Analytical Strategies to Analyze the Oxidation Products of Phytosterols, and Formulation-Based Approaches to Reduce Their Generation
Abstract
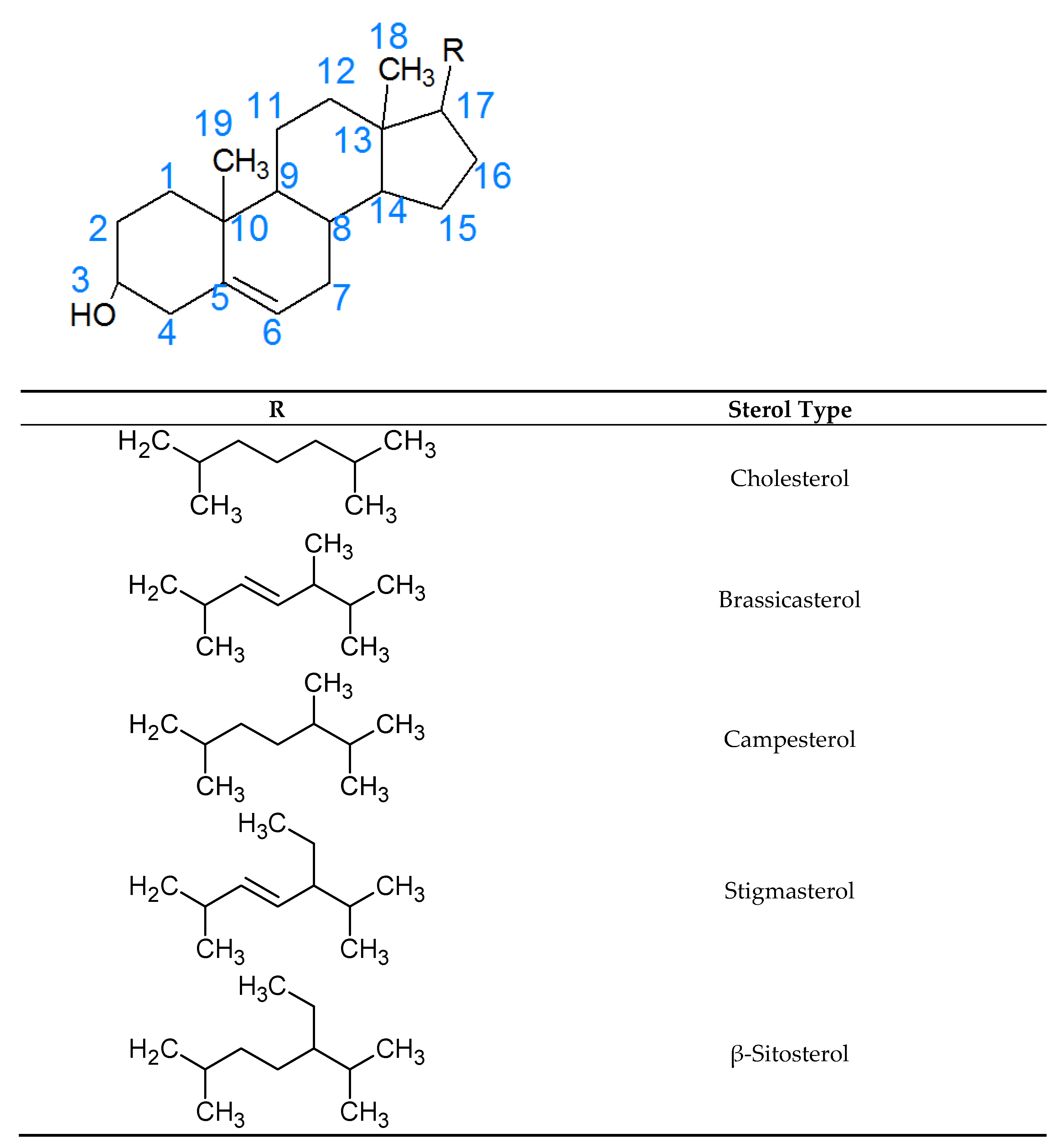
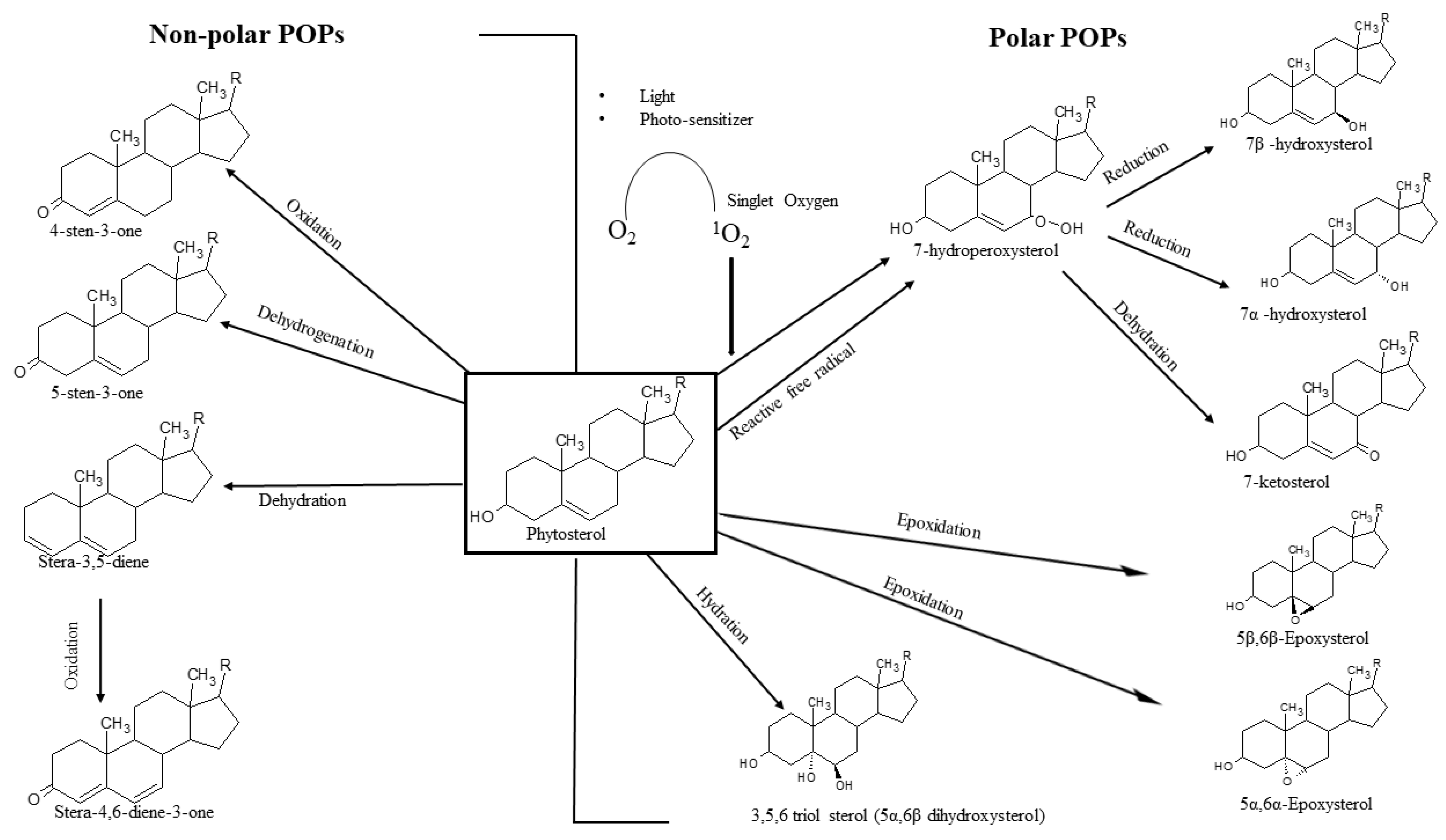
:1. Introduction
2. Analysis of Phytosterol Oxidation Products
2.1. Extraction and Enrichment of POPs
2.1.1. Solid Phase Extraction (SPE)
2.1.2. Thin Layer Chromatography (TLC) and Preparative Liquid Chromatography
2.2. Gas Chromatography
GC-MS/MS
2.3. Liquid Chromatography
Liquid Chromatography–Mass Spectrometry
3. Possible Formulation Strategies to Prevent the Formation of POPs
3.1. Formulation of Phytosterols in Suitable Delivery Systems
3.2. The Addition of Antioxidants to the Formulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vlahakis, C.; Hazebroek, J. Phytosterol accumulation in canola, sunflower, and soybean oils: Effects of genetics, planting location, and temperature. J. Am. Oil Chem. Soc. 2000, 77, 49–53. [Google Scholar] [CrossRef]
- Rowlands, J.C.; Hoadley, J.E. FDA perspectives on health claims for food labels. Toxicology 2006, 221, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Canada, H. Summary of Health Canada’s Assessment of a Health Claim about Vegetables and Fruit and Heart Disease; Bureau of Nutritional Sciences, Food Directorate, Health Products and Food Branch: Ottawa, ON, Canada, 2016. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to 3 g/day plant stanols as plant stanol esters and lowering blood LDL-cholesterol and reduced risk of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2693. [Google Scholar] [CrossRef] [Green Version]
- Alhassan, S.; Reese, K.A.; Mahurin, J.; Plaisance, E.P.; Hilson, B.D.; Garner, J.C.; Wee, S.O.; Grandjean, P.W. Blood lipid responses to plant stanol ester supplementation and aerobic exercise training. Metabolism 2006, 55, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H.; Maki, K.C.; Umporowicz, D.M.; Ingram, K.A.; Dicklin, M.R.; Schaefer, E.; Lane, R.W.; McNamara, J.R.; Ribaya-Mercado, J.D.; Perrone, G.; et al. Safety and Tolerability of Esterified Phytosterols Administered in Reduced-Fat Spread and Salad Dressing to Healthy Adult Men and Women. J. Am. Coll. Nutr. 2001, 20, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Autret, B.; Jialal, I. Reduced-calorie orange juice beverage with plant sterols lowers C-reactive protein concentrations and improves the lipid profile in human volunteers. Am. J. Clin. Nutr. 2006, 84, 756–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demonty, I.; Ras, R.; van Der Knaap, H.; Duchateau, G.; Meijer, L.; Zock, P.; Geleijnse, J.; Trautwein, E. Continuous Dose-Response Relationship of the LDL-Cholesterol-Lowering Effect of Phytosterol Intake1,2. J. Nutr. 2009, 139, 271–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abumweis, S.; Barake, R.; Jones, P. Plant sterols/stanols as cholesterol lowering agents: A meta-analysis of randomized controlled trials. Food Nutr. Res. 2008, 52, 1811. [Google Scholar] [CrossRef]
- Ras, R.T.; Hiemstra, H.; Lin, Y.; Vermeer, M.A.; Duchateau, G.S.M.J.E.; Trautwein, E.A. Consumption of plant sterol-enriched foods and effects on plasma plant sterol concentrations—A meta-analysis of randomized controlled studies. Atherosclerosis 2013, 230, 336–346. [Google Scholar] [CrossRef] [Green Version]
- Paul, D.; Dey, T.K.; Chakraborty, A.; Dhar, P. Promising Functional Lipids for Therapeutic Applications. Role of Materials Science in Food Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 413–449. [Google Scholar]
- Rozner, S.; Garti, N. The activity and absorption relationship of cholesterol and phytosterols. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282, 435–456. [Google Scholar] [CrossRef]
- Su, Y.; Wang, Z.; Yang, H.; Cao, L.; Liu, F.; Bai, X.; Ruan, C. Clinical and molecular genetic analysis of a family with sitosterolemia and co-existing erythrocyte and platelet abnormalities. Haematologica 2006, 91, 1392–1395. [Google Scholar] [PubMed]
- Izar, M.; Tegani, D.; Kasmas, S.; Fonseca, F. Phytosterols and phytosterolemia: Gene–diet interactions. Stud. Relatsh. Genet. Nutr. Improv. Hum. Health 2011, 6, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemany, L.; Barbera, R.; Alegría, A.; Laparra, J.M. Plant sterols from foods in inflammation and risk of cardiovascular disease: A real threat? Food Chem. Toxicol. 2014, 69, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Scholz, B.; Guth, S.; Engel, K.H.; Steinberg, P. Phytosterol oxidation products in enriched foods: Occurrence, exposure, and biological effects. Mol. Nutr. Food Res. 2015, 59, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Grün, C.H.; Besseau, S. Normal-phase liquid chromatography-atmospheric-pressure photoionization-mass spectrometry analysis of cholesterol and phytosterol oxidation products. J. Chromatogr. A 2016, 1439, 74–81. [Google Scholar] [CrossRef]
- Lu, B.; Zhao, Y. Photooxidation of phytochemicals in food and control: A review. Ann. N. Y. Acad. Sci. 2017, 1398, 72–82. [Google Scholar] [CrossRef]
- Scholz, B.; Wocheslander, S.; Lander, V.; Engel, K.-H. On-line liquid chromatography–gas chromatography: A novel approach for the analysis of phytosterol oxidation products in enriched foods. J. Chromatogr. A 2015, 1396, 98–108. [Google Scholar] [CrossRef]
- Menéndez-Carreño, M.; Steenbergen, H.; Janssen, H.-G. Development and validation of a comprehensive two-dimensional gas chromatography–mass spectrometry method for the analysis of phytosterol oxidation products in human plasma. Anal. Bioanal. Chem. 2012, 402, 2023–2032. [Google Scholar] [CrossRef]
- Johnsson, L.; Andersson, R.E.; Dutta, P.C. Side-chain autoxidation of stigmasterol and analysis of a mixture of phytosterol oxidation products by chromatographic and spectroscopic methods. J. Am. Oil Chem. Soc. 2003, 80, 777–783. [Google Scholar] [CrossRef]
- Vanmierlo, T.; Husche, C.; Schött, H.F.; Pettersson, H.; Lütjohann, D. Plant sterol oxidation products—Analogs to cholesterol oxidation products from plant origin? Biochimie 2013, 95, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Otaegui-Arrazola, A.; Menéndez-Carreño, M.; Ansorena, D.; Astiasarán, I. Oxysterols: A world to explore. Food Chem. Toxicol. 2010, 48, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Hovenkamp, E.; Demonty, I.; Plat, J.; Lütjohann, D.; Mensink, R.P.; Trautwein, E.A. Biological effects of oxidized phytosterols: A review of the current knowledge. Prog. Lipid Res. 2008, 47, 37–49. [Google Scholar] [CrossRef]
- Plat, J.; Theuwissen, E.; Husche, C.; Lütjohann, D.; Gijbels, M.J.J.; Jeurissen, M.; Shiri-Sverdlov, R.; van der Made, I.; Mensink, R.P. Oxidised plant sterols as well as oxycholesterol increase the proportion of severe atherosclerotic lesions in female LDL receptor +/− mice. Br. J. Nutr. 2014, 111, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.T.; Wong, W.T.; Guan, L.; Tian, X.Y.; Ma, K.Y.; Huang, Y.; Chen, Z.-Y. Effect of phytosterols and their oxidation products on lipoprotein profiles and vascular function in hamster fed a high cholesterol diet. Atherosclerosis 2011, 219, 124–133. [Google Scholar] [CrossRef]
- Lin, Y.; Knol, D.; Trautwein, E.A. Phytosterol oxidation products (POP) in foods with added phytosterols and estimation of their daily intake: A literature review. Eur. J. Lipid Sci. Technol. 2016, 118, 1423–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’callaghan, Y.; McCarthy, F.O.; O’brien, N.M. Recent advances in Phytosterol Oxidation Products. Biochem. Biophys. Res. Commun. 2014, 446, 786–791. [Google Scholar] [CrossRef]
- Wang, M.; Lu, B. How do oxyphytosterols affect human health? Trends Food Sci. Technol. 2018, 79, 148–159. [Google Scholar] [CrossRef]
- Barriuso, B.; Ansorena, D.; Astiasarán, I. Oxysterols formation: A review of a multifactorial process. J. Steroid Biochem. Mol. Biol. 2017, 169, 39–45. [Google Scholar] [CrossRef]
- Sosińska, E.; Przybylski, R.; Aladedunye, F.; Hazendonk, P. Spectroscopic characterisation of dimeric oxidation products of phytosterols. Food Chem. 2014, 151, 404–414. [Google Scholar] [CrossRef]
- Raith, K.; Brenner, C.; Farwanah, H.; Müller, G.; Eder, K.; Neubert, R.H.H. A new LC/APCI-MS method for the determination of cholesterol oxidation products in food. J. Chromatogr. A 2005, 1067, 207–211. [Google Scholar] [CrossRef]
- Helmschrodt, C.; Becker, S.; Schröter, J.; Hecht, M.; Aust, G.; Thiery, J.; Ceglarek, U. Fast LC–MS/MS analysis of free oxysterols derived from reactive oxygen species in human plasma and carotid plaque. Clin. Chim. Acta 2013, 425, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inchingolo, R.; Cardenia, V.; Rodriguez-Estrada, M.T. Analysis of phytosterols and phytostanols in enriched dairy products by fast gas chromatography with mass spectrometry. J. Sep. Sci. 2014, 37, 2911–2919. [Google Scholar] [CrossRef] [PubMed]
- Pataj, Z.; Liebisch, G.; Schmitz, G.; Matysik, S. Quantification of oxysterols in human plasma and red blood cells by liquid chromatography high-resolution tandem mass spectrometry. J. Chromatogr. A 2016, 1439, 82–88. [Google Scholar] [CrossRef]
- Almeida, C.A.S.; Baggio, S.R.; Mariutti, L.R.B.; Bragagnolo, N. One-step rapid extraction of phytosterols from vegetable oils. Food Res. Int. 2020, 130, 108891. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Carreño, M.; García-Herreros, C.; Astiasarán, I.; Ansorena, D. Validation of a gas chromatography–mass spectrometry method for the analysis of sterol oxidation products in serum. J. Chromatogr. B 2008, 864, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Carreño, M.; Knol, D.; Janssen, H.-G. Development and validation of methodologies for the quantification of phytosterols and phytosterol oxidation products in cooked and baked food products. J. Chromatogr. A 2016, 1428, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Schött, H.-F.; Lütjohann, D. Validation of an isotope dilution gas chromatography–mass spectrometry method for combined analysis of oxysterols and oxyphytosterols in serum samples. Steroids 2015, 99, 139–150. [Google Scholar] [CrossRef]
- Husche, C.; Weingärtner, O.; Pettersson, H.; Vanmierlo, T.; Böhm, M.; Laufs, U.; Lütjohann, D. Validation of an isotope dilution gas chromatography–mass spectrometry method for analysis of 7-oxygenated campesterol and sitosterol in human serum. Chem. Phys. Lipids 2011, 164, 425–431. [Google Scholar] [CrossRef]
- Plat, J.; Brzezinka, H.; Lütjohann, D.; Mensink, R.P.; von Bergmann, K. Oxidized plant sterols in human serum and lipid infusions as measured by combined gas-liquid chromatography-mass spectrometry. J. Lipid Res. 2001, 42, 2030–2038. [Google Scholar] [CrossRef]
- Grandgirard, A.; Martine, L.; Joffre, C.; Juaneda, P.; Berdeaux, O. Gas chromatographic separation and mass spectrometric identification of mixtures of oxyphytosterol and oxycholesterol derivatives: Application to a phytosterol-enriched food. J. Chromatogr. A 2004, 1040, 239–250. [Google Scholar] [CrossRef]
- Lampi, A.-M.; Juntunen, L.; Toivo, J.; Piironen, V. Determination of thermo-oxidation products of plant sterols. J. Chromatogr. B 2002, 777, 83–92. [Google Scholar] [CrossRef]
- Decloedt, A.; Van Landschoot, A.; Watson, H.; Vanderputten, D.; Vanhaecke, L. Plant-Based Beverages as Good Sources of Free and Glycosidic Plant Sterols. Nutrients 2018, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Balme, S.; Gülaçar, F.O. Rapid screening of phytosterols in orange juice by solid-phase microextraction on polyacrylate fibre derivatisation and gas chromatographic–mass spectrometric. Food Chem. 2012, 132, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane-Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boselli, E.; Velazco, V.; Caboni, M.F.; Lercker, G. Pressurized liquid extraction of lipids for the determination of oxysterols in egg-containing food. J. Chromatogr. A 2001, 917, 239–244. [Google Scholar] [CrossRef]
- Azadmard-Damirchi, S.; Dutta, P.C. A single step solid-phase extraction method for complete separation of sterol oxidation products in food lipids. J. Chromatogr. A 2009, 1216, 36–42. [Google Scholar] [CrossRef]
- Conchillo, A.; Cercaci, L.; Ansorena, D.; Rodriguez-Estrada, M.T.; Lercker, G.; Astiasarán, I. Levels of phytosterol oxides in enriched and nonenriched spreads: Application of a thin-layer chromatography− gas chromatography methodology. J. Agric. Food Chem. 2005, 53, 7844–7850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnsson, L.; Dutta, P.C. Determination of phytosterol oxides in some food products by using an optimized transesterification method. Food Chem. 2006, 97, 606–613. [Google Scholar] [CrossRef]
- Dean, L.; Fenner, G.; Boyd, L. Characterization of lipids and their oxidation products in baked or fried breaded shrimp products. Open Food Sci. J. 2009, 3, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Soupas, L.; Juntunen, L.; Säynäjoki, S.; Lampi, A.M.; Piironen, V. GC-MS method for characterization and quantification of sitostanol oxidation products. J. Am. Oil Chem. Soc. 2004, 81, 135–141. [Google Scholar] [CrossRef]
- Dutta, P.C.; Appelqvist, L.Å. Studies on phytosterol oxides. I: Effect of storage on the content in potato chips prepared in different vegetable oils. J. Am. Oil Chem. Soc. 1997, 74, 647–657. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, G.; Huang, W.; Lai, S.; Ren, Y.; Huang, B.; Zhang, L.; Li, P.; Lu, B. Development and validation of a gas chromatography-mass spectrometry method for determination of sterol oxidation products in edible oils. RSC Adv. 2015, 5, 41259–41268. [Google Scholar] [CrossRef]
- Johnsson, L.; Dutta, P.C. Characterization of side-chain oxidation products of sitosterol and campesterol by chromatographic and spectroscopic methods. J. Am. Oil Chem. Soc. 2003, 80, 767–776. [Google Scholar] [CrossRef]
- Lütjohann, D.; Björkhem, I.; Friedrichs, S.; Kerksiek, A.; Lövgren-Sandblom, A.; Geilenkeuser, W.-J.; Ahrends, R.; Andrade, I.; Ansorena, D.; Astiasarán, I. First international descriptive and interventional survey for cholesterol and non-cholesterol sterol determination by gas-and liquid-chromatography–Urgent need for harmonisation of analytical methods. J. Steroid Biochem. Mol. Biol. 2019, 190, 115–125. [Google Scholar] [CrossRef]
- Soupas, L.; Juntunen, L.; Lampi, A.-M.; Piironen, V. Effects of sterol structure, temperature, and lipid medium on phytosterol oxidation. J. Agric. Food Chem. 2004, 52, 6485–6491. [Google Scholar] [CrossRef]
- Matysik, S.; Klünemann, H.; Schmitz, G. Gas chromatography–tandem mass spectrometry method for the simultaneous determination of oxysterols, plant sterols, and cholesterol precursors. Clin. Chem. 2012, 58, 1557–1564. [Google Scholar] [CrossRef] [Green Version]
- Rudziñska, M.; Korczak, J.; Wasowicz, E. Changes in phytosterols and their oxidation products during frying of French fries in rapeseed oil. Pol. J. Food Nutr. Sci. 2005, 14, 381. [Google Scholar]
- Derewiaka, D.; Obiedziński, M. Oxysterol content in selected meats and meat products. Acta Sci. Pol. Technol. Aliment. 2009, 8, 5–13. [Google Scholar]
- Jiang, K.; Gachumi, G.; Poudel, A.; Shurmer, B.; Bashi, Z.; El-Aneed, A. The establishment of tandem mass spectrometric fingerprints of phytosterols and tocopherols and the development of targeted profiling strategies in vegetable oils. J. Am. Soc. Mass Spectrom. 2019, 30, 1700–1712. [Google Scholar] [CrossRef]
- González-Larena, M.; García-Llatas, G.; Vidal, M.C.; Sánchez-Siles, L.M.; Barberá, R.; Lagarda, M.J. Stability of plant sterols in ingredients used in functional foods. J. Agric. Food Chem. 2011, 59, 3624–3631. [Google Scholar] [CrossRef] [PubMed]
- Alemany-Costa, L.; González-Larena, M.; García-Llatas, G.; Alegría, A.; Barberá, R.; Sánchez-Siles, L.M.; Lagarda, M.J. Sterol stability in functional fruit beverages enriched with different plant sterol sources. Food Res. Int. 2012, 48, 265–270. [Google Scholar] [CrossRef]
- Gonzalez-Larena, M.; Garcia-Llatas, G.; Clemente, G.; Barberá, R.; Lagarda, M.J. Plant sterol oxides in functional beverages: Influence of matrix and storage. Food Chem. 2015, 173, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Sala, A.; Blanco-Morales, V.; Cilla, A.; Garcia-Llatas, G.; Sánchez-Siles, L.M.; Barberá, R.; Lagarda, M.J. Safe intake of a plant sterol-enriched beverage with milk fat globule membrane: Bioaccessibility of sterol oxides during storage. J. Food Compost. Anal. 2018, 68, 111–117. [Google Scholar] [CrossRef]
- Baumgartner, S.; Mensink, R.P.; Konings, M.; Schött, H.-F.; Friedrichs, S.; Husche, C.; Lütjohann, D.; Plat, J. Postprandial plasma oxyphytosterol concentrations after consumption of plant sterol or stanol enriched mixed meals in healthy subjects. Steroids 2015, 99, 281–286. [Google Scholar] [CrossRef]
- Baumgartner, S.; Ras, R.T.; Trautwein, E.A.; Konings, M.C.; Mensink, R.P.; Plat, J. Plasma oxyphytosterol concentrations are not associated with CVD status in Framingham Offspring Study participants. J. Lipid Res. 2019, 60, 1905–1911. [Google Scholar] [CrossRef]
- Säynäjoki, S.; Sundberg, S.; Soupas, L.; Lampi, A.-M.; Piironen, V. Determination of stigmasterol primary oxidation products by high-performance liquid chromatography. Food Chem. 2003, 80, 415–421. [Google Scholar] [CrossRef]
- Foley, D.A.; O’Callaghan, Y.; O’Brien, N.M.; McCarthy, F.O.; Maguire, A.R. Synthesis and characterization of stigmasterol oxidation products. J. Agric. Food Chem. 2010, 58, 1165–1173. [Google Scholar] [CrossRef]
- Poudel, A.; Gachumi, G.; Badea, I.; Bashi, Z.D.; El-Aneed, A. The simultaneous quantification of phytosterols and tocopherols in liposomal formulations using validated atmospheric pressure chemical ionization- liquid chromatography –tandem mass spectrometry. J. Pharm. Biomed. Anal. 2020, 183, 113104. [Google Scholar] [CrossRef]
- Kemmo, S.; Ollilainen, V.; Lampi, A.-M.; Piironen, V. Liquid chromatography mass spectrometry for plant sterol oxide determination in complex mixtures. Eur. Food Res. Technol. 2008, 226, 1325–1334. [Google Scholar] [CrossRef]
- Kemmo, S.; Ollilainen, V.; Lampi, A.-M.; Piironen, V. Determination of stigmasterol and cholesterol oxides using atmospheric pressure chemical ionization liquid chromatography/mass spectrometry. Food Chem. 2007, 101, 1438–1445. [Google Scholar] [CrossRef]
- Ng, A.W.K.; Lukic, T.; Pritchard, P.H.; Wasan, K.M. Development and Characterization of Liposomal Disodium Ascorbyl Phytostanyl Phosphates (FM-VP4). Drug Dev. Ind. Pharm. 2004, 30, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Looije, N.A.; Risovic, V.; Stewart, D.J.; Debeyer, D.; Kutney, J.; Wasan, K.M. Disodium Ascorbyl Phytostanyl Phosphates (FM-VP4) reduces plasma cholesterol concentration, body weight and abdominal fat gain within a dietary-induced obese mouse model. J. Pharm. Pharm. Sci. Publ. Can. Soc. Pharm. Sci. Soc. Can. Sci. Pharm. 2005, 8, 400. [Google Scholar]
- Acevedo-Estupiñan, M.V.; Gutierrez-Lopez, G.F.; Cano-Sarmiento, C.; Parra-Escudero, C.O.; Rodriguez-Estrada, M.T.; Garcia-Varela, R.; García, H.S. Stability and characterization of O/W free phytosterols nanoemulsions formulated with an enzymatically modified emulsifier. LWT 2019, 107, 151–157. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ciftci, O.N. In vitro bioaccessibility of novel low-crystallinity phytosterol nanoparticles in non-fat and regular-fat foods. Food Res. Int. 2019, 123, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kmiecik, D.; Korczak, J.; Rudzińska, M.; Kobus-Cisowska, J.; Gramza-Michałowska, A.; Hęś, M. β-Sitosterol and campesterol stabilisation by natural and synthetic antioxidants during heating. Food Chem. 2011, 128, 937–942. [Google Scholar] [CrossRef]
- Kmiecik, D.; Korczak, J.; Rudzińska, M.; Gramza-Michałowska, A.; Hęś, M.; Kobus-Cisowska, J. Stabilisation of phytosterols by natural and synthetic antioxidants in high temperature conditions. Food Chem. 2015, 173, 966–971. [Google Scholar] [CrossRef]
- Shin, M.-J.; Lee, J.H.; Jang, Y.; Lee-Kim, Y.C.; Park, E.; Kim, K.M.; Chung, B.C.; Chung, N. Micellar Phytosterols Effectively Reduce Cholesterol Absorption at Low Doses. Ann. Nutr. Metab. 2005, 49, 346–351. [Google Scholar] [CrossRef]
- Amir Shaghaghi, M.; Harding, S.V.; Jones, P.J.H. Water dispersible plant sterol formulation shows improved effect on lipid profile compared to plant sterol esters. J. Funct. Foods 2014, 6, 280–289. [Google Scholar] [CrossRef]
- Coimbra, M.; Isacchi, B.; van Bloois, L.; Torano, J.S.; Ket, A.; Wu, X.; Broere, F.; Metselaar, J.M.; Rijcken, C.J.F.; Storm, G.; et al. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int. J. Pharm. 2011, 416, 433–442. [Google Scholar] [CrossRef]
- Patel, M.; Patel, R.; Parikh, J.; Solanki, A.; Patel, B. Effect of Formulation Components on the In Vitro Permeation of Microemulsion Drug Delivery System of Fluconazole. AAPS PharmSciTech 2009, 10, 917–923. [Google Scholar] [CrossRef] [Green Version]
- Haham, M.; Ish-shalom, S.; Nodelman, M.; Duek, I.; Segal, E.; Kustanovich, M.; Livney, Y.D. Stability and bioavailability of vitamin D nanoencapsulated in casein micelles. Food Funct. 2012, 3, 737–744. [Google Scholar] [CrossRef]
- Naksuriya, O.; Steenbergen, M.; Torano, J.; Okonogi, S.; Hennink, W. A Kinetic Degradation Study of Curcumin in Its Free Form and Loaded in Polymeric Micelles. AAPS J. 2016, 18, 777–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Battista, C.A.; Constenla, D.; Ramírez-Rigo, M.V.; Piña, J. The use of arabic gum, maltodextrin and surfactants in the microencapsulation of phytosterols by spray drying. Powder Technol. 2015, 286, 193–201. [Google Scholar] [CrossRef]
- Tolve, R.; Condelli, N.; Can, A.; Tchuenbou-Magaia, F. Development and Characterization of Phytosterol-Enriched Oil Microcapsules for Foodstuff Application. Int. J. 2018, 11, 152–163. [Google Scholar] [CrossRef]
- Ostlund, R.E.; Spilburg, C.A.; Stenson, W.F. Sitostanol administered in lecithin micelles potently reduces cholesterol absorption in humans. Am. J. Clin. Nutr. 1999, 70, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fröhlich, M.; Brecht, V.; Peschka-Süss, R. Parameters influencing the determination of liposome lamellarity by 31P-NMR. Chem. Phys. Lipids 2001, 109, 103–112. [Google Scholar] [CrossRef]
- Pan, Y.; Tikekar, R.V.; Nitin, N. Effect of antioxidant properties of lecithin emulsifier on oxidative stability of encapsulated bioactive compounds. Int. J. Pharm. 2013, 450, 129–137. [Google Scholar] [CrossRef]
- Toniazzo, T.; Berbel, I.F.; Cho, S.; Fávaro-Trindade, C.S.; Moraes, I.C.F.; Pinho, S.C. β-carotene-loaded liposome dispersions stabilized with xanthan and guar gums: Physico-chemical stability and feasibility of application in yogurt. LWT Food Sci. Technol. 2014, 59, 1265–1273. [Google Scholar] [CrossRef]
- Chen, H.; Guan, Y.; Zhong, Q. Microemulsions based on a sunflower lecithin-tween 20 blend have high capacity for dissolving peppermint oil and stabilizing coenzyme Q 10. J. Agric. Food Chem. 2015, 63, 983–989. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, Q. Thermal and UV stability of β-carotene dissolved in peppermint oil microemulsified by sunflower lecithin and Tween 20 blend. Food Chem. 2015, 174, 630–636. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Food-grade microemulsions and nanoemulsions: Role of oil phase composition on formation and stability. Food Hydrocoll. 2012, 29, 326–334. [Google Scholar] [CrossRef]
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014, 112, 214–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Ma, C.; Gao, Z.; Zheng, J.; He, L.; McClements, D.J.; Xiao, H. Characterization of the Interactions between Titanium Dioxide Nanoparticles and Polymethoxyflavones Using Surface-Enhanced Raman Spectroscopy. J. Agric. Food Chem. 2016, 64, 9436–9441. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Xiao, H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci. Food 2017, 1, 6. [Google Scholar] [CrossRef]
- Xu, G.; Guan, L.; Sun, J.; Chen, Z.-Y. Oxidation of cholesterol and beta-sitosterol and prevention by natural antioxidants. J. Agric. Food Chem. 2009, 57, 9284–9292. [Google Scholar] [CrossRef]
- Amazan, D.; Cordero, G.; López-Bote, C.J.; Lauridsen, C.; Rey, A.I. Effects of oral micellized natural vitamin E (d—α-tocopherol) vs. synthetic vitamin E (dl—α-tocopherol) in feed on α -tocopherol levels, stereoisomer distribution, oxidative stress and the immune response in piglets. Animal 2014, 8, 410–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poudel, A.; Gachumi, G.; Wasan, K.M.; Dallal Bashi, Z.; El-Aneed, A.; Badea, I. Development and Characterization of Liposomal Formulations Containing Phytosterols Extracted from Canola Oil Deodorizer Distillate along with Tocopherols as Food Additives. Pharmaceutics 2019, 11, 185. [Google Scholar] [CrossRef] [Green Version]
| Sample Matrix | Pretreatment Step | Extraction Solvent | References |
|---|---|---|---|
| Vegetable fat spread | Transesterification | Chloroform | [19] |
| Cooked/baked products | Lipid extraction/Saponification | Various b/Dichloromethane | [38] |
| Serum | Saponification | Dichloromethane | [39,40,41] |
| Vegetable fat spread | Lipid extraction/Saponification | Dichloromethane/methanol (2:1) a/Dichloromethane | [42] |
| Beverages | NA | Chloroform/methanol (2:1) a | [44] |
| Hazelnut oil | Lipid extraction/Transesterification | Hexane:isopropanol (3:2) a/Chloroform | [49] |
| Rapeseed oil | Saponification | Dichloromethane | [49] |
| Vegetable fat spread | Lipid extraction/Saponification | Hexane:isopropanol (3:2) a/Diethyl ether | [50] |
| Maize oil | Transesterification | Dichloromethane | [51] |
| Breaded shrimp | Saponification | Dichloromethane | [52] |
| Phytosterols Formulations | Composition of Delivery Vehicle | Assessment of Oxidative Stability of Phytosterols | References |
|---|---|---|---|
| Nanoemulsion | Phosphatidylcholine Medium chain triglyceride Glycerol | Not conducted | [76] |
| Nanoparticles | Starch aerogel | Not conducted | [77] |
| Microparticles | Arabic gum, maltodextrin, polysorbate Tween 20, sodium lauryl sulphate | Not conducted | [86] |
| Microcapsules | Asolectin | (i) Low peroxide value (ii) POPs not screened | [87] |
| Micelles | Soy lecithin | Not conducted | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gachumi, G.; Poudel, A.; Wasan, K.M.; El-Aneed, A. Analytical Strategies to Analyze the Oxidation Products of Phytosterols, and Formulation-Based Approaches to Reduce Their Generation. Pharmaceutics 2021, 13, 268. https://doi.org/10.3390/pharmaceutics13020268
Gachumi G, Poudel A, Wasan KM, El-Aneed A. Analytical Strategies to Analyze the Oxidation Products of Phytosterols, and Formulation-Based Approaches to Reduce Their Generation. Pharmaceutics. 2021; 13(2):268. https://doi.org/10.3390/pharmaceutics13020268
Chicago/Turabian StyleGachumi, George, Asmita Poudel, Kishor M. Wasan, and Anas El-Aneed. 2021. "Analytical Strategies to Analyze the Oxidation Products of Phytosterols, and Formulation-Based Approaches to Reduce Their Generation" Pharmaceutics 13, no. 2: 268. https://doi.org/10.3390/pharmaceutics13020268
APA StyleGachumi, G., Poudel, A., Wasan, K. M., & El-Aneed, A. (2021). Analytical Strategies to Analyze the Oxidation Products of Phytosterols, and Formulation-Based Approaches to Reduce Their Generation. Pharmaceutics, 13(2), 268. https://doi.org/10.3390/pharmaceutics13020268







