6-Gingerol, a Bioactive Compound of Ginger Attenuates Renal Damage in Streptozotocin-Induced Diabetic Rats by Regulating the Oxidative Stress and Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Experimental Design
2.4. Measurement of Body Weight and Biochemical Parameters
2.5. Measurement of Lipid Peroxidation
2.6. Determination of Antioxidant Enzymes Levels
2.7. Measurement of Pro-Inflammatory Parameters
2.8. Histopathological Examination of Kidney
2.9. Immunohistochemical Evaluation of TNF-alpha
2.10. Statistical Analysis
3. Results
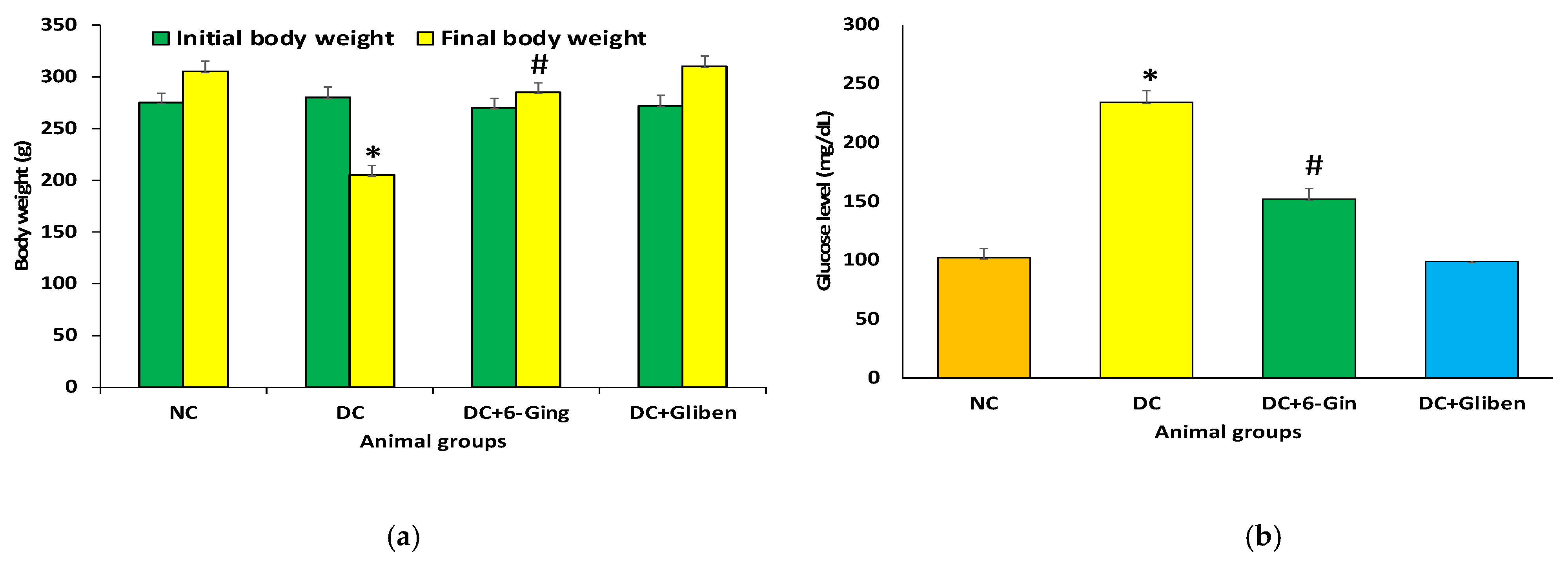
3.1. Effect of 6-Gingerol on Body Weight and Blood Glucose Levels
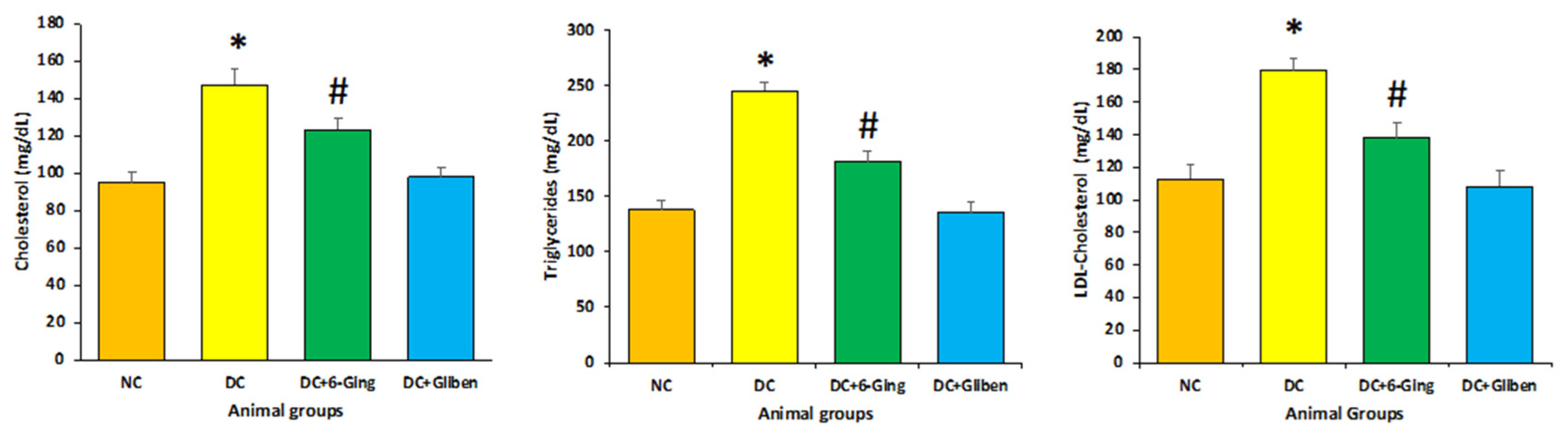
3.2. Effect of 6-Gingerol on Lipid Profile
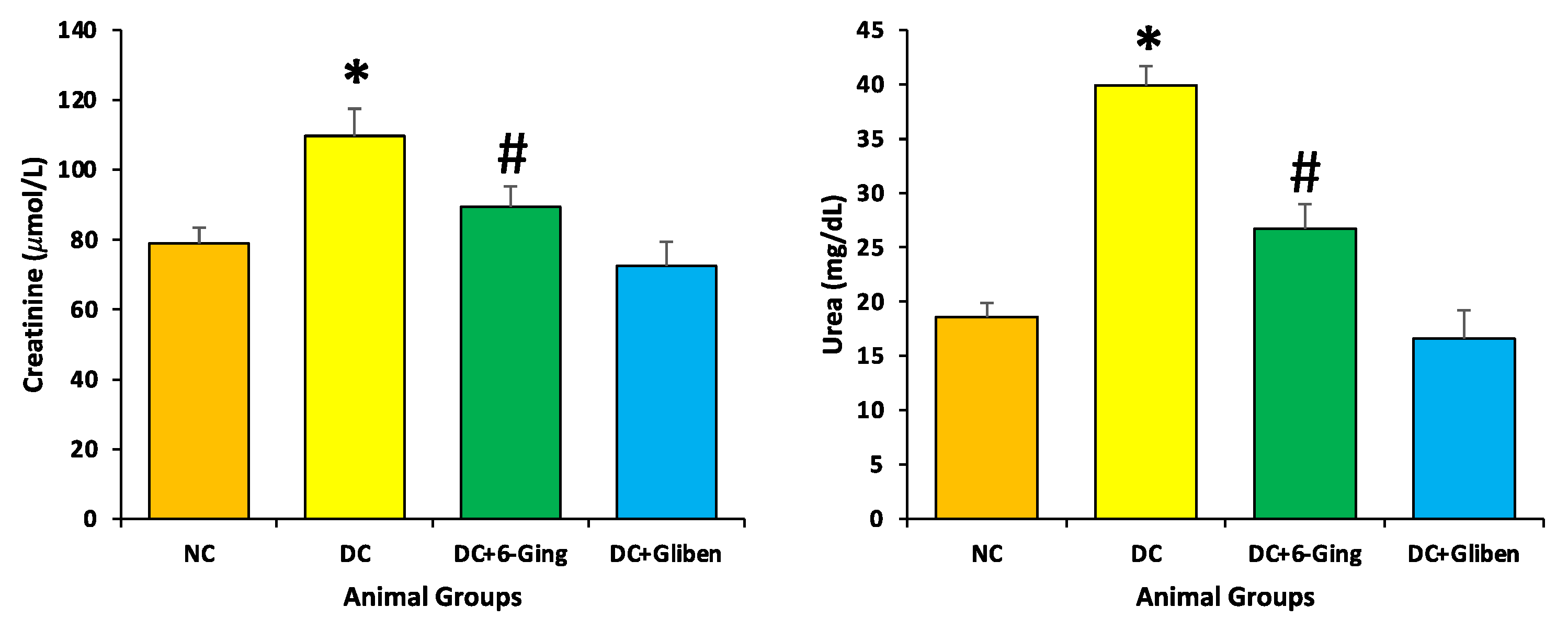
3.3. Effect of 6-Gingerol on Serum Creatinine, and Blood Urea Level
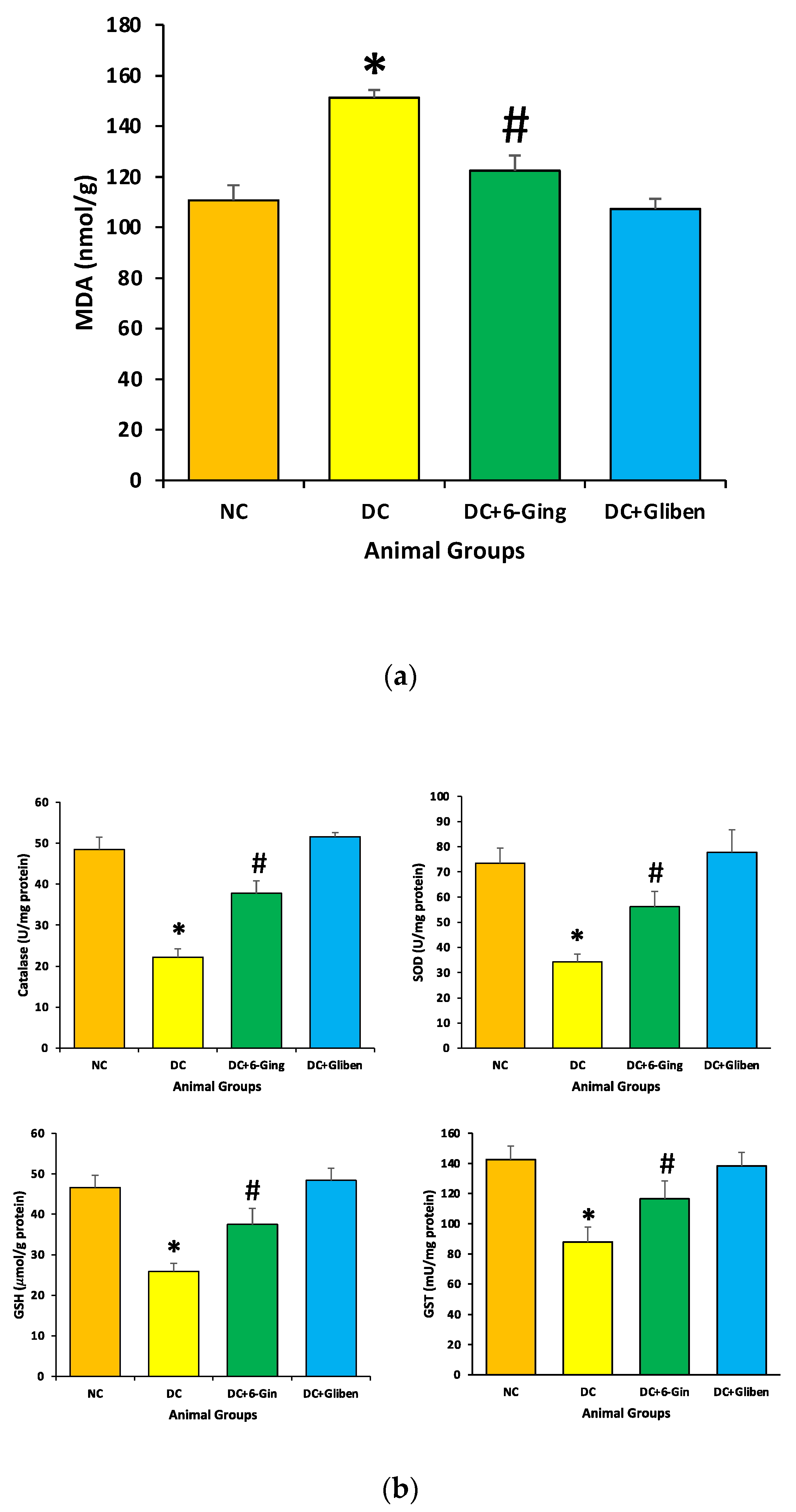
3.4. Effect of 6-Gingerol on Oxidative Stress
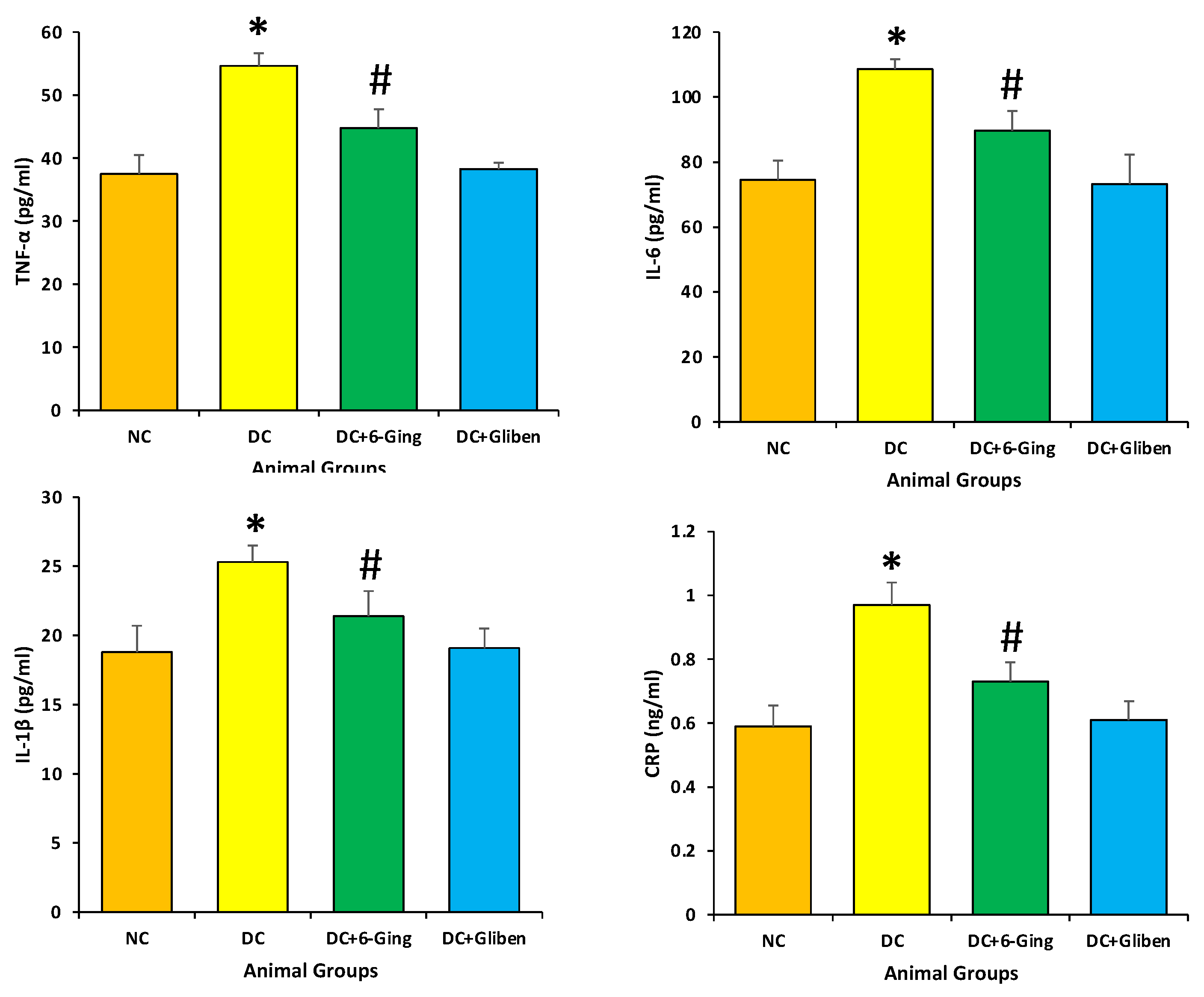
3.5. 6-Gingerol Decrease the Levels of CRP, IL-6, IL-1β and TNF-α
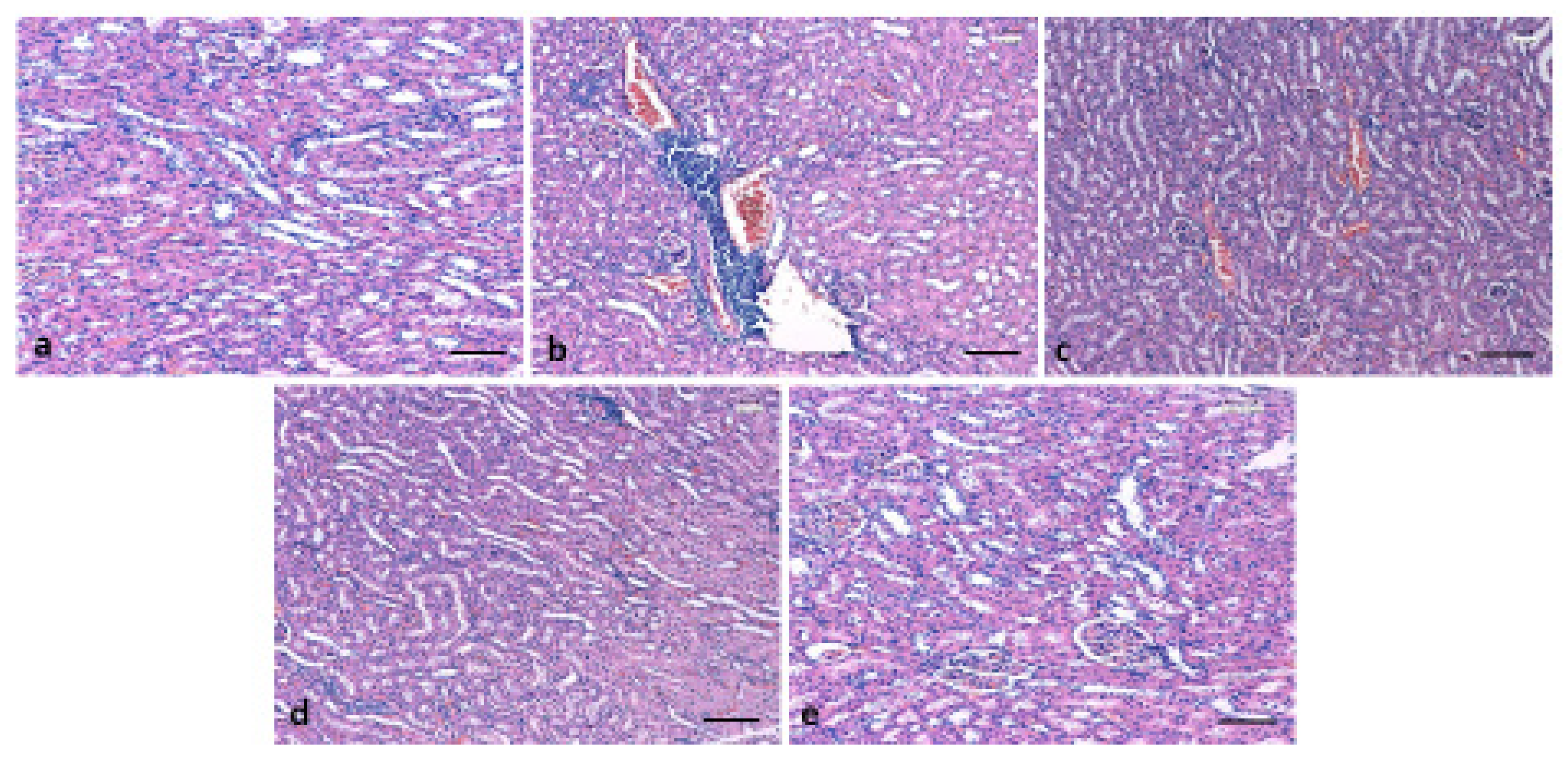
3.6. Effect of 6-Gingerol on Kidney Architecture
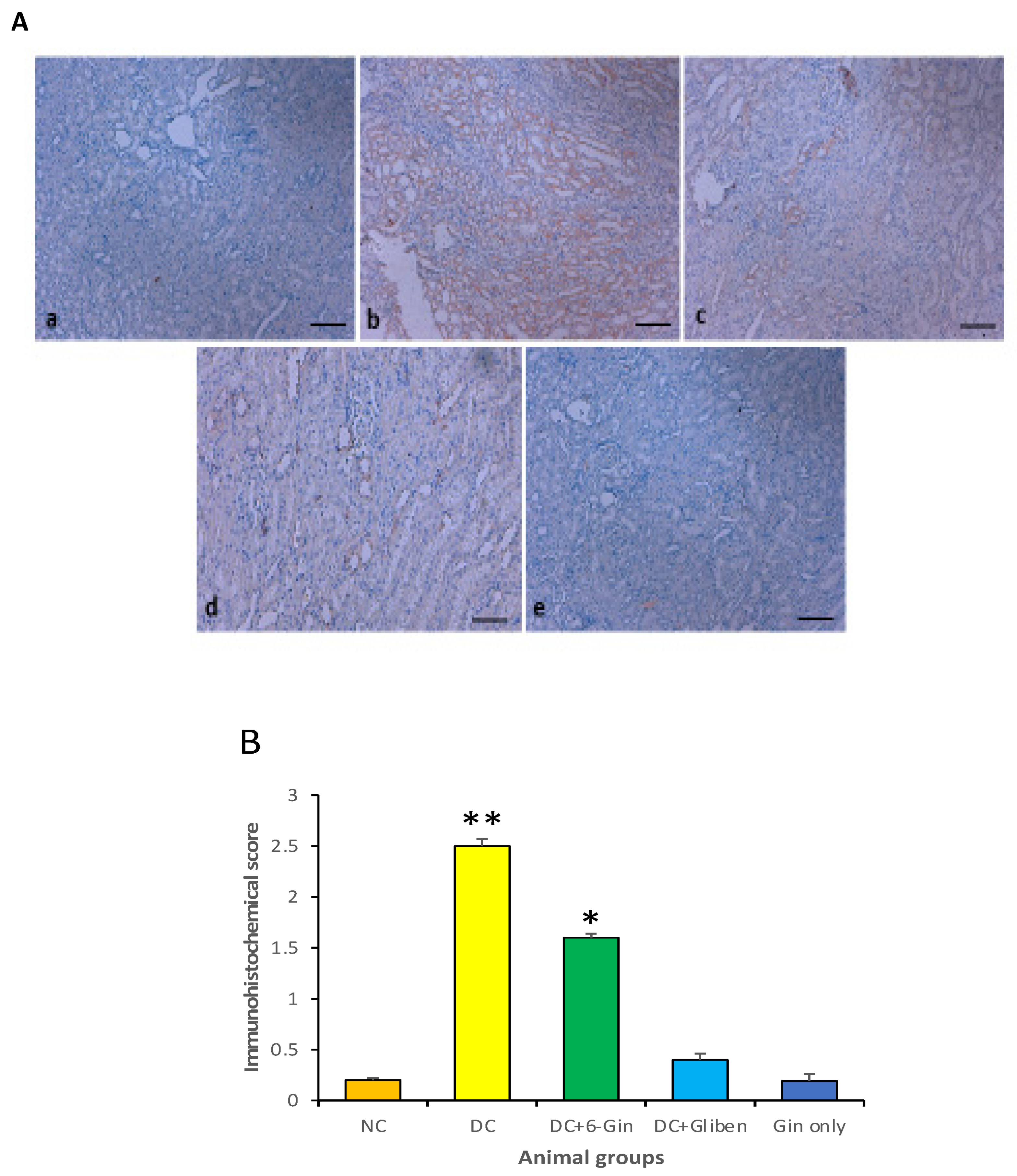
3.7. Effects of 6-Gingerol on TNF-α Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mokdad, A.H.; Ford, E.S.; Bowman, B.A.; Dietz, W.H.; Vinicor, F.; Bales, V.S.; Marks, J.S. Prevalence of Obesity, Diabetes, and Obesity-Related Health Risk Factors, 2001. JAMA 2003, 289, 76–79. [Google Scholar] [CrossRef]
- Juarez-Reyes, K.; Brindis, F.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Bye, R.; Linares, E.; Mata, R. Hypoglycemic, antihyperglycemic, and antioxidant effects of the edible plant Anoda cristata. J. Ethnopharmacol. 2015, 161, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.; Maxwell, A.P. Managing diabetic nephropathy. Clin. Med. 2010, 10, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, T.; Abe, H.; Ueda, O.; Goto, C.; Nakahara, K.; Murakami, T.; Matsubara, T.; Mima, A.; Nagai, K.; Araoka, T.; et al. Activation of Bone Morphogenetic Protein 4 Signaling Leads to Glomerulosclerosis That Mimics Diabetic Nephropathy. J. Biol. Chem. 2011, 286, 20109–20116. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Kishikawa, H.; Araki, E.; Miyata, T.; Isami, S.; Motoyoshi, S.; Kojima, Y.; Furuyoshi, N.; Shichiri, M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: A randomized prospective 6-year study. Diabetes Res. Clin. Pract. 1995, 28, 103–117. [Google Scholar] [CrossRef]
- Shah, S.V.; Baliga, R.; Rajapurkar, M.; Fonseca, V.A. Oxidants in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2007, 18, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Ohshiro, Y.; Takasu, N. [Molecular mechanism of diabetic nephropathy]. Nihon Rinsho. Jpn. J. Clin. Med. 2006, 64, 997–1003. [Google Scholar]
- Ward, D.T.; Yau, S.K.; Mee, A.P.; Mawer, E.B.; A Miller, C.; O Garland, H.; Riccardi, D. Functional, molecular, and biochemical characterization of streptozotocin-induced diabetes. J. Am. Soc. Nephrol. 2001, 12, 779–790. [Google Scholar]
- Davidson, E.P.; Kleinschmidt, T.L.; Oltman, C.L.; Lund, D.D.; Yorek, M.A. Treatment of streptozotocin-induced diabetic rats with AVE7688, a vasopeptidase inhibitor: Effect on vascular and neural disease. Diabetes 2007, 56, 355–362. [Google Scholar] [CrossRef][Green Version]
- Lallemand, F.; De Witte, P. Taurine concentration in the brain and in the plasma following intraperitoneal injections. Amino Acids 2004, 26, 111–116. [Google Scholar] [CrossRef]
- Amaral, F.G.D.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free. Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, R.R.; Li, J.H.; Ren, M.; Ren, Z.X.; Shi, J.H.; Pan, Q.Z.; Ren, S.P. Radix Astragali lowers kidney oxidative stress in diabetic rats treated with insulin. Endocrinology 2012, 42, 592–598. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free. Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Antonio, C.; Roberto, T. Antioxidant anti-inflammatory treatment in type 2 diabetes. Diabetes Care 2009, 32, S232–S236. [Google Scholar]
- Klein, G.; Kim, J.; Himmeldirk, K.; Cao, Y.; Chen, X. Antidiabetes and Anti-Obesity Activity ofLagerstroemia speciosa. Evid.-Based Complement. Altern. Med. 2007, 4, 401–407. [Google Scholar] [CrossRef]
- Spiller, H.A.; Sawyer, T.S. Toxicology of oral antidiabetic medications. Am. J. Health Pharm. 2006, 63, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Meerza, D.; Naseem, I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014, 109, 8–14. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and an-ti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Yagihashi, S.; Miura, Y.; Yagasaki, K. Inhibitory effect of gingerol on the proliferation and invasion of hepatoma cells in culture. Cytotechnology 2008, 57, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, Z.M.; Thomson, M.; Al-Qattan, K.K.; Peltonen-Shalaby, R.; Ali, M. Anti-diabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br. J. Nutr. 2006, 96, 660–666. [Google Scholar] [CrossRef]
- Chakraborty, D.; Mukherjee, A.; Sikdar, S.; Paul, A.; Ghosh, S.; Khuda-Bukhsh, A.R. [6]-Gingerol isolated from ginger attenuates sodium arsenite induced oxidative stress and plays a corrective role in improving insulin signaling in mice. Toxicol. Lett. 2012, 210, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.B.; Akanksha Singh, N.; Maurya, R.; Srivastava, A.K. Anti-hyperglycaemic, lipid lowering and anti-oxidant properties of [6]-gingerol in db/db mice. Int. J. Med. Med. Sci. 2009, 1, 536–544. [Google Scholar]
- Zafar, M.; Naqvi, S.N.-U.-H. Effects of STZ-Induced Diabetes on the Relative Weights of Kidney, Liver and Pancreas in Albino Rats: A Comparative Study. Int. J. Morphol. 2010, 28, 135–142. [Google Scholar] [CrossRef]
- Kim, C.Y.; Seo, Y.; Lee, C.; Park, G.H.; Jang, J.H. Neuroprotective effect and molecular mechanism of [6]-gingerol against scopola-mine-induced amnesia in C57BL/6 mice. Evid. Based Complement. Altern. Med. 2018, 2018, 8941564. [Google Scholar]
- Kim, J.D.; Kang, S.M.; Seo, B.I.; Choi, H.Y.; Choi, H.S.; Ku, S.K. Anti-diabetic Activity of SMK001, a Poly Herbal Formula in Streptozotocin Induced Diabetic Rats: Therapeutic Study. Biol. Pharm. Bull. 2006, 29, 477–482. [Google Scholar] [CrossRef]
- Song, Y.; Liu, W.; Tang, K.; Zang, J.; Li, D.; Gao, H. Mangiferin Alleviates Renal Interstitial Fibrosis in Streptozotocin-Induced Diabetic Mice through Regulating the PTEN/PI3K/Akt Signaling Pathway. J. Diabetes Res. 2020, 2020, 9481720. [Google Scholar] [CrossRef] [PubMed]
- Bidani, A.K.; Picken, M.; Hacioglu, R.; Williamson, G.; Griffin, K.A. Spontaneously reduced BP load in the rat streptozotocin-induced diabetes model: Potential pathogenetic relevance. Am. J. Physiol. Renal Physiol. 2007, 292, F647–F654. [Google Scholar] [CrossRef] [PubMed]
- Miric, G.; Dallemagne, C.; Endre, Z.; Margolin, S.; Taylor, S.M.; Brown, L. Reversal of cardiac and renal fibrosis by pirfenidine and spironolactone in strepto-zotocin-diabetic rats. Br. J. Pharmacol. 2001, 133, 687–694. [Google Scholar] [CrossRef]
- American Diabetes Association Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2008, 32, S62–S67. [CrossRef]
- Masuda, Y.; Kikuzaki, H.; Hisamoto, M.; Nakatani, N. Antioxidant properties of gingerol related compounds from ginger. BioFactors 2004, 21, 293–296. [Google Scholar] [CrossRef]
- Samad, M.B.; Mohsin, M.N.A.B.; Razu, B.A.; Hossain, M.T.; Mahzabeen, S.; Unnoor, N.; Muna, I.A.; Akhter, F.; Kabir, A.U.; Hannan, J.A.M. [6]-Gingerol, from Zingiber officinale, potentiates GLP-1 mediated glu-cose-stimulated insulin secretion pathway in pancreatic β-cells and increases RAB8/RAB10-regulated membrane presentation of GLUT4 transporters in skeletal muscle to improve hyperglycemia in Leprdb/db type 2 diabetic mice. BMC Complement. Altern. Med. 2017, 17, 395. [Google Scholar]
- Rodrigues, F.A.P.; Prata, M.M.G.; Oliveira, I.C.M.; Alves, N.T.Q.; Freitas, R.E.M.; Monteiro, H.S.A.; Silva, J.A.; Vieira, P.C.; Viana, D.A.; Libório, A.B.; et al. Gingerol Fraction from Zingiber officinale Protects against Gentamicin-Induced Nephrotoxicity. Antimicrob. Agents Chemother. 2014, 58, 1872–1878. [Google Scholar] [CrossRef]
- Song, S.; Dang, M.; Kumar, M. Anti-inflammatory and renal protective effect of gingerol in high-fat di-et/streptozotocin-induced diabetic rats via inflammatory mechanism. Inflammopharmacol 2019, 27, 1243–1254. [Google Scholar] [CrossRef]
- Spencer, E.A.; Pirie, K.L.; Stevens, R.J.; Beral, V.; Brown, A.; Liu, B.; Green, J.; Reeves, G.K. Million Women Study Collaborators Diabetes and modifiable risk factors for cardiovascular disease: The prospective Million Women Study. Eur. J. Epidemiol. 2008, 23, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Pihlajamäki, J.; Gylling, H.; Miettinen, T.A.; Laakso, M. Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption in normoglycemic men. J. Lipid Res. 2004, 45, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Alsahli, M.A.; Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Anwar, S.; Almutary, A.G.; Alrumaihi, F.; Rahmani, A.H. 6-Gingerol, a Major Ingredient of Ginger Attenuates Diethylnitrosamine-Induced Liver Injury in Rats through the Modulation of Oxidative Stress and Anti-Inflammatory Activity. Mediat. Inflamm. 2021, 6661937. [Google Scholar] [CrossRef]
- Sifuentes-Franco, S.; Padilla-Tejeda, D.E.; Carrillo-Ibarra, S.; Miranda-Díaz, A.G. Oxidative Stress, Apoptosis, and Mitochondrial Function in Diabetic Nephropathy. Int. J. Endocrinol. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodnia, L.; Aghadavod, E.; Beigrezaei, S.; Rafieian-Kopaei, M. An update on diabetic kidney disease, oxidative stress and antioxidant agents. J. Ren. Inj. Prev. 2017, 6, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Yu, M.-R.; Yang, Y.; Jiang, Z.; Ha, H. Reactive Oxygen Species-Regulated Signaling Pathways in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2003, 14, S241–S245. [Google Scholar] [CrossRef]
- Levy, Y.; Zaltzberg, H.; Ben-Amotz, A.; Kanter, Y.; Aviram, M. β-Carotene affects antioxidant status in non-insulin-dependent diabetes mellitus. Pathophysiology 1999, 6, 157–161. [Google Scholar] [CrossRef]
- Al Hroob, A.M.; Abukhalil, M.H.; Alghonmeen, R.D.; Mahmoud, A.M. Ginger alleviates hyperglycemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed. Pharmacother. 2018, 106, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.M.; Mosaed, M.M.; Elshafey, S.H.; Bayomy, N.A. 6-gingerol ameliorates gentamicin induced renal cortex oxidative stress and apoptosis in adult male albino rats. Tissue Cell. 2016, 48, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Wada, J.; Makino, H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. 2013, 124, 139–152. [Google Scholar] [CrossRef]
- Tzeng, T.-F.; Liou, S.-S.; Chang, C.J.; Liu, I.-M. The Ethanol Extract ofZingiber zerumbetAttenuates Streptozotocin-Induced Diabetic Nephropathy in Rats. Evid. Based Complement. Altern. Med. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Afshari, A.T.; Shirpoor, A.; Farshid, A.; Saadatian, R.; Rasmi, Y.; Saboory, E.; Ilkhanizadeh, B.; Allameh, A. The effect of ginger on diabetic nephropathy, plasma antioxidant capacity and lipid peroxidation in rats. Food Chem. 2007, 101, 148–153. [Google Scholar] [CrossRef]
- Lim, A.K.H.; Tesch, G.H. Inflammation in Diabetic Nephropathy. Mediat. Inflamm. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Al-Quraishy, S.; Dkhil, M.A.; Abdel Moneim, A.E. Anti-hyperglycemic activity of selenium nanoparticles in streptozoto-cin-induced diabetic rats. Int. J. Nanomed. 2015, 10, 6741–6756. [Google Scholar]
- Almatroodi, S.A.; Anwar, S.; Almatroudi, A.; Khan, A.A.; Alrumaihi, F.; Alsahli, M.A.; Rahmani, A.H. Hepatoprotective Effects of Garlic Extract against Carbon Tetrachloride (CCl4)-Induced Liver Injury via Modulation of Antioxidant, An-ti-Inflammatory Activities and Hepatocyte Architecture. Appl. Sci. 2020, 10, 6200. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; AlRumaihi, F.; Alsahli, M.A.; Alhommrani, M.F.; Khan, A.; Rahmani, A.H. Curcumin, an Active Constituent of Turmeric Spice: Implication in the Prevention of Lung Injury Induced by Benzo(a) Pyrene (BaP) in Rats. Molecules 2020, 25, 724. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Alharbi, H.M.; Khan, A.A.; Husain Rahmani, A. Epigallocatechin-3-Gallate (EGCG), An Active Constituent of Green Tea: Implications in the Prevention of Liver Injury Induced by Diethylnitrosamine (DEN) in Rats. Appl. Sci. 2019, 9, 4821. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Alsahli, M.A.; Aljasir, M.A.; Syed, M.A.; Rahmani, A.H. Epigallocatechin-3-Gallate (EGCG), an Active Compound of Green Tea Attenuates Acute Lung Injury Regulating Macrophage Polarization and Krüp-ple-Like-Factor 4 (KLF4) Expression. Molecules 2020, 25, 2853. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.C.; Gopal, V.; Mandal, V. Biochemical Investigation of Standardized Wattakaka volubilis Leaf Petroleum Ether Cold Macerated Extract Against Experimentally Induced Diabetes in the Rat. Pharmacologia 2013, 4, 391–399. [Google Scholar] [CrossRef][Green Version]
- Payami, S.; Babaahmadi-Rezaei, H.; Ghaffari, M.; Mansouri, E.; Mohammadzadeh, G. Hydroalcoholic Extract of Zingiber officinale Improves STZ-Induced Diabetic Nephropathy in Rats by Reduction of NF-κB Activation. Jundishapur J. Nat. Pharm. Prod. 2019, 14, e55063. [Google Scholar]
- Xu, H.-L.; Wang, X.-T.; Cheng, Y.; Zhao, J.-G.; Zhou, Y.-J.; Yang, J.-J.; Qi, M.-Y. Ursolic acid improves diabetic nephropathy via suppression of oxidative stress and inflammation in streptozotocin-induced rats. Biomed. Pharmacother. 2018, 105, 915–921. [Google Scholar] [CrossRef] [PubMed]

| Group Number | Group | Short Name | Treatment Plan |
|---|---|---|---|
| I | Normal Control | NC | Rats were allowed to have free access to a standard pellet diet |
| II | Diabetes Control | DC | The rats were intraperitoneally injected with freshly prepared STZ (55 mg/kg b.w.) in citrate buffer pH 4.5 |
| III | Diabetes Control + 6-Gingerol | DC + 6-Ging | The diabetic rats were treated with 6-gingerol (10 mg/kg b.w.) [26] |
| IV | Diabetes Control + Glibenclamide | DC + Gliben | The diabetic rats were treated with Glibenclamide (5 mg/kg b.w.) [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almatroodi, S.A.; Alnuqaydan, A.M.; Babiker, A.Y.; Almogbel, M.A.; Khan, A.A.; Husain Rahmani, A. 6-Gingerol, a Bioactive Compound of Ginger Attenuates Renal Damage in Streptozotocin-Induced Diabetic Rats by Regulating the Oxidative Stress and Inflammation. Pharmaceutics 2021, 13, 317. https://doi.org/10.3390/pharmaceutics13030317
Almatroodi SA, Alnuqaydan AM, Babiker AY, Almogbel MA, Khan AA, Husain Rahmani A. 6-Gingerol, a Bioactive Compound of Ginger Attenuates Renal Damage in Streptozotocin-Induced Diabetic Rats by Regulating the Oxidative Stress and Inflammation. Pharmaceutics. 2021; 13(3):317. https://doi.org/10.3390/pharmaceutics13030317
Chicago/Turabian StyleAlmatroodi, Saleh A., Abdullah M. Alnuqaydan, Ali Yousif Babiker, Mashael Abdullah Almogbel, Amjad Ali Khan, and Arshad Husain Rahmani. 2021. "6-Gingerol, a Bioactive Compound of Ginger Attenuates Renal Damage in Streptozotocin-Induced Diabetic Rats by Regulating the Oxidative Stress and Inflammation" Pharmaceutics 13, no. 3: 317. https://doi.org/10.3390/pharmaceutics13030317
APA StyleAlmatroodi, S. A., Alnuqaydan, A. M., Babiker, A. Y., Almogbel, M. A., Khan, A. A., & Husain Rahmani, A. (2021). 6-Gingerol, a Bioactive Compound of Ginger Attenuates Renal Damage in Streptozotocin-Induced Diabetic Rats by Regulating the Oxidative Stress and Inflammation. Pharmaceutics, 13(3), 317. https://doi.org/10.3390/pharmaceutics13030317







