Boosting Antimicrobial Activity of Ciprofloxacin by Functionalization of Mesoporous Silica Nanoparticles
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
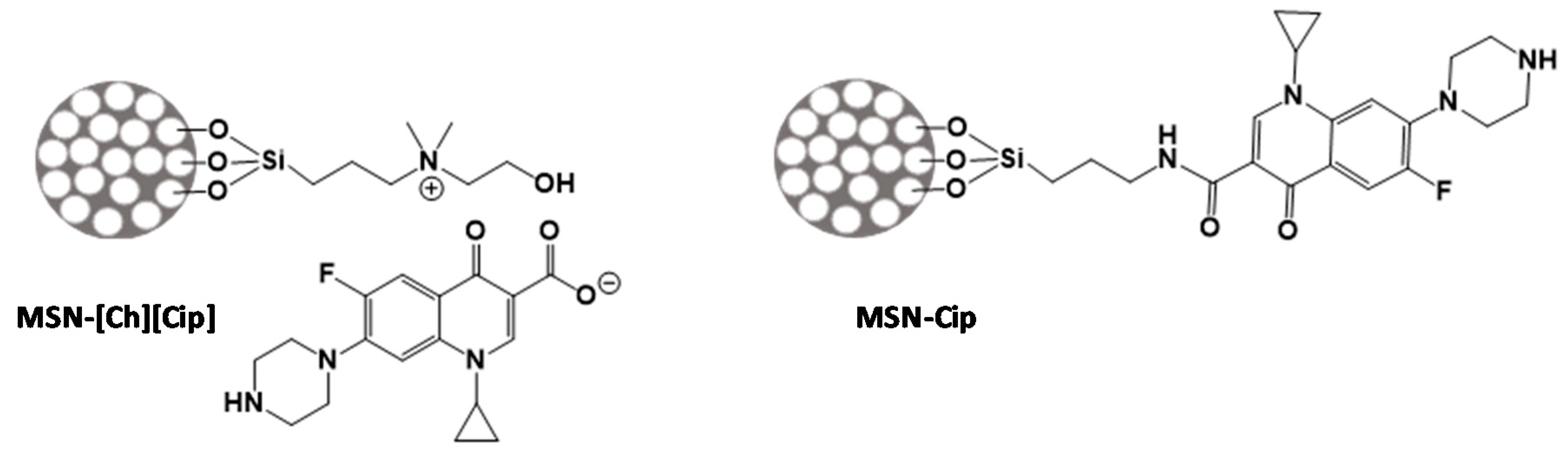
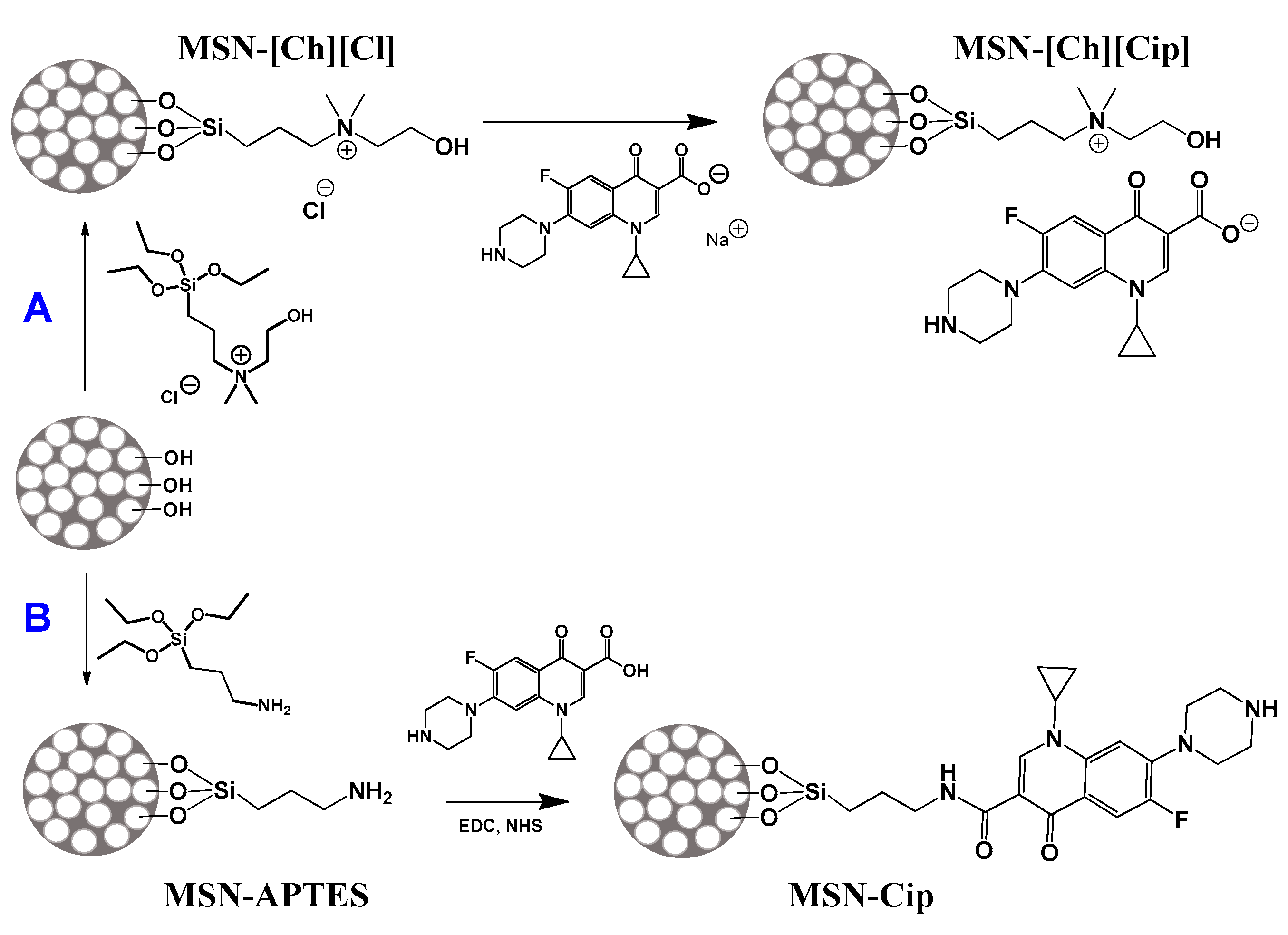
2.2. Description of the Synthesis
2.2.1. Synthesis of Triethoxysilane Cholinium Derivative (Si-[Ch][Cl])
2.2.2. Synthesis of Sodium Salt of Ciprofloxacin ([Na][Cip])
2.2.3. Synthesis of Mesoporous Silica Nanoparticles (MSNs) (MSN-OH)
2.2.4. Synthesis of Mesoporous Silica Nanoparticles Functionalized with Si-[Ch][Cl] (MSN-[Ch][Cl])
2.2.5. Synthesis of Mesoporous Silica Nanoparticles Functionalized with [Ch][Cip] (MSN-[Ch][Cip])
2.2.6. Synthesis of Mesoporous Silica Nanoparticles Functionalized with (3-Aminopropyl)triethoxysilane (APTES) (MSN-APTES)
2.2.7. Modification of MSN-APTES with Ciprofloxacin (MSN-Cip)
2.3. Cytotoxicity of Compounds
2.4. Antimicrobial Activities
2.5. Data and Statistical Analysis
3. Results and Discussion
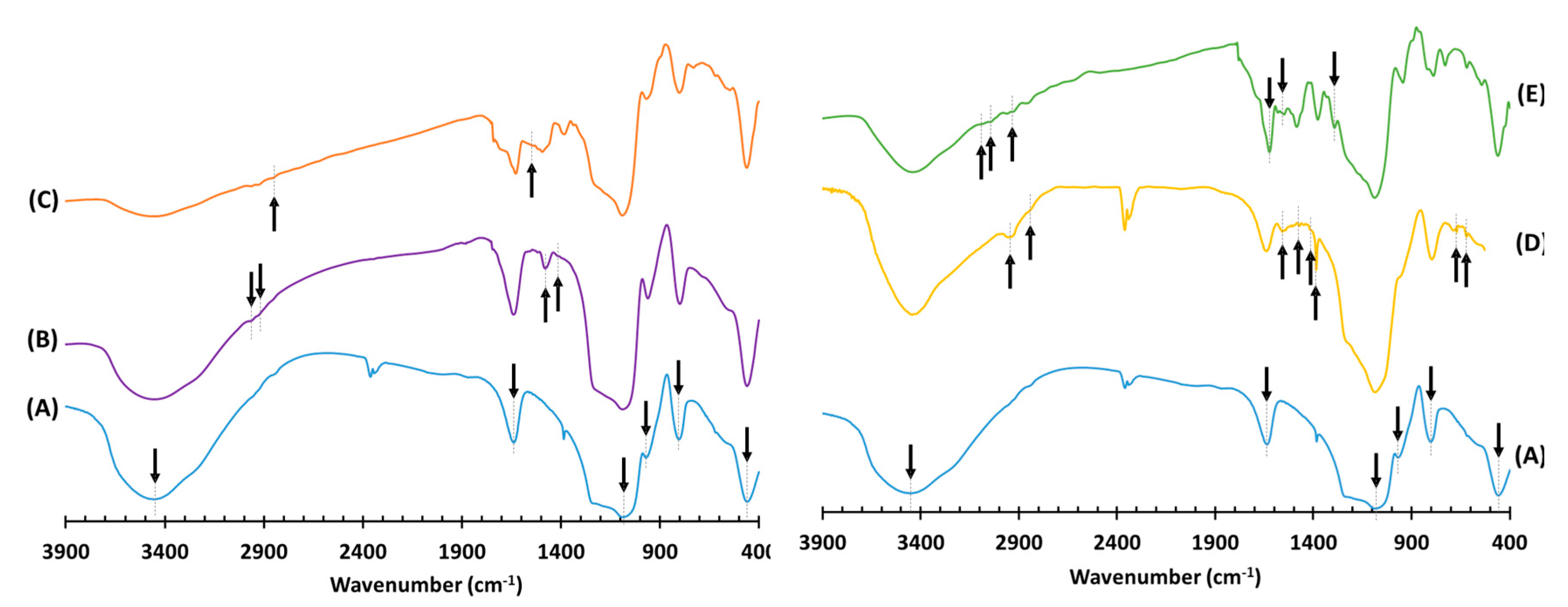
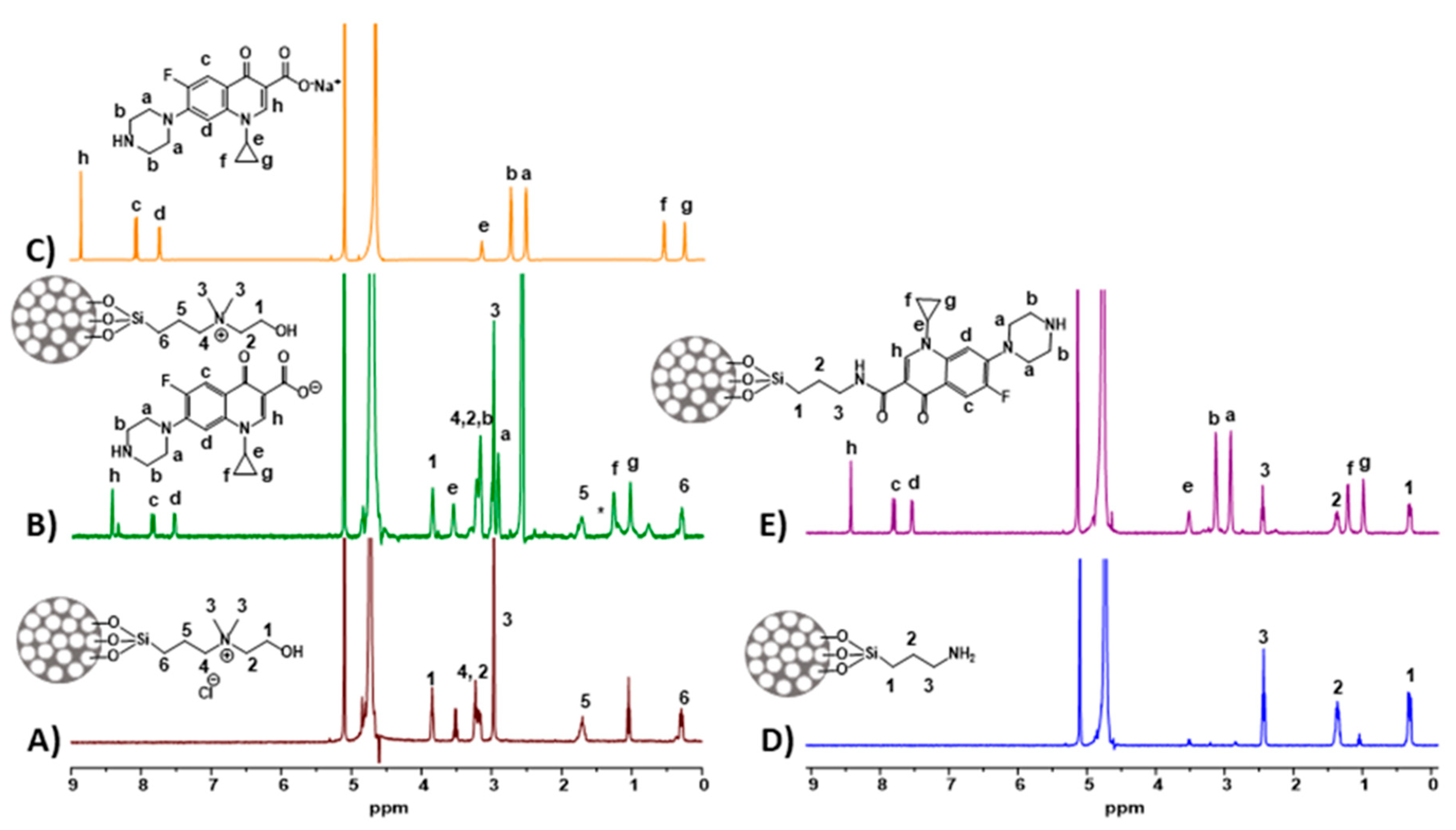
3.1. Synthesis and Characterization
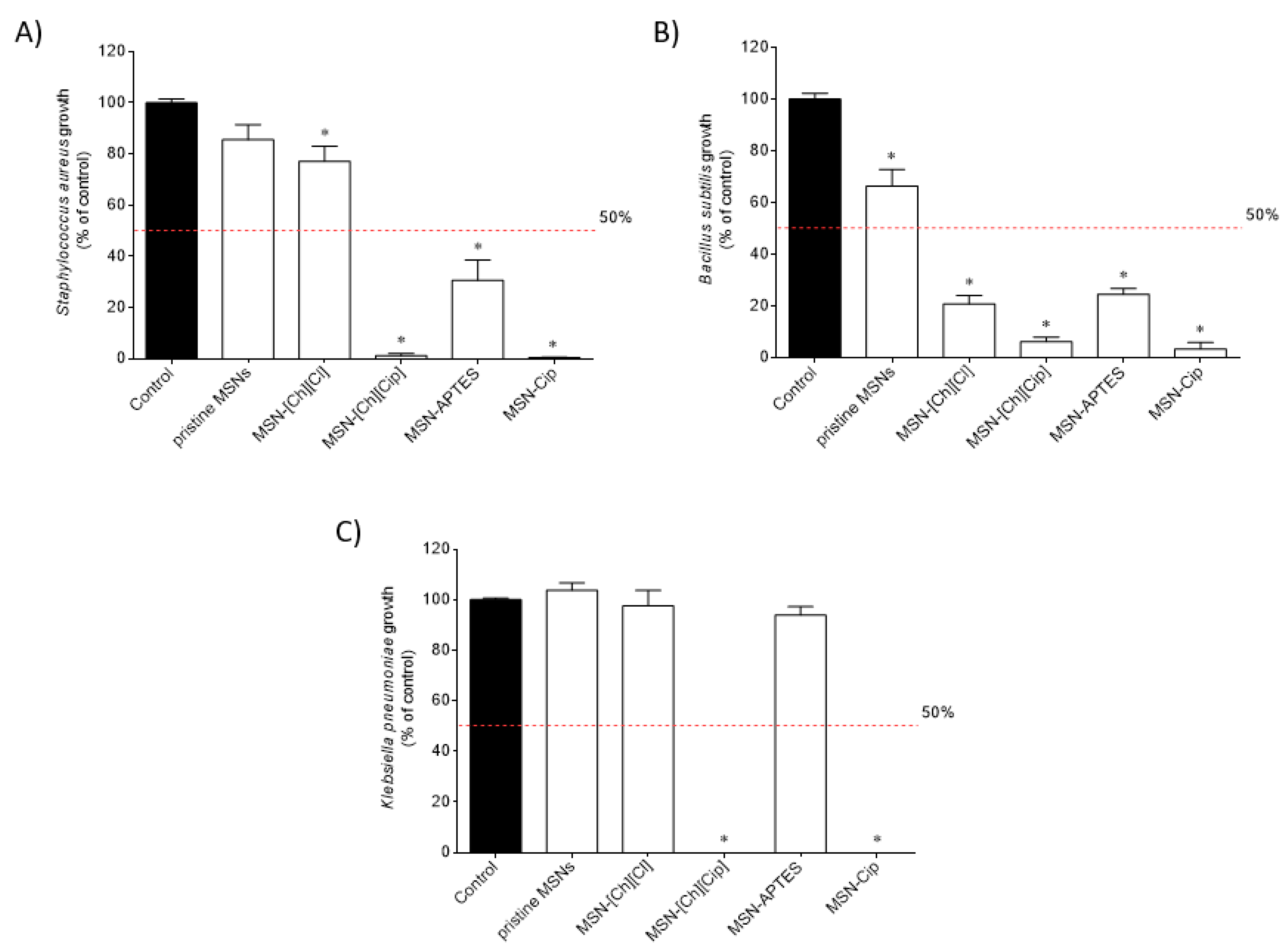
3.2. Cytotoxic and Antimicrobial Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Santos, M.M.; Branco, L.C. Ionic Liquids and Deep Eutectic Solvents for Application in Pharmaceutics. Pharmaceutics 2020, 12, 909. [Google Scholar] [CrossRef]
- Ferraz, R.; Branco, L.C.; Marrucho, I.M.; Araújo, J.M.M.; Rebelo, L.P.N.; da Ponte, M.N.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž. Development of novel ionic liquids based on ampicillin. Medchemcomm 2012, 3, 494. [Google Scholar] [CrossRef]
- Florindo, C.; Araújo, J.M.M.; Alves, F.; Matos, C.; Ferraz, R.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž.; Branco, L.; Rebelo, L.P.N.; et al. Evaluation of solubility and partition properties of ampicillin-based ionic liquids. Int. J. Pharm. 2013, 456, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.; Teixeira, V.; Rodrigues, D.; Fernandes, R.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž.; Branco, L.C. Antibacterial activity of Ionic Liquids based on ampicillin against resistant bacteria. RSC Adv. 2014, 4, 4301–4307. [Google Scholar] [CrossRef]
- Ferraz, R.; Silva, D.; Dias, A.R.; Dias, V.; Santos, M.M.; Pinheiro, L.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž.; Branco, L.C. Synthesis and Antibacterial Activity of Ionic Liquids and Organic Salts Based on Penicillin G and Amoxicillin hydrolysate Derivatives against Resistant Bacteria. Pharmaceutics 2020, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Carrera, G.V.S.M.; Santos, M.M.; Costa, A.; Rebelo, L.P.N.; Marrucho, I.M.; da Ponte, M.N.; Branco, L.C. Highly water soluble room temperature superionic liquids of APIs. New J. Chem. 2017, 41, 6986–6990. [Google Scholar] [CrossRef]
- Florindo, C.; Costa, A.; Matos, C.; Nunes, S.L.; Matias, A.N.; Duarte, C.M.M.; Rebelo, L.P.N.; Branco, L.C.; Marrucho, I.M. Novel organic salts based on fluoroquinolone drugs: Synthesis, bioavailability and toxicological profiles. Int. J. Pharm. 2014, 469, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.M.; Alves, C.; Silva, J.; Florindo, C.; Costa, A.; Petrovski, Ž.; Marrucho, I.M.; Pedrosa, R.; Branco, L.C. Antimicrobial Activities of Highly Bioavailable Organic Salts and Ionic Liquids from Fluoroquinolones. Pharmaceutics 2020, 12, 694. [Google Scholar] [CrossRef]
- Santos, M.M.; Raposo, L.R.; Carrera, G.V.S.M.; Costa, A.; Dionísio, M.; Baptista, P.V.; Fernandes, A.R.; Branco, L.C. Ionic Liquids and Salts from Ibuprofen as Promising Innovative Formulations of an Old Drug. ChemMedChem 2019, 14, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.; Santos, M.M.; Ferraz, R.; Prudêncio, C.; Fernandes, M.H.; Costa-Rodrigues, J.; Branco, L.C. A Novel Approach for Bisphosphonates: Ionic Liquids and Organic Salts from Zoledronic Acid. ChemMedChem 2019, 14, 1767–1770. [Google Scholar] [CrossRef]
- Teixeira, S.; Santos, M.M.; Fernandes, M.H.; Costa-Rodrigues, J.; Branco, L.C. Alendronic Acid as Ionic Liquid: New Perspective on Osteosarcoma. Pharmaceutics 2020, 12, 293. [Google Scholar] [CrossRef]
- Bernardos, A.; Piacenza, E.; Sancenón, F.; Hamidi, M.; Maleki, A.; Turner, R.J.; Martínez-Máñez, R. Mesoporous Silica-Based Materials with Bactericidal Properties. Small 2019, 15, 1–34. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; González, B.; Izquierdo-Barba, I. Nanomaterials as Promising Alternative in the Infection Treatment. Int. J. Mol. Sci. 2019, 20, 3806. [Google Scholar] [CrossRef]
- Cicuéndez, M.; Izquierdo-Barba, I.; Portolés, M.T.; Vallet-Regí, M. Biocompatibility and levofloxacin delivery of mesoporous materials. Eur. J. Pharm. Biopharm. 2013, 84, 115–124. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, X.; Chen, Y.; Guo, M.; Zhang, Y.; Guo, X.; Gu, H. Antibiotic-loaded, silver core-embedded mesoporous silica nanovehicles as a synergistic antibacterial agent for the treatment of drug-resistant infections. Biomaterials 2016, 101, 207–216. [Google Scholar] [CrossRef]
- Song, Z.; Ma, Y.; Xia, G.; Wang, Y.; Kapadia, W.; Sun, Z.; Wu, W.; Gu, H.; Cui, W.; Huang, X. In vitro and in vivo combined antibacterial effect of levofloxacin/silver co-loaded electrospun fibrous membranes. J. Mater. Chem. B 2017, 5, 7632–7643. [Google Scholar] [CrossRef]
- Cicuéndez, M.; Doadrio, J.C.; Hernández, A.; Portolés, M.T.; Izquierdo-Barba, I.; Vallet-Regí, M. Multifunctional pH sensitive 3D scaffolds for treatment and prevention of bone infection. Acta Biomater. 2018, 65, 450–461. [Google Scholar] [CrossRef] [PubMed]
- González, B.; Colilla, M.; Díez, J.; Pedraza, D.; Guembe, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous silica nanoparticles decorated with polycationic dendrimers for infection treatment. Acta Biomater. 2018, 68, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Tahmasbi, L.; Sedaghat, T.; Motamedi, H.; Kooti, M. Mesoporous silica nanoparticles supported copper(II) and nickel(II) Schiff base complexes: Synthesis, characterization, antibacterial activity and enzyme immobilization. J. Solid State Chem. 2018, 258, 517–525. [Google Scholar] [CrossRef]
- Tamanna, T.; Landersdorfer, C.B.; Ng, H.J.; Bulitta, J.B.; Wood, P.; Yu, A. Prolonged and continuous antibacterial and anti-biofilm activities of thin films embedded with gentamicin-loaded mesoporous silica nanoparticles. Appl. Nanosci. 2018, 8, 1471–1482. [Google Scholar] [CrossRef]
- Hao, N.; Jayawardana, K.W.; Chen, X.; Yan, M. One-Step Synthesis of Amine-Functionalized Hollow Mesoporous Silica Nanoparticles as Efficient Antibacterial and Anticancer Materials. ACS Appl. Mater. Interfaces 2015, 7, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jayawardana, K.W.; Kong, N.; Ren, Y.; Hao, N.; Yan, M.; Ramström, O. Trehalose-Conjugated, Photofunctionalized Mesoporous Silica Nanoparticles for Efficient Delivery of Isoniazid into Mycobacteria. ACS Biomater. Sci. Eng. 2015, 1, 1250–1255. [Google Scholar] [CrossRef]
- Lee, B.Y.; Li, Z.; Clemens, D.L.; Dillon, B.J.; Hwang, A.A.; Zink, J.I.; Horwitz, M.A. Redox-Triggered Release of Moxifloxacin from Mesoporous Silica Nanoparticles Functionalized with Disulfide Snap-Tops Enhances Efficacy Against Pneumonic Tularemia in Mice. Small 2016, 12, 3690–3702. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Pethe, K.; Kim, R.; Ballell, L.; Barros, D.; Cechetto, J.; Jeon, H.; Kim, K.; Garcia-Bennett, A. Encapsulation of Anti-Tuberculosis Drugs within Mesoporous Silica and Intracellular Antibacterial Activities. Nanomaterials 2014, 4, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Clemens, D.L.; Lee, B.-Y.; Dillon, B.J.; Horwitz, M.A.; Zink, J.I. Mesoporous Silica Nanoparticles with pH-Sensitive Nanovalves for Delivery of Moxifloxacin Provide Improved Treatment of Lethal Pneumonic Tularemia. ACS Nano 2015, 9, 10778–10789. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Xiao, Y.; Chen, F.; Xiao, F. A multifunctional nanoplatform based on mesoporous silica nanoparticles for imaging-guided chemo/photodynamic synergetic therapy. RSC Adv. 2017, 7, 31133–31141. [Google Scholar] [CrossRef]
- Karaman, D.S.; Sarwar, S.; Desai, D.; Björk, E.M.; Odén, M.; Chakrabarti, P.; Rosenholm, J.M.; Chakraborti, S. Shape engineering boosts antibacterial activity of chitosan coated mesoporous silica nanoparticle doped with silver: A mechanistic investigation. J. Mater. Chem. B 2016, 4, 3292–3304. [Google Scholar] [CrossRef]
- Koneru, B.; Shi, Y.; Wang, Y.C.; Chavala, S.H.; Miller, M.L.; Holbert, B.; Conson, M.; Ni, A.; Di Pasqua, A.J. Tetracycline-containing MCM-41 mesoporous silica nanoparticles for the treatment of Escherichia coli. Molecules 2015, 20, 19690–19698. [Google Scholar] [CrossRef]
- Sharmiladevi, S.; Priya, A.S.; Sujitha, M.V. Synthesis of mesoporous silica nanoparticles and drug loading for gram positive and gram-negative bacteria. Int. J. Pharm. Pharm. Sci. 2016, 8, 196–201. [Google Scholar]
- Rakhshaei, R.; Namazi, H. A potential bioactive wound dressing based on carboxymethyl cellulose/ZnO impregnated MCM-41 nanocomposite hydrogel. Mater. Sci. Eng. C 2017, 73, 456–464. [Google Scholar] [CrossRef]
- Ehlert, N.; Badar, M.; Christel, A.; Lohmeier, S.J.; Luessenhop, T.; Stieve, M.; Lenarz, T.; Mueller, P.P.; Behrens, P. Mesoporous silica coatings for controlled release of the antibiotic ciprofloxacin from implants. J. Mater. Chem. 2011, 21, 752–760. [Google Scholar] [CrossRef]
- Stanton, M.M.; Park, B.W.; Vilela, D.; Bente, K.; Faivre, D.; Sitti, M.; Sánchez, S. Magnetotactic Bacteria Powered Biohybrids Target E. coli Biofilms. ACS Nano 2017, 11, 9968–9978. [Google Scholar] [CrossRef] [PubMed]
- Mudakavi, R.J.; Vanamali, S.; Chakravortty, D.; Raichur, A.M. Development of arginine based nanocarriers for targeting and treatment of intracellular: Salmonella. RSC Adv. 2017, 7, 7022–7032. [Google Scholar] [CrossRef]
- Mudakavi, R.J.; Raichur, A.M.; Chakravortty, D. Lipid coated mesoporous silica nanoparticles as an oral delivery system for targeting and treatment of intravacuolar Salmonella infections. RSC Adv. 2014, 4, 61160–61166. [Google Scholar] [CrossRef]
- Lensing, R.; Bleich, A.; Smoczek, A.; Glage, S.; Ehlert, N.; Luessenhop, T.; Behrens, P.; Müller, P.P.; Kietzmann, M.; Stieve, M. Efficacy of nanoporous silica coatings on middle ear prostheses as a delivery system for antibiotics: An animal study in rabbits. Acta Biomater. 2013, 9, 4815–4825. [Google Scholar] [CrossRef] [PubMed]
- Ghaith, E.-S.; Connolly, S. Evaluation of mesoporous SBA-15 for the controlled delivery of ciprofloxacin hydrochloride. Bioinspired Biomim. Nanobiomater. 2014, 3, 199–207. [Google Scholar] [CrossRef]
- Skwira, A.; Szewczyk, A.; Prokopowicz, M. The effect of polydimethylsiloxane-ethylcellulose coating blends on the surface characterization and drug release of ciprofloxacin-loaded mesoporous silica. Polymers 2019, 11, 1450. [Google Scholar] [CrossRef]
- Skwira, A.; Szewczyk, A.; Konopacka, A.; Górska, M.; Majda, D.; Sadej, R.; Prokopowicz, M. Silica-polymer composites as the novel antibiotic delivery systems for bone tissue infection. Pharmaceutics 2020, 12, 28. [Google Scholar]
- Korzeniowska, A.; Strzempek, W.; Makowski, W.; Menaszek, E.; Roth, W.J.; Gil, B. Incorporation and release of a model drug, ciprofloxacin, from non-modified SBA-15 molecular sieves with different pore sizes. Microporous Mesoporous Mater. 2020, 294, 109903. [Google Scholar] [CrossRef]
- Andrade, G.F.; Gomide, V.S.; da Silva Júnior, A.C.; Goes, A.M.; de Sousa, E.M.B. An in situ synthesis of mesoporous SBA-16/hydroxyapatite for ciprofloxacin release: In vitro stability and cytocompatibility studies. J. Mater. Sci. Mater. Med. 2014, 25, 2527–2540. [Google Scholar] [CrossRef]
- Bouchoucha, M.; Côté, M.F.; C-Gaudreault, R.; Fortin, M.A.; Kleitz, F. Size-Controlled Functionalized Mesoporous Silica Nanoparticles for Tunable Drug Release and Enhanced Anti-Tumoral Activity. Chem. Mater. 2016, 28, 4243–4258. [Google Scholar] [CrossRef]
- Patra, P.; Mitra, S.; Debnath, N.; Pramanik, P.; Goswami, A. Ciprofloxacin conjugated zinc oxide nanoparticle: A camouflage towards multidrug resistant bacteria. Bull. Mater. Sci. 2014, 37, 199–206. [Google Scholar] [CrossRef]
- Malba, C.; Sudhakaran, U.P.; Borsacchi, S.; Geppi, M.; Enrichi, F.; Natile, M.M.; Armelao, L.; Finotto, T.; Marin, R.; Riello, P.; et al. Structural and photophysical properties of rare-earth complexes encapsulated into surface modified mesoporous silica nanoparticles. Dalt. Trans. 2014, 43, 16183–16196. [Google Scholar] [CrossRef]
- Zawisza, I.; Lachenwitzer, A.; Zamlynny, V.; Horswell, S.L.; Goddard, J.D.; Lipkowski, J. Electrochemical and Photon Polarization Modulation Infrared Reflection Absorption Spectroscopy Study of the Electric Field Driven Transformations of a Phospholipid Bilayer Supported at a Gold Electrode Surface. Biophys. J. 2003, 85, 4055–4075. [Google Scholar] [CrossRef]
- Vieira, L.; Schennach, R.; Gollas, B. In situ PM-IRRAS of a glassy carbon electrode/deep eutectic solvent interface. Phys. Chem. Chem. Phys. 2015, 17, 12870–12880. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Zhao, B.; Chen, X.B.; Birbilis, N.; Yang, H. Effect of water presence on choline chloride-2urea ionic liquid and coating platings from the hydrated ionic liquid. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vaz-Ramos, J.; Cordeiro, R.; Castro, M.M.C.A.; Geraldes, C.F.G.C.; Costa, B.F.O.; Faneca, H.; Durães, L. Supercritically dried superparamagnetic mesoporous silica nanoparticles for cancer theranostics. Mater. Sci. Eng. C 2020, 115, 111124. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Chakraborti, C.K.; Mishra, S.C.; Nanda, U.N.; Naik, S. FTIR and XRD investigations of some fluoroquinolones. Int. J. Pharm. Pharm. Sci. 2011, 3, 165–170. [Google Scholar]
- Hezma, A.M.; Elkhooly, T.A.; El-Bahy, G.S. Fabrication and characterization of bioactive chitosan microspheres incorporated with mesoporous silica nanoparticles for biomedical applications. J. Porous Mater. 2020, 27, 555–562. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Baleizão, C.; Farinha, J.P.S. Functional Group Coverage and Conversion Quantification in Nanostructured Silica by 1H NMR. Anal. Chem. 2017, 89, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W. Adsorption by Powders and Porous Solids; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 1999; ISBN 9780125989206. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Salis, A.; Fanti, M.; Medda, L.; Nairi, V.; Cugia, F.; Piludu, M.; Sogos, V.; Monduzzi, M. Mesoporous Silica Nanoparticles Functionalized with Hyaluronic Acid and Chitosan Biopolymers. Effect of Functionalization on Cell Internalization. ACS Biomater. Sci. Eng. 2016, 2, 741–751. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Popat, A.; Hartono, S.B.; Stahr, F.; Liu, J.; Qiao, S.Z.; Lu, G.Q. Mesoporous silica nanoparticles for bioadsorption, enzyme immobilisation, and delivery carriers. Nanoscale 2011, 3, 2801–2818. [Google Scholar] [CrossRef]




| Sample | ABET (m2 g−1) | Dp (nm) | Vp (cm3 g−1) |
|---|---|---|---|
| MSN-OH | 821 | 3.30 | 0.46 |
| MSN-[Ch][Cl] | 485 | 3.06 | 0.21 |
| MSN-[Ch][Cip] | 56 | - | 0 |
| MSN-APTES | 257 | 3.54 | 0.09 |
| MSN-Cip | 80 | - | 0.01 |
| Nanomaterials | S. aureus | B. subtilis | K. pneumoniae | 3T3 |
|---|---|---|---|---|
| Pristine MSNs | >100,000 | >100,000 | >100,000 | >100,000 |
| MSN-[Ch][Cl] | >100,000 | 25,037 (19,856.0–31,571.0) | >100,000 | >100,000 |
| MSN-[Ch][Cip] | 35.85 (31.95–40.21) | 23.33 (19.79–27.51) | 172.2 (128.6–230.6) | >100,000 |
| MSN-APTES | >100,000 | >100,000 | >100,000 | 9630 (1360–68,150) |
| MSN-Cip | 34.55 (23.98–49.78) | 25.55 (21.74–30.04) | 159.6 (121.8–209.1) | >100,000 |
| Compounds | S. aureus | B. subtilis | K. pneumonia |
|---|---|---|---|
| Cip [9] | 29.16 | 3.84 | 196.5 |
| [Ch][Cip] [9] | 181.4 | 20.60 | 51.12 |
| MSN-[Ch][Cip] | 13.98 | 9.09 | 67.16 |
| MSN-Cip | 20.38 | 15.07 | 94.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Juan Mora, B.; Filipe, L.; Forte, A.; Santos, M.M.; Alves, C.; Teodoro, F.; Pedrosa, R.; Ribeiro Carrott, M.; Branco, L.C.; Gago, S. Boosting Antimicrobial Activity of Ciprofloxacin by Functionalization of Mesoporous Silica Nanoparticles. Pharmaceutics 2021, 13, 218. https://doi.org/10.3390/pharmaceutics13020218
de Juan Mora B, Filipe L, Forte A, Santos MM, Alves C, Teodoro F, Pedrosa R, Ribeiro Carrott M, Branco LC, Gago S. Boosting Antimicrobial Activity of Ciprofloxacin by Functionalization of Mesoporous Silica Nanoparticles. Pharmaceutics. 2021; 13(2):218. https://doi.org/10.3390/pharmaceutics13020218
Chicago/Turabian Stylede Juan Mora, Blanca, Luís Filipe, Andreia Forte, Miguel M. Santos, Celso Alves, Fernando Teodoro, Rui Pedrosa, Manuela Ribeiro Carrott, Luís C. Branco, and Sandra Gago. 2021. "Boosting Antimicrobial Activity of Ciprofloxacin by Functionalization of Mesoporous Silica Nanoparticles" Pharmaceutics 13, no. 2: 218. https://doi.org/10.3390/pharmaceutics13020218
APA Stylede Juan Mora, B., Filipe, L., Forte, A., Santos, M. M., Alves, C., Teodoro, F., Pedrosa, R., Ribeiro Carrott, M., Branco, L. C., & Gago, S. (2021). Boosting Antimicrobial Activity of Ciprofloxacin by Functionalization of Mesoporous Silica Nanoparticles. Pharmaceutics, 13(2), 218. https://doi.org/10.3390/pharmaceutics13020218













