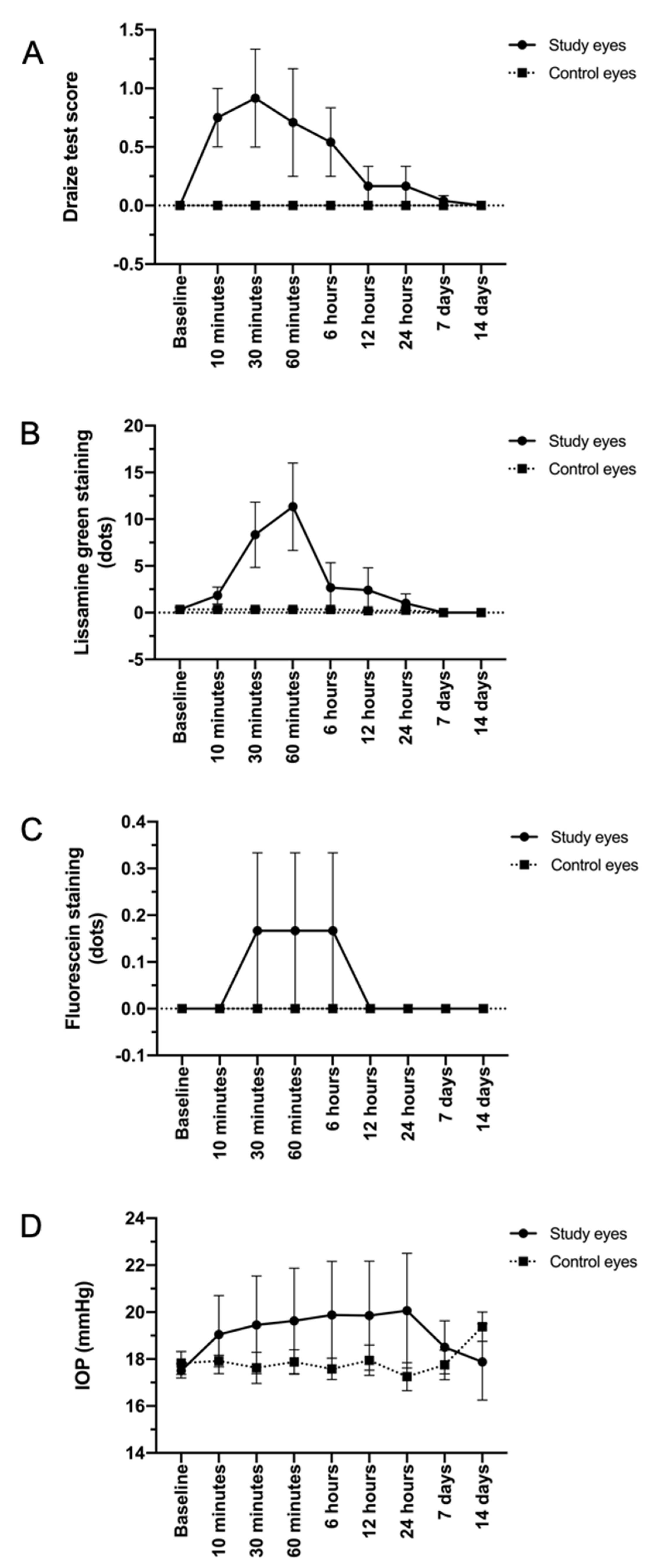
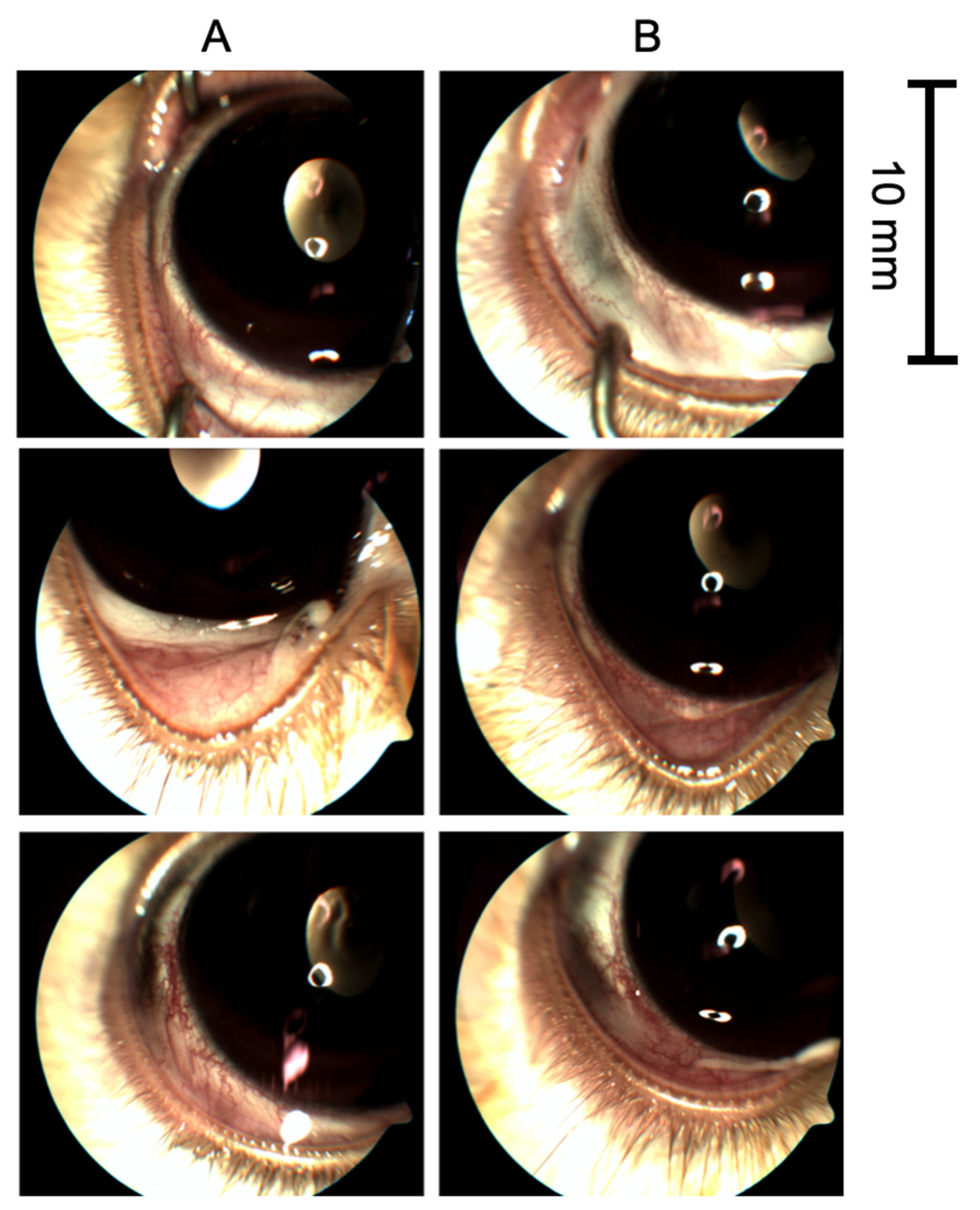
3.1. TALF Is Well Tolerated in the Preclinical Model
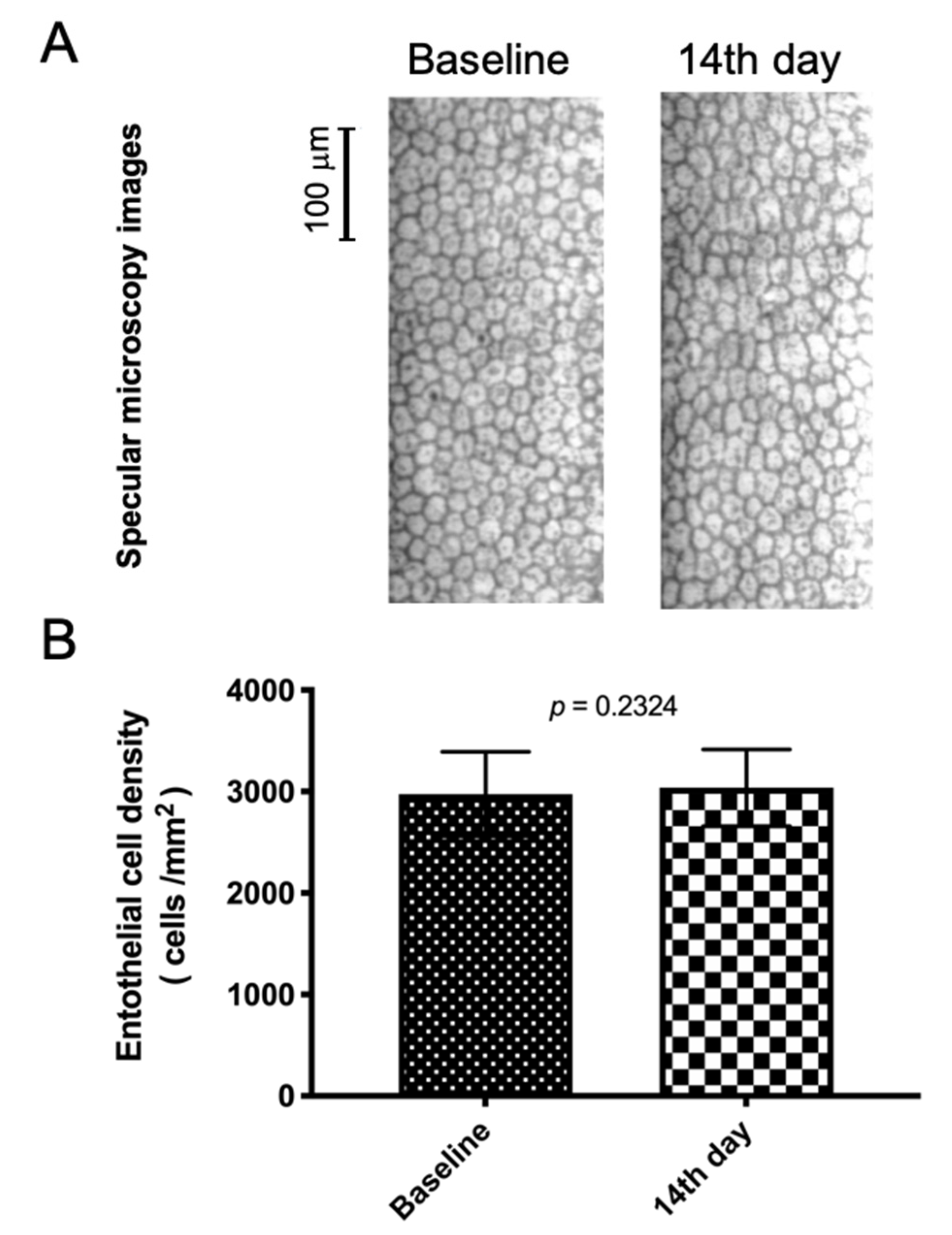
3.2. TALF Is Safe for Healthy Volunteers
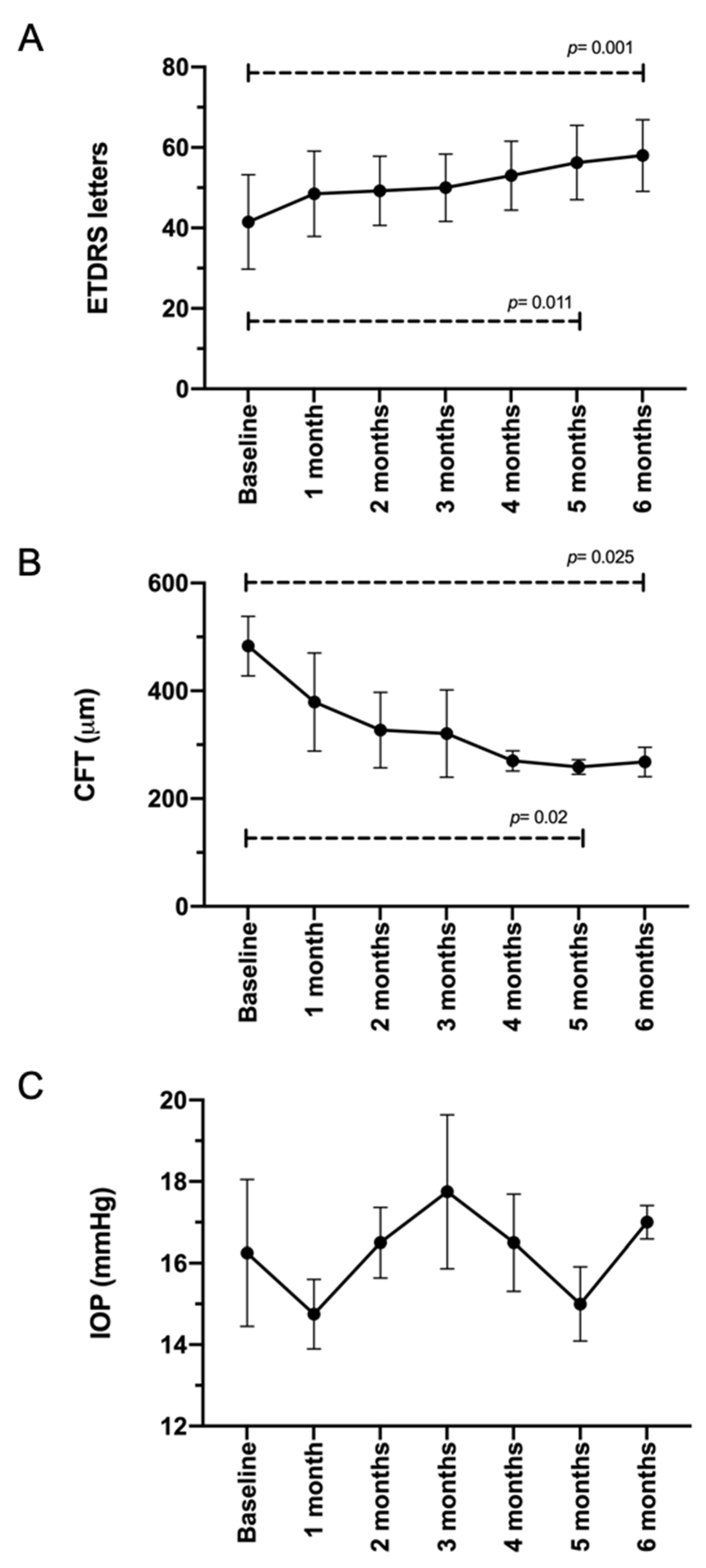
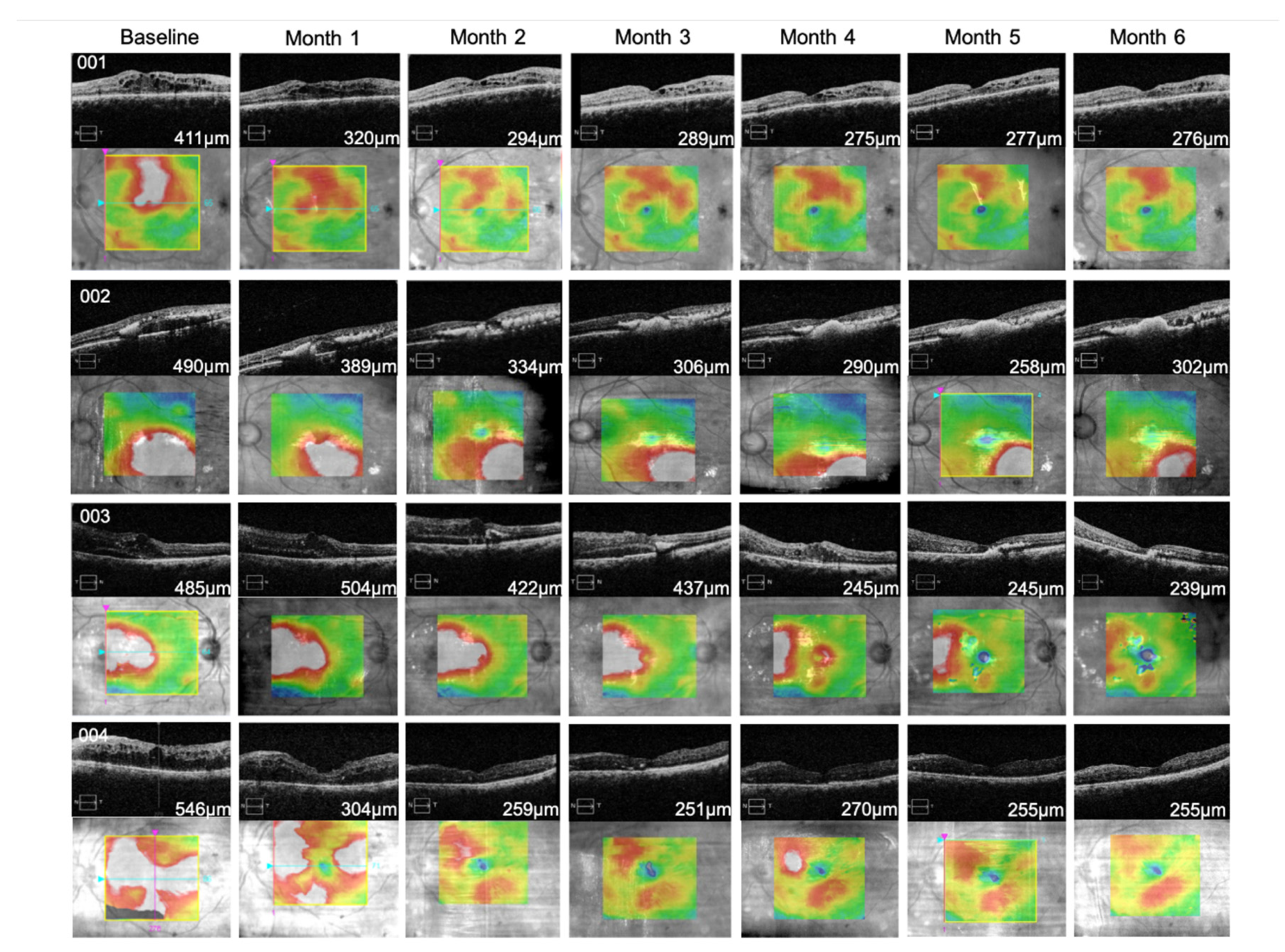
3.3. TALF Improves BCVA and Reduces CFT in Patients with DME
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kwon, S.I.; Kim, Y.W.; Bang, Y.W.; Lee, J.Y.; Park, I.W. Comparison of natural course, intravitreal triamcinolone, and intravitreal bevacizumab for treatment of macular edema secondary to branch retinal vein occlusion. J. Ocul. Pharmacol. Ther. 2013, 29, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Habot-Wilner, Z.; Sallam, A.; Pacheco, P.A.; Do, H.H.; McCluskey, P.; Lightman, S. Intravitreal triamcinolone acetonide as adjunctive treatment with systemic therapy for uveitic macular edema. Eur. J. Ophthalmol. 2011, 21 (Suppl. 6), S56–S61. [Google Scholar] [CrossRef]
- Yalcinbayir, O.; Gelisken, O.; Kaderli, B.; Avci, R. Intravitreal versus sub-tenon posterior triamcinolone injection in bilateral diffuse diabetic macular edema. Ophthalmologica 2011, 225, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Arikan, G.; Osman Saatci, A.; Hakan Oner, F. Immediate intraocular pressure rise after intravitreal injection of ranibizumab and two doses of triamcinolone acetonide. Int. J. Ophthalmol. 2011, 4, 402–405. [Google Scholar]
- Chan, C.K.; Fan, D.S.; Chan, W.M.; Lai, W.W.; Lee, V.Y.; Lam, D.S. Ocular-hypertensive response and corneal endothelial changes after intravitreal triamcinolone injections in Chinese subjects: A 6-month follow-up study. Eye 2005, 19, 625–630. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Veritti, D.; Di Giulio, A.; Sarao, V.; Lanzetta, P. Drug safety evaluation of intravitreal triamcinolone acetonide. Expert Opin. Drug Saf. 2012, 11, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.K.; Chung, E.J.; Kwon, O.W.; Lee, J.H.; Koh, H.J. Objective evaluation of cataract progression associated with a high dose intravitreal triamcinolone injection. Eye 2008, 22, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Quiram, P.A.; Gonzales, C.R.; Schwartz, S.D. Severe steroid-induced glaucoma following intravitreal injection of triamcinolone acetonide. Am. J. Ophthalmol. 2006, 141, 580–582. [Google Scholar] [CrossRef]
- Viola, F.; Morescalchi, F.; Staurenghi, G. Argon laser trabeculoplasty for intractable glaucoma following intravitreal triamcinolone. Arch. Ophthalmol. 2006, 124, 133–134. [Google Scholar] [CrossRef][Green Version]
- Agrawal, S.; Agrawal, J.; Agrawal, T.P. Management of intractable glaucoma following intravitreal triamcinolone acetonide. Am. J. Ophthalmol. 2005, 139, 575–576. [Google Scholar] [CrossRef] [PubMed]
- Lyall, D.A.; Tey, A.; Foot, B.; Roxburgh, S.T.; Virdi, M.; Robertson, C.; MacEwen, C.J. Post-intravitreal anti-VEGF endophthalmitis in the United Kingdom: Incidence, features, risk factors, and outcomes. Eye 2012, 26, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Poku, E.; Rathbone, J.; Wong, R.; Everson-Hock, E.; Essat, M.; Pandor, A.; Wailoo, A. The safety of intravitreal bevacizumab monotherapy in adult ophthalmic conditions: Systematic review. BMJ Open 2014, 4, e005244. [Google Scholar] [CrossRef]
- Azad, R.; Chandra, P.; Gupta, R. The economic implications of the use of anti-vascular endothelial growth factor drugs in age-related macular degeneration. Indian J. Ophthalmol. 2007, 55, 441–443. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, T.; Tian, Q.; Cheng, Y.; Zhao, L.; Zhang, X.; Qu, Y. A more efficient ocular delivery system of triamcinolone acetonide as eye drop to the posterior segment of the eye. Drug Deliv. 2019, 26, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Raval, N.; Khunt, D.; Misra, M. Microemulsion-based delivery of triamcinolone acetonide to posterior segment of eye using chitosan and butter oil as permeation enhancer: An in vitro and in vivo investigation. J. Microencapsul. 2018, 35, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Santer, V.; Chen, Y.; Kalia, Y.N. Controlled non-invasive iontophoretic delivery of triamcinolone acetonide amino acid ester prodrugs into the posterior segment of the eye. Eur. J. Pharm. Biopharm. 2018, 132, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Altamirano-Vallejo, J.C.; Navarro-Partida, J.; Gonzalez-De la Rosa, A.; Hsiao, J.H.; Olguin-Gutierrez, J.S.; Gonzalez-Villegas, A.C.; Keller, B.C.; Bouzo-Lopez, L.; Santos, A. Characterization and Pharmacokinetics of Triamcinolone Acetonide-Loaded Liposomes Topical Formulations for Vitreoretinal Drug Delivery. J. Ocul. Pharmacol. Ther. 2018, 34, 416–425. [Google Scholar] [CrossRef]
- Meza-Rios, A.; Navarro-Partida, J.; Armendariz-Borunda, J.; Santos, A. Therapies Based on Nanoparticles for Eye Drug Delivery. Ophthalmol. Ther. 2020, 9, 1–14. [Google Scholar] [CrossRef]
- Gonzalez-De la Rosa, A.; Navarro-Partida, J.; Altamirano-Vallejo, J.C.; Jauregui-Garcia, G.D.; Acosta-Gonzalez, R.; Ibanez-Hernandez, M.A.; Mora-Gonzalez, G.F.; Armendáriz-Borunda, J.; Santos, A. Novel Triamcinolone Acetonide-Loaded Liposomal Topical Formulation Improves Contrast Sensitivity Outcome After Femtosecond Laser-Assisted Cataract Surgery. J. Ocul. Pharmacol. Ther. 2019, 35, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-De la Rosa, A.; Navarro-Partida, J.; Altamirano-Vallejo, J.C.; Hernandez-Gamez, A.G.; Garcia-Banuelos, J.J.; Armendariz-Borunda, J.; Santos, A. Novel triamcinolone acetonide-loaded liposomes topical formulation for the treatment of cystoid macular edema after cataract surgery: A Pilot Study. J. Ocul. Pharmacol. Ther. 2019, 35, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Partida, J.; Altamirano-Vallejo, J.C.; Lopez-Naranjo, E.J.; Gonzalez-De la Rosa, A.; Manzano-Ramírez, A.; Apatiga-Castro, L.M.; Armendáriz-Borunda, J.; Santos, A. Topical Triamcinolone Acetonide-Loaded Liposomes as Primary Therapy for Macular Edema Secondary to Branch Retinal Vein Occlusion: A Pilot Study. J. Ocul. Pharmacol. Ther. 2020, 36, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Karasawa, K.; Hironaka, K.; Tahara, K.; Tozuka, Y.; Takeuchi, H. Retinal drug delivery using eyedrop preparations of poly-L-lysine-modified liposomes. Eur. J. Pharm. Biopharm. 2013, 83, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.M.; Normando, E.M.; Guo, L.; Turner, L.A.; Nizari, S.; O’Shea, P.; Moss, S.E.; Somavarapu, S.; Cordeiro, M.F. Topical delivery of Avastin to the posterior segment of the eye in vivo using annexin A5-associated liposomes. Small 2014, 10, 1575–1584. [Google Scholar] [CrossRef]
- Inokuchi, Y.; Hironaka, K.; Fujisawa, T.; Tozuka, Y.; Tsuruma, K.; Shimazawa, M.; Takeuchi, H.; Hara, H. Physicochemical properties affecting retinal drug/coumarin-6 delivery from nanocarrier systems via eyedrop administration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3162–3170. [Google Scholar] [CrossRef]
- Fujisawa, T.; Miyai, H.; Hironaka, K.; Tsukamoto, T.; Tahara, K.; Tozuka, Y.; Ito, M.; Takeuchi, H. Liposomal diclofenac eye drop formulations targeting the retina: Formulation stability improvement using surface modification of liposomes. Int. J. Pharm. 2012, 436, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, H.; Hironaka, K.; Fujisawa, T.; Tsuruma, K.; Tozuka, Y.; Shimazawa, M.; Takeuchi, H.; Hara, H. Edaravone-loaded liposome eyedrops protect against light-induced retinal damage in mice. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7289–7297. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Tsuchiya, T.; Igarashi, Y.; Kanazawa, T.; Okada, H.; Urtti, A. Non-invasive ophthalmic liposomes for nucleic acid delivery to posterior segment of eye. Yakugaku Zasshi 2012, 132, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Hironaka, K.; Fujisawa, T.; Sasaki, H.; Tozuka, Y.; Tsuruma, K.; Shimazawa, M.; Hara, H.; Takeuchi, H. Fluorescence investigation of the retinal delivery of hydrophilic compounds via liposomal eyedrops. Biol. Pharm. Bull. 2011, 34, 894–897. [Google Scholar] [CrossRef]
- Hironaka, K.; Inokuchi, Y.; Tozuka, Y.; Shimazawa, M.; Hara, H.; Takeuchi, H. Design and evaluation of a liposomal delivery system targeting the posterior segment of the eye. J. Control. Release 2009, 136, 247–253. [Google Scholar] [CrossRef]
- Masuda, I.; Matsuo, T.; Yasuda, T.; Matsuo, N. Gene transfer with liposomes to the intraocular tissues by different routes of administration. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1914–1920. [Google Scholar]
- Abelson, M.B.; Udell, I.J.; Weston, J.H. Normal human tear pH by direct measurement. Arch. Ophthalmol. 1981, 99, 301. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.O.; Shadduck, J.A. Eye Irritation; Wiley & Sons: New York, NY, USA, 1977; Volume 4. [Google Scholar]
- Kinyoun, J.; Barton, F.; Fisher, M.; Hubbard, L.; Aiello, L.; Ferris, F., III; Group, E.R. Detection of diabetic macular edema: Ophthalmoscopy versus photography—Early Treatment Diabetic Retinopathy Study report number 5. Ophthalmology 1989, 96, 746–751. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F.; Veiga, F.; Silva, A.M.; Souto, E.B. Ocular Drug Delivery—New Strategies for Targeting Anterior and Posterior Segments of the Eye. Curr. Pharm. Des. 2016, 22, 1135–1146. [Google Scholar] [CrossRef]
- Kim, S.H.; Lutz, R.J.; Wang, N.S.; Robinson, M.R. Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res. 2007, 39, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Told, R.; Sacu, S.; Bandello, F.; Moisseiev, E.; Loewenstein, A.; Schmidt-Erfurth, U.; Euretina, B. 2018 Update on Intravitreal Injections: Euretina Expert Consensus Recommendations. Ophthalmologica 2018, 239, 181–193. [Google Scholar] [CrossRef]
- Raghava, S.; Hammond, M.; Kompella, U.B. Periocular routes for retinal drug delivery. Expert Opin. Drug Deliv. 2004, 1, 99–114. [Google Scholar] [CrossRef]
- Martin, D.F.; Maguire, M.G.; Fine, S.L.; Ying, G.S.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Redford, M.; Ferris, F.L., 3rd; Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology 2012, 119, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.E.; Rosenfeld, P.J.; Reichel, E. The International Intravitreal Bevacizumab Safety Survey: Using the internet to assess drug safety worldwide. Br. J. Ophthalmol. 2006, 90, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Droege, K.M.; Muether, P.S.; Hermann, M.M.; Caramoy, A.; Viebahn, U.; Kirchhof, B.; Fauser, S. Adherence to ranibizumab treatment for neovascular age-related macular degeneration in real life. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Mousa, S.S.; Mousa, S.A. Comparative effectiveness of aflibercept for the treatment of patients with neovascular age-related macular degeneration. Clin. Ophthalmol. 2013, 7, 495–501. [Google Scholar]
- Pacella, E.; Vestri, A.R.; Muscella, R.; Carbotti, M.R.; Castellucci, M.; Coi, L.; Turchetti, P.; Pacella, F. Preliminary results of an intravitreal dexamethasone implant (Ozurdex(R)) in patients with persistent diabetic macular edema. Clin. Ophthalmol. 2013, 7, 1423–1428. [Google Scholar] [CrossRef]
- Pearson, P.A.; Comstock, T.L.; Ip, M.; Callanan, D.; Morse, L.S.; Ashton, P.; Levy, B.; Mann, E.S.; Eliott, D. Fluocinolone acetonide intravitreal implant for diabetic macular edema: A 3-year multicenter, randomized, controlled clinical trial. Ophthalmology 2011, 118, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Berezovsky, D.E.; McCarey, B.E.; Zarnitsyn, V.; Edelhauser, H.F.; Prausnitz, M.R. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4433–4441. [Google Scholar] [CrossRef]
- Lo, R.; Li, P.Y.; Saati, S.; Agrawal, R.; Humayun, M.S.; Meng, E. A refillable microfabricated drug delivery device for treatment of ocular diseases. Lab Chip 2008, 8, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Saati, S.; Lo, R.; Li, P.Y.; Meng, E.; Varma, R.; Humayun, M.S. Mini drug pump for ophthalmic use. Trans. Am. Ophthalmol. Soc. 2009, 107, 60–70. [Google Scholar] [CrossRef]
- Gutierrez-Hernandez, J.C.; Caffey, S.; Abdallah, W.; Calvillo, P.; Gonzalez, R.; Shih, J.; Brennan, J.; Zimmerman, J.; Martinez-Camarillo, J.C.; Rodriguez, A.R.; et al. One-Year Feasibility Study of Replenish MicroPump for Intravitreal Drug Delivery: A Pilot Study. Transl. Vis. Sci. Technol. 2014, 3, 8. [Google Scholar] [CrossRef]
- Humayun, M.; Santos, A.; Altamirano, J.C.; Ribeiro, R.; Gonzalez, R.; de la Rosa, A.; Shih, J.; Pang, C.; Jiang, F.; Calvillo, P.; et al. Implantable MicroPump for Drug Delivery in Patients with Diabetic Macular Edema. Transl. Vis. Sci. Technol. 2014, 3, 5. [Google Scholar] [CrossRef]
- Hans, M.L.; Lowman, A.M. Biodegradable Nanoparticles for Drug Delivery and Targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Moya-Ortega, M.D.; Alves, T.F.; Alvarez-Lorenzo, C.; Concheiro, A.; Stefansson, E.; Thorsteinsdottir, M.; Loftsson, T. Dexamethasone eye drops containing gamma-cyclodextrin-based nanogels. Int. J. Pharm. 2013, 441, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Garg, A.; Singla, A.K.; Aggarwal, D. Vesicular systems in ocular drug delivery: An overview. Int. J. Pharm. 2004, 269, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, M.; Kim, Y.; Park, C.; Lee, J.; Chung, I.; Yoo, J.; Choi, W.; Cho, G.; Kang, S. Triamcinolone acetonide protects the rat retina from STZ-induced acute inflammation and early vascular leakage. Life Sci. 2007, 81, 1167–1173. [Google Scholar] [CrossRef]
- Zhang, S.X.; Wang, J.J.; Gao, G.; Shao, C.; Mott, R.; Ma, J.X. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006, 20, 323–325. [Google Scholar] [CrossRef]
- Tsaprouni, L.G.; Ito, K.; Punchard, N.; Adcock, I.M. Triamcinolone Acetonide and Dexamethasome Suppress TNF-α-Induced Histone H4 Acetylation on Lysine Residues 8 and 12 in Mononuclear Cells. Ann. N. Y. Acad. Sci. 2002, 973, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Bandello, F.; Parodi, M.B.; Lanzetta, P.; Loewenstein, A.; Massin, P.; Menchini, F.; Veritti, D. Diabetic macular edema. In Macular Edema; Karger Publishers: Basel, Switzerland, 2017; Volume 58, pp. 102–138. [Google Scholar]
- Umscheid, C.A.; Margolis, D.J.; Grossman, C.E. Key concepts of clinical trials: A narrative review. Postgrad. Med. 2011, 123, 194–204. [Google Scholar] [CrossRef]
- Akula, S.K.; Ma, P.E.; Peyman, G.A.; Rahimy, M.H.; Hyslop, N.E., Jr.; Janney, A.; Ashton, P. Treatment of cytomegalovirus retinitis with intravitreal injection of liposome encapsulated ganciclovir in a patient with AIDS. Br. J. Ophthalmol. 1994, 78, 677–680. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeng, S.; Hu, C.; Wei, H.; Lu, Y.; Zhang, Y.; Yang, J.; Yun, G.; Zou, W.; Song, B. Intravitreal pharmacokinetics of liposome-encapsulated amikacin in a rabbit model. Ophthalmology 1993, 100, 1640–1644. [Google Scholar] [CrossRef]
- Cannon, J.P.; Fiscella, R.; Pattharachayakul, S.; Garey, K.W.; De Alba, F.; Piscitelli, S.; Edward, D.P.; Danziger, L.H. Comparative toxicity and concentrations of intravitreal amphotericin B formulations in a rabbit model. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2112–2117. [Google Scholar] [CrossRef] [PubMed]
- Shabir, G.A. Validation of high-performance liquid chromatography methods for pharmaceutical analysis. Understanding the differences and similarities between validation requirements of the US Food and Drug Administration, the US Pharmacopeia and the International Conference on Harmonization. J. Chromatogr. A 2003, 987, 57–66. [Google Scholar] [PubMed]
- Purslow, C.; Wolffsohn, J.S. Ocular surface temperature: A review. Eye Contact Lens 2005, 31, 117–123. [Google Scholar] [CrossRef] [PubMed]
| (w or v) | (%) | ||
|---|---|---|---|
| Triamcinolone acetonide | 2.0 mg | 0.2 | w/v |
| Kolliphor HS 15 | 50 mg | 5 | w/v |
| PEG-12 glyceryl dimyristate | 100 mg | 10 | w/v |
| Ethyl alcohol | 14 mL | 1.40 | v/v |
| Citric acid anhydrous | 0.8 mg | 0.08 | w/v |
| Sodium citrate dihydrate | 4.675 mg | 0.4675 | w/v |
| Benzalkonium chloride | 0.1 mg | 0.01 | w/v |
| Grade 2 purified water | Q.S.1.0 ml | NA | NA |
| Baseline | 14th Day | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Gender | Study Eye | Age | BCVA | Contrast | IOP | CFT | BCVA | Contrast | IOP | CFT |
| (Years) | (ETDRS Letters) | (L/Contrast) | (mmHg) | (μm) | (ETDRS Letters) | (L/Contrast) | (mmHg) | (μm) | |||
| 1 | F | OD | 25 | 85 | 1.65 | 10 | 250 | 85 | 1.65 | 12 | 248 |
| 2 | F | OS | 25 | 85 | 1.65 | 11 | 264 | 85 | 1.5 | 13 | 269 |
| 3 | F | OD | 26 | 85 | 1.35 | 10 | 256 | 85 | 1.35 | 16 | 260 |
| 4 | F | OS | 26 | 85 | 1.35 | 10 | 259 | 85 | 1.35 | 16 | 261 |
| 5 | M | OD | 25 | 83 | 1.35 | 13 | 242 | 84 | 1.5 | 14 | 243 |
| 6 | M | OS | 25 | 84 | 1.35 | 12 | 252 | 85 | 1.5 | 12 | 254 |
| 7 | F | OD | 24 | 84 | 1.35 | 11 | 243 | 85 | 1.5 | 8 | 247 |
| 8 | F | OS | 24 | 85 | 1.35 | 11 | 247 | 85 | 1.5 | 7 | 241 |
| 9 | F | OD | 24 | 85 | 1.5 | 16 | 255 | 85 | 1.5 | 11 | 258 |
| 10 | F | OS | 24 | 85 | 1.35 | 17 | 257 | 85 | 1.65 | 9 | 256 |
| 11 | M | OD | 56 | 85 | 1.5 | 16 | 257 | 85 | 1.65 | 15 | 256 |
| 12 | M | OD | 35 | 85 | 1.65 | 17 | 263 | 85 | 1.65 | 14 | 265 |
| 13 | F | OD | 47 | 85 | 1.65 | 13 | 246 | 85 | 1.65 | 15 | 245 |
| 14 | F | OD | 35 | 85 | 1.65 | 18 | 245 | 85 | 1.65 | 16 | 247 |
| 15 | F | OD | 63 | 83 | 1.35 | 12 | 258 | 85 | 1.65 | 14 | 260 |
| 16 | M | OD | 56 | 85 | 1.65 | 15 | 260 | 85 | 1.65 | 14 | 262 |
| 17 | F | OD | 40 | 85 | 1.65 | 16 | 262 | 84 | 1.65 | 14 | 259 |
| 18 | F | OD | 38 | 84 | 1.5 | 12 | 252 | 85 | 1.5 | 11 | 255 |
| 19 | M | OD | 63 | 84 | 1.5 | 16 | 249 | 84 | 1.5 | 13 | 248 |
| 20 | M | OD | 59 | 85 | 1.35 | 15 | 263 | 85 | 1.65 | 16 | 260 |
| X ± s | 37 ± 14.8 | 84.6 ± 0.6 | 1.5 ± 0.1 | 13.5 ± 2.7 | 254 ± 7 | 84.9 ± 0.3 ‡ | 1.6 ± 0.1 † | 13 ± 2.7 ‡ | 254.7 ± 7.8 ‡ | ||
| in | F = 13 M = 7 | OD = 15 OS = 5 | |||||||||
| p | 0.096 | 0.014 | 0.4853 | 0.2825 | |||||||
| Adverse Events | Categories | Dry Eyes | Burning | Discharge | Tearing | Blurred Vision | None |
|---|---|---|---|---|---|---|---|
| Safety Variables | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Frequency | Not presented | 14 (70) | 14 (70) | 18 (90) | 17 (85) | 18 (90) | 8 (40) |
| Rare | 6 (30) | 4 (20) | |||||
| Occasionally | 2 (10) | 3 (15) | |||||
| Most of the time | 2 (10) | 2 (10) | |||||
| All the time | |||||||
| Severity | Not presented | 14 (70) | 14 (70) | 18 (90) | 17 (85) | 18 (90) | 8 (40) |
| Mild | 6 (30) | 6 (30) | 3 (15) | 2 (10) | |||
| Moderate | 2 (10) | ||||||
| Severe |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Partida, J.; Altamirano-Vallejo, J.C.; Gonzalez-De la Rosa, A.; Armendariz-Borunda, J.; Castro-Castaneda, C.R.; Santos, A. Safety and Tolerability of Topical Ophthalmic Triamcinolone Acetonide-Loaded Liposomes Formulation and Evaluation of Its Biologic Activity in Patients with Diabetic Macular Edema. Pharmaceutics 2021, 13, 322. https://doi.org/10.3390/pharmaceutics13030322
Navarro-Partida J, Altamirano-Vallejo JC, Gonzalez-De la Rosa A, Armendariz-Borunda J, Castro-Castaneda CR, Santos A. Safety and Tolerability of Topical Ophthalmic Triamcinolone Acetonide-Loaded Liposomes Formulation and Evaluation of Its Biologic Activity in Patients with Diabetic Macular Edema. Pharmaceutics. 2021; 13(3):322. https://doi.org/10.3390/pharmaceutics13030322
Chicago/Turabian StyleNavarro-Partida, Jose, Juan Carlos Altamirano-Vallejo, Alejandro Gonzalez-De la Rosa, Juan Armendariz-Borunda, Carlos Rodrigo Castro-Castaneda, and Arturo Santos. 2021. "Safety and Tolerability of Topical Ophthalmic Triamcinolone Acetonide-Loaded Liposomes Formulation and Evaluation of Its Biologic Activity in Patients with Diabetic Macular Edema" Pharmaceutics 13, no. 3: 322. https://doi.org/10.3390/pharmaceutics13030322
APA StyleNavarro-Partida, J., Altamirano-Vallejo, J. C., Gonzalez-De la Rosa, A., Armendariz-Borunda, J., Castro-Castaneda, C. R., & Santos, A. (2021). Safety and Tolerability of Topical Ophthalmic Triamcinolone Acetonide-Loaded Liposomes Formulation and Evaluation of Its Biologic Activity in Patients with Diabetic Macular Edema. Pharmaceutics, 13(3), 322. https://doi.org/10.3390/pharmaceutics13030322





